Abstract
Purpose
Previous studies have used near-infrared spectroscopy (NIRS) to measure skeletal muscle mitochondrial capacity. This study tested the hypothesis that NIRS measured mitochondrial capacity would improve with endurance exercise training and decline with detraining.
Methods
Nine, young, participants performed four weeks of progressively increasing endurance exercise training of the wrist flexor muscles followed by approximately five weeks of inactivity. The rate of recovery of muscle oxygen consumption (mVO2) was measured with NIRS every 3-7 days, indicating mitochondrial oxidative capacity.
Results
A linear increase in mitochondrial capacity (NIRS rate constant) was found with a group average of 64 ± 37% improvement after four weeks of exercise training (p < 0.05). Mitochondrial capacity declined exponentially upon cessation of exercise training, with a mean half-time of ~7.7 days.
Conclusion
Both the magnitude and time course of mitochondrial adaptations to exercise training and detraining measured with NIRS was consistent with previous studies using both in vitro and in vivo techniques. These findings show that NIRS based measurements can detect meaningful changes in mitochondrial capacity.
Keywords: NIRS, mitochondrial capacity, oxidative metabolism, endurance training, detraining
Introduction
The effects of exercise training on skeletal muscle mitochondrial oxidative capacity have been well-known for a number of years (21). Skeletal muscle contractile activity initiates several cellular signals that results in increased nuclear and mitochondrial gene transcription, followed by translation into mitochondrial proteins (24). Over time, repeated bouts of exercise results in increased mitochondrial enzyme concentrations and activities (14, 19, 23), which have been termed mitochondrial biogenesis. In contrast, a lack of physical activity, aging, and several pathological conditions are associated with reduced mitochondrial function (28, 29, 31).
Historically, skeletal muscle mitochondrial function has been measured using muscle tissue samples that require surgical removal (21-23). The concentration and activity levels of key mitochondrial enzymes such as citrate synthase or succinate dehydrogenase are commonly used as a measure of mitochondrial function (14, 19, 23). A major limitation of these assays is that mitochondrial function is inferred from a very small amount of tissue and the evaluation of a single mitochondrial enzyme. High-resolution respirometry can provide more information about the specific function of the various complexes in the electron transport chain, but the isolated tissue used is subjected to non-physiological conditions (i.e. higher oxygen concentrations). Non-invasive assessments of mitochondrial function have advantages for testing human subjects. In vivo techniques allow for repeated measurements with little, if any, discomfort while circulatory and other regulatory systems remain intact. Phosphorus magnetic resonance spectroscopy (31P-MRS) is the most commonly used in vivo technique for assessing mitochondrial function (6).
Recent advances in optical spectroscopy have led to improved optical devices and applications for studying muscle physiology (12). Near-infrared spectroscopy (NIRS) has been used to measure various aspects of muscle physiology including: muscle blood flow and perfusion (10), muscle oxygen consumption (9, 10, 33), and muscle oxygenation (1, 15). The recovery of muscle oxygen consumption (mVO2) after exercise, measured with NIRS, has been used as an index of skeletal muscle oxidative capacity (5, 36). Recently, this method has been improved by correcting for the small changes in observed heme concentrations that often occur during these measurements (38). Recent studies have shown the approach to be reproducible, and that the increase in muscle metabolic rate needed for the study can be produced either voluntary or electrical stimulation (37). Furthermore, a recent study from our lab demonstrated that NIRS measured skeletal muscle mitochondrial capacity of endurance-trained cyclists was higher than sedentary control subjects, and that the relative magnitude of difference in mitochondrial capacity was similar to more established techniques (4).
The purpose of this study was to use NIRS measurements of the recovery rate of mVO2 after exercise to measure changes in skeletal muscle mitochondrial capacity induced by endurance exercise training and detraining. It was hypothesized that mitochondrial capacity would increase with endurance exercise training, and would return to baseline with detraining.
Materials and Methods
Participants
Nine healthy college-aged men and women volunteered to participate in this study (5M/4F; Age = 23 ± 2.3 yr; Height = 172.4 ± 9.8 cm; Weight = 63.4 ± 12.5 kg). Participants were included if they had not been diagnosed with any chronic disease known to influence muscle metabolism, or were not taking medications that could alter muscle mitochondrial function, or if they were not currently performing forearm exercise training more than one day per week. The study was conducted with the approval of the Institutional Review Board at the University of Georgia (Athens, GA), and was carried out in accordance with the Declaration of Helsinki (2008). All participants gave written, informed consent before testing.
Experimental Design
This was a longitudinal study design where participants performed four weeks of unilateral wrist flexor exercise of the non-dominant arm. The wrist flexor muscles were chosen because they are not involved in locomotion and should be reasonably untrained in comparison to the musculature of the thigh or calf. The dominant arm was not trained and served as the control arm. NIRS measurements of mitochondrial capacity were made every 3-7 days throughout the entire length of the study. Maximal voluntary isometric contractions (MVIC) were performed once per week to determine the appropriate weight for exercise training and testing (~30% MVIC).
Wrist flexion exercise was performed 5 days per week for four weeks (20 total sessions) of the non-dominant arm only. Each session consisted of continuous wrist flexion exercise for 30 minutes. Participants performed the exercise on a padded, flat surface with the elbow at 90 degrees of flexion. Gloves were provided to prevent any discomfort to the hands or skin. Participants trained with dumbbell weights adjusted to ~30% MVIC. Progressive increases in the contraction frequency occurred as tolerated, with the goal of inducing the largest change in mitochondrial capacity. Participants began training with a contraction frequency of 0.3 – 0.5 Hz (600 - 900 contractions per session) and increases to 1.0 - 1.2 Hz (1800 - 2160 contractions per session). During the final one minute of the each exercise training session, participants performed a high-intensity “sprint”, which consisted of performing wrist flexions at a maximal rate. This one-minute period was included in an attempt to maximize the stimulus for mitochondrial biogenesis (11). Following the 20th session of exercise, participants were instructed to not perform any forearm exercise for the remaining duration of the study.
Experimental Procedures
NIRS testing was performed on both the experimental (training) and control arm every 3-7 days throughout both the training and detraining portions of the study. Each participant was placed supine, on a padded table with the tested arm extended (90 degrees from the body). For each testing session, the NIRS protocol was performed on both the control and experimental arm, which last approximately 45 minutes. The NIRS probe was placed over the superficial wrist flexor muscles (flexor carpi radialis, palmaris longus, and flexor carpi ulnaris) approximately 2-3 cm distal to the medial epicondyle of the humerus. A blood pressure cuff (Hokanson SC5, Bellevue, WA) was placed proximally to the elbow joint, and was attached to rapid cuff-inflation system (Hokanson E20 cuff inflator, Bellevue, WA) powered by a 30-gallon commercial air compressor (Husky VT6315, Kenosha, WI).
NIRS signals were obtained using a continuous wave NIRS device (Oxymon MK III, Artinis Medical Systems, The Netherlands), which consisted of 2 channels (2 equivalent pulsed light sources, 2 avalanche photodiode detectors, shielding from ambient light), uses intensity-modulated light at a frequency of 1 MHz and laser diodes at 3 wavelengths (905, 850, and 770 nm) corresponding to the absorption wavelengths of oxyhemoglobin (O2Hb) and deoxyhemoglobin (HHb), with an autosensing power supply (approximately 40 W at 110-240 V). The probe was set for one source-detector separation distance after the measurement of adipose tissue thickness. The source-detector distance was set to the closest available distance (choices available were 25, 30, 35, 40, 45, and 50 mm) that was at least twice the adipose tissue thickness. Adipose tissue thickness (ATT) was measured at the site of the NIRS probe using B-mode ultrasound (LOGIQe; GE HealthCare, USA). NIRS data was collected at 10 Hz. NIRS signals that represent oxygenated (O2Hb) and deoxygenated (HHb) hemoglobin/myoglobin were corrected for blood volume changes as previously described (38). Once corrected, the Hbdifference signal was calculated from the difference of O2Hb and HHb, which effectively increases the signal to noise ratio by a factor of two.
NIRS Measurements
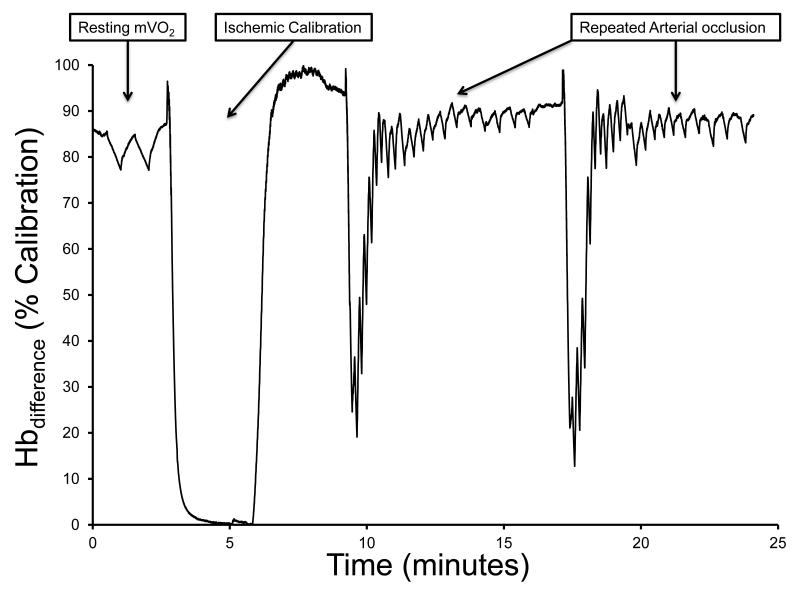
The NIRS protocol used was based on a previous study (4). All NIRS measurements were made using the calculated Hbdifference signal (difference between O2Hb and HHB, after correction for blood volume shifts). Resting muscle oxygen consumption (mVO2) was measured as the decline in muscle oxygenation (Hbdifference signal) during inflation of a blood pressure cuff to 250 – 300 mmHg. Two resting measurements were made using 30 seconds of arterial occlusion. Resting mVO2 was calculated using simple linear regression with the first 20 seconds of each occlusion (200 data points). Following the resting measurements, mitochondrial capacity was measured as the rate of recovery of mVO2 after voluntary wrist flexion exercise. Short duration (~10 seconds) wrist flexion exercise (30% MVIC) was used to increase mVO2. A series of short duration arterial occlusions was performed immediately following the exercise. The cuff protocol is as follows: cuffs 1-10 = 3 seconds on, 3 seconds off; cuffs 11-15 = 7 seconds on, 7 seconds off; cuffs 16-20 = 10 seconds on, 10 seconds off; cuffs 21+ = 10 seconds on, 20 seconds off. This cuffing protocol was designed to optimize our ability to characterize the recovery of mVO2 while minimizing any discomfort to the participants. The exercise/cuff protocol was performed twice and the two tests were averaged. An ischemia/hyperemia calibration was used to normalize NIRS signals as previously described (37). Briefly, 5-seconds of voluntary wrist flexion exercise was performed, followed by inflation of the blood pressure cuff to 250 – 300 mmHg for 3-6 minutes (until the NIRS signals plateau). Upon release of the cuff, a 1-3 minutes period of hyperemia occurs. This calibration was used to scale the NIRS signals to this ‘physiological’ range.
Calculation of Muscle Oxygen Consumption
mVO2 was calculated as the slope of change in Hbdifference signal during the arterial occlusion using simple linear regression. The post-exercise repeated measurements of mVO2 were fit to a mono-exponential curve according to the formula below:
| (Equation 1) |
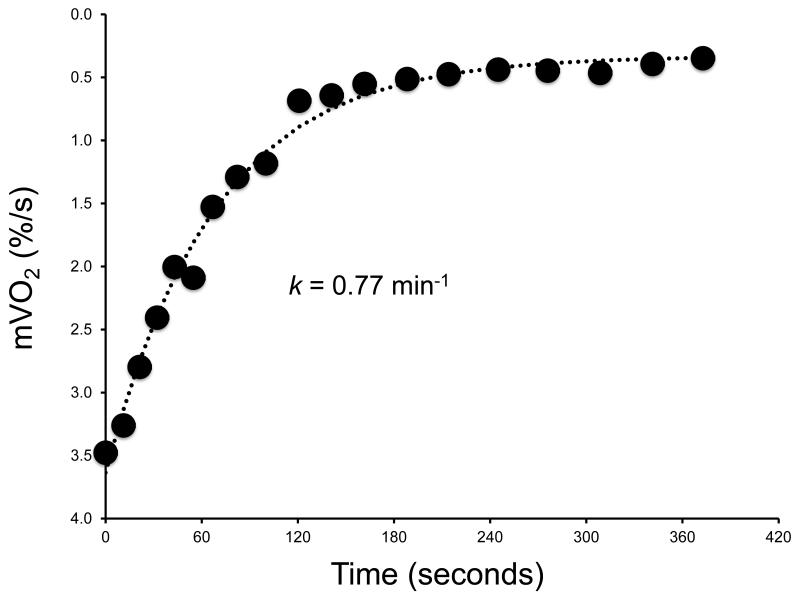
For this equation, y represents relative mVO2 during the arterial occlusion, End is the mVO2 immediately after the cessation of exercise, Delta is the change in mVO2 from rest to end exercise, t is time, and k is the fitting rate constant. The recovery rate constant (k) of mVO2 after exercise is proportional to the maximal oxidative capacity. We choose to report the NIRS rate constant because this value is directly proportional to mitochondrial capacity. Time constants can be calculated as 1/rate constant.
Statistical Analysis
Data are presented as means ± SD. Statistical analyses were performed using SPSS 19.0 (IBM®, Armonk, NY). A two-way mixed model ANOVA with a within-subjects factor (time) and between-subjects factor (control arm vs. training arm) was performed on the NIRS rate constants. When a significant interaction effect was found, a post hoc analysis was performed using pairwise comparisons of the main effect (time) with a Bonferroni adjustment. An A Priori power calculation was performed using G*Power 3 (Heinrich Heine, Düsseldorf, Germany) and yielded a total sample size of 6 based on the interaction term for repeated ANOVA with a 20% improvement in mitochondrial capacity, α = 0.05, and power (1−β) = 0.8.
Results
All participants completed the testing and exercise training without any adverse events. The physical characteristics of the participants in this study are shown in Table 1. All participants increased the number of contractions performed throughout the training portion of the study (Session 1 ~ 800 ± 160 contractions, Session 20 ~ 1800 ± 130 contractions; p < 0.001). Weekly training progression for the group is shown in Table 2. Resting mVO2 was not altered by training (p = 0.790) and was not different between control and training arms (0.29 ± 0.03 vs. 0.31 ± 0.02 %/s, p = 0.461). MVIC did not change over time in either the control arm (p = 0.833) or the training arm (p = 0.537).
Table 1.
Physical characteristics of the participants.
| Height | Weight | Age | ATT (mm) | ||
|---|---|---|---|---|---|
| (cm) | (kg) | (yr) | Control Arm | Training Arm | |
| Males (n = 5) | 178.8 ± 7.3 | 72.3 ± 5.3 | 24.0 ± 2.1 | 3.4 ± 0.4 | 3.4 ± 0.5 |
| Females (n = 4) | 164.5 ± 5.6 | 52.3 ± 9.2 | 21.8 ± 2.2 | 5.2 ± 3.1 | 5.1 ± 3.2 |
|
| |||||
| Total (n = 9) | 172.4 ± 9.8 | 63.4 ± 12.5 | 23.0 ± 2.3 | 4.2 ± 2.1 | 4.2 ± 2.2 |
Note: Data presented as means ± SD. Statistical comparisons between genders were not performed.
Table 2.
Weekly progression in training intensity.
| Week 1 | Week 2 | Week 3 | Week4 | |
|---|---|---|---|---|
|
Number of Contractions
(n=9) |
4200 ± 350 | 5300±450 | 6500±300 | 8400 ± 600 |
Note: Data are presented as means ± SD. The number of contractions performed per week (5 sessions of exercise; each session 30 minutes) was increased throughout the training by increasing the contraction frequency as tolerated.
NIRS Mitochondrial Capacity
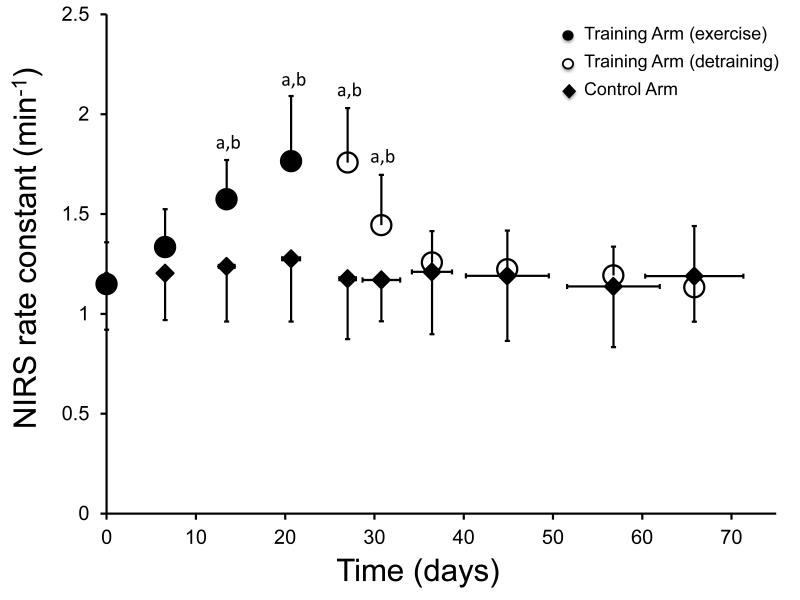
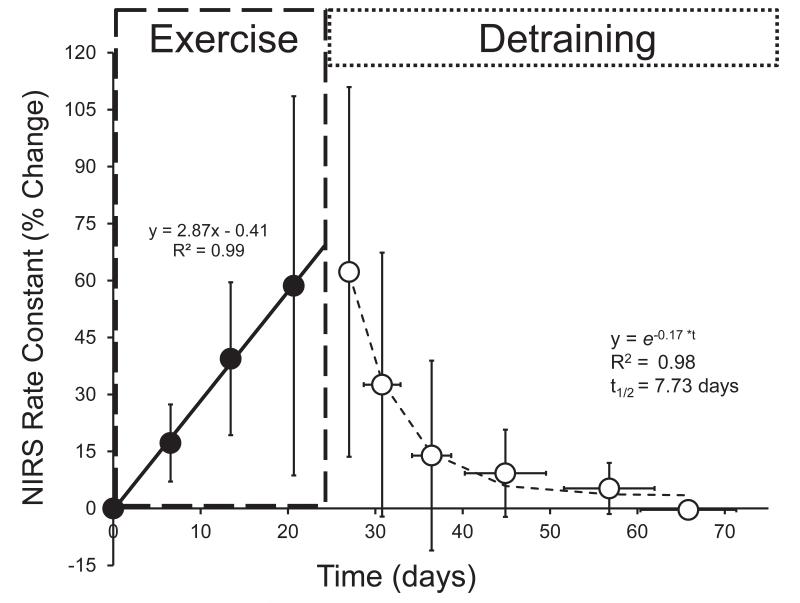
Representative NIRS raw data and the recovery kinetics of mVO2 are shown in Figure 1a and 1b for descriptive purposes. All participants showed improvements in skeletal muscle oxidative capacity in the endurance trained wrist flexors, as indicated by an increase in the rate constant (k) for the recovery of mVO2 (p < 0.001) (Figure 2). There was no change in the rate constant (k) in the control arm (p = 0.757) (Figure 2). The mean coefficient of variation for the NIRS rate constant in the control arm was 10.4% (range = 6 – 16%). A two-way ANOVA found a main effect of group, F(1,160) = 22.7, p < 0.001, indicating that the trained and control arm NIRS rate constants were different. There was also a significant main effect for time, F(9,160) = 4.54, p < 0.001, indicating that the NIRS rate constants changed over time. The interaction effect (time*group) was also significant F(9,160) = 3.085, p = 0.002. Post-hoc analysis of the interaction was performed using pairwise comparisons (control arm vs. training arm) with a Bonferroni correction. These pairwise comparisons found significant differences (p < 0.05) in the NIRS rate constants between the control arm and training arm at the following time points: 3-6; as well as a significant differences (p < 0.05) between time points 3-6 and baseline (time = 0) for the training arm only (Figure 2). We also found that the initial (baseline) NIRS rate constant was not different between the dominant and non-dominant arms (1.17 ± 0.23 vs. 1.15 ± 0.21, p = 0.717). The normalized changes in mitochondrial capacity (percent change from baseline) are shown in Figure 3. We found a wide range of improvement in mitochondrial capacity in this study (31 – 151 %). Because of this wide range in responses to training protocol, we conducted a regression analysis to determine the relationship between the improvement (percent change) and the initial mitochondrial capacity (rate constant at baseline). This relationship was significant [F(1,8) = 7.447, p = 0.029, r = −0.718]. The initial (end-exercise) mVO2 also increased with exercise training. The initial mVO2 from the first testing session was 4.45 ± 1.88 %/s and peaked at 6.67 ± 1.34 %/s in testing session 5 (p = 0.09).
Figure 1.
A representative testing protocol showing the NIRS Hbdifference signal during resting measurements of mVO2, an ischemic calibration, and two exercise/recovery trials for the measurement of post-exercise kinetics for mVO2 is shown in panel A. Each arterial occlusion is fit to a mono-exponential function and the rate constant (proportional to mitochondrial oxidative capacity) is calculated (Panel B).
Figure 2.
NIRS rate constants (k) for the recovery of mVO2 during exercise training (closed circles) and detraining (open circles). The control arm (closed triangles) did not perform exercise training. aRate constants were statistically different between the training and control arms (all points p < 0.05). bRate constants from the training arm were significantly different from the baseline (time = 0 days) at testing sessions 3-6 (all points p < 0.05).
Figure 3.
Percentage change in NIRS rate constant (proportional to mitochondrial capacity) from baseline (time = 0 days) of the training arm. Data from all participants were averaged prior to the calculation of fitting parameters. Data from the training portion were characterized using simple linear regression. Detraining data were fit to a monoexponential decay function and half-times were calculated (t1/2). Data are presented as the mean ± SD (error bars).
We also calculated rates of adaptation and de-adaptation in mitochondrial oxidative capacity using the NIRS data. During the exercise training, the mean change in mitochondrial capacity increase linearly over time reaching a peak improvement of approximately 64 ± 37% measured ~48-72 hours after the last training session. Individual training and detraining responses are shown in Table 3. Pooled training and detraining responses were characterized with linear regression and monoexponential decay functions respectively (Figure 3). A rapid decline in mitochondrial capacity occurred during the detraining portion of the study, which was well-characterized by a monoexponential decay (Figure 3). The calculated half-time for the decay in the NIRS rate constant was 7.7 days. There was no significant difference between the starting mitochondrial capacity and mitochondrial capacity after five weeks of detraining (p = 1.000).
Table 3.
Individual responses to exercise training and detraining
| Training Response (% Change) (linear regression) |
Detraining Response (% Change) (exponential decay) |
||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Participant Number |
Slope (m) |
Intercept (b) |
Magnitude (%) |
R2 | kdecay | Half-time (days) |
R2 |
| 1 | 8.8 | −18.0 | 151 | 0.90 | 0.06 | 14.4 | 0.97 |
| 2 | 3.0 | −1.1 | 100 | 0.86 | 0.21 | 6.2 | 0.99 |
| 3 | 1.8 | 6.7 | 53 | 0.80 | 0.09 | 9.9 | 0.86 |
| 4 | 2.5 | −0.6 | 52 | 0.99 | 0.17 | 6.9 | 0.99 |
| 5 | 2.1 | −3.8 | 57 | 0.98 | 0.11 | 9.2 | 0.98 |
| 6 | 2.8 | 7.3 | 63 | 0.82 | 0.16 | 7.2 | 0.77 |
| 7 | 1.6 | −3.4 | 31 | 0.94 | 0.16 | 7.2 | 0.80 |
| 8 | 2.1 | −2.9 | 48 | 0.86 | 0.17 | 6.9 | 0.92 |
| 9 | 2.1 | −1.5 | 40 | 0.97 | 0.19 | 6.5 | 0.85 |
|
| |||||||
| MEAN | 2.9 | −1.9 | 66 | 0.90 | 0.15 | 8.3 | 0.90 |
| SD | 2.2 | 7.3 | 37 | 0.07 | 0.05 | 2.6 | 0.09 |
Values presented are for each individual participant. Training responses (% change in NIRS rate constant) were fit using simple linear regression. Detraining responses (% change in NIRS rate constant) were fit using a mono-exponential decay. Half-times were calculated from the decay rate constant (kdecay) and the time delay between the first detraining measurement and the last exercise session (~2-3 days).
Discussion
This study found that mitochondrial capacity measured by NIRS improved with exercise endurance training and returned to baseline values with detraining. We are not aware of previous studies that have used the rate constant (k) for the recovery of mVO2 measured with NIRS to assess training and detrained in skeletal muscle. The magnitude and rate of adaptation in mitochondrial capacity found in this study are in agreement with previous studies which used in vitro measurements from muscle biopsies (14, 16, 18, 19, 21, 39) and in vivo measurements of phosphocreatine resynthesis (13, 35). Moreover, the time course of de-adaptation is also consistent with previous studies (2, 7, 20, 27, 30, 35). The ability to detect training and detrained induced changes support the validity of NIRS based measurements as a technique for assessing mitochondrial oxidative capacity.
In this study, we report a 64 ± 37% increase in mitochondrial oxidative capacity (indicated by the rate constant for the recovery of mVO2) in response to four weeks of endurance exercise training of the wrist flexor muscles. It is difficult to compare the relative magnitudes of increase in mitochondrial capacity to previous studies due to methodological differences in both the measurement of mitochondrial function and the exercise training protocols. However, the magnitude of change is within the expected range from previous studies. For example, Gollnick et al. (14) reported a 95% increase in succinate dehydrogenase (SDH) activity after 5 months of cycling exercise training in the vastus lateralis muscle. After 2 months of unilateral cycling exercise training, Henriksson et al. (19) reported a 27% increase in SDH activity in the vastus lateralis muscle. Shorter duration exercise training also causes increased mitochondrial function. Spina et al. (39) found that 7-10 days of endurance cycling exercise (~2 hours per day) increased citrate synthase (CS) concentrations by approximately 30% in the vastus lateralis muscle. A similar study by Green and colleagues (18) was published a few years earlier. The authors of this study reported that 10-12 days of endurance exercise increased SDH and CS activities by 14% and 23% respectively in the vastus lateralis muscle, although these changes were not considered statistically significant. Exercise training-induced increases in mitochondrial capacity have also been reported using the in vivo 31P-MRS (13, 34, 35).
Participants in the current study performed continuous wrist flexion exercise for 30 minutes per day, five days per week, for four weeks. Progressive increases in the exercise intensity were made as tolerated by the participants (~2 ¼ increase in contraction number per training session by the end of the study). The short duration of training, in combination with the increasing stimulus, resulted in a linear increase in mitochondrial capacity during the training portion. This relationship is not unexpected as Green et al. (17) found a linear increase in SDH activity through six weeks of cycling exercise training. Previous studies have suggested that a fixed and unchanging training stimulus might result in a first-order increase in oxidative capacity with a similar rates of adaptation and de-adaptation, which would level off given the appropriate training duration (2, 11, 40).
The participants in this study had a wide range of responses to the exercise training (31% - 151% improvement). The heterogeneity in responses to exercise training was consistent with previous studies suggesting genetic influences on training responses (3). While our study was not designed to determine the mechanisms behind this wide range, several factors could influence the magnitude of change in mitochondrial capacity. We did find a statistically significant relationship between the baseline mitochondrial capacity (NIRS rate constant) and the percent improvement, which accounted for ~50% of the variance in the percent improvement. This suggests that greater improvements were seen in the participants with the lowest rate constant at baseline. Another potential factor could be differences in the training intensity between participants (11). There were small differences in the number of contractions performed between participants, but all participants increased the number of contractions performed. We did not find a statistically significant relationship between the magnitude of improvement and either the number of contractions performed or the rate of increase in training intensity (i.e. rate of increase in the number of contractions). It is possible that the difference in training intensity between participants was not large enough to detect an effect on the magnitude of adaptation. In addition, there are numerous wrist flexor muscles capable of contributing to the exercise, thus the motor unit recruit patterns (of the superficial wrist flexor muscles where the NIRS device was placed) during training are unknown. We also found no gender differences in the magnitude of improvement in mitochondrial oxidative capacity, consistent with previous reports (32).
Skeletal muscle mitochondrial capacity declined rapidly after the cessation of exercise training. We characterized this decline by fitting the group data to a monoexponential decay function, and calculated the half-time to be 7.7 days. All participants roughly followed this time course, given the expected variation in NIRS rate constants (~10-15%). Both the exponential pattern, and the rate of decline in mitochondrial capacity are consistent with previous studies using in vitro techniques. Booth and Holloszy (2) reported half-times for the turnover of cytochrome c in fast and slow rat muscles were 7 and 8 days respectively. This rapid decline in mitochondrial enzyme concentration/activity in human skeletal muscle has been shown to be somewhere between 2 and 6 weeks (7, 20, 25-27, 30).
In the current study, NIRS was used to measure the rate of recovery of muscle oxygen consumption after exercise. The rate constant for the recovery is proportional to mitochondrial oxidative capacity, such that higher rate constants are related to higher maximal rates of oxygen consumption (i.e. State 3 respiration). The relative contributions of myoglobin and hemoglobin to the NIRS signal remains controversial (8). However, with the NIRS device used in the current study (as well as all commercially available NIRS devices), separation of the hemoglobin and myoglobin absorption spectra is not possible. Future studies are needed to determine whether the contributions of hemoglobin and myoglobin influence the current measurements, but these studies will require a device that can separate the absorption spectra of these two heme sources.
In conclusion, this study reports changes in skeletal muscle mitochondrial oxidative capacity in response to endurance exercise training and detraining measured in the wrist flexor muscles with near-infrared spectroscopy (NIRS). Both the magnitude and time course of changes in mitochondrial capacity were consistent with previous studies using both in vitro and in vivo methods. NIRS based assessments of mitochondrial capacity have previously been shown to be reproducible, independent of the type or magnitude of exercise needed to perform the measurements, and to detect differences in training status in cross-sectional studies. In addition, the NIRS measurements are inexpensive, portable, and easy to perform. Future studies could employ NIRS to examine mitochondrial capacity in both research and clinical settings, especially when repeated-measures on human participants are of interest.
Acknowledgements
The authors would like to thank the participants of this study for their enthusiastic commitment to this study.
The results of the present study do not constitute endorsement by the American College of Sports Medicine.
Disclosure of Funding
This study was funded in part by National Institutes of Health Grant (HD-039676) and the University of Georgia Graduate School intramural grants.
Footnotes
Conflict of interest statement
The authors report no conflicts of interest.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boone J, Koppo K, Barstow TJ, Bouckaert J. Pattern of deoxy[Hb+Mb] during ramp cycle exercise: influence of aerobic fitness status. European Journal Of Applied Physiology. 2009;105:851–859. doi: 10.1007/s00421-008-0969-2. [DOI] [PubMed] [Google Scholar]
- 2.Booth FW, Holloszy JO. Cytochrome c turnover in rat skeletal muscles. J Biol Chem. 1977;252:416–419. [PubMed] [Google Scholar]
- 3.Bouchard C, Rankinen T. Individual differences in response to regular physical activity. Med Sci Sports Exerc. 2001;33:S446–451. doi: 10.1097/00005768-200106001-00013. discussion S452-443. [DOI] [PubMed] [Google Scholar]
- 4.Brizendine JT, Ryan TE, Larson RD, McCully KK. Skeletal Muscle Metabolism in Endurance Athletes with Near-Infrared Spectroscopy. Med Sci Sports Exerc. 2013;45(5):869–75. doi: 10.1249/MSS.0b013e31827e0eb6. [DOI] [PubMed] [Google Scholar]
- 5.Buchheit M, Ufland P, Haydar B, Laursen PB, Ahmaidi S. Reproducibility and sensitivity of muscle reoxygenation and oxygen uptake recovery kinetics following running exercise in the field. Clin Physiol Funct Imaging. 2011;31:337–346. doi: 10.1111/j.1475-097X.2011.01020.x. [DOI] [PubMed] [Google Scholar]
- 6.Chance B, Im J, Nioka S, Kushmerick M. Skeletal muscle energetics with PNMR: personal views and historic perspectives. NMR In Biomedicine. 2006;19:904–926. doi: 10.1002/nbm.1109. [DOI] [PubMed] [Google Scholar]
- 7.Chi MM, Hintz CS, Coyle EF, Martin WH, 3rd, Ivy JL, Nemeth PM, Holloszy JO, Lowry OH. Effects of detraining on enzymes of energy metabolism in individual human muscle fibers. Am J Physiol. 1983;244:C276–287. doi: 10.1152/ajpcell.1983.244.3.C276. [DOI] [PubMed] [Google Scholar]
- 8.Davis ML, Barstow TJ. Estimated contribution of hemoglobin and myoglobin to near infrared spectroscopy. Respir Physiol Neurobiol. 2013;186:180–187. doi: 10.1016/j.resp.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 9.De Blasi RA, Almenrader N, Aurisicchio P, Ferrari M. Comparison of two methods of measuring forearm oxygen consumption (mVO2) by near infrared spectroscopy. Journal Of Biomedical Optics. 1997;2:171–175. doi: 10.1117/12.269893. [DOI] [PubMed] [Google Scholar]
- 10.Deblasi RA, Ferrari M, Natali A, Conti G, Mega A, Gasparetto A. Noninvasive Measurement of Forearm Blood-Flow and Oxygen-Consumption by near-Infrared Spectroscopy. Journal of Applied Physiology. 1994;76:1388–1393. doi: 10.1152/jappl.1994.76.3.1388. [DOI] [PubMed] [Google Scholar]
- 11.Dudley GA, Abraham WM, Terjung RL. Influence of exercise intensity and duration on biochemical adaptations in skeletal muscle. J Appl Physiol. 1982;53:844–850. doi: 10.1152/jappl.1982.53.4.844. [DOI] [PubMed] [Google Scholar]
- 12.Ferrari M, Mathalib M, Quaresima V. The use of near-infrared spectroscopy in understanding skeletal muscle physiology: recent developments. Philos Transact A Math Phys Eng Sci. 2011;369:4577–4590. doi: 10.1098/rsta.2011.0230. [DOI] [PubMed] [Google Scholar]
- 13.Forbes SC, Slade JM, Meyer RA. Short-term high-intensity interval training improves phosphocreatine recovery kinetics following moderate-intensity exercise in humans. Applied Physiology, Nutrition, And Metabolism = Physiologie Appliquée, Nutrition Et Métabolisme. 2008;33:1124–1131. doi: 10.1139/H08-099. [DOI] [PubMed] [Google Scholar]
- 14.Gollnick PD, Armstrong RB, Saltin B, Saubert CWt, Sembrowich WL, Shepherd RE. Effect of training on enzyme activity and fiber composition of human skeletal muscle. J Appl Physiol. 1973;34:107–111. doi: 10.1152/jappl.1973.34.1.107. [DOI] [PubMed] [Google Scholar]
- 15.Grassi B, Pogliaghi S, Rampichini S, Quaresima V, Ferrari M, Marconi C, Cerretelli P. Muscle oxygenation and pulmonary gas exchange kinetics during cycling exercise on-transitions in humans. Journal of Applied Physiology. 2003;95:149–158. doi: 10.1152/japplphysiol.00695.2002. [DOI] [PubMed] [Google Scholar]
- 16.Green HJ, Helyar R, Ball-Burnett M, Kowalchuk N, Symon S, Farrance B. Metabolic adaptations to training precede changes in muscle mitochondrial capacity. J Appl Physiol. 1992;72:484–491. doi: 10.1152/jappl.1992.72.2.484. [DOI] [PubMed] [Google Scholar]
- 17.Green HJ, Jones S, Ball-Burnett M, Farrance B, Ranney D. Adaptations in muscle metabolism to prolonged voluntary exercise and training. J Appl Physiol. 1995;78:138–145. doi: 10.1152/jappl.1995.78.1.138. [DOI] [PubMed] [Google Scholar]
- 18.Green HJ, Jones S, Ball-Burnett ME, Smith D, Livesey J, Farrance BW. Early muscular and metabolic adaptations to prolonged exercise training in humans. J Appl Physiol. 1991;70:2032–2038. doi: 10.1152/jappl.1991.70.5.2032. [DOI] [PubMed] [Google Scholar]
- 19.Henriksson J. Training induced adaptation of skeletal muscle and metabolism during submaximal exercise. J Physiol. 1977;270:661–675. doi: 10.1113/jphysiol.1977.sp011974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henriksson J, Reitman JS. Time course of changes in human skeletal muscle succinate dehydrogenase and cytochrome oxidase activities and maximal oxygen uptake with physical activity and inactivity. Acta Physiol Scand. 1977;99:91–97. doi: 10.1111/j.1748-1716.1977.tb10356.x. [DOI] [PubMed] [Google Scholar]
- 21.Holloszy JO. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem. 1967;242:2278–2282. [PubMed] [Google Scholar]
- 22.Holloszy JO, Booth FW. Biochemical adaptations to endurance exercise in muscle. Annu Rev Physiol. 1976;38:273–291. doi: 10.1146/annurev.ph.38.030176.001421. [DOI] [PubMed] [Google Scholar]
- 23.Holloszy JO, Oscai LB, Don IJ, Mole PA. Mitochondrial citric acid cycle and related enzymes: adaptive response to exercise. Biochem Biophys Res Commun. 1970;40:1368–1373. doi: 10.1016/0006-291x(70)90017-3. [DOI] [PubMed] [Google Scholar]
- 24.Hood DA. Mechanisms of exercise-induced mitochondrial biogenesis in skeletal muscle. Appl Physiol Nutr Metab. 2009;34:465–472. doi: 10.1139/H09-045. [DOI] [PubMed] [Google Scholar]
- 25.Houmard JA, Hortobagyi T, Johns RA, Bruno NJ, Nute CC, Shinebarger MH, Welborn JW. Effect of short-term training cessation on performance measures in distance runners. Int J Sports Med. 1992;13:572–576. doi: 10.1055/s-2007-1024567. [DOI] [PubMed] [Google Scholar]
- 26.Houmard JA, Hortobagyi T, Neufer PD, Johns RA, Fraser DD, Israel RG, Dohm GL. Training cessation does not alter GLUT-4 protein levels in human skeletal muscle. J Appl Physiol. 1993;74:776–781. doi: 10.1152/jappl.1993.74.2.776. [DOI] [PubMed] [Google Scholar]
- 27.Houston ME, Bentzen H, Larsen H. Interrelationships between skeletal muscle adaptations and performance as studied by detraining and retraining. Acta Physiol Scand. 1979;105:163–170. doi: 10.1111/j.1748-1716.1979.tb06328.x. [DOI] [PubMed] [Google Scholar]
- 28.Joseph AM, Joanisse DR, Baillot RG, Hood DA. Mitochondrial dysregulation in the pathogenesis of diabetes: potential for mitochondrial biogenesis-mediated interventions. Exp Diabetes Res. 2012;2012:642038. doi: 10.1155/2012/642038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.KENT-BRAUN JA, MILLER RG, WEINER MW. Human Skeletal Muscle Metabolism in Health and Disease: Utility of Magnetic Resonance Spectroscopy. Exercise and Sport Sciences Reviews. 1995;23:305–348. [PubMed] [Google Scholar]
- 30.Klausen K, Andersen LB, Pelle I. Adaptive changes in work capacity, skeletal muscle capillarization and enzyme levels during training and detraining. Acta Physiol Scand. 1981;113:9–16. doi: 10.1111/j.1748-1716.1981.tb06854.x. [DOI] [PubMed] [Google Scholar]
- 31.Lanza IR, Nair KS. Muscle mitochondrial changes with aging and exercise. Am J Clin Nutr. 2009;89:467S–471S. doi: 10.3945/ajcn.2008.26717D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis DA, Kamon E, Hodgson JL. Physiological differences between genders. Implications for sports conditioning. Sports Med. 1986;3:357–369. doi: 10.2165/00007256-198603050-00005. [DOI] [PubMed] [Google Scholar]
- 33.Malagoni AM, Felisatti M, Mandini S, Mascoli F, Manfredini R, Basaglia N, Zamboni P, Manfredini F. Resting muscle oxygen consumption by near-infrared spectroscopy in peripheral arterial disease: A parameter to be considered in a clinical setting? Angiology. 2010;61:530–536. doi: 10.1177/0003319710362975. [DOI] [PubMed] [Google Scholar]
- 34.McCully K, Posner J. Measuring exercise-induced adaptations and injury with magnetic resonance spectroscopy. International Journal of Sports Medicine. 1992;13(Suppl 1):S147–S149. doi: 10.1055/s-2007-1024621. [DOI] [PubMed] [Google Scholar]
- 35.Mccully KK, Kakihira H, Vandenborne K, Kentbraun J. Noninvasive Measurements of Activity-Induced Changes in Muscle Metabolism. J Biomech. 1991;24:153–161. doi: 10.1016/0021-9290(91)90385-z. [DOI] [PubMed] [Google Scholar]
- 36.Motobe M, Murase N, Osada T, Homma T, Ueda C, Nagasawa T, Kitahara A, Ichimura S, Kurosawa Y, Katsumura T, Hoshika A, Hamaoka T. Noninvasive monitoring of deterioration in skeletal muscle function with forearm cast immobilization and the prevention of deterioration. Dyn Med. 2004;3:2. doi: 10.1186/1476-5918-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ryan TE, Brizendine JT, McCully KK. A comparison of exercise type and intensity on the noninvasive assessment of skeletal muscle mitochondrial function using near-infrared spectroscopy. J Appl Physiol. 2013;114:230–237. doi: 10.1152/japplphysiol.01043.2012. [DOI] [PubMed] [Google Scholar]
- 38.Ryan TE, Erickson ML, Brizendine JT, Young HJ, McCully KK. Noninvasive evaluation of skeletal muscle mitochondrial capacity with near-infrared spectroscopy: correcting for blood volume changes. Journal of Applied Physiology. 2012;113:175–183. doi: 10.1152/japplphysiol.00319.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spina RJ, Chi MM, Hopkins MG, Nemeth PM, Lowry OH, Holloszy JO. Mitochondrial enzymes increase in muscle in response to 7-10 days of cycle exercise. J Appl Physiol. 1996;80:2250–2254. doi: 10.1152/jappl.1996.80.6.2250. [DOI] [PubMed] [Google Scholar]
- 40.Terjung RL. The turnover of cytochrome c in different skeletal-muscle fibre types of the rat. Biochem J. 1979;178:569–574. doi: 10.1042/bj1780569. [DOI] [PMC free article] [PubMed] [Google Scholar]