Abstract
Background
The impact of physical activity on survival outcomes of recurrent colon cancer has not been studied. We tested the association between the level of post-diagnosis physical activity and survival outcome of patients with recurrent colon cancer.
Materials and Methods
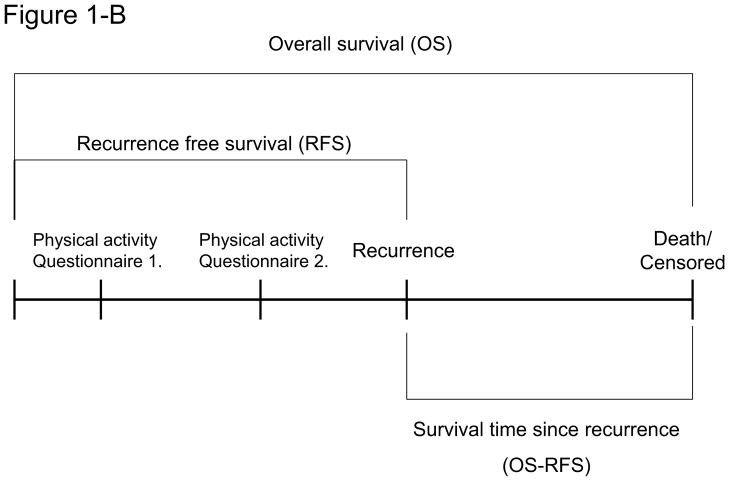
We conducted a prospective observational study of 237 stage III colon cancer patients who had a recurrence. Physical activity was measured approximately six months after the completion of therapy (14 months after the surgical resection) but before detection of recurrent disease. The primary endpoint of the study was survival time after recurrence.
Results
The hazard ratio comparing patients who reported at least 18 metabolic equivalent task (MET)-hours per week of physical activity to those engaging in less than 3 MET-hours / week was 0.71(95% confidence interval 0.46–1.11). Increasing total MET-hours per week of physical activity was associated with a borderline statistical significance trend for improved survival after recurrence (P=0.052). The benefit of physical activity on survival was not significantly modified by sex, body mass index, number of positive lymph nodes, age, baseline performance status, adjuvant chemotherapy regimen or recurrence-free survival period.
Conclusion
To our knowledge, this is the first study that studied the association of physical activity with survival outcome of recurrent colon cancer patients. While the association exceeded our pre-defined P trend <0.05 for statistical significance, these findings warrant further studies of physical activity in patients with recurrent colorectal cancer.
Keywords: Physical activity, Exercise, Recurrent colon cancer, Cancer recurrence, Survival
Introduction
Physically active individuals have a reduced risk of colorectal cancer development(1). Physical activity further reduces the risk of recurrence and mortality in colon cancer patients (2–6). We have previously reported the influence of exercise on recurrence and survival in patients with stage III colon cancer (4). Individuals engaging in more than 18 total metabolic equivalent task (MET) hour per week experienced approximately 50% reduction in recurrence or death compared to those who were inactive. Similarly, among 573 stage I–III colorectal cancer patients in the Nurses’ Health Study, post-diagnosis physical activity was inversely associated with colorectal cancer-specific mortality and overall mortality (3).
Advancements in surgery and perioperative therapies have significantly improved survival of colon cancer patients without metastases at the time of diagnosis (7); however, 5-year recurrence rates for patients with stage I, II and III colon cancer are still 10%, 20% and 30–50%, respectively.(8) Many colon cancer patients still develop tumor recurrence and the median survival following recurrence is less than 2 year (9, 10). Factors such as initial tumor stage, tumor grade, performance status, weight loss, and anatomic sites of metastatic disease have been known to contribute to survival after recurrence. A few studies have investigated the effects of physical activity on palliative care and quality of life in metastatic cancer patients (11–13). However, the impact of physical activity on survival time once colon cancer recurs has not been studied. Therefore, it is important to identify whether physical activity would have positive impact on the outcome of recurrent colon cancer. The current study aims to identify the association between post-diagnosis physical activity and overall survival after the recurrence of colon cancer.
Materials and Methods
Study Population
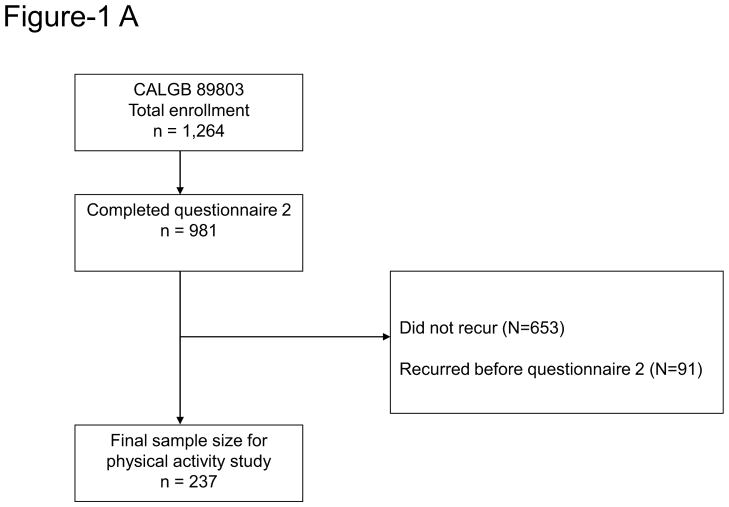
Patients in this prospective cohort study were participants in the NCI-sponsored Cancer and Leukemia Group B (CALGB) adjuvant therapy trial for stage III colon cancer comparing therapy with weekly fluorouracil and leucovorin to weekly irinotecan, fluorouracil, and leucovorin (CALGB 89803) (14). Between April 1999 and May 2001, a total of 1,264 patients were recruited for the trial. The detailed study methods have been described previously (4). In brief, a self-administered questionnaire including diet and lifestyle habits was obtained from patients midway through their adjuvant therapy (Questionnaire 1: 4 months after surgical resection) and again 6 months after completion of adjuvant therapy (Questionnaire 2: 14 months after surgical resection). As the purpose of this current study is to examine the effects of post-diagnosis physical activity on outcome of recurrent colon cancer, patients whose colon cancer recurred after the completion of questionnaire 2 were included in the analysis. Among 1,264 patients, 981 patients completed questionnaire 2 (Figure 1A). Of these 981 patients, we excluded from these analyses the 653 patients who did not recur and 91 patients who recurred before the completion of the second questionnaire (Figure 1B). The final sample size for this study was 237. All patients signed informed consent, approved by the internal review boards of the local institution.
Figure 1.
Physical Activity Assessment
The physical activity questions used in this study have been described and validated previously (3, 4, 15). The method for calculation of scores for MET hour per week based on the physical activity questionnaire was explained in detail in a previous publication (4). Categories of MET-hours per week were defined as less than 3, 3 to 17.9, and 18 or more, consistent with prior analyses (3, 4).
Study End Points and Covariates
In this ancillary study, the primary end point was survival after recurrence, defined as the time from the recurrence to death as a result of any cause. Covariates included gender, age, body mass index (BMI), depth of invasion through bowel wall (T stage), the number of positive lymph nodes, and baseline performance status (at time of adjuvant therapy).
Statistical Analyses
Cox proportional hazards regression was used to determine association between physical activity and survival outcome, controlling potential confounders which may influence the outcome. Total MET-hours was considered as a continuous variable in tests of trend and interaction. A categorical variable based on total MET-hours was defined as <3, 3–17.9, and ≥ 18 MET-hours per week. In survival comparisons, the physical activity less than 3 MET-hours per week category was the reference group. P values less than or equal to 0.05 were considered statistically significant. Tests of interaction between physical activity categories and potential confounders were assessed by entering the cross product of the physical activity and the dichotomized covariate. Patient registration and clinical data collection were managed by the CALGB (Alliance) Statistics and Data Center, and all analyses were performed by CALGB (Alliance) statisticians based on the study database, frozen on November 9th, 2009.
Results
Baseline Characteristics by Physical Activity Category
Baseline characteristics by activity levels for the 237 participants with colon cancer recurrence included in this analysis are shown in Table 1. Patients with higher physical activity tended to be male and have a higher performance status (measured at the time of initiation of adjuvant therapy). Other characteristics did not differ significantly across physical activity levels.
Table 1.
Baseline characteristics
| Characteristic | Total Met-Hours per Week
|
|||
|---|---|---|---|---|
| < 3 | 3 – 17.9 | ≥ 18 | overall | |
| Number. of patients | 81 | 96 | 60 | 237 |
|
| ||||
| Median total MET-hours per week | 0.6 | 8.3 | 36 | 7.7 |
|
| ||||
| Median age (years) | 63 | 60 | 59.5 | 61 |
|
| ||||
| Gender, n (%) | ||||
| Male | 38 (46.9) | 59 (61.5) | 43 (71.7) | 140 (59.1) |
| Female | 43 (53.1) | 37 (38.5) | 17 (28.3) | 97 (40.9) |
|
| ||||
| Race, n ( %) | ||||
| White | 70 (86.4) | 91 (94.8) | 55 (91.7) | 216 (91.1) |
| Black | 8 (9.9) | 3 (3.1) | 3 (5) | 14 (5.9) |
| Other | 3 (3.7) | 2 (2.1) | 2 (3.3) | 7 (3.0) |
|
| ||||
| Time from diagnosis to recurrence, years, n (%) | ||||
| < 2 years | 34 (42.0) | 45 (46.9) | 28 (46.7) | 107 (45.1) |
| ≥ 2 years | 47 (58.0) | 51 (53.1) | 32 (53.3) | 130 (54.9) |
|
| ||||
| BMI, median | 30.3 | 28 | 29.6 | 28.8 |
|
| ||||
| Depth of invasion through bowel wall, n (%) | ||||
| T1–2 | 6 (7.4) | 7 (7.3) | 5 (8.3) | 18 (7.6) |
| T3–4 | 72 (88.9) | 88 (91.7) | 54 (90) | 214 (90.3) |
| missing | 3 (3.7) | 1 (1) | 1 (1.7) | 5 (2.1) |
|
| ||||
| Number of positive lymph nodes, n (%) | ||||
| 1–3 (N1) | 54 (66.7) | 54 (56.3) | 32 (53.3) | 140 (59.1) |
| 4+ (N2) | 24 (29.6) | 41 (42.7) | 27 (45.0) | 92 (38.8) |
| Missing | 3 (3.7) | 1 (1) | 1 (1.7) | 5 (2.1) |
|
| ||||
| Baseline performance status, n (%) | ||||
| 0 | 52 (64.2) | 70 (72.9) | 48 (80) | 170 (71.7) |
| 1,2 | 26 (32.1) | 25 (26.0) | 11 (18.3) | 62 (26.2) |
| Missing | 3 (3.7) | 1 (1) | 1 (1.7) | 5 (2.1) |
|
| ||||
| Treatment, n (%) | ||||
| 5FU/LV | 41 (50.6) | 44 (45.8) | 27 (45) | 112 (47.3) |
| CPT-11/5FU/LV | 40 (49.4) | 52 (54.2) | 33 (55.0) | 125 (52.7) |
5-FU = 5-fluorouracil; LV = leucovorin; IFL = irinotecan,; kg = kilograms; m2 = meters squared; MET = metabolic equivalent tasks
Baseline performance status: PS 0 = fully active; PS 1 = restricted in physically strenuous activity but ambulatory and able to carry out light work; PS 2 = ambulatory and capable of all self-care but unable to carry out any work activities, up and about more than 50% of waking hours.
T1–2 = level of invasion through the bowel wall not beyond the muscle layer; T3–4 = level of invasion through the bowel wall beyond the muscle layer.
Survival after recurrence by level of physical activity
Of the 237 participants, 169 had confirmed deaths as of November 9th 2009, the date the CALGB 89803 database was frozen. The overall median follow-up time for the entire CALGB 89803 cohort was 7.3 years. The primary endpoint of this study was survival time after recurrence. Post-diagnosis physical activity was inversely associated with the risk of death after recurrence, though the P trend = 0.052 exceeded our pre-specified level of statistical significance (Table 2). Compared with patients who reported less than 3 total MET-hours per week of physical activity, those reporting 18 or more MET-hours per week had a multivariate hazard ratio of 0.71 (95% CI, 0.46–1.11).
Table 2.
Association between post-diagnosis physical activity and overall survival in patients with recurrent colon cancer
| Total Met-Hours per Week
|
P for Trend b | |||
|---|---|---|---|---|
| <3 | 3 – 17.9 | 18 + | ||
| No. of events / No. at risk | 58/81 | 72/96 | 39/60 | |
| Unadjusted | 1.0 (Referent) | 0.95 (0.67–1.35) | 0.84 (0.56–1.26) | 0.172 |
| Adjusted a | 1.0 (Referent) | 0.85 (0.58–1.23) | 0.71 (0.46–1.11) | 0.052 |
MET = metabolic equivalent task
Adjusted for sex, age, BMI, depth of invasion through bowel wall, number of positive lymph nodes, baseline performance status and treatment group
Based on the Cox model with continuous total METs per week
We examined the influence of physical activity across strata of other predictors of cancer recurrence and mortality. No significant interactions were detected on the association between physical activity and survival after recurrence by gender (P interaction =.97), baseline performance status (0 vs. 1–2, P interaction =.51), extent of invasion through bowel wall (T 1–2 vs. 3–4, P interaction =.58), number of positive nodes (≤3 vs. ≥4, P interaction = 0.32), body mass index (≤25 vs. >25, P interaction =0.98) or adjuvant chemotherapy regimen (P interaction = 0.89). Further, there was no interaction between the level of physical activity and survival after recurrence when tested by time from diagnosis to recurrence (<2 years or ≥ 2 years; P interaction = 0.94).
Discussion
Despite improvements in the treatment of colorectal cancer, over 30% of patients with stage III colon cancer will recur identification of prognostic markers that influence survival rate in recurrent colon cancer patients is of a major importance. We previously demonstrated that physical activity significantly reduces the likelihood of recurrence in colorectal cancer survivors, and thus increases overall and colorectal cancer specific survival (3–5). No prior studies have reported the impact of physical activity after the diagnosis on the survival of patients who experience recurrence of colon cancer. In the current study, we observed that the level of physical activity after the diagnosis of cancer may be associated with survival of recurrent colon cancer patients, though the association just exceeded our pre-specified level of statistical significance. Recurrent colon cancer patients who engaged in at least 18 MET-hours per week of activity had a non-significant 29% improvement in mortality compared with inactive patients after controlling for potential factors which may influence survival.
O’Connell and colleagues reported that patients who recur later live longer than those who recur earlier (16). To control for this factor, we have stratified our participants into two groups: those who had recurrence-free survival less than two years and those who had a recurrence-free survival of two years or more. We did not observe a significant interaction in overall survival between the level of physical activity and earlier or later recurrence time from diagnosis (P=0.94).
There are only two studies that have investigated the association between either functional capacity or physical activity behavior and survival in recurrent cancer (13, 17). One study (13) found that functional capacity measured by a 6-minute walk test and the level of physical activity (more versus less than 9 MET hours per week) were predictors for survival in metastatic non-small cell lung cancer patients. In another study (17), the level of physical activity but not functional capacity was a significant predictor for patients with malignant recurrent glioma. The result from the current study is in agreement with previously reported studies in recurrent cancer patients; however, the current study was the first performed in colon cancer patients. Taken together with previously reported studies, we may be able to suggest that our patients with recurrent colon cancer should increase or at least maintain their level of physical activity if there is no contraindication to exercise.
There are limitations in this study that are important to consider. First, the sample size is limited both by the number of recurrences detected overall in this adjuvant therapy study and the requirement that patients did not recur prior to the second questionnaire (approximately 14 months after surgery). While the point estimated for overall survival for 18 or more MET-hours per week of physical activity suggests a benefit, the confidence interval crosses 1; this could be due to sample size or lack of a true benefit. In addition, the P value for the test for trend with increasing physical activity on survival time after recurrence was at the borderline significance at 0.052. Second, physical activity was measured before the recurrence and it is not known if that level of activity was maintained after recurrence and for how long. Further studies of the effects of physical activity on survival of recurrent colon cancer patients, as well as the associated biological mechanism, are needed.
In summary, physical activity after completion of therapy but prior to recurrence seems to be a factor that influences the prognosis of recurrent colon cancer patients.
Conclusion
The impact of physical activity after cancer recurrence on the risk of mortality in colon cancer patients was first investigated in the current study. In the current study, a trend toward statistical significance in association between the physical activity after completion of therapy but prior to recurrence and the risk of mortality was observed. In the future, we need a larger prospective cohort study as well as randomized controlled trial with physical activity intervention and control groups to confirm the findings of our study.
Clinical Practice Points.
-
What is already known about this subject?
The positive impact of physical activity participation on cancer prevention, prevention of recurrence and survival in colorectal cancer patients has been reported. In addition, the safety and efficacy of physical activity and exercise have been tested adequately and oncologists can recommend physical activity and exercise to their patients. However, the association between physical activity and mortality has not been tested in metastatic colorectal cancer patients and there is no evidence on whether physical activity and exercise would have a positive impact of survival of recurrent colorectal cancer.
-
What are the new findings?
The current study is the first study which investigated the association between physical activity levels and survival outcome in recurrent colon cancer patients. We have observed that increasing total MET-hours per week of physical activity was associated with a borderline statistical significance trend for improved survival after recurrence (P=0.052).
-
How might it impact on clinical practice in the foreseeable future?
Although it was not statistically significant, this study provides some evidence that physical activity may have positive impact on survival of recurrent colorectal cancer.
Acknowledgments
The research for CALGB 89803 was supported, in part, by grants from the National Cancer Institute (CA31946) to the Cancer and Leukemia Group B (Monica M. Bertagnolli, M.D., Chairman) and to the CALGB Statistical Center (Daniel J. Sargent, Ph.D., CA33601), as well as support from Pharmacia & Upjohn Company, now Pfizer Oncology. JYJ was supported by the national research foundation of Korea (NRF), Ministry of Education, (No.2011-0004892) and the National R & D program for Cancer Control, Ministry of Health and Welfare, Republic of Korea (1120230).
The research for CALGB 89803 was supported, in part, by grants from the National Cancer Institute (CA31946) to the Cancer and Leukemia Group B (Monica M. Bertagnolli, M.D., Chairman) and to the CALGB Statistical Center (Daniel J. Sargent, Ph.D., CA33601), as well as support from Pharmacia & Upjohn Company, now Pfizer Oncology. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
The following institutions participated in this study:
Baptist Cancer Institute CCOP, Memphis, TN, Lee S. Schwartzberg, M.D., supported by CA71323
Cancer Centers of the Carolinas, Greenville, SC, Jeffrey K. Giguere, M.D, supported by CA29165
Christiana Care Health Services, Inc. CCOP, Wilmington, DE, Stephen Grubbs, M.D., supported by CA45418
Dana-Farber Cancer Institute, Boston, MA, Harold J. Burstein, M.D., Ph.D., supported by CA32291
Dartmouth Medical School, Norris Cotton Cancer Center, Lebanon, NH, Konstantin Dragnev, M.D., supported by CA04326
Duke University Medical Center, Durham, NC, Jeffrey Crawford, M.D., supported by CA47577
Georgetown University Medical Center, Washington, DC, Bruce Cheson, M.D., supported by CA77597
Hematology-Oncology Associates of Central New York CCOP, Syracuse, NY, Jeffrey Kirshner, M.D., supported by CA45389
Massachusetts General Hospital, Boston, MA, Jeffrey W. Clark, M.D., supported by CA32291
Memorial Sloan-Kettering Cancer Center, New York, NY, Clifford A. Hudis, M.D., supported by CA77651
Missouri Baptist Medical Center, St. Louis, MO, Alan P. Lyss, M.D., supported by CA114558-02
Monter Cancer Center of North Shore - LIJ Health Systems, Lake Success, NY, Daniel Budman, MD, supported by CA35279
Mount Sinai Medical Center, Miami, FL, Michael A. Schwartz, M.D., supported by CA45564
Mount Sinai School of Medicine, New York, NY, Lewis R. Silverman, M.D., supported by CA04457
Nevada Cancer Research Foundation CCOP, Las Vegas, NV, John A. Ellerton, M.D., supported by CA35421
North Shore-Long Island Jewish Health System, New Hyde Park, NY, Daniel Budman, MD, supported by CA35279
Rhode Island Hospital, Providence, RI, William Sikov, M.D., supported by CA08025
Roswell Park Cancer Institute, Buffalo, NY, Ellis Levine, M.D., supported by CA59518
Southeast Cancer Control Consortium Inc. CCOP, Goldsboro, NC, James N. Atkins, M.D., supported by CA45808
State University of New York Upstate Medical University, Syracuse, NY, Stephen L. Graziano, M.D., supported by CA21060
The Ohio State University Medical Center, Columbus, OH, Clara D Bloomfield, M.D., supported by CA77658
University of California at San Diego, San Diego, CA, Barbara A. Parker, M.D., supported by CA11789
University of California at San Francisco, San Francisco, CA, Charles J. Ryan, M.D., supported by CA60138
University of Chicago, Chicago, IL, Hedy L. Kindler, M.D., supported by CA41287
University of Illinois MBCCOP, Chicago, IL, David J. Peace, M.D., supported by CA74811
University of Iowa, Iowa City, IA, Daniel A. Vaena, M.D., supported by CA47642
University of Maryland Greenebaum Cancer Center, Baltimore, MD, Martin Edelman, M.D., supported by CA31983
University of Massachusetts Medical School, Worcester, MA, William V. Walsh, M.D., supported by CA37135
University of Minnesota, Minneapolis, MN, Bruce A Peterson, M.D., supported by CA16450
University of Missouri/Ellis Fischel Cancer Center, Columbia, MO, Michael C Perry, M.D., supported by CA12046
University of Nebraska Medical Center, Omaha, NE, Apar Ganti, M.D., supported by CA77298
University of North Carolina at Chapel Hill, Chapel Hill, NC, Thomas C. Shea, M.D., supported by CA47559
University of Tennessee Memphis, Memphis, TN, Harvey B. Niell, M.D., supported by CA47555
University of Vermont, Burlington, VT, Steven M. Grunberg, M.D., supported by CA77406
Wake Forest University School of Medicine, Winston-Salem, NC, David D. Hurd, M.D., supported by CA03927
Walter Reed Army Medical Center, Washington, DC, David C. Van Echo, M.D., supported by CA26806
Washington University School of Medicine, St. Louis, MO, Nancy Bartlett, M.D., supported by CA77440
Weill Medical College of Cornell University, New York, NY, John Leonard, M.D., supported by CA07968
Footnotes
Disclosures
All authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wolin KY, Yan Y, Colditz GA, Lee IM. Physical activity and colon cancer prevention: a meta-analysis. Br J Cancer. 2009;100:611–6. doi: 10.1038/sj.bjc.6604917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haydon AM, Macinnis RJ, English DR, Giles GG. Effect of physical activity and body size on survival after diagnosis with colorectal cancer. Gut. 2006;55:62–7. doi: 10.1136/gut.2005.068189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyerhardt JA, Giovannucci EL, Holmes MD, Chan AT, Chan JA, Colditz GA, et al. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol. 2006;24:3527–34. doi: 10.1200/JCO.2006.06.0855. [DOI] [PubMed] [Google Scholar]
- 4.Meyerhardt JA, Heseltine D, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol. 2006;24:3535–41. doi: 10.1200/JCO.2006.06.0863. [DOI] [PubMed] [Google Scholar]
- 5.Meyerhardt JA, Giovannucci EL, Ogino S, Kirkner GJ, Chan AT, Willett W, et al. Physical activity and male colorectal cancer survival. Arch Intern Med. 2009;169:2102–8. doi: 10.1001/archinternmed.2009.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baade PD, Meng X, Youl PH, Aitken JF, Dunn J, Chambers SK. The impact of body mass index and physical activity on mortality among patients with colorectal cancer in Queensland, Australia. Cancer Epidemiol Biomarkers Prev. 2011;20:1410–20. doi: 10.1158/1055-9965.EPI-11-0079. [DOI] [PubMed] [Google Scholar]
- 7.Wolpin BM, Meyerhardt JA, Mamon HJ, Mayer RJ. Adjuvant treatment of colorectal cancer. CA Cancer J Clin. 2007;57:168–85. doi: 10.3322/canjclin.57.3.168. [DOI] [PubMed] [Google Scholar]
- 8.Gunderson LL, Jessup JM, Sargent DJ, Greene FL, Stewart AK. Revised TN categorization for colon cancer based on national survival outcomes data. J Clin Oncol. 2010;28:264–71. doi: 10.1200/JCO.2009.24.0952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Gramont A, Hubbard J, Shi Q, O’Connell MJ, Buyse M, Benedetti J, et al. Association between disease-free survival and overall survival when survival is prolonged after recurrence in patients receiving cytotoxic adjuvant therapy for colon cancer: simulations based on the 20,800 patient ACCENT data set. J Clin Oncol. 2010;28:460–5. doi: 10.1200/JCO.2009.23.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Connell MJ, Campbell ME, Goldberg RM, Grothey A, Seitz JF, Benedetti JK, et al. Survival following recurrence in stage II and III colon cancer: findings from the ACCENT data set. J Clin Oncol. 2008;26:2336–41. doi: 10.1200/JCO.2007.15.8261. [DOI] [PubMed] [Google Scholar]
- 11.Palacio A, Calmels P, Genty M, Le-Quang B, Beuret-Blanquart F. Oncology and physical medicine and rehabilitation. Ann Phys Rehabil Med. 2009;52:568–78. doi: 10.1016/j.rehab.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Oldervoll LM, Loge JH, Paltiel H, Asp MB, Vidvei U, Wiken AN, et al. The effect of a physical exercise program in palliative care: A phase II study. J Pain Symptom Manage. 2006;31:421–30. doi: 10.1016/j.jpainsymman.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Dahele M, Skipworth RJ, Wall L, Voss A, Preston T, Fearon KC. Objective physical activity and self-reported quality of life in patients receiving palliative chemotherapy. J Pain Symptom Manage. 2007;33:676–85. doi: 10.1016/j.jpainsymman.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 14.Saltz LB, Niedzwiecki D, Hollis D, et al. Irinotecan plus fluorouracil/leucovorin (IFL) versus fluorouracil/leucovorin alone (FL) in stage III colon cncer (intergroup trial CALGB C89803) J Clin Oncol. 2004;22:245S. (Suppl; abstr 3500) [Google Scholar]
- 15.Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. Jama. 2005;293:2479–86. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 16.O’Connell MJ, Campbell ME, Goldberg RM, Grothey A, Seitz JF, Benedetti JK, et al. Survival following recurrence in stage II and III colon cancer: findings from the ACCENT data set. J Clin Oncol. 2008;26:2336–41. doi: 10.1200/JCO.2007.15.8261. [DOI] [PubMed] [Google Scholar]
- 17.Ruden E, Reardon DA, Coan AD, Herndon JE, 2nd, Hornsby WE, West M, et al. Exercise behavior, functional capacity, and survival in adults with malignant recurrent glioma. J Clin Oncol. 2011;29:2918–23. doi: 10.1200/JCO.2011.34.9852. [DOI] [PMC free article] [PubMed] [Google Scholar]