Abstract
Each year, over one million people in the United States are affected by traumatic brain injury (TBI). Symptoms of both acute and chronic neuroinflammation follow TBI, coinciding with a robust immune response and activation of the brain’s endogenous repair mechanisms. TBI can lead to endocrine failure as a result of damage to the thalamic region of the brain, evidenced by excessive thirst and polyuria often accompanying TBI. These symptoms indicate the presence of diabetes insipidus (DI), a disruption of water homeostasis due to antidiuretic hormone deficiency. This deficiency accompanies a mechanical or neuroinflammatory damage to the thalamic region during TBI, evidenced by increased expression of inflammatory microglial marker MHCII in this brain region. Excessive thirst and urinations, which are typical DI symptoms, in our chronic TBI rats also suggest a close connection between TBI and DI. We seek to bridge this gap between TBI and DI through investigation of the Cluster of Differentiation 36 (CD36) receptor. This receptor is associated with Low-Density Lipoprotein (LDL) deregulation, proinflammatory events, and innate immunity regulation. We posit that CD36 exacerbates TBI through immune activation and subsequent neuroinflammation. Indeed, scientific evidence already supports pathological interaction of CD36 in other neurological disorders including stroke and Alzheimer’s disease. We propose that DI contributes to TBI pathology via CD36 neuroinflammation. Use of CD36 as a biomarker may provide insights into treatment and disease pathology of TBI and DI. This unexplored avenue of research holds potential for a better understanding and treatment of TBI and DI.
Keywords: Diabetes Insipidus, Traumatic brain injury, Neuroinflammation, CD36
Introduction
Traumatic brain injury (TBI) affects an estimated 1.7 million people in the United States annually, accounting for 1.365 million emergency room visits and 52,000 deaths each year—one third of all injury-related deaths in the United States [1]. Disruptions of water balance and osmolality are common following TBI [2-6]. Diabetes insipidus (DI), a condition in which the kidneys fail to reabsorb water, occur in 21.6% to 26% of patients diagnosed with TBI, 6% to 6.9% of whom experienced chronic DI symptoms [3,7]. Other studies report that most, but not all, DI cases are transient; symptoms usually appear 1 to 3 days post TBI and disappear within a month [2,5]. This link between DI and TBI has been recognized for many years [8].
TBI is usually associated with central diabetes insipidus (CDI), a failure of the kidneys to reabsorb water due to antidiuretic hormone (ADH) deficiency [4]. This deficiency results in increased blood osmolality and increased excretion (polyuria) due to decreased water reabsorption in the distal tubules and collecting ducts [2,3,9]. Other hypothalamus and pituitary dysfunction has been observed after TBI as well. In fact, approximately 30% to 40% of patients show some type of hormone deficiency following TBI [6,10]. These symptoms, including CDI, are likely due to hypothalamus or pituitary damage sustained during injury, and studies have identified a direct relationship of the severity of injury with likelihood of developing chronic hormone deficiencies [5-7]. Therefore, a strong pathological interaction exists between TBI and CDI. Here, we discuss these two disease conditions and examine the Cluster of Differentiation 36 (CD36) receptor as a major pathological link between them.
A Neuroinflammatory Response in TBI
Neuroinflammation, both acute and chronic, is widely recognized as a symptom following TBI. Immediately following TBI, the brain mounts a robust inflammatory response, including activation of the immune cells and secretion of regulatory cytokines and chemokines [11-13]. CD36 may directly and indirectly modulate cytokine release. Following a neurotoxic protein stimulant (i.e., prion) a CD36-directed microglial activation is achieved involving an immediate but transient increase in the mRNA expression of CD36 that upregulates mRNA and protein levels of proinflammatory cytokines (IL-1β, IL-6 and TNF-α), resulting in increased inducible nitric oxide synthase expression and nitric oxide production, which in turn activate NF-κB and caspase-1, and elevated Fyn activity [14]. Indirectly, CD36 participates in the development of atherosclerosis by mediating the uptake of oxidized low-density lipoproteins (oxLDL) by macrophages, thus leading to foam cell formation, with progression of the pathogenesis of atherosclerosis determined by ongoing inflammatory reactions [15]. Furthermore, CD36 in platelet binding of hypochlorite-oxidized LDL. CD36 plays a prominent role as the major receptor responsible for hypochlorite-oxidized LDL-induced platelet activation that accumulates in the release of CD40L-mediated proinflammatory role of platelets, especially in conditions of oxidative stress [16,17]. CD36 modulation of these cytokine pathways has also been shown to be influenced by serum amyloid and dietary cholesterol, both of which have been associated with several pathological diseases [18,19]. The brain also attempts to repair itself via endogenous repair mechanisms such as cell proliferation [20]; however, these repair mechanisms cannot fully ameliorate the secondary cell death, neuroinflammation [11,20], and apoptosis [21,22] following TBI. Chronic neuroinflammation from TBI results in neuron loss, impaired cell proliferation, and an upregulation of activated microglia cells [13,23]. Therefore, neuroinflammation interferes with the brain’s endogenous repair mechanisms, which in turn impedes recovery from TBI [23]. Neuroinflammation and its associated cell death events linger over time following injury; in fact, neuroinflammation has been observed up to 17 years post TBI [24]. Both the primary injury itself and neuroinflammation following TBI can damage the thalamic region of the brain, leading to subsequent endocrine system failure [25] and among other endocrine-related deficits associated with neurodegeneration of the thalamus. In particular, excessive thirst and excretion of large amounts of severely diluted urine, have been documented in TBI with thalamic damage, symptoms reminiscent of CDI [2,4].
The Thalamic Region and DI
Hypopituitarism frequently follows TBI, either as a result of brain damage or the body’s endocrine response to the injury. Still, present data do not define the exact time frame of hypopituitarism [3,6]. Regardless, evidence of both chronic and acute pituitary damage following TBI is abundant. Autopsy of 496 patients who died from acute TBI revealed hemorrhage or necrosis at the anterior pituitary in 21% of cases and at the posterior pituitary in 22% of cases [6]. In a large series of cases, a 7% prevalence of diabetes insipidus in long-term TBI survivors was observed [26]. Consequences of endocrine dysfunction following TBI have been routinely explored in adults, but juvenile studies are limited and show an incidence rate of endocrine dysfunction in 16-61% in patients 1-5 years after injury [28]. In addition, evidence of hypothalamic or pituitary vascular lesions via CT or MRI scan has been noted in about 79% of patients with post traumatic hypopituitarism [27]. Normally, ADH is secreted by the posterior pituitary, and ADH production is monitored by the hypothalamus through osmoregulatory cells [2,4]. Therefore, mechanical or neuroinflammatory damage to these regions or the pituitary stalk can inhibit the body’s osmotic balance, thus causing post-traumatic DI [2,3,5,7]. Reports suggest that disruption of blood flow to these hypothalamic osmoreceptor cells disrupts water homeostasis in the body [2]. Irreversible damage can result in permanent DI [7]. We seek to draw the connection between TBI and DI through studying this neuroinflammation in tandem with the CD36 receptor.
CD36: An Overview
CD36 or Fatty Acid Translocase (FAT) is a scavenger receptor that supports a large extracellular domain and two short cytoplasmic tails [29]. This extracellular region includes a hydrophobic region, a proline-cysteine rich domain, and ten potential N-linked glycosylation sites. These glycosylation sites allow the cell to recognize various lipid-based ligands, which further increases CD36’s highly diverse cellular responses [29]. This class B scavenger receptor can be found in hematopoietic cells, which include erythroid precursors, platelets, monocytes, macrophages, and megakaryocytes [30]. CD36 is mainly associated with Low-Density Lipoprotein (LDL) deregulation, regulating inflammation, innate immunity, and lipid uptake in peripheral tissue including retina, breast, kidney, and tongue. Once the CD36 receptors have been activated in response to Fibrillar β amyloid (fAβ), it causes proinflammatory events.
CD36-Mediated Neuroinflammation: A Link between TBI and DI
CD36 contributes to neuroinflammation in patients with post-ischemic inflammation [32]. Reduction in free radical production and injury size in CD36 KO mice further prove that CD36 induces an increase in brain injury. CD36 ligands such as Fibrillar β amyloid (fAβ), modified/oxidizes low density proteins (mLDL, oxLDL), among others are elevated following ischemia [33-35]. Since CD36 works in a feed forward manner [36], the existence of CD36 ligands in the infarct area insinuates that excess CD36 pathways are activated. Certain CD36 ligands influence vascular dysfunction, such as amyloid β, oxLDLs, lipid based proteins, and advanced glycogen end products (AGE) LDL [29].
The plethora of modified/oxidized low-density proteins (mLDL, oxLDL) in hyperlipidemic plasma suggests the connection of lipid-based ligands in intensifying CD36-mediated function (Figure 1). Foam cell formation occurs when there is an uptake of excess lipid ligands by monocyte/macrophage CD36, which is a key event that leads to atherosclerotic lesion development in vessels [30]. The involvement of CD36 in hyperlipidemia-induced exacerbation in ischemia is further supported by laboratory observations demonstrating that CD36 knockout animals displayed smaller infarct sizes, reduced expression of inflammatory cytokines MCP-1 and CCR2, and a decreased number of foam cells [29]. Along this line of investigations, previous laboratory evidence implicates an interaction between CD36 and insulin resistance [36,37]. (AGE) LDL has a high affinity for CD36 in that the exposure of (AGE) LDL induced a pro-oxidant and proinflammatory state in smooth muscle cells, increasing lipid accumulation [38]. This directly compromises endothelial function and promotes proinflammatory responses.
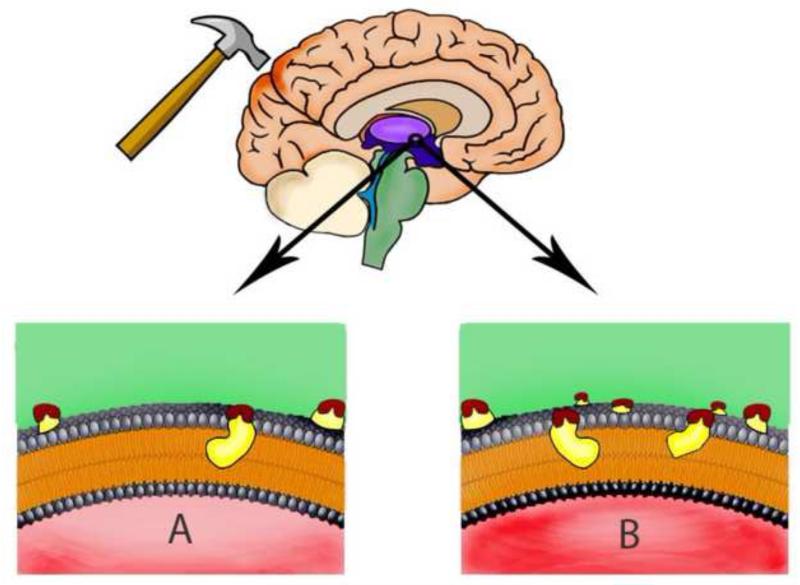
Figure 1. CD36 neuroinflammation in the thalamic/hypothalamic region - a pathological link between DI and TBI.
(A). This picture depicts a brain that has suffered TBI, demonstrating a modest level of inflammation. (B). A person who suffers from diabetes insipidus has more ligands available that activate CD36, consequently causing more inflammation.
Fibrillar β amyloid (fAβ) is one of many examples about how CD36 brings about a neurological disease as a result of neuroinflammation. In this specific case, Alzheimer’s Disease (AD) is brought about. The pileup of β amyloid in the plaque attributes AD. fAβ binds to many biomolecules such as proteins and membrane lipids. Consequently, this binding further advances fibrillation of fAβ, which reconfigures the structure and function of the membrane [39]. CD36 activates signaling cascades, which trigger innate host responses [40-42], in response to fAβ.
Altogether, the body of laboratory evidence advances the notion of an intimate pathological interaction between CD36 and neurological disorders, such as stroke and Alzheimer’s disease, characterized by robust neuroinflammation. To this end, because neuroinflammation is a hallmark pathological feature of TBI, as discussed above, there is high likelihood that CD36 also participates in the disease pathology of TBI. As noted above, CD36 has been widely associated with the development of atherosclerosis [43]. Recent scientific advances have implicated a key role for CD36 in stroke in hyperlipidemia [44]. Here, we advance that CD36 may serve as a pathological link between DI and TBI. Moreover, we envision that CD36 can be used as a biomarker of disease onset and progression, as well as an outcome measure of therapeutic intervention, in that a high level of CD36 either in plasma or CSF may indicate disease pathology, while a reduced CD36 level may suggest effectiveness of therapy.
Hypothesis
We propose that DI following TBI contributes to disease pathology via CD36 neuroinflammation. Investigations into CD36 as a biomarker of DI/TBI may reveal the acute, subacute, and/or chronic neuroinflammation associated with both DI and TBI, allowing for a better understanding of disease pathology that may provide insights into the development of novel treatments for DI and TBI. Evidence suggests that following a TBI incident, CD36 is activated, causing an upregulation of activated microglia cells and neuroinflammation. Detailed previously, this neuroinflammation can damage the thalamus region of the brain, thus causing the onset of post-traumatic DI. Therefore, CD36 bridges the gap between these two conditions.
Our recent laboratory observations revealed a rampant neuroinflammation in the thalamic region of the brain in our chronic TBI animals, as evidenced by the overexpression of the inflammatory microglial marker MHCII. With this new knowledge, we next assessed whether CD36 colocalized with the MHCII-stained thalamic cells. Interestingly, we found that CD36 expression was increased in the same thalamic area populated by the inflammatory MHCII cells (Figure 2). Equally compelling in support of our hypothesis is the demonstration that this histopathological detection of CD36-neuroinflammation in the thalamic region in our chronic TBI animals coincided with the observation that the majority of the rats displayed excessive thirst and urinations, which are routine CDI behavioral pathologic outcomes. What previously seemed like a usual side effect to TBI is actually a hallmark feature of CDI. These underexplored and novel brain pathological and behavioral symptoms of TBI advance our hypothesis linking TBI to the phenotypic characteristics of CDI.
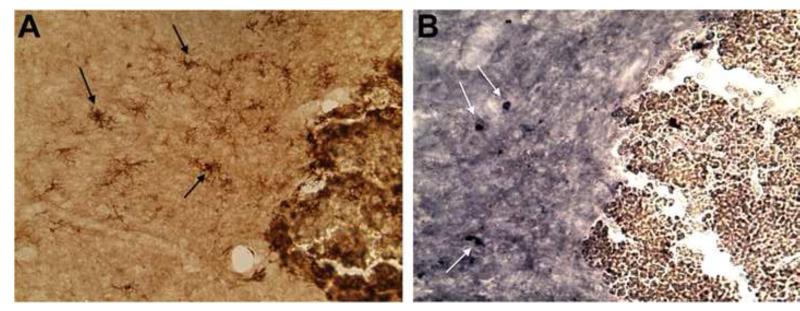
Figure 2. CD36 expression in TBI rat brain.
A representative microphotograph (20x) showing activated microglial cells in the thalamus/hypothalamus of an animal subjected at 30 days post-injury after controlled cortical impact model of TBI. MHCII+ stained cells (black arrows in A), with adjacent coronal section demonstrating CD36+ (white arrows in B).
Acknowledgments
Funding: CVB is supported by James and Esther King Foundation for Biomedical Research Program 1KG01-33966 and NIH NINDS RO1 1R01NS071956-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
None
References
- 1.Faul M, Xu L, Wald MM, Coronado VG. [Accessed July 14, 2013];Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. 2010 http://www.cdc.gov/traumaticbraininjury/pdf/blue_book.pdf.
- 2.Hannon MJ, Finucane FM, Sherlock M, Agha A, Thompson CJ. Disorders of water homeostasis in Neurosurgical Patients. J Clin Endocrinol Metab. 2012;97(5):1423–33. doi: 10.1210/jc.2011-3201. [DOI] [PubMed] [Google Scholar]
- 3.Agha A, Sherlock M, Phillips J, Tormey W, Thompson CJ. The natural history of post-traumatic neurohypophysial dysfunction. Eur J Endocrinol. 2005;52(3):371–7. doi: 10.1530/eje.1.01861. [DOI] [PubMed] [Google Scholar]
- 4.Tisdal M, Crocker M, Watkiss J, Smith M. Disturbances of sodium in critically ill adult neurologic patients: a clinical review. J Neurosurg Anesthesiol. 2006;18(1):57–63. doi: 10.1097/01.ana.0000191280.05170.0f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsagarkis S, Tzanela M, Dimopoulou I. Diabetes insipidus, secondary hypoadenalism and hypothyroidism after traumatic brain injury: clinical implications. Pituitary. 2005;8(3-4):251–4. doi: 10.1007/s11102-006-6049-x. [DOI] [PubMed] [Google Scholar]
- 6.Bondanelli M, Ambrosio MR, Zatelli MC, De Marinis L, Degli Uberti EC. Hypopituitarism after traumatic brain injury. J Neurotrauma. 2004;21(6):865–96. doi: 10.1089/0897715041269713. [DOI] [PubMed] [Google Scholar]
- 7.Agha A, Thornton E, O’Kelly P, Tormey W, Phillips J, Thompson CJ. Posterior pituitary dysfunction after traumatic brain injury. J Clin Endocrinol Metab. 2004;89(12):5987–92. doi: 10.1210/jc.2004-1058. [DOI] [PubMed] [Google Scholar]
- 8.Griffin JM, Hartley JH, Jr, Crow RW, Schatten WE. Diabetes insipidus caused by craniofacial trauma. J Trauma. 1976;16(12):979–84. doi: 10.1097/00005373-197612000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Makaryus AN, McFarlane SI. Diabetes insipidus: diagnosis and treatment of a complex disease. Cleve Clin J Med. 2006;73(1):65–71. doi: 10.3949/ccjm.73.1.65. [DOI] [PubMed] [Google Scholar]
- 10.Bavisetty S, Bavisetty S, McArthur DL, et al. Chronic hypopituitarism after traumatic brain injury: risk assessment and relationship to outcome. Neurosurgery. 2008;62(5):1080–93. doi: 10.1227/01.neu.0000325870.60129.6a. [DOI] [PubMed] [Google Scholar]
- 11.Woodcok T, Morganti-Kossmann MC. The role of markers of inflammation in traumatic brain injury. Front Neurol. 2013;4:18. doi: 10.3389/fneur.2013.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giunta B, Obregon D, Velisetty R, Sanberg PR, Borlongan CV, Tan J. The immunology of traumatic brain injury: a prime target for Alzheimer’s disease prevention. J Neuoinflammation. 2012;9:185. doi: 10.1186/1742-2094-9-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernandez-Ontiveros DG, Tajiri N, Acosta SA, Giunta B, Tan J, Borlongan CV. Microglia activation as a biomarker for traumatic brain injury. Front Neurol. 2013;4:30. doi: 10.3389/fneur.2013.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kouadir M, Yang L, Tan R, et al. CD36 participates in PrP(106-126)-induced activation of microglia. PLoS One. 2012;7(1):e30756. doi: 10.1371/journal.pone.0030756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiurchiu V, Izzi V, D’Aquilio F, et al. Endomorphine-1 prevents lipid accumulation via CD36 down-regulation and modulates cytokines release from human lipid-laden macrophages. Peptides. 2011;32(1):80–5. doi: 10.1016/j.peptides.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 16.Assinger A, Koller F, Schmid W, et al. Hypochlorite-oxidized LDL induces intraplatelet ROS formation and surface exposure of CD40L—a prominent role of CD36. Atherosclerosis. 2010;213(1):129–34. doi: 10.1016/j.atherosclerosis.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 17.Assinger A, Koller F, Schmid W, et al. Specific binding of hypochlorite-oxidized HDL to platelet CD36 triggers proinflammatory and procoagulant effects. Atherosclerosis. 2010;212(1):153–60. doi: 10.1016/j.atherosclerosis.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 18.Baranova IN, Bocharov AV, Vishnyakova TG, et al. CD36 is a novel serum amyloid A (SAA) receptor mediating SAA binding and SAA-induced signaling in human and rodent cells. J Biol Chem. 2010;285(11):8492–506. doi: 10.1074/jbc.M109.007526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kennedy DJ, Kuchibhotla SD, Guy E, et al. Dietary cholesterol plays a role in CD36-mediated atherogenesis in LDLR-knockout mice. Artherioscler Thromb Vasc Biol. 2009;29(10):1481–7. doi: 10.1161/ATVBAHA.109.191940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaneko Y, Tajiri N, Yu S, et al. Nestin overexpression precedes capase-3 upregulation in rats exposed to controlled cortical impact traumatic brain injury. Cell Med. 2012;4(2):55–63. doi: 10.3727/215517912X639306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu S, Kaneko Y, Bae E, et al. Severity of controlled cortical impact traumatic brain injury in rats and mice dictates degree of behavioral deficits. Brain Res. 2009;1287:157–63. doi: 10.1016/j.brainres.2009.06.067. [DOI] [PubMed] [Google Scholar]
- 22.Shojo H, Kaneko Y, Mabuchi T, Kibayashi K, Adachi N, Borlongan CV. Genetic and histologic evidence implicates role of inflammation in traumatic brain injury-induced apoptosis in the rat cerebral cortex following moderate fluid percussion injury. Neuroscience. 2010;171(4):1273–82. doi: 10.1016/j.neuroscience.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 23.Acosta SA, Tajiri N, Shinozuka K, et al. Long-term upregulation of inflammation and suppression of cell proliferation in the brain of adult rats exposed to traumatic brain injury using the controlled cortical impact model. PLoS One. 2013;8(1):e53376. doi: 10.1371/journal.pone.0053376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramlackhansingh AF, Brooks DJ, Greenwood RJ, et al. Inflammation after trauma: microglial activation and traumatic brain injury. Ann Neurol. 2011;70:374–383. doi: 10.1002/ana.22455. [DOI] [PubMed] [Google Scholar]
- 25.Powner DJ, Boccalandro C, Alp MS, Vollmer DG. Endocrine failure after traumatic brain injury in adults. Neurocrit Care. 2006;5(1):61–70. doi: 10.1385/ncc:5:1:61. [DOI] [PubMed] [Google Scholar]
- 26.Glynn N, Agha A. Which patient requires neuroendocrine assessment following traumatic brain injury, when and how? Clin Endocrinal (Oxf) 2013;78(1):17–20. doi: 10.1111/cen.12010. [DOI] [PubMed] [Google Scholar]
- 27.Greco T, Hovda D, Prins M. The effects of repeat traumatic brain injury on the pituitary in adolescent rats. J Neurotrauma. 2013 doi: 10.1089/neu.2013.2990. PH D. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benvenga S, Campenni A, Ruggeri RM, Trimarchi F. Hypopituitarism secondary to head trauma. J Clin Endocrin and Metab. 2000;85:1353–61. doi: 10.1210/jcem.85.4.6506. [DOI] [PubMed] [Google Scholar]
- 29.Cho S. CD36 as a therapeutic target for endothelial dysfunction in stroke. Curr Pharm Des. 2012;18(25):3721–30. doi: 10.2174/138161212802002760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Febbraio M, Haijar DP, Silverstein RL. CD36: a class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation, and lipid metabolism. J Clin Invest. 2001;108:785–791. doi: 10.1172/JCI14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nihashi T, Inao S, Kajita Y, et al. Expression and distribution of beta amyloid precursor protein and beta amyloid peptide in reactive astrocytes after transient middle cerebral artery occlusion. Acta Neurochir (Wein) 2001;143:287–95. doi: 10.1007/s007010170109. [DOI] [PubMed] [Google Scholar]
- 32.Hayashi T, Noshita N, Sugawara T, Chan PH. Temporal profile of angiogenesis and expression of related genes in the brain after ischemia. J Cereb Blood Flow Metab. 2003;23:166–80. doi: 10.1097/01.WCB.0000041283.53351.CB. [DOI] [PubMed] [Google Scholar]
- 33.Pilitis JG, Coplin WM, O’Regan MH, et al. Measurement of free fatty acids in cerebrospinal fluids from patients with hemorrhagic and ischemic stroke. Brain Res. 2003;985:198–201. doi: 10.1016/s0006-8993(03)03044-0. [DOI] [PubMed] [Google Scholar]
- 34.Uno M, Kitazato KT, Nishi K, Itabe H, Nagahiro S. Raised plasma oxidized LDL in acute cerebral infraction. J Neurol Neurosurg Psychiatry. 2003;74:312–6. doi: 10.1136/jnnp.74.3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tontonoz P, Nagy L, Alvarez JG, Thomazy VA, Evans RM. PPARgamma promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell. 1998;93:241–52. doi: 10.1016/s0092-8674(00)81575-5. [DOI] [PubMed] [Google Scholar]
- 36.Aitman TJ, Glazier AM, Wallace CA, et al. Identification of CD36 (Fat) as an insulin-resistance gene causing defective fatty acid and glucose metabolism in hypertensive rats. Nat Genet. 1999;21:76–83. doi: 10.1038/5013. [DOI] [PubMed] [Google Scholar]
- 37.Griffin E, RE A, Hamel N, et al. A link between diabetes and atherosclerosis: Glucose regulates expression of CD36 at the level of translation. Nat Med. 2001;7:840–6. doi: 10.1038/89969. [DOI] [PubMed] [Google Scholar]
- 38.Sima AV, Botez GM, Stancu CS, Manea A, RAicu M, Simionescu M. Effect of irreversibility glycated LDL in human vascular smooth muscle cells: lipid loading, oxidative and inflammatory stress. J Cell Mol Med. 2010;14:2790–802. doi: 10.1111/j.1582-4934.2009.00933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verdier Y, Zarandi M, Penke B. Amyloid beta-peptide interactions with neuronal and glial cell plasma membrane: binding sites and implications for Alzheimer’s disease. J Pept Sci. 2004;10:229–48. doi: 10.1002/psc.573. [DOI] [PubMed] [Google Scholar]
- 40.El Khoury JB, Moore KJ, Means TK, Leung J, Terada K, Toft M, Freeman MW, Luster AD. CD36 mediates the innate host response to beta-amyloid. J Exp Med. 2003;197:1657–66. doi: 10.1084/jem.20021546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moore KJ, El Khoury J, Medeiros LA, Terada K, Geula C, Luster AD, Freeman MW. A CD36-initiated signaling cascade mediates inflammatory effects of beta-amyloid. J Biol Chem. 2002;277:47373–9. doi: 10.1074/jbc.M208788200. [DOI] [PubMed] [Google Scholar]
- 42.Silverstein RL, Febbraio M. CD36 and atherosclerosis. Curr Opin Lipidol. 2000;11:483–91. doi: 10.1097/00041433-200010000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Cho S, Kim E. CD36: a multi-modal target for acute stroke therapy. J Neurochem. 2009;109(Suppl 1):126–32. doi: 10.1111/j.1471-4159.2009.05801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim E, Febbraio M, Bao Y, et al. CD36 in the periphery and brain synergizes in stroke injury in hyperlipidemia. Ann Neurol. 2012;71(6):753–64. doi: 10.1002/ana.23569. [DOI] [PMC free article] [PubMed] [Google Scholar]