Abstract
Background
In altricial species, maternal stimuli have powerful effects on amygdala development and attachment-related behaviors. In humans, maternal deprivation has been associated with both “indiscriminate friendliness” towards non-caregiving adults and altered amygdala development. We hypothesized that maternal deprivation would be associated with reduced amygdala discrimination between mothers and strangers and increased parent report of indiscriminate friendliness behaviors.
Methods
67 youths [33 previously-institutionalized (PI); 34 Comparison (Comp); age-at-scan 4-17 y] participated in an fMRI experiment designed to examine amygdala response to mother versus stranger faces. In-scanner behavior was measured. Indiscriminate friendliness was assessed using parental report.
Results
Comparison youth showed an amygdala response that clearly discriminated mother versus stranger stimuli. PI youths, by contrast, exhibited reduced amygdala discrimination between mothers and strangers. Reduced amygdala differentiation correlated with greater reports of indiscriminate friendliness. These effects correlated with age-at-adoption, with later adoptions being associated with reduced amygdala discrimination and more indiscriminate friendliness.
Conclusions
Our results suggest that early maternal deprivation is associated with reduced amygdala discrimination between mothers and strangers, and reduced amygdala discrimination was associated with greater reports of indiscriminate friendliness. Moreover, these effects increased with age-at-adoption. These data suggest that the amygdala, in part, is associated with indiscriminate friendliness, and that there may be a dose-response relationship between institutional rearing and indiscriminate friendliness.
Keywords: Indiscriminate friendliness, attachment, institutional rearing, maternal deprivation, affective salience, amygdala development
Introduction
The impact of maternal deprivation in the formation of attachment-related behaviors has been explored in the animal (1-4) and human literature (5-8). Early maternal separation and institutional rearing (e.g., orphanages) has implications for mental health outcomes (9-12). One common outcome in previously-institutionalized (PI) children is a behavior often called “indiscriminate friendliness,” which includes reduced reticence and atypical approach behaviors towards all adults, including strangers (13). It is important to note that the term is a misnomer, as the behavior noted in these children has been noted to be “neither ‘friendly’ nor ‘sociable (14).’” Tizard and Hodges note that this behavior was the greatest source of complaints from teachers, as the children engaged in attention-seeking behaviors, attempting to engage in social approach towards teachers too frequently and at inappropriate times, in a way that disrupted the classroom environment (15). This common phenotype following deprivation may be associated with Reactive Attachment Disorder, Indiscriminate Type (16), or may be present in the absence of dysfunctional attachment (17-20).
Under most circumstances, the early human environment is highly constrained in that a caregiver will typically remain present. Caregiver presence is a necessary and species-expected environmental agent (21), which instantiates a developmental learning process that includes: (1) approaching the caregiver; (2) learning to recognize the caregiver; (3) forming a preference for the caregiver and avoiding non-caregiver adults (2). Thus, experience with a primary caregiver facilitates a process whereby infants show preference for that caregiver over and above all other adults. In contrast, indiscriminate friendliness is characterized by attenuated affective discrimination between caregiver and strangers. Caregiver preference development is profoundly influenced by stability of care. Several factors work against this process in an institutional environment, including fluctuating staff, lack of caregiver sensitivity, and physical deprivation (22). If presence of a stable caregiver is required for typical attachment-related behaviors, including discrimination between mothers and strangers, then it is not surprising that PI children are at elevated risk for displaying indiscriminate behaviors (23).
Work in humans and non-human animals suggests that the amygdala plays an important role in representing affective relevance of the caregiver. Maternal absence alters the trajectory of amygdala development (24-26). In its broader role, the amygdala represents motivational salience of stimuli (27-31). For this reason, the amygdala may be well-suited to mediate affective discrimination of attachment figures; that is, the amygdala’s role in detecting affective salience and motivating behavior may also serve to represent the importance of the maternal stimulus. Work in nonhuman primates has demonstrated that the amygdala is necessary for expression of caregiver preference; infants with amygdala lesions showed lack of maternal preference after maternal separation, despite initially demonstrating species-typical bonding behaviors with mothers (32, 33). Similarly, children’s amygdala is preferentially engaged by the mother stimulus over and above that for an unfamiliar adult, and this amygdala response has been found to mediate specific approach behaviors to caregivers (21). These findings suggest that amygdala response is associated with intense emotional relationships. The hypothesis that amygdala activity supports attachment-related behaviors is substantiated by findings that mothers also show increased amygdala activation by their own child, an effect that does not seem to merely reflect familiarity (34, 35). Taken together, these data suggest a role for amygdala in the dyadic and intense interaction between mother and child, perhaps in recognizing affective salience of the primary caregiver.
Notwithstanding evidence for the amygdala’s involvement in human attachment representation, little is known about the mechanism by which deprivation-induced brain development gives rise to indiscriminate friendliness behaviors. Of note, PI children have been shown to have atypical amygdala development, with children adopted later having larger amygdala volumes compared to early-adopted/non-adopted children (36, 37). In addition, PI children have been shown to exhibit amygdala hyperactivation to emotionally arousing faces (38). These findings with human samples mirror the effects of maternal deprivation observed in several other altricial species (24, 25, 39, 40).
We utilized a previously-published fMRI paradigm (21) to examine neural responses to mother and stranger stimuli in PI youth and a typically-raised comparison (Comp) group. Given the amygdala’s role inselectively representing affective/motivational salience of caregivers (21, 41), we hypothesized that children with a history of maternal deprivation would show indiscriminate amygdala response to all social stimuli that would mirror the indiscriminate friendliness seen both by parents and in laboratory settings in this population. We predicted that, unlike typically-raised children who show more robust amygdala response to their mothers relative to strangers [21], PI children would show reduced amygdala discrimination between mothers and strangers, a prediction based on previous work showing hyperactivity of the amygdala [38]. We anticipated that amygdala reactivity would be atypically high to strangers in the PI group, despite the non-fearful nature of our stimuli. Moreover, we anticipated that children with less amygdala discrimination would exhibit more indiscriminate friendliness. Based on previous findings of age-at-adoption associations with indiscriminate friendliness (10, 42), we hypothesized that children adopted at a later age would show more indiscriminate friendliness and less differential amygdala response to mothers and strangers.
Methods and Materials
Participants
Functional MRI data were collected from 75 youths. Comp youth (N=37), living with biological parents, and PI youth (N=38) with a history of institutional rearing and resultant deprivation were studied. All PI youths were adopted by families in the United States via international adoption. Although all youths in institutional care experience maternal deprivation (43), institutional care is also commonly associated with physical, nutritional, and sensory deprivation, in addition to adverse prenatal exposures (43).
Of the 75 participants for whom data was collected, 67 were included in our study (Comp N=34, mean age-at-scan=11 ± 4 years, range 4-17 years; PI N=33, mean age-at-scan=10 ± 3 years, range 6-15 years). Twenty-five Comps have been previously published (21), while all PI data have never been published. There was no significant difference in number excluded or reason for exclusion by group (Comp=3, PI=5, p>0.05): motion artifacts (Comp=0, PI=1, p>0.05), clinical imaging findings (Comp=0, PI=1, p>0.05), imaging outliers (Comp=3, PI=2, p>0.05)1. Parents completed a series of questionnaires, including an indiscriminate friendliness questionnaire (detailed below), the Security Scale to assess attachment-related behaviors (44), the Child Behavior Checklist (45), and a telephone interview regarding medical and psychiatric history. Relevant demographic data, including country of origin (Supplement: Table S1) and age-at-orphanage/adoption were collected for each PI participant. To address variability in pre-adoption quality of care and possible prenatal exposure to alcohol, we included additional data (Supplement: Figure S1) related to preadoption parameters in our PI population: (1) measures of orphanage quality of care and (2) prevalence of typical Fetal Alcohol dysmorphological facial features by photographs, that may suggest prenatal alcohol exposures. Modified version of the Hoyme criteria (46) as well as the Astley photographic scale (47), were utilized to quantify upper lip and philtrum characteristics on a scale of 1-5. However, no definitive FAS diagnoses can be made based on these data alone (47).
Youths with a history of serious medical illness, including head trauma, seizure disorder, or with borderline intellectual functioning (IQ<70) were excluded from the study. All participants were right-handed. Families had incomes above the US median annual household income ($48,451) (US Census Bureau, 2006). This study was approved by the UCLA IRB, and informed consent and assent were obtained.
Questionnaires
Indiscriminate Friendliness scale
To examine stranger-related behaviors, we adapted indiscriminate friendliness measures of multiple labs (17, 42, 48, 49), which have been shown to have convergent validity (19). Previous work has shown that parental report of indiscriminate friendliness correlates well with observation of children and families by clinical psychology staff (10). Parent-administered questionnaire (1-10 scale) assessed the following: (1) How likely do you think it is that your child would willingly go home with a stranger? (2) How likely do you think it is that your child would wander off (and not be distressed)? (3) How trusting is your child with new adults?
Attachment Security
In order to examine mother-related behaviors in our sample, youths completed the Security Scale (44), which provides a continuous measure of their perception of security in parent-child relationships in middle childhood and early adolescence. Although frequency and intensity of caregiver-directed attachment-related behaviors decline after infancy, these behaviors continue to be observed during childhood and adolescence, particularly during stress (44). Items are rated on a 4-point scale, with higher scores signifying more secure attachment. The instrument provides scores for three subscales: 1) children’s belief that attachment figure is responsive and available; 2) children’s reliance on attachment figure in times of stress; and 3) children’s ease and interest in communicating with attachment figure. Kerns et al. (1996) demonstrated good internal consistency (Cronbach’s α = .84 and .88, respectively), and the measure was highly correlated (p<0.01) with children’s self-esteem, peer acceptance, observer ratings of friendship quality, and behavioral conduct, but longitudinal studies have not been performed to test its concordance with infant measures of attachment security (50).
Additional Questionnaires
The Child Behavior Checklist (CBCL) (51) was utilized to examine anxiety, mood, and inattention symptoms of subjects (Supplement: Table S1). Psychiatric disorders were reported via history by parents. The Wechsler Abbreviated Scale of Intelligence was also administered to participants over 5 years old (52).
Experimental Task
We used a previously-published fMRI block-design task (21). Participants viewed color pictures of their mother (adopted or biological) and an age- and ethnicity-matched unfamiliar individual, who was another participant’s mother (stranger) in alternating 28-second blocks. Mother2 and stranger stimuli posed happy and neutral expressions, with one exemplar of each emotional state per stimulus set. These images were taken by the experimenter in a set location, and standardized for size and luminance. Color images had a vertical visual angle of approximately 15°. Participants were instructed to respond quickly (within 1500 milliseconds) by pressing a button for happy expressions (regardless of model), which were presented 50% of the time with fixed random order. Thus, the task required responses for target expressions (happy) and inhibiting response for distracter (neutral). Four blocks each of mother and stranger, and 3 fixation blocks, were presented in alternation (+MSMS+SMSM+), counterbalanced across subjects. Each block contained 18 identical mother- or stranger-stimuli (with happy or neutral expressions), resulting in 144 total stimuli—(72 mother, 72 stranger). Each stimulus was presented for 500 milliseconds followed by 1-second fixation. Video goggles (Resonance Technology, Inc., model: VisuaStim Digital) were utilized to present stimuli and a response pad (Current Designs, Inc., model 932 fORP) to record behavioral responses. The task lasted 4:54 minutes. Prior to scanning, participants were given the opportunity to practice to ensure that they understood and could perform the task.
Image Acquisition
Images were acquired using a Siemens Trio 3T MRI scanner (Malvern, PA). Foam padding placed around the head reduced motion artifacts. Whole-brain, high-resolution structural T1 images were acquired as follows: MP-RAGE, 1 × 1 × 1 mm resolution, 256 mm FOV, 192 sagittal slices. Functional T2*-weighted echoplanar images (EPI) were acquired during the behavioral task at 30-degree oblique angle as follows: 34 slices, 4 mm slice thickness (skip 0), TR=2000 ms, TE=30 ms, flip angle=90°, matrix 64 × 64.
Procedure
Participants attended two sessions: 1) behavioral measures were collected, and participants were acclimated to the scanner environment with an MRI replica; 2) the fMRI task was administered.
Behavioral Data Analysis
Out of Scanner Behavioral Measures
Because total indiscriminate friendliness scores were skewed (skewness=1.22), we log-transformed these values for analyses. To examine group differences in indiscriminate friendliness, we performed a univariate ANCOVA on total indiscriminate friendliness score, controlling for age-at-scan and IQ. To determine if there was a dose-response effect of time spent in institutional care on level of indiscriminate friendliness, we correlated indiscriminate friendliness score and age-at-adoption, controlling for age-at-scan and IQ.
In-Scanner Behavioral Measures
Reaction time and behavioral response rates were analyzed in SPSS. Average reaction times, correct hit rate and false alarm rates were calculated for each group. Subjects (Comp=4, PI=5) were excluded from behavioral analysis for correct hit rate <50%. Since the task was employed mostly to ensure engagement (e.g., not sleeping), we justify these rather lenient compliance thresholds. No subjects were excluded from the imaging analysis based on correct hit rate. Repeated-measures ANCOVAs for each variable (reaction time, false alarm rate, correct hit rate) were performed in SPSS using within-subject variable of stimulus type and between-subjects variable of group, with age-at-scan and mean reaction time (or false alarm or correct hit rate) as covariates.
fMRI Data Analysis
Preprocessing and Single-subject Analysis
Functional imaging data were analyzed using Analysis of Functional Neuro Images (AFNI) software (53). All data with motion artifact of greater than 2.5 mm in any direction were removed. Slice-timing correction Talairach spatial normalization (54), and smoothing with an anisotropic 6 mm Gaussian kernel were performed. Single-subject models included repeated measures for stimulus types (mother, stranger), as well as six motion parameters that were convolved with the hemodynamic response function. General linear model (GLM) was performed to fit beta weights to each regressor, modeling correlated drift using linear and quadratic factors within each voxel. Additionally, psychophysiological interaction analyses (PPI) were performed using the functionally-defined amygdala as a seed region (see Supplemental Methods).
Group-level Analysis
We performed a linear mixed effect (LME) voxel-wise whole-brain AFNI analysis, with within-subjects factor of stimulus type and between-subjects factor of group, with age-at-scan as a covariate. Correction for multiple comparisons was applied at cluster-level for the functionally-derived left amygdala ROI following Monte Carlo simulations conducted in AFNI’s AlphaSim (p<0.01). This method offers reasonable multiple-comparisons correction during group-level analyses in small ROIs (55).
Initial analyses to decompose the interaction in the AFNI GLM utilized cluster-level statistics, but correlation analyses were performed with an anatomical ROI (defined by a right amygdala mask in the Talairach-Tournoux atlas implemented in AFNI) in order to avoid redundancy (56). Extracted beta weights were analyzed using a repeated-measures ANCOVA with the within-subjects factor of stimulus type (mother, stranger) and between-subjects factor of age-at-adoption (values designated as 0 for Comps, in order to simulate a continuous rather than categorical variable), controlling for age-at-scan and IQ. To examine dose-response relationships, we correlated amygdala response and age-at-adoption, controlling for age-at-scan, age-at-adoption, and IQ. We also correlated indiscriminate friendliness with differential amygdala response (mother – stranger).
Habituation analysis was performed by extracting beta weights from the functionally-defined amygdala ROI during the first and second half of the experiment separately. Change scores were calculated (second-first block) for both stimulus types and subjected to a repeated-measures ANCOVA (age-at-scan, age-at-adoption, and IQ as covariates), with the within-subject factor of stimulus type.
Results
Participants
Of the 67 participants included in analyses, there was a trend for more PI females (p=0.07). There was no significant group difference in age-at-scan. There was a group difference in IQ, with Comps having higher IQ (p<0.05); neither group had below-average IQ (Table 1). Region of origin data are provided in Table 2.
Table 1.
Demographic Data
| Comp | PI | Sig. | |
|---|---|---|---|
| N | 34 | 33 | — |
| Age at scan (y), Mean (SD) | 11 (4) | 10 (3) | p>0.05 |
| Range | 4-17 | 6-15 | |
| Months at adoption, Mean (SD) | — | 37 (31) | — |
| Range | — | 6-120 | — |
| Age orphaned (mo), Mean (SD) | — | 12 (18)a | — |
| Range | — | 0-72 | — |
| Time (mo) in orphanage | — | 26 (19)b | — |
| Range | — | 5-65 | — |
| Parent’s rating – quality of care | — | 0.93 (0.86) c | — |
| Parent’s rating – quantity of care | — | 1.11 (0.80) c | — |
| Gender (% male) | 65% | 42% | p>0.05 |
| Mean Full-Scale IQ, Mean (SD) | 110 (17) d | 100 (15) e | p<0.05 |
| Presence of Any Psych Dx, N (%) | 1 (3%) | 11 (34%) f | p<0.01 |
N=3 missing data from PI group.
N=5 missing data from PI group.
N=6 missing data from PI group.
N=8 missing data from Comp group.
N=2 missing data from PI group.
N=1 missing data from PI group.
Table 2.
Region of origin – PI children
| Region of Origin | % of PI Sample |
|---|---|
| Eastern Europe | 76 |
| East and South Asia | 24 |
N=4 missing data from PI group.
Behavioral Findings
Indiscriminate Friendliness scale
Indiscriminate friendliness score differed between groups (controlling for age-at-scan and IQ) (F=4.33, p<0.05), with PI being more indiscriminately friendly per parent-report. There was a positive correlation between age-at-adoption and indiscriminate friendliness score (controlling for IQ and age-at-scan) (r=0.37, p<0.05)3, which became under-powered when we examined the PI group alone (Figure 1).
Figure 1.
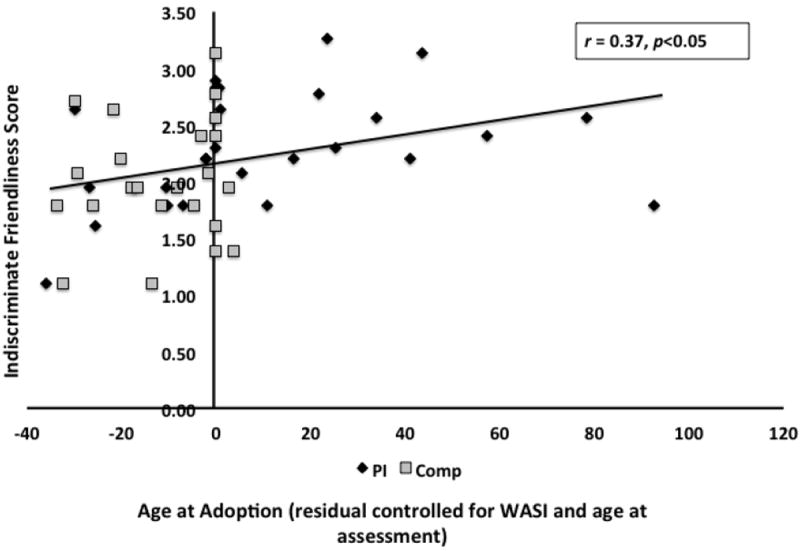
Older age-at-adoption was associated with higher parent report of indiscriminate friendliness (adjusted for IQ and age-at-scan). Pearson correlation (r=0.37, p<0.05). Missing IF data N=11 from Comparison group and N=4 from PI group. Total N=40.
Attachment Security
There were no group differences in the Security Scale score, (controlling for age-at-scan and IQ) (p>0.05). The Security Scale score (controlling for age-at-scan and IQ) did not correlate with age-at-adoption (r=-0.39, p>0.05).
Behavioral Data Analysis
Four Comp and 5 PI were excluded for low hit rates, which did not differ between groups (p>0.05). Correct hits (to happy), errors of commission (to neutral), and reaction time (correct trials) were measured. There were no significant differences between groups for any of these variables. There was no effect of age except in the case of reaction time (F=8.23, p<0.05, partial η2=0.112); age-at-scan was associated with faster reaction times.
fMRI Findings
Whole-brain analysis
LME analysis revealed a Group × Stimulus Type interaction (F=4.003, p<0.05, small-volume-corrected): left amygdala ([-27 -3 -19]; k=47; Figure 2A). Other activated regions are provided in Supplemental Data (Table S2).
Figure 2.
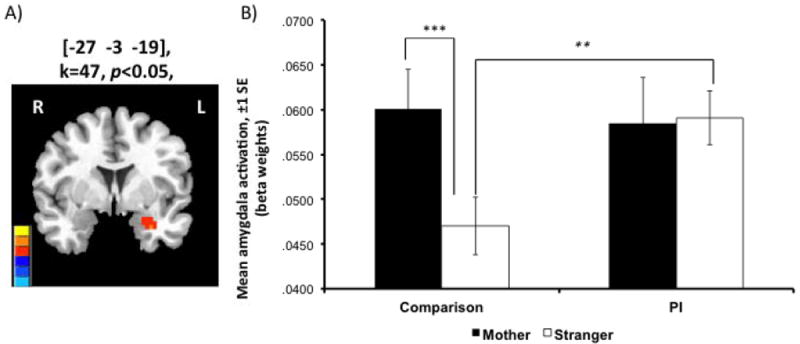
A) Whole-brain LME analysis revealed a Group × Stimulus Type interaction (F=4.003, p<0.05, small-volume-corrected): left amygdala ROI (peak [-27 -3 -19]; k=47. B) Unlike the Comp group, who showed greater amygdala signal for mother than stranger stimuli, the PI group showed equivalent signal across stimuli (controlling for age-at-adoption, age-at-scan, and IQ). Stars indicate post hoctests: Mother vs. Stranger – Comp: **p<0.001, PI: p>0.05; Comp vs. PI – Mother: p>0.05, *Stranger: p<0.05.
Amygdala response to mothers and strangers as a function of early maternal deprivation
We used predicted values from a repeated-measures GLM in SPSS with within-subjects factor of stimulus type and covariates of age-at-adoption, age-at-scan, and IQ to examine effects by stimulus and group. Post hoc t-test showed that Comps exhibited higher amygdala signal for mother than stranger stimuli (t=7.00 p<0.05), whereas, PI did not differ (t=-0.09, p>0.05) (Figure 2B). There was no significant difference in response to mothers between groups (p>0.05), though PI children did exhibit increased response to strangers versus Comparisons (t=-2.74, p<0.05)4.
Habituation Analysis
Repeated-measures GLM showed a main effect of group (F=5.42, p<.025), such that Comps decreased amygdala response by late trials, but PI did not (Figure 3).
Figure 3.
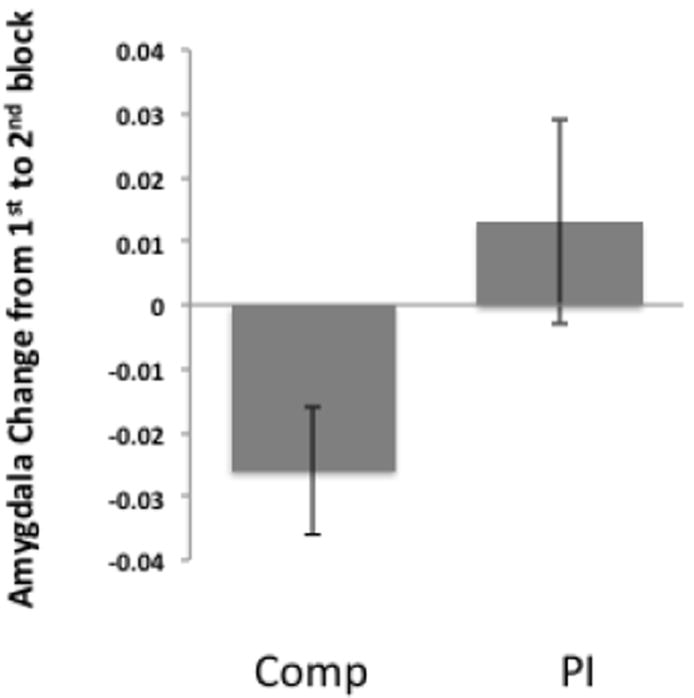
Amygdala habituation. Comparison youth exhibited a greater decrease in amygdala signal to facial stimuli over the course of the scan session relative to PI youth.
PPI analysis
An ANOVA comparing the two groups in the difference between mothers and strangers revealed significant group differences in connectivity between the left amygdala and several cortical regions (Supplement: Table S3), most notably the ventral anterior cingulate. This was the only region where amygdala connectivity was greater in the comparison group than in the PI group.
Correlations with amygdala discrimination
There was a negative correlation between age-at-adoption and values extracted from left anatomically-defined amygdala (mother - stranger) in PI children, with those adopted later exhibiting attenuated amygdala discrimination (r=-0.39, p<0.05) (Figure 4). Finally, children with higher indiscriminate friendliness exhibited more attenuated amygdala (anatomically-defined) discrimination between mothers and strangers (r=0.28, p<0.05), controlling for age-at-scan, age-at-adoption, and IQ (Figure 5). Indiscriminate friendliness correlations became underpowered when examining only the PI group, due to missing data as noted in the figures. There was no relationship between amygdala discrimination and the Security Scale.
Figure 4.
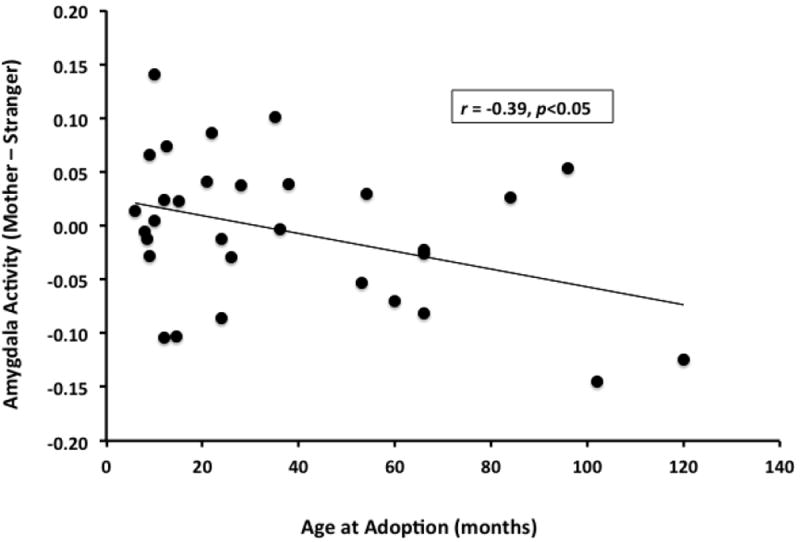
Older age-at-adoption in PI group was associated with less typical amygdala discrimination between mother and stranger stimuli. Pearson correlation r=-0.39, p<0.05. PI group N=31.
Figure 5.
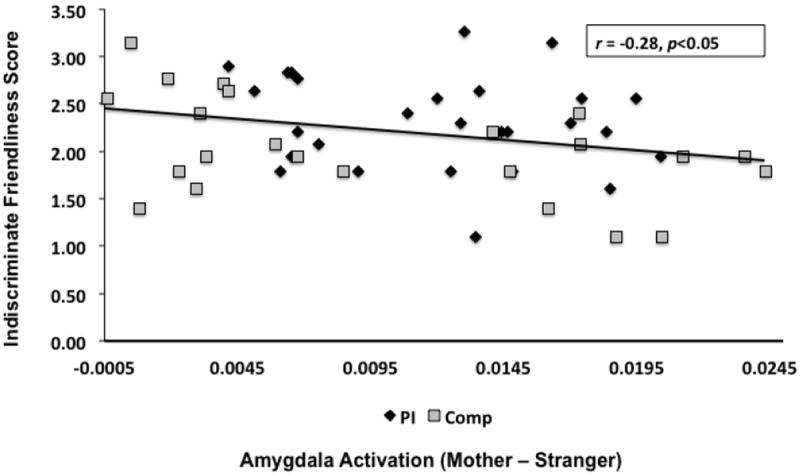
Association between amygdala discrimination and indiscriminate friendliness. Participants with more attenuated amygdala discrimination between mother and stranger stimuli tended to exhibit more indiscriminate behaviors as reported by parents (r=-0.28, p<0.05; controlling for IQ, age-at-scan, and age-at-adoption). Missing IF data N=11 from Comparison group and N=4 from PI group. Total N=40.
Discussion
We tested the hypothesis that early-life maternal deprivation would be associated with attenuated amygdala discrimination between mothers and strangers and parent-report of indiscriminate friendly behaviors. We focused on the amygdala because of its role in representing intense relationships (21, 32, 33, 35). Consistent with our predictions, we observed that relative to the typically-raised comparison group, PI youth exhibited equivalent amygdala response to mothers and strangers. This lack of discrimination was the result of atypically high amygdala response to strangers in PI youth, whereas responses to mother stimuli were equivalent across groups. Moreover, the amygdala response in PI youth did not attenuate over the course of the scan session as evidenced by habituation analysis and showed decreased functional coupling with the ventral anterior cingulate. This prefrontal region has been associated with regulatory skill [69], suggesting that the current neural findings may support previous work associating indiscriminate behaviors with low inhibitory control abilities [70]. Amygdala findings were associated with age-at-adoption, such that younger age-at-adoption was associated with more typical differentiation between mother and stranger stimuli and older age-at-adoption was associated with reduced discrimination. Additionally, PI demonstrated more parent-reported indiscriminately-friendly behaviors, which correlated with amygdala discrimination; participants with reduced amygdala mother-stranger discrimination tended to be rated as exhibiting more indiscriminate friendly behaviors.
The association of amygdala response with indiscriminate affective behaviors in PI youth suggests that the amygdala detects affective salience appropriately (mother) and inappropriately (stranger), unlike typically-raised comparison youth, who showed higher amygdala activation to mothers. The current findings suggest that highly-affiliative behaviors directed towards unfamiliar adults, may in part be explained by inappropriate amygdala response to strangers. Indiscriminate friendliness is observed during institutional care (23) and has been described as an adaptive behavior in that setting (perhaps eliciting maternal care from unfamiliar adults) (17). However, these behaviors often continue after adoption, and it has been suggested that because of their enduring nature, they may be understood in terms of biological adaptations at the level of brain development (1).
The process of distinguishing primary caregivers and strangers typically occurs during a sensitive period soon after birth. In rat pups, maternal odor learning (57, 58) has been shown to develop within the first 10 days of life. In humans, this process requires more ontological time, and typically the discrimination emerges within the first year (59, 60). How this affective discrimination is then maintained over the course of development is not yet well-understood, although work in typical children and adults suggests that the amygdala plays an important role in representing the affective salience of intimate relationships (21, 35, 61) and may be part of the maintenance process. In the current study, we observed associations with age-at-adoption for both amygdala response to mothers versus strangers and indiscriminate friendliness behaviors, where earlier removal from institutional care was associated with more typical phenotypes. Therefore, it is possible that the neural and behavioral phenotypes observed in the current study are constrained by a sensitive period for mother-stranger discrimination. Maternal deprivation may have removed opportunities to learn about mother-stranger discrimination in infancy, resulting in PI children continuing to detect affective salience inappropriately.
We examined indiscriminate friendliness as a dimensional construct rather than examining dysfunctional attachment as a diagnosis (Reactive Attachment Disorder, Indiscriminate Type). We chose this route because there have been several studies suggesting that attachment type and indiscriminate friendliness are independent of one another (17-19). One investigation of the phenomenology of RAD, disinhibited type recently demonstrated that children can have organized attachment despite presence of indiscriminate friendliness (62). Consistent with previous studies, we observed group differences in indiscriminate behaviors, but not in subjects’ reports of attachment to parents, suggesting a dissociation between indiscriminate friendliness and attachment representations in the current sample. Additionally, the imaging data suggest that it was the response to stranger stimuli, rather than mother stimuli, that distinguished PI from Comps. The current study may be useful in explaining the behavioral dissociation between attachment to parent and indiscriminate friendliness.
Our study has several limitations. First, psychiatric diagnoses were assessed by parental report. We did not perform a structured diagnostic interview. Parent-reported diagnoses may be inaccurate. Since we chose to study indiscriminate friendliness as a behavioral construct rather than the specific phenomenology of attachment disorders, this limitation may be mitigated. There is no question that degree of psychopathology varied by group (with PI children exhibiting more dysfunctional behaviors in general); in fact, much of the rationale for studying this population is the possibility of early intervention. We have thus provided in the Supplementary Data section a comparison of CBCL scores by group as an exploratory finding as well as repeated all analyses covarying for the presence of mental illness. Another limitation is lack of access to prenatal/developmental histories for PI. This is a common issue for investigators studying this population. Randomized control intervention work suggests that institutionalization itself may be the most significant factor in children’s developmental histories (63). The experimental benefit to studying this population is knowledge of the timing of deprivation. The observed dose-response associations with age-at-adoption provide additional confidence that observed associations with group were influenced by maternal deprivation. However, given that indiscriminate friendliness behaviors also are related to time with adoptive family (they decrease with more time), it is impossible to rule out that this factor, too, may play a role. Furthermore, it is important to note, that although other patient populations, including those with Williams syndrome [6-8] and children who have experienced maltreatment [9], are known to exhibit undifferentiated approach behaviors, it is unclear to what extent the neural correlates would be similar to that in the PI population, although both populations have been associated with amygdala anomalies [6, 7, 10-13].
We investigated the neural correlates of indiscriminate friendliness; amygdala discrimination between mother and stranger stimuli was attenuated in PI children. Importantly, attenuated amygdala discrimination between mother and stranger stimuli correlated with indiscriminate friendliness. Characterizing the pathophysiology of indiscriminate friendliness behaviors may provide important insight into understanding how early deprivation contributes to aberrant behaviors. By studying these pathways longitudinally, we may further describe the relationship between risk and resilience from a developmental perspective. Describing these basic processes is critical for implementing early intervention strategies to improve psychiatric outcomes in children.
Supplementary Material
Acknowledgments
This work was supported by NIMH R01MH091864 (NT).
Footnotes
In the supplemental analysis using anatomical ROI, there were two additional imaging outliers from PI group excluded for >2.5 SD from mean.
One child viewed images of his father and an ethnically-matched male stranger.
Of note, there was also a negative correlation of indiscriminate friendliness with time spent in adoptive families (r=-0.31, p<0.05).
Of note, primary repeated-measures analysis was re-performed with gender as a factor, and demonstrated no significant effect of this variable.
Financial Disclosures: All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rutter M, O’Connor TG. Are there biological programming effects for psychological development? Findings from a study of Romanian adoptees. Dev Psychol. 2004;40:81–94. doi: 10.1037/0012-1649.40.1.81. [DOI] [PubMed] [Google Scholar]
- 2.Insel TR, Young LJ. The neurobiology of attachment. Nat Rev Neurosci. 2001;2:129–136. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- 3.O’Connor TG, Cameron JL. Translating research findings on early experience to prevention: animal and human evidence on early attachment relationships. Am J Prev Med. 2006;31:S175–181. doi: 10.1016/j.amepre.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Sabatini MJ, Ebert P, Lewis DA, Levitt P, Cameron JL, Mirnics K. Amygdala gene expression correlates of social behavior in monkeys experiencing maternal separation. J Neurosci. 2007;27:3295–3304. doi: 10.1523/JNEUROSCI.4765-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowlby J. The effect of separation from the mother in early life. Ir J Med Sci. 1954:121–126. doi: 10.1007/BF02952876. [DOI] [PubMed] [Google Scholar]
- 6.Bowlby J. Developmental psychiatry comes of age. Am J Psychiatry. 1988;145:1–10. doi: 10.1176/ajp.145.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Ainsworth M, Boston M, Bowlby J, Rosenbluth D. The effects of mother-child separation: a follow-up study. Br J Med Psychol. 1956;29:211–247. doi: 10.1111/j.2044-8341.1956.tb00915.x. [DOI] [PubMed] [Google Scholar]
- 8.Beckett C, Bredenkamp D, Castle J, Groothues C, O’Connor TG, Rutter M. Behavior patterns associated with institutional deprivation: a study of children adopted from Romania. J Dev Behav Pediatr. 2002;23:297–303. doi: 10.1097/00004703-200210000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Colvert E, Rutter M, Beckett C, Castle J, Groothues C, Hawkins A, et al. Emotional difficulties in early adolescence following severe early deprivation: findings from the English and Romanian adoptees study. Dev Psychopathol. 2008;20:547–567. doi: 10.1017/S0954579408000278. [DOI] [PubMed] [Google Scholar]
- 10.Rutter M, Colvert E, Kreppner J, Beckett C, Castle J, Groothues C, et al. Early adolescent outcomes for institutionally-deprived and non-deprived adoptees. I: disinhibited attachment. J Child Psychol Psychiatry. 2007;48:17–30. doi: 10.1111/j.1469-7610.2006.01688.x. [DOI] [PubMed] [Google Scholar]
- 11.Pinheiro P. World Report on Violence Against Children. UNICEF; 2006. [Google Scholar]
- 12.Rutter M, Sonuga-Barke EJ, Castle J. I. Investigating the impact of early institutional deprivation on development: background and research strategy of the English and Romanian Adoptees (ERA) study. Monogr Soc Res Child Dev. 2010;75:1–20. doi: 10.1111/j.1540-5834.2010.00548.x. [DOI] [PubMed] [Google Scholar]
- 13.Hodges J, Tizard B. Social and family relationships of ex-institutional adolescents. J Child Psychol Psychiatry. 1989;30:77–97. doi: 10.1111/j.1469-7610.1989.tb00770.x. [DOI] [PubMed] [Google Scholar]
- 14.O’Connor TG, B D, Rutter M the English and Romanian Adoptees (ERA) Study Team. Attachment disturbances and disorders in children exposed to early severe deprivation. Infant Mental Health Journal. 1999;20:10–29. [Google Scholar]
- 15.Tizard B, Hodges J. The effect of early institutional rearing on the development of eight year old children. Journal of child psychology and psychiatry, and allied disciplines. 1978;19:99–118. doi: 10.1111/j.1469-7610.1978.tb00453.x. [DOI] [PubMed] [Google Scholar]
- 16.O’Connor TG, Rutter M. Attachment disorder behavior following early severe deprivation: extension and longitudinal follow-up. English and Romanian Adoptees Study Team. J Am Acad Child Adolesc Psychiatry. 2000;39:703–712. doi: 10.1097/00004583-200006000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Chisholm K. A three year follow-up of attachment and indiscriminate friendliness in children adopted from Romanian orphanages. Child Dev. 1998;69:1092–1106. [PubMed] [Google Scholar]
- 18.Smyke AT, Dumitrescu A, Zeanah CH. Attachment disturbances in young children. I: The continuum of caretaking casualty. J Am Acad Child Adolesc Psychiatry. 2002;41:972–982. doi: 10.1097/00004583-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Zeanah CH, Smyke AT, Dumitrescu A. Attachment disturbances in young children. II: Indiscriminate behavior and institutional care. J Am Acad Child Adolesc Psychiatry. 2002;41:983–989. doi: 10.1097/00004583-200208000-00017. [DOI] [PubMed] [Google Scholar]
- 20.American Psychiatric Association., American Psychiatric Association. Diagnostic and statistical manual of mental disorders : DSM-IV. 4. Washington, DC: American Psychiatric Association; 1994. Task Force on DSM-IV. [Google Scholar]
- 21.Tottenham N, Shapiro M, Telzer EH, Humphreys KL. Amygdala response to mother. Dev Sci. 2012;15:307–319. doi: 10.1111/j.1467-7687.2011.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van ijzendoorn MH, Palacios J, Sonuga-Barke EJS, Gunnar MR, Vorria P, McCall RB, et al. I. Children in Insitutional Care: Delayed Development and Resilience. Monogr Soc Res Child Dev. 2011;76:8–30. doi: 10.1111/j.1540-5834.2011.00626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bakermans-Kranenburg MJ, Steele H, Zeanah CH, Muhamderahimov RJ, Vorria P, Dobrova-Krol NA, et al. III. Attachment and Emotional Development in Institutional Care: Characteristics and Catch Up. Monogr Soc Res Child Dev. 2011;76:62–91. doi: 10.1111/j.1540-5834.2011.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ono M, Kikusui T, Sasaki N, Ichikawa M, Mori Y, Murakami-Murofushi K. Early weaning induces anxiety and precocious myelination in the anterior part of the basolateral amygdala of male Balb/c mice. Neuroscience. 2008;156:1103–1110. doi: 10.1016/j.neuroscience.2008.07.078. [DOI] [PubMed] [Google Scholar]
- 25.Ali I, Salzberg MR, French C, Jones NC. Electrophysiological insights into the enduring effects of early life stress on the brain. Psychopharmacology (Berl) 2011;214:155–173. doi: 10.1007/s00213-010-2125-z. [DOI] [PubMed] [Google Scholar]
- 26.Moriceau S, Sullivan RM. Maternal presence serves as a switch between learning fear and attraction in infancy. Nat Neurosci. 2006;9:1004–1006. doi: 10.1038/nn1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sander D, Grafman J, Zalla T. The human amygdala: an evolved system for relevance detection. Rev Neurosci. 2003;14:303–316. doi: 10.1515/revneuro.2003.14.4.303. [DOI] [PubMed] [Google Scholar]
- 28.Hamann S, Mao H. Positive and negative emotional verbal stimuli elicit activity in the left amygdala. Neuroreport. 2002;13:15–19. doi: 10.1097/00001756-200201210-00008. [DOI] [PubMed] [Google Scholar]
- 29.Ousdal OT, Jensen J, Server A, Hariri AR, Nakstad PH, Andreassen OA. The human amygdala is involved in general behavioral relevance detection: evidence from an event-related functional magnetic resonance imaging Go-NoGo task. Neuroscience. 2008;156:450–455. doi: 10.1016/j.neuroscience.2008.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paton JJ, Belova MA, Morrison SE, Salzman CD. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature. 2006;439:865–870. doi: 10.1038/nature04490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adolphs R. What does the amygdala contribute to social cognition? Ann N Y Acad Sci. 2010;1191:42–61. doi: 10.1111/j.1749-6632.2010.05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bauman MD, Lavenex P, Mason WA, Capitanio JP, Amaral DG. The development of social behavior following neonatal amygdala lesions in rhesus monkeys. J Cogn Neurosci. 2004;16:1388–1411. doi: 10.1162/0898929042304741. [DOI] [PubMed] [Google Scholar]
- 33.Bauman MD, Lavenex P, Mason WA, Capitanio JP, Amaral DG. The development of mother-infant interactions after neonatal amygdala lesions in rhesus monkeys. J Neurosci. 2004;24:711–721. doi: 10.1523/JNEUROSCI.3263-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swain JE, Lorberbaum JP, Kose S, Strathearn L. Brain basis of early parent-infant interactions: psychology, physiology, and in vivo functional neuroimaging studies. J Child Psychol Psychiatry. 2007;48:262–287. doi: 10.1111/j.1469-7610.2007.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leibenluft E, Gobbini MI, Harrison T, Haxby JV. Mothers’ neural activation in response to pictures of their children and other children. Biol Psychiatry. 2004;56:225–232. doi: 10.1016/j.biopsych.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 36.Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T, et al. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev Sci. 2010;13:46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mehta MA, Golembo NI, Nosarti C, Colvert E, Mota A, Williams SC, et al. Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: the English and Romanian Adoptees study pilot. J Child Psychol Psychiatry. 2009;50:943–951. doi: 10.1111/j.1469-7610.2009.02084.x. [DOI] [PubMed] [Google Scholar]
- 38.Tottenham N, Hare TA, Millner A, Gilhooly T, Zevin JD, Casey BJ. Elevated amygdala response to faces following early deprivation. Dev Sci. 2011;14:190–204. doi: 10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caldji C, Diorio J, Meaney MJ. Variations in maternal care in infancy regulate the development of stress reactivity. Biol Psychiatry. 2000;48:1164–1174. doi: 10.1016/s0006-3223(00)01084-2. [DOI] [PubMed] [Google Scholar]
- 40.Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ. Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology. 2005;30:2192–2204. doi: 10.1038/sj.npp.1300769. [DOI] [PubMed] [Google Scholar]
- 41.Todd RM, Cunningham WA, Anderson AK, Thompson E. Affect-biased attention as emotion regulation. Trends Cogn Sci. 2012;16:365–372. doi: 10.1016/j.tics.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 42.O’Connor TG, Marvin RS, Rutter M, Olrick JT, Britner PA. Child-parent attachment following early institutional deprivation. Dev Psychopathol. 2003;15:19–38. doi: 10.1017/s0954579403000026. [DOI] [PubMed] [Google Scholar]
- 43.Gunnar MR, Bruce J, Grotevant HD. International adoption of institutionally reared children: research and policy. Dev Psychopathol. 2000;12:677–693. doi: 10.1017/s0954579400004077. [DOI] [PubMed] [Google Scholar]
- 44.Kerns KA, Aspelmeier JE, Gentzler AL, Grabill CM. Parent-child attachment and monitoring in middle childhood. J Fam Psychol. 2001;15:69–81. doi: 10.1037//0893-3200.15.1.69. [DOI] [PubMed] [Google Scholar]
- 45.Achenbach TM, Edelbrock CS. Behavioral problems and competencies reported by parents of normal and disturbed children aged four through sixteen. Monogr Soc Res Child Dev. 1981;46:1–82. [PubMed] [Google Scholar]
- 46.Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, et al. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 institute of medicine criteria. Pediatrics. 2005;115:39–47. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Astley SJ. Comparison of the 4-digit diagnostic code and the Hoyme diagnostic guidelines for fetal alcohol spectrum disorders. Pediatrics. 2006;118:1532–1545. doi: 10.1542/peds.2006-0577. [DOI] [PubMed] [Google Scholar]
- 48.Tizard B, Hodges J. The effect of early institutional rearing on the development of eight year old children. J Child Psychol Psychiatry. 1978;19:99–118. doi: 10.1111/j.1469-7610.1978.tb00453.x. [DOI] [PubMed] [Google Scholar]
- 49.Hodges J, Tizard B. Social and Family Relationships of Ex-Institutional Adolescents. J Child Psychol Psyc. 1989;30:77–97. doi: 10.1111/j.1469-7610.1989.tb00770.x. [DOI] [PubMed] [Google Scholar]
- 50.Kerns KA, Klepac L, Cole AK. Peer relationships and preadolescents’ perceptions of security in the mother–child relationships. Dev Psychol. 1996;32:457–466. [Google Scholar]
- 51.Achenbach TM, Ruffle TM. The Child Behavior Checklist and related forms for assessing behavioral/emotional problems and competencies. Pediatr Rev. 2000;21:265–271. doi: 10.1542/pir.21-8-265. [DOI] [PubMed] [Google Scholar]
- 52.Clements GR. An Abbreviated Form of the Wechsler Intelligence Scale for Children. J Consult Psychol. 1965;29:92. doi: 10.1037/h0020970. [DOI] [PubMed] [Google Scholar]
- 53.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 54.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain : an approach to medical cerebral imaging Stuttgart. New York New York: G. Thieme; Thieme Medical Publishers; 1988. [Google Scholar]
- 55.Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biol Psychiatry. 2006;59:424–429. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 56.Poldrack RA, Mumford JA. Independence in ROI analysis: where is the voodoo? Social cognitive and affective neuroscience. 2009;4:208–213. doi: 10.1093/scan/nsp011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moriceau S, Sullivan RM. Neurobiology of infant attachment. Dev Psychobiol. 2005;47:230–242. doi: 10.1002/dev.20093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moriceau S, Sullivan RM. Unique neural circuitry for neonatal olfactory learning. J Neurosci. 2004;24:1182–1189. doi: 10.1523/JNEUROSCI.4578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carlson EA, Sampson MC, Sroufe LA. Implications of attachment theory and research for developmental-behavioral pediatrics. J Dev Behav Pediatr. 2003;24:364–379. doi: 10.1097/00004703-200310000-00010. [DOI] [PubMed] [Google Scholar]
- 60.Ainsworth MD, Bell SM. Attachment, exploration, and separation: illustrated by the behavior of one-year-olds in a strange situation. Child Dev. 1970;41:49–67. [PubMed] [Google Scholar]
- 61.Todd RM, Evans JW, Morris D, Lewis MD, Taylor MJ. The changing face of emotion: age-related patterns of amygdala activation to salient faces. Soc Cogn Affect Neurosci. 2011;6:12–23. doi: 10.1093/scan/nsq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gleason MM, Fox NA, Drury S, Smyke A, Egger HL, Nelson CA, 3rd, et al. Validity of evidence-derived criteria for reactive attachment disorder: indiscriminately social/disinhibited and emotionally withdrawn/inhibited types. J Am Acad Child Adolesc Psychiatry. 2011;50:216–231. e213. doi: 10.1016/j.jaac.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smyke AT, Zeanah CH, Jr, Fox NA, Nelson CA., 3rd A new model of foster care for young children: the Bucharest early intervention project. Child Adolesc Psychiatr Clin N Am. 2009;18:721–734. doi: 10.1016/j.chc.2009.03.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


