Abstract
Aims
Determine the extent to which buprenorphine injectors continue treatment with buprenorphine-naloxone or methadone, and the impact of these treatments on substance use and HIV risk in the Republic of Georgia.
Methods
Randomized controlled 12-week trial of daily-observed methadone or buprenorphine-naloxone followed by a dose taper, referral to ongoing treatment, and follow-up at week 20 at the Uranti Clinic in Tbilisi, Republic of Georgia. Eighty consenting treatment-seeking individuals (40/group) aged 25 and above who met ICD-10 criteria for opioid dependence with physiologic features and reported injecting buprenorphine 10 or more times in the past 30 days. Opioid use according to urine tests and self-reports, treatment retention, and HIV risk behavior as determined by the Risk Assessment Battery.
Results
Mean age of participants was 33.7 (SD5.7), 4 were female, mean history of opioid injection use was 5.8 years (SD4.6), none were HIV+ at intake or at the 12-week assessment and 73.4% were HCV+. Sixty-eight participants (85%) completed the 12-week medication phase (33 from methadone and 35 from buprenorphine/naloxone group); 37 (46%) were in treatment at the 20-week follow-up (21 from methadone and 16 from the buprenorphine/naloxone group). In both study arms, treatment resulted in a marked reduction in unprescribed buprenorphine, other opioid use, and HIV injecting risk behavior with no clinically significant differences between the two treatment arms.
Conclusions
Daily observed methadone or buprenorphine-naloxone are effective treatments for non-medical buprenorphine and other opioid use in the Republic of Georgia and likely to be useful for preventing HIV infection.
Keywords: buprenorphine abuse, treatment, Republic of Georgia
1. Introduction
Buprenorphine is available for opioid addiction treatment as a sublingual tablet or a sublingual tablet or film composed of 4 parts buprenorphine to one part naloxone; one or more of these products is available in at least 44 countries (Carrieri et al., 2006). The buprenorphine-naloxone combination was developed to reduce diversion and injecting use and it appears to have had that effect (Simojoki et al., 2008), however, these problems continue to occur (Bruce et al., 2009; Vicknasingam et al., 2010). In the US, almost all addiction treatment is done using buprenorphine- naloxone and approximately 640,000 patients received it in 2009, mostly in office based settings (Clark, 2010). In France, where buprenorphine has been the main product used in addiction treatment, more than 100,000 patients have received it (Diaz-Gomez et al., 2010). In September 2012 Reckitt Benckiser, the developer and manufacturer of buprenorphine-naloxone and buprenorphine announced it will discontinue distribution of tablets in the US and replace them with buprenorphine- naloxone film after reports of six overdose deaths in children who inadvertently took buprenorphine-naloxone tablets belonging to adults (Reckitt Benckiser Group, 2012).
Similar to other opioids, buprenorphine has reinforcing and subjective effects similar to methadone (Comer et al., 2005) with the potential for abuse and addiction (Comer and Collins, 2002; Comer et al., 2008; Pickworth et al., 1993; Zacny et al., 1997), particularly when administered intravenously where its effects are comparable to those of morphine and heroin (Sporer, 2004). Most cases of non-medical use have involved crushing buprenorphine, mixing it with water and injecting it (Chua and Lee, 2006; Jenkinson et al., 2005; Otiashvili et al., 2010; Singh et al., 2004). However, inhaling crushed tablets has also been reported, particularly in France (Roux et al., 2008).
Following the expansion of buprenorphine treatment in Europe after France introduced it in 1995 (Verster and Buning, 2005), non-medical use has been reported in at least twelve countries (EMCDDA, 2005) and identified as the main reason for entering treatment by 40% of opioid addicted patients in Finland and 8% in France (EMCDDA, 2008). In the Czech Republic, 42% of problem opioid users were using buprenorphine (Mravcik et al., 2010); in Australia, 11% of a national sample of injecting users reported recent injection of prescribed buprenorphine and 20% reported injecting illicitly-obtained buprenorphine (O’Brien et al., 2006). Non-medical buprenorphine use has also occurred in the U.S. where a post-marketing study found that about 22% of people seeking treatment for prescription opioid addiction used buprenorphine-naloxone during the past month and for about 2%, buprenorphine-naloxone was their primary drug of abuse (Cicero et al., 2007). Though these data are of concern, studies have also shown that significant portions of buprenorphine injectors use it to cope with withdrawal and not primarily for its reinforcing effects (Daniulaityte et al., 1988; Uosukainen et al., 2012). For example, self-treatment was reported as the main reason for non-prescription use of buprenorphine by 77.7% of users in Finland (Alho et al., 2007), 57% in France (EMCDDA, 2005), and 87% in Sweden (Hakansson et al., 2007).
1.1. Buprenorphine injection in Georgia
The first reports of buprenorphine injection in Georgia appeared in early 2000 (Gamkrelidze et al., 2004) and showed that buprenorphine tablets, reportedly smuggled from Europe, were sold for US $100 per 8 mg tablet and injected after crushing and dissolving them in water (Gamkrelidze et al., 2005). In 2005, methadone maintenance was available but access was extremely limited, and the share of buprenorphine injectors among drug users admitted for inpatient treatment reached 39% (Javakhishvili et al., 2006) even though buprenorphine was not available legally in Georgia at the time. Over the following 5 years, 95.5% of out-of-treatment opioid addicted individuals reported having injected buprenorphine at some time in their life with 75% injecting it in the last month (Otiashvili et al., 2010), and buprenorphine had become the most prevalent of all injected drugs. Consistent with data from Europe and the U.S., almost half of the buprenorphine injectors reported that they did it to relieve withdrawal or to stop use of other opioids (Otiashvili et al., 2010).
One theory about why widespread buprenorphine use occurred in Georgia was that increased police activity with random urine drug testing forced drug users to turn to buprenorphine because it was not part of the drug testing panel (Otiashvili et al., 2008). In addition, many users thought that the relatively long-lasting effect of buprenorphine with less obvious signs of intoxication, compared to other opioids, reduced the chances of being spotted by the police (Otiashvili et al., 2010).
1.2. Treatment of buprenorphine-addicted injectors
Despite a growing number of reports about buprenorphine addiction, there have been only a few reports focusing on its treatment. One was an Iranian study that compared 50 mg of daily methadone to 5 mg of daily sublingual buprenorphine or 50 mg of daily oral naltrexone over 24 weeks and found that the methadone had the best retention, followed by sublingual buprenorphine, followed by naltrexone (J. Ahmadi et al., 2003). In another study patients were randomized to 40 mg of methadone, 4 mg of buprenorphine, or 0.4 mg of daily clonidine and outcomes measured over 12 weeks (M. Ahmadi et al., 2003) and retention on methadone was significantly better than buprenorphine. However both medications were well accepted by buprenorphine injectors and outcomes on both were significantly better than if treated only with clonidine. In Finland, a naturalistic follow-up study found an 83% retention rate in opioid dependent patients that were injecting buprenorphine and treated with sublingual buprenorphine (Aalto et al., 2011).
In view of these limited data on treatment for buprenorphine injectors, we conducted a randomized 12-week pilot study that aimed to determine: 1) the extent to which buprenorphine injectors accept and respond to treatment with daily observed dosing using Buprenorphine-naloxone or methadone; and 2) to examine the impact of these treatments on HIV risk, injection use, and other addiction treatment outcomes.
2. METHODS
2.1. Treatment setting
The study was done at the Addiction Research Centre, Alternative Georgia, a independent non-profit research institution located in a residential area in one of the central districts of Tbilisi. It is closely affiliated with the nearby Centre for Medical, Socio-economic and Cultural Issues, the second largest addiction program in the country and one that provides in-patient detoxification, psychosocial-based outpatient treatment, and methadone maintenance.
2.2. Participants
Patients were recruited through word of mouth, fliers and advertisements in addiction clinics, harm reduction programs, and other facilities frequented by injection drug users. All screening, assessment and follow-up evaluations were done at Uranti. The clinical director (ZS) was responsible for enrolling participants, as well as for assigning them to interventions following randomization. The consent form included information about the purpose of the study, pharmacology of methadone and Buprenorphine-naloxone, the importance of taking medication as prescribed and keeping appointments, the conditions under which medication can be stopped, and the times when assessments are needed. Eligibility criteria included: opioid dependent with physiological features for the past three or more years according to ICD-10; age 25 or above as per Georgian regulations; injecting buprenorphine 10 or more times in the past 30 days; buprenorphine and/or opioid positive urine test; not on methadone maintenance in last 4 weeks; stable address within Tbilisi area and not planning to move; home or cellular telephone number at which the participant can be reached; and willingness and ability to give informed consent and otherwise participate, including daily clinic attendance since take-home dosing is not permitted by Georgian law. Institutional Review Boards at the University of Pennsylvania and the Georgian National Council on Bioethics approved the study.
2.3. Randomization
Patients were randomized 1:1 to methadone or Buprenorphine-naloxone in blocks of four and stratified according to gender and age (over 30/30 or below). The study statistician generated the random allocation sequence using Statistical Computer Program R version 2.6.2 (www.r-project.org).
2.4. Procedures
Methadone and Buprenorphine-naloxone were administered 7 days/week at Uranti under direct observation and patients were offered weekly individual drug counseling and group therapy. Counseling sessions lasted approximately 45 minutes and were delivered by trained and experienced staff using procedures described in a drug counseling that has been modified for opioid use and translated into Russian (which is well understood by Georgians), and whose original version is available on the NIDA web site: (http://archives.drugabuse.gov/TXManuals/IDCA/IDCA1.html). Detailed assessments were done at baseline and at weeks 4, 8, 12 and 20; brief assessments were done in weeks 1–12 and at week 20. Patients who requested methadone treatment at the end of 12 weeks were switched to methadone after completing the medication phase. There was only one Buprenorphine-naloxone program in Tbilisi at the time and it had limited capacity thus most patients that did not want to be treated with methadone were placed on a 3-week dose taper with followup at Uranti.
2.5. Measures
At intake each patient had a physical examination that included a CBC, glucose, bilirubin, liver enzymes, ECG, testing for HIV and hepatitis B and C; a urine drug screen using an on-site kit that tested for opioids, benzodiazepines, amphetamine, buprenorphine, methadone and THC; the Addiction Severity Index (ASI) 5th edition (McLellan et al., 1992); a timeline follow-back (TLFB) for self-reported drug use with a timeframe of past 30 days (Sobell and Sobell, 1992); a visual analogue scale for current opioid craving (0–100; 0=not at all, 100=very much); and the Risk Assessment Battery (RAB), a self-reported measure of drug and sexual HIV risk behaviors using the timeframe of past six months.
Urine drug screens, opioid craving and the TLFB with a timeframe of past 7 days were repeated in weeks 1–12 and at week 20. Attendance at counseling sessions was recorded weekly and doses of study medication were available from pharmacy dispensing records. Adverse Events (AEs) and use of concomitant medications were assessed at all visits, and the severity of AEs and their potential relationship to study procedures were assessed and monitored until the event resolved. The ASI and RAB were repeated at weeks 4, 8, 12 and 20 using the timeframe of past 30 days.
Interviews and assessments were conducted in Georgian. Study instruments were translated into Georgian and back translated into English to ensure correct interpretation in the Georgian language and that the Georgian and English case-report forms matched each other. Data were entered into a web-based system developed by the data management unit at the Penn/VA Center for Studies on Addiction and analyzed by GK in Tbilisi. Buprenorphine and other opioid use, study retention, and HIV drug risk behavior were the focus of the analyses presented here.
2.6. Statistical analyses
Statistical analysis was performed using statistical package SPSS 20.0. The study was designed to have 80% power to detect an effect size of Cohen’s d=0.7 at a two-sided significance level of 0.05. For a comparison of binary outcomes, 80% power was set to detect a difference of about 30% in rates of use. For the quantitative endpoints the Student’s t test (for the comparison of two groups) or ANOVA or Univariate General Linear Model approach (for more than two groups) were used. The categorical endpoints were compared using χ2 test or Fisher’s exact test (where appropriate according to the size of the outcome).
3. RESULTS
3.1. Participant characteristics
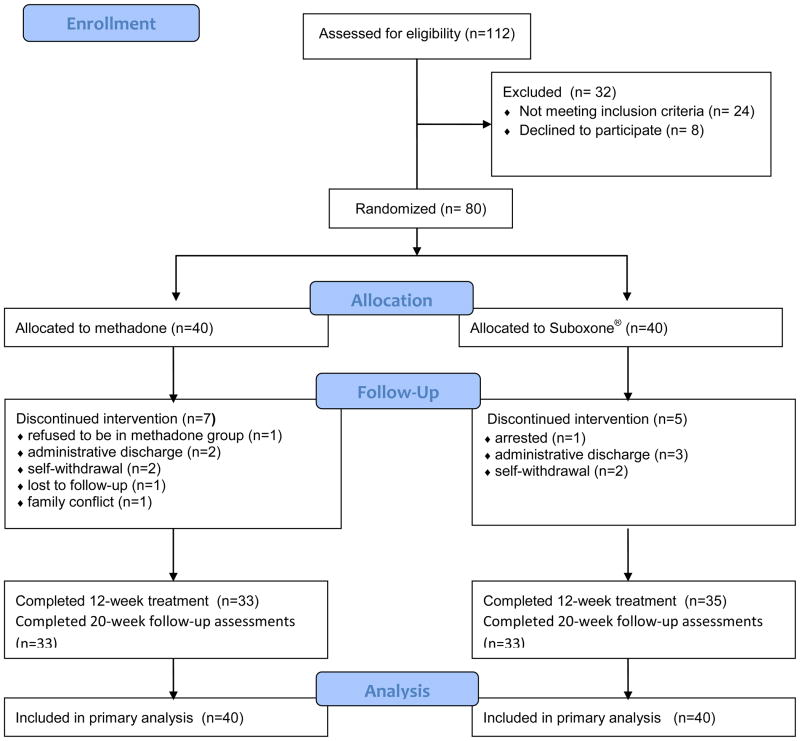
In total, 112 potential subjects were screened between January 25 and September 27, 2011, of which 80 (4 females) were randomly assigned to methadone or Buprenorphine-naloxone. Of the 112 that were screened, 32 were excluded from study participation for the reasons seen in Figure 1.
Figure 1.
Study flow diagram.
Subjects were all Caucasian; average age was 34; mean years of opioid injection use was 5.8 (SD4.6); and heroin, buprenorphine, other opioids (opium, desomorphine) and home-produced amphetamine type stimulants were the main drugs reported to have been injected. Injecting more than one drug was reported by 68.4% of methadone patients and 72.5% of buprenorphine-naloxone patients. None were HIV positive however 73.4%were positive for hepatitis C. There were no significant differences in socio-demographic and clinical characteristics between two groups (Table 1).
Table 1.
Background characteristics of participants
| Total sample (n=80) | Met (n=40) | Sub (n=40) | Test statistics | p | |
|---|---|---|---|---|---|
| Male, n (%) | 76.0 (95.0) | 37.0 (92.5) | 39.0 (97.5) | χ2=1.05 | 0.305 |
| Age, M (SD) | 33.7 (5.7) | 34.3 (6.1) | 33.1 (5.2) | t=0.94 | 0.35 |
| Education (years), M (SD) | 14.8 (2.9) | 14.9 (2.7) | 14.7 (3.0) | t=0.33 | 0.74 |
| Unemployed, n (%) | 46.0 (57.5) | 24.0 (60.0) | 22.0 (55.0) | χ2=0.51 | 0.47 |
| Married, n (%) | 38.0 (47.5) | 20.0 (50.0) | 18.0 (48.0) | χ2=0.08 | 0.78 |
| Drug use history (years), M (SD) | 5.8 (4.6) | 6.2 (5.3) | 5.3 (3.8) | t=0.83 | 0.41 |
|
| |||||
| Days drugs used in last 30 days (self reported), M (SD) | |||||
| Heroine | 3.2 (5.8) | 3.4 (5.9) | 3.0 (5.8) | t=0.27 | 0.78 |
| Subutex® | 15.2 (5.8) | 15.3 (6.6) | 15.0 (5.0) | t=0.24 | 0.81 |
| Other opioids | 10.2 (10.3) | 10.6 (10.3) | 10.5 (10.2) | t=0.40 | 0.70 |
| Stimulants | 1.5 (2.4) | 1.4 (2.3) | 1.7 (2.5) | t=0.41 | 0.68 |
| Benzodiazepines | 4.2 (7.3) | 3.7 (7.2) | 4.7 (7.5) | t=0.58 | 0.56 |
| Marijuana | 2.1 (6.2) | 0.8 (1.7) | 3.4 (8.3) | t=1.9 | 0.06 |
|
| |||||
| Opioid craving scale, M (SD) | 81 (20.54) | 84(20.46) | 77.5(20.35) | t=0.32 | 0.75 |
|
| |||||
| HIV status1, n (%) | |||||
| Positive | 0 (0) | 0 (0) | 0 (0) | ||
| Negative | 79 (100) | 39 (100) | 40 (100) | χ2=0.00 | 1.00 |
|
| |||||
| HCV status1, n (%) | |||||
| Positive | 58 (73.4) | 33 (84.6) | 25 (62.5) | χ2=4.95 | 0.03 |
| Negative | 21 (26.6) | 6 (15.4) | 15 (37.5) | ||
data provided for 79 participants, 1 refused testing
3.2. Outcomes
Of the 80 study participants, 68 (85%) completed 12-weeks of treatment; 12 left the study for the reasons seen in Figure 1. Average number of days in treatment was 87 and average number of individual counseling sessions attended was 13.8 with no difference between groups. Mean dose of methadone at treatment midpoint (six weeks) was 39mg (SD17.8; 17 to 80) and mean dose of Buprenorphine-naloxone was 8.5 mg (SD3.5; 4 to 16).
Over the 12-week medication phase, 837 weekly, observed urine samples were collected and tested (see Table 2). During this period 123 of the 960 scheduled tests (12.8%) were missing with 74 of 480 (15.4%) in the methadone group and 49 of 480 (10.2%) in the buprenorphine-naloxone group; 108 of the missing tests were due to early termination. The overall level of opioid-positive urine samples was very low but there were significantly more positive opioid tests in methadone than buprenorphine-naloxone patients (6 vs. 1, or 1.5% vs. 0.2%; p=0.03). Other drug use was also low and will be described in a later paper.
Table 2.
Treatment impact
| Met (n=40) | Sub (n=40) | Test statistics | P | |
|---|---|---|---|---|
| Assessments conducted, n (%) | ||||
| 0 week | 40 (100) | 40 (100) | ||
| 4 week | 34 (85.0) | 38 (95.0) | ||
| 8 week | 35 (87.5) | 36 (90.0) | χ2=4.95 | 0.99 |
| 12 week | 33 (82.5) | 35 (87.5) | ||
| 20 week | 33 (82.5) | 33 (82.5) | ||
|
| ||||
| Days in treatment over 12 weeks, M (SD) | 85.4 (34.2) | 88.8 (26.6) | t=0.5 | 0.6 |
| Counseling sessions attended, n | 414 | 443 | ||
| Sessions attended per participant, M (SD) | 13.8 (5.2) | 13.8 (5.4) | t=0.05 | 0.96 |
|
| ||||
| Urine samples collected (1–12 weeks), n | 406 | 431 | ||
|
| ||||
| Opioid positive urine samples, n (%) | 6 (1.5) | 1 (0.2) | χ2=4.87 | 0.03 |
| Buprenorphine positive urine samples1, n (%) | 3 (0.7) | -- | -- | -- |
Buprenorphine positive urine samples not shown for Suboxone® group.
Of the 843 weekly TLFB responses on opioid use that were obtained, 836 were matched with urine tests performed on the same patient in the same week and 96.7% were in agreement. Consistent with the reduction in opioid use, there was a marked reduction in opioid craving with no significant difference between groups.
There was a significant reduction in reported HIV risk injection behaviors over the 12-week treatment period in both groups, with improvements persisting by the 20-week follow-up. In most cases unsafe injecting risk behavior was virtually eliminated (see Table 3). Sexual risk behavior did not change over the course of treatment with about half of the sample never using condoms during sex, and about a third of participants having 2 or 3 sexual partners over the past 30 days.
Table 3.
Injection risk behavior in past four weeks
| Met (n=40)1 | Sub (n=40) | Test statistics | P | |
|---|---|---|---|---|
| Did not share needles or works or did not inject2, n (%) | ||||
| 0 week | 35 (91.2) | 35 (87.5) | 0 | 1.00 |
| 4 week | 34 (100) | 38 (100) | 2.22 | 0.14 |
| 8 week | 35 (100) | 36 (100) | 0.13 | 0.72 |
| 12 week | 33 (100) | 34 (100) | 0.09 | 0.76 |
| 20 week | 33 (100) | 33 (100) | 0 | 1.00 |
|
| ||||
| Did not share cooker or did not inject, n (%) | ||||
| 0 week | 24 (63.2) | 22 (55.0) | 0.20 | 0.65 |
| 4 week | 34 (100) | 37 (97.4) | 0.44 | 0.51 |
| 8 week | 35 (100) | 36 (100) | 0.13 | 0.72 |
| 12 week | 33 (100) | 34 (100) | 0.09 | 0.76 |
| 20 week | 32 (97.0) | 32 (97.0) | 0 | 1.00 |
|
| ||||
| Did not shared cotton or did not inject, n (%) | ||||
| 0 week | 30 (78.9) | 33 (82.5) | 0.67 | 0.41 |
| 4 week | 34 (100) | 37 (97.4) | 1.13 | 0.29 |
| 8 week | 35 (100) | 36 (100) | 0.13 | 0.72 |
| 12 week | 33 (100) | 34 (100) | 0.09 | 0.76 |
| 20 week | 33 (100) | 33 (100) | 0 | 1.00 |
|
| ||||
| Did not divide or share drugs with others using one syringe or did not inject, n (%) | ||||
| 0 week | 9 (23.7) | 5 (12.5) | 1.39 | 0.24 |
| 4 week | 33 (97.1) | 35 (92.1) | 0.39 | 0.53 |
| 8 week | 34 (97.1) | 35 (97.2) | 0.11 | 0.75 |
| 12 week | 32 (97.0) | 34 (100) | 0.35 | 0.56 |
| 20 week | 28 (84.8) | 28 (84.8) | 0 | 1.00 |
|
| ||||
| Did not practice unsafe injection behavior or did not inject3, n (%) | ||||
| 0 week | 9 (23.7) | 4 (10.0) | 2.63 | 0.1 |
| 4 week | 33 (97.1) | 35 (92.1) | 0.84 | 0.36 |
| 8 week | 34 (97.1) | 35 (97.2) | 0.00 | 0.98 |
| 12 week | 32 (97.0) | 35 (100) | 1.05 | 0.31 |
| 20 week | 28 (84.8) | 28 (84.2) | 0.00 | 1.00 |
baseline data provided for 38 participants; 2 cases are missing data.
for the purpose of current analyses we focus on four types of injection risk behavior characteristic to the population studied
presents data on participants who did not practice any of risk behaviors shown in rows above
Sixty-six participants were evaluated at the 20-week follow up and of these, 37 were receiving agonist maintenance with 34 on methadone and 3 on Buprenorphine-naloxone. Based on the results of urine tests at this assessment point, significantly fewer participants who remained in treatment used illicit opioids (5.6% vs 27.6%; p<0.001) or used illicit buprenorphine (2.7% vs 13.8%; p=0.005), benzodiazepines (13.5% vs 34.5%; p<0.001), or marijuana (2.8% vs 20.7%; p<0.001, compared to those who were not in treatment.
Significantly more Buprenorphine-naloxone than methadone patients experienced at least one adverse event (p=0.003). Insomnia, constipation and depression were the most frequent events reported in both groups and constipation was the event most often judged possibly related to study medication. All 80 adverse events in the methadone group, and 108 in the Buprenorphine-naloxone group, were judged to be mild or moderate and 10 were deemed to be definitely related to study medication. There were no deaths, overdoses, suicide attempts or other serious adverse events.
4. DISCUSSION
In this randomized controlled trial of opioid addicted individuals that had injected buprenorphine 10 or more times in the last month, daily observed methadone and buprenorphine-naloxone were well accepted and 85% remained in treatment throughout the 12 week dosing period. In both study arms treatment participation resulted in a marked reduction in opioid use, a reduction in opioid craving, and a reduction or elimination of unsafe HIV risk injecting behaviors.
The vast majority of study participants were using more than one psychoactive substance prior to study inclusion, as previously documented in Georgia and regionally (Booth et al., 2009, 2008; Javakhishvili et al., 2011; Kruse et al., 2009; Tiihonen et al., 2012). It has been suggested that this poly-substance use that involves mixing buprenorphine and other opioids with sedatives and amphetamine-type stimulants is related to the ever fluctuating availability and high price of drugs on the Georgian black market and/or attempts by users to increase the euphoric effects and potency of injection preparations (Javakhishvili et al., 2012; Otiashvili et al., 2010).
Study participants reported paying as much as US $100 for an 8 mg buprenorphine tablet and then dividing it four ways with friends. This high cost and low-dose use could explain the low mean daily doses of medications prescribed to study participants, namely 39 mg for methadone and 8.5 mg for buprenorphine-naloxone at the six-week mid-point. These dosing levels are consistent with a previous report where the average daily dose of buprenorphine in a sample of needle exchange participants was as low as 1–2 mg (Otiashvili et al., 2010). In contrast, the daily dose of illicit buprenorphine injected in other countries varies between 6–10 mg (Aitken et al., 2008; Alho et al., 2007; Winslow et al., 2006; Winstock et al., 2008). These local conditions could well explain the fact that desirable clinical effects were achieved in our sample with comparatively moderate doses of treatment medications though higher doses may be needed to improve the relatively low level of participation in longer term treatment, as seen by the 20-week follow up data.
One of the most striking findings was the 76% prevalence of hepatitis C but the absence of HIV. Similar to other recent reports (Chikovani et al., 2011) direct needle sharing was not high among study participants (Table 3); the most common unsafe injection behavior at baseline was sharing a cooker and dividing the solution using one syringe. Buprenorphine injection in Georgia generally occurs in groups of 3–4 people who dissolve one 8 mg tablet in a water and then, using a large volume syringe, divide the solution by front- or back-loading into smaller individual syringes (Javakhishvili et al., 2011; Otiashvili et al., 2010). Home preparation of meth/amphetamine type stimulants (—vint and —jeff ) and opioids (—crocodile ) both involve using a common cooker to process ingredients through often complicated chemical refinement, and using a large-volume syringe to divide the final product into smaller syringes for injection.
In both cases drug preparation is a group activity with predetermined division of roles and contributions (money, ingredients, space for production) but with little direct sharing of injection equipment, behaviors that could be due to long-term efforts to educate drug users about the risks of direct needle sharing. Nevertheless, indirect sharing, in this case through use of a common container and common syringe for drug division has not been sufficiently acknowledged and targeted, and may account for the high prevalence of HCV since it is more easily transmitted than HIV (Doerrbecker et al., 2013; Thibault et al., 2011). In addition, given the relatively high mean age of study participants, HCV prevalence is likely a function of sharing injection equipment over their extended injection careers.
Findings of the study must be considered in light of some limitations. We could not objectively measure buprenorphine misuse in the buprenorphine-naloxone group, however, in the methadone group only 3 of 406 urine samples were positive for buprenorphine during weeks 1–12 and TLFB data were highly consistent with urine tests results, a finding supporting the validity of self-reports and the conclusion that non-prescribed use of buprenorphine in both groups was extremely low.
Daily, observed dosing eliminated diversion and ensured medication compliance. Results might be different if take-home doses were allowed. However, the costs associated with provision of buprenorphine in specialized clinics under daily direct observation may limit dissemination of a useful treatment in resource limited settings and in locations where patients must pay for their own treatment, which is the case in Georgia. In this regard, using higher buprenorphine-naloxone doses with alternate day dosing may reduce the cost of buprenorphine maintenance treatment while also controlling diversion (Johnson et al., 2003).
In a number of studies flexible-dosing methadone treatment was found to be somehow less expensive than buprenorphine treatment (Connock et al., 2007; Doran et al., 2003). This difference in the cost of treatment was largely attributed to a significant difference in the cost of medications. In Georgian reality, both methadone and buprenorphine/naloxone are provided in specialized clinics that provide daily-supervised dosing with no take-homes allowed, procedures that raise the cost of both treatments (Kirtadze et al., 2012). With agonist maintenance rapidly expanding – about 2500 were receiving methadone and more than 200 were on buprenorphine-naloxone in 2011 (Javakhishvili et al., 2012), and with data showing that they are highly effective, there is an opportunity to consider how costs might be reduced and treatment expanded while retaining control over diversion.
The sample size was relatively small and not chosen based on a power analysis since this trial was primarily a feasibility study to collect initial data on treatment engagement and retention and its impact on drug injection and risk behavior. Importantly, although we did not focus on retaining participants in maintenance treatment after the study completion, 56% of participants assessed at the 20-week follow-up (46% of the initial sample) were continuing on agonist treatment and assessments showed that significantly fewer patients who remained in treatment had urine samples positive for opioids, and significantly fewer reported HIV risk behavior compared to those who were not in treatment.
Daily observed doses methadone and buprenorphine-naloxone were effective in reducing illicit buprenorphine and other opioid use, and reducing HIV risk behavior among non-medical buprenorphine and other opioid users in Georgia. These results suggest that increasing the availability and accessibility of opiate agonist treatment with methadone and buprenorphine-naloxone would be an effective public health approach for treating non-medical use of buprenorphine and other opioids in Georgia.
Acknowledgments
Role of Funding Source
This study was supported by an unrestricted, unsolicited investigator initiated request from Reckitt Benckiser Pharmaceuticals Inc. who provided Suboxone® for this trial. Research was supported by grants R21-DA-026754, U10 DA-13043 and KO5 DA-17009 from the National Institute on Drug Abuse (George E. Woody, PI). Neither NIDA nor Reckitt Benckiser had any role in study design; collection, analysis and interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication.
Footnotes
The study is registered with ClinicalTrials.gov– ID NCT01131273
Contributors
GEW, DO and GP designed the initial study and protocol, while SP and ZS provided input into the refinement of the protocol. DO and GP were Principal Investigators at the Republic of Georgia site where the protocol was implemented and were responsible for overseeing data collection and management. ZS was responsible for the medical screening and medication treatment and monitoring. GK conducted the data analyses. DO wrote the first draft of the manuscript. All authors discussed the results and implications and commented on the manuscript at all stages.
All authors have approved the final manuscript.
No honorarium, grant, or other form of payment was given to any author or any other individual to produce the manuscript.
Conflict of Interest
The authors report no conflicts of interest regarding this manuscript. The authors alone are responsible for the content and writing of the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aalto M, Visapaa JP, Halme JT, Fabritius C, Salaspuro M. Effectiveness of buprenorphine maintenance treatment as compared to a syringe exchange program among buprenorphine misusing opioid-dependent patients. Nord J Psychiatry. 2011;65:238–243. doi: 10.3109/08039488.2010.531762. [DOI] [PubMed] [Google Scholar]
- Ahmadi J, Ahmadi K, Ohaeri J. Controlled, randomized trial in maintenance treatment of intravenous buprenorphine dependence with naltrexone, methadone or buprenorphine: a novel study. Eur J Clin Invest. 2003;33:824–829. doi: 10.1046/j.1365-2362.2003.01218.x. [DOI] [PubMed] [Google Scholar]
- Ahmadi M, Maany I, Ahmadi J. Treatment of intravenous buprenorphine dependence. A randomized open clinical trial. Ger J Psychiatry. 2003;6:23–29. [Google Scholar]
- Aitken CK, Higgs PG, Hellard ME. Buprenorphine injection in Melbourne, Australia--an update. Drug Alcohol Rev. 2008;27:197–199. doi: 10.1080/09595230701829553. [DOI] [PubMed] [Google Scholar]
- Alho H, Sinclair D, Vuori E, Holopainen A. Abuse liability of buprenorphine-naloxone tablets in untreated IV drug users. Drug Alcohol Depend. 2007;88:75–78. doi: 10.1016/j.drugalcdep.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Booth RE, Lehman WE, Dvoryak S, Brewster JT, Sinitsyna L. Interventions with injection drug users in Ukraine. Addiction. 2009;104:1864–1873. doi: 10.1111/j.1360-0443.2009.02660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth RE, Lehman WE, Kwiatkowski CF, Brewster JT, Sinitsyna L, Dvoryak S. Stimulant injectors in Ukraine: the next wave of the epidemic? AIDS Behav. 2008;12:652–661. doi: 10.1007/s10461-008-9359-3. [DOI] [PubMed] [Google Scholar]
- Bruce RD, Govindasamy S, Sylla L, Kamarulzaman A, Altice FL. Lack of reduction in buprenorphine injection after introduction of co-formulated buprenorphine/naloxone to the Malaysian market. Am J Drug Alcohol Abuse. 2009;35:68–72. doi: 10.1080/00952990802585406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrieri MP, Amass L, Lucas GM, Vlahov D, Wodak A, Woody GE. Buprenorphine use: the international experience. Clin Infect Dis. 2006;43(Suppl 4):S197–215. doi: 10.1086/508184. [DOI] [PubMed] [Google Scholar]
- Chikovani I, Bozicevic I, Goguadze K, Rukhadze N, Gotsadze G. Unsafe injection and sexual risk behavior among injecting drug users in Georgia. J Urban Health. 2011;88:736–748. doi: 10.1007/s11524-011-9571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua SM, Lee TS. Abuse of prescription buprenorphine, regulatory controls and the role of the primary physician. Ann Acad Med Singapore. 2006;35:492–495. [PubMed] [Google Scholar]
- Cicero TJ, Surratt HL, Inciardi J. Use and misuse of buprenorphine in the management of opioid addiction. J Opioid Manag. 2007;3:302–308. doi: 10.5055/jom.2007.0018. [DOI] [PubMed] [Google Scholar]
- Clark HW. The State of Buprenorphine Treatment. Buprenorphine in the Treatment of Opioid Addiction: Reassessment 2010. 2010 Retrieved August 20 2012, from http://buprenorphine.samhsa.gov/bwns/2010_presentations_pdf/01_Clark_508.pdf.
- Comer SD, Collins ED. Self-administration of intravenous buprenorphine and the buprenorphine/naloxone combination by recently detoxified heroin abusers. J Pharmacol Exp Ther. 2002;303:695–703. doi: 10.1124/jpet.102.038141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Walker EA. Comparison of intravenous buprenorphine and methadone self-administration by recently detoxified heroin-dependent individuals. J Pharmacol Exp Ther. 2005;315:1320–1330. doi: 10.1124/jpet.105.090423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Whittington RA, Vosburg SK, Kowalczyk WJ. Abuse liability of prescription opioids compared to heroin in morphine-maintained heroin abusers. Neuropsychopharmacology. 2008;33:1179–1191. doi: 10.1038/sj.npp.1301479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connock M, Juarez-Garcia A, Jowett S, Frew E, Liu Z, Taylor R. Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluatio. Health Technol Assess. 2007;11:1–171. doi: 10.3310/hta11090. [DOI] [PubMed] [Google Scholar]
- Daniulaityte R, Falck R, Carlson RG. Illicit use of buprenorphine in a community sample of young adult non-medical users of pharmaceutical opioids. Drug Alcohol Depend. 2011;122:201–207. doi: 10.1016/j.drugalcdep.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Gomez C, Obradovic I, Le Nezet O, Spilka S, Cadet-Tairou A, Mutatayi C. 2010 NATIONAL REPORT TO THE EMCDDA. Reitox National Focal Point; Paris: 2010. [Google Scholar]
- Doerrbecker J, Behrendt P, Mateu-Gelabert P, Ciesek S, Riebesehl N, Wilhelm C, Steinmann J, Pietschmann T, Steinmann E. Transmission of hepatitis C virus among people who inject drugs: viral stability and association with drug preparation equipment. J Infect Dis. 2013;207:281–287. doi: 10.1093/infdis/jis677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran CM, Shanahan M, Mattick RP, Ali R, White J, Bell J. Buprenorphine versus methadone maintenance: a cost-effectiveness analysis. Drug Alcohol Depend. 2003;71:295–302. doi: 10.1016/s0376-8716(03)00169-8. [DOI] [PubMed] [Google Scholar]
- EMCDDA. Buprenorphine — treatment, misuse and prescription practices. 2005 http://issues05.emcdda.europa.eu/en/page031-en.html.
- EMCDDA. The State of the Drugs Problem In Europe. EMCDDA; Lisbon: 2008. [Google Scholar]
- Gamkrelidze A, Javakhishvili J, Kariauli D, Lejava G, Stvilia K, Todadze K. Drug Situation in Georgia - 2003. Southern Caucasus Anti-Drug Programme; Tbilisi, Georgia: 2004. [Google Scholar]
- Gamkrelidze A, Javakhishvili J, Kariauli D, Lejava G, Stvilia K, Todadze K. Drug Situation in Georgia - 2004. Southern Caucasus Anti-Drug Programme; Tbilisi, Georgia: 2005. [Google Scholar]
- Hakansson A, Medvedeo A, Andersson M, Berglund M. Buprenorphine misuse among heroin and amphetamine users in Malmo, Sweden: purpose of misuse and route of administration. Eur Addict Res. 2007;13:207–215. doi: 10.1159/000104883. [DOI] [PubMed] [Google Scholar]
- Javakhishvili DJ, Balanchivadze N, Kirtadze I, Sturua L, Otiashvili D, Zabransky T. Global Initiative on Psychiatry/Alternative Georgia, Tbilisi. 2012. Overview of the Drug Situation in Georgia, 2012. [Google Scholar]
- Javakhishvili DJ, Sturua L, Otiashvili D, Kirtadze I, Zabransky T. Overview of the drug situation in Georgia. Adictologie. 2011;11:42–51. [Google Scholar]
- Javakhishvili J, Kariauli D, Lejava G, Stvilia K, Todadze K, Tsintsadze M. Drug Situation in Georgia - 2005. Southern Caucasus Anti-Drug Programme; Tbilisi, Georgia: 2006. [Google Scholar]
- Jenkinson RA, Clark NC, Fry CL, Dobbin M. Buprenorphine diversion and injection in Melbourne, Australia: an emerging issue? Addiction. 2005;100:197–205. doi: 10.1111/j.1360-0443.2004.00958.x. [DOI] [PubMed] [Google Scholar]
- Johnson RE, Strain EC, Amass L. Buprenorphine: how to use it right. Drug Alcohol Depend. 2003;70(2 Suppl):S59–77. doi: 10.1016/s0376-8716(03)00060-7. [DOI] [PubMed] [Google Scholar]
- Kirtadze I, Menon V, Beardsley K, Forsythe S. Futures Group, USAID Health Policy Initiative Costing Task Order. Washington, DC: 2012. Assessing the Costs of Medication-Assisted Treatment for HIV Prevention in Georgia. [Google Scholar]
- Kruse G, Barbour R, Heimer R, Shaboltas A, Toussova O, Hoffman I, Kozlo AP. Drug choice, spatial distribution, HIV risk, and HIV prevalence among injection drug users in St. Petersburg, Russia Harm Reduct J. 2009;6:22. doi: 10.1186/1477-7517-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Necas V, Skarupova K, Stastna L. Annual Report: The Czech Republic – 2009 Drug Situation. Government of the Czech Republic; Prague: 2010. [Google Scholar]
- O’Connor JJ, Moloney E, Travers R, Campbell A. Buprenorphine abuse among opiate addicts. Br J Addict. 1988;83:1085–1087. doi: 10.1111/j.1360-0443.1988.tb00536.x. [DOI] [PubMed] [Google Scholar]
- O’Brien S, Black E, Degenhardt L, Roxburgh A, Campbell G, de Graaff B. Australian Drug Trends 2006 Findings from the Illicit Drug Reporting System (IDRS) National Drug Research Institute; Perth: 2006. [Google Scholar]
- Otiashvili D, Srosi P, Somogyi G. The Beckley Foundation Drug Policy Program, Briefing Paper 15. Oxford: 2008. Drug control in Georgia:drug testing and the reduction of drug use? [Google Scholar]
- Otiashvili D, Zabransky T, Kirtadze I, Piralishvili G, Chavchanidze M, Miovsky M. Why do the clients of Georgian needle exchange programmes inject buprenorphine? Eur Addict Res. 2010;16:1–8. doi: 10.1159/000253858. [DOI] [PubMed] [Google Scholar]
- Pickworth WB, Johnson RE, Holicky BA, Cone EJ. Subjective and physiologic effects of intravenous buprenorphine in humans. Clin Pharmacol Ther. 1993;53:570–576. doi: 10.1038/clpt.1993.72. [DOI] [PubMed] [Google Scholar]
- Reckitt Benckiser Group. US RB Pharmaceuticals Announcement. 2012 Retrieved 5 March, 2013, from http://www.rb.com/site/rkbr/templates/mediainvestorsgeneral2.aspx?pageid=1332andcc=gb.
- Roux P, Villes V, Bry D, Spire B, Feroni I, Marcellin F, Carrieri MP. Buprenorphine sniffing as a response to inadequate care in substituted patients: results from the Subazur survey in south-eastern France. Addict Behav. 2008;33:1625–1629. doi: 10.1016/j.addbeh.2008.07.018. [DOI] [PubMed] [Google Scholar]
- Simojoki K, Vorma H, Alho H. A retrospective evaluation of patients switched from buprenorphine (Subutex) to the buprenorphine/naloxone combination (Suboxone) Subst Abuse Treat Prev Policy. 2008;3:16. doi: 10.1186/1747-597X-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J, Grover S, Basu D. Very High-Dose Intravenous Buprenorphine Dependence A Case Report. Ger J Psychiatry. 2004;7:58–59. [Google Scholar]
- Sobell LC, Sobell MB. Timeline Follow-Back: a technique for assessing self-reported alcohol consumption. In: Litten R, Allen J, editors. Measuring Alcohol Consumption. Humana Press; Clifton, NJ: 1992. [Google Scholar]
- Sporer KA. Buprenorphine: a primer for emergency physicians. Ann Emerg Med. 2004;43:580–584. doi: 10.1016/j.annemergmed.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Thibault V, Bara JL, Nefau T, Duplessy-Garson C. Hepatitis C transmission in injection drug users: could swabs be the main culprit? J Infect Dis. 2011;204:1839–1842. doi: 10.1093/infdis/jir650. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Krupitsky E, Verbitskaya E, Blokhina E, Mamontova O, Fohr J, tuomola P, Kuoppasalmi K, Kiviniemi V, Zwartau E. Naltrexone implant for the treatment of polydrug dependence: a randomized controlled trial. Am J Psychiatry. 2012;169:531–536. doi: 10.1176/appi.ajp.2011.11071121. [DOI] [PubMed] [Google Scholar]
- Uosukainen H, Kauhanen J, Voutilainen S, Fohr J, Paasolainen M, Tiihonen J, Laitinen K, Onyeka IN, Bell JS. Twelve-year trend in treatment seeking for buprenorphine abuse in Finland. Drug Alcohol Depend. 2012 doi: 10.1016/j.drugalcdep.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Verster A, Buning E. Buprenophine, critical questions examined. Euro-Methwork; Ireland: 2005. [Google Scholar]
- Vicknasingam B, Mazlan M, Schottenfeld RS, Chawarski MC. Injection of buprenorphine and buprenorphine/naloxone tablets in Malaysia. Drug Alcohol Depend. 2010;111:44–49. doi: 10.1016/j.drugalcdep.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Winslow M, Ng WL, Mythily S, Song G, Yiong HC. Socio-demographic profile and help-seeking behaviour of buprenorphine abusers in Singapore. Ann Acad Med Singapore. 2006;35:451–456. [PubMed] [Google Scholar]
- Winstock AR, Lea T, Sheridan J. Prevalence of diversion and injection of methadone and buprenorphine among clients receiving opioid treatment at community pharmacies in New South Wales, Australia. Int J Drug Policy. 2008;19:450–458. doi: 10.1016/j.drugpo.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Zacny JP, Conley K, Galinkin J. Comparing the subjective, psychomotor and physiological effects of intravenous buprenorphine and morphine in healthy volunteers. J Pharmacol Exp Ther. 1997;282:1187–1197. [PubMed] [Google Scholar]