Abstract
Until very recently addiction-research was limited by existing tools and strategies that were inadequate for studying the inherent complexity at each of the different phenomenological levels. However, powerful new tools (e.g., optogenetics and designer drug receptors) and high throughput protocols are starting to give researchers the potential to systematically interrogate “all” genes, epigenetic marks, and neuronal circuits. These advances, combined with imaging technologies (both for preclinical and clinical studies) and a paradigm shift towards open access have spurred an unlimited growth of datasets transforming the way we investigate the neurobiology of substance use disorders (SUD) and the factors that modulate risk and resilience.
Introduction
“Between stimulus and response there is a space. In that space is our power to choose our response. In our response lie our growth and our freedom.”
Viktor E. Frankl
Frankl’s statement distills much of his life-long efforts to wring happiness out of an often agonizing human experience (Frankl, 1959). But the quote is also relevant to addiction for the very “space” Viktor Frankl is talking about, a space whose topology changes naturally throughout life, influences addiction risk and is also changed by addiction. A heuristics model that captures this space starts by defining the relational boundaries between competing cognitive and visceral processes within a triangular space (Figure 1) (Yang et al, 2012). The cognitive axis has been proposed to fluctuate between the arbitrarily termed system 1 and 2 processes: Because system 1 is largely based on perceptions, intuitions, and emotions, it tends to operate quickly, effortlessly, and automatically. In contrast, system 2 is based more on critical, in depth reasoning, so it is slower, more effortful, and deliberate (Kahneman and Frederick, 2002). The visceral axis, which operates in a range between “cold” and “hot” extremes, exerts powerful effects on cognitive operations (Loewenstein, 1996). Such visceral influences are the driving force behind urges, such as hunger, thirst, pain, and sexual arousal, the immediate satisfaction of which helps explain why people sometimes make unhealthy choices.
Figure 1.
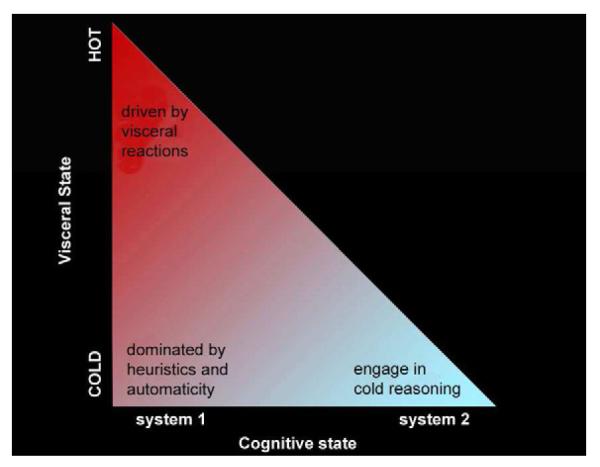
The triangular space of the framework proposed by (Yang et al, 2012) portrays the relationship between cognitive processes and visceral influences. System 2 processing and the hot visceral state are two extremes of a conceptual continuum. Experiencing hot visceral reactions (e.g., sexual arousal, extreme fear, hunger) inhibit system 2 processing. Conversely, using cold reasoning (careful pondering) through system 2 before visceral reaction kick in can help circumvent the onset or reduce the power of visceral urges, reducing the likelihood that a hot state will take over. Figure reprinted with permission from (Yang et al, 2012).
At one extreme, integration within this triangular space becomes manifest in “hot” executive function processes that are engaged during situations with stronger affective salience and recruit areas of the brain that control emotions and the brain’s reward systems (e.g., orbitofrontal cortex, ventral striatum, and the limbic system). At the other extreme, “cold” executive functions have been associated with more purely cognitive processing and the activation of the dorsolateral parts of the prefrontal cortex (Castellanos et al, 2006). Addiction research is creating a more detailed and multileveled map of addiction trajectories in this triangular space
Research on addiction trajectories has shown that, while initial experimentation with drugs of abuse is largely a voluntary behavior, continued drug use gradually impairs neural function, eventually impacting the very capacity to exert free will. In persons with genetic vulnerabilities, suffering from chronic stress or comorbid psychiatric conditions, or who have been exposed to drugs, these processes can eventually turn drug use into the automatic and compulsive behaviors that characterize addiction. We now know that addictive drugs can trigger epigenetic mechanisms that modulate (up or down) the expression of genes implicated in neuroplasticity ultimately perturbing the intracellular level of key proteins, and modifying neurotransmitter signaling (both strengthening and blunting) and information processing in various neuronal circuits in the brain (reward/antireward, executive function/control, interoception/awareness, mood/stress reactivity among others). Therefore, the resultant behavioral dysfunctions in addiction reflect the emergent property of complex systems that are disrupted at multiple, interacting levels.
Here, we highlight some of the most significant and recent findings in addiction research and assess their impact on our understanding of substance use disorders (SUD) and their implications for prevention, treatment and public health policy. A common target that emerges for prevention and treatment is need to balance the critical space that exists “between stimulus and response” and that becomes increasingly disrupted as the severity of a SUD deepens.
Genetics
It has been known for a long time that addiction has a prominent hereditary component. Yet, the goal of finding genes with definite contributions to this disorder has been elusive. Indeed, genome wide association studies (GWAS) have yielded several polymorphic variations with very modest contributions to the overall addiction vulnerability (Bierut et al, 2006; Rutter, 2006). However, this is a recurring theme when investigating other complex phenotypes, such as depression (Hamet and Tremblay, 2005) and schizophrenia (Doherty et al, 2012), which can, incidentally, also modulate risk for SUD. This is consistent with the notion that addiction (like other psychiatric disorders) is a polygenic disease that hinges on a vast number of genes with an ability to impact the risk of abuse and addiction (Drgon et al, 2010; Tyndale and Sellers, 2002; Uhl et al, 2008). Such genes are likely to operate through their influence, either direct or indirect, on brain development, relevant neurotransmitter systems, drug metabolic pathways, neural circuitry, cellular physiology, behavioral patterns, and the responses to environmental stimuli (i.e., stress, social support, social deprivation) and an individual’s personality traits (e.g., novelty seeking, impulsivity, stress reactivity).
Thus, teasing apart the causal relationships, timing, strength and contingent nature of any genetic contribution to SUD is a challenging endeavor. Yet, for the past decade or so, the steady characterization of hundreds of genes that interact among themselves and with the environment has begun to coalesce into an increasingly coherent, albeit highly nuanced narrative on the role of genes in SUD.
A genetic foundation of personality
Several genes, whose proteins have a central role in brain function have been independently identified at influencing an individual’s susceptibility to different psychiatric conditions, including depression, anxiety, antisocial behavior, and SUD (Caspi et al, 2002; Caspi et al, 2003; Lau et al, 2009; Nilsson et al, 2006; Nilsson et al, 2008) as opposed to being specific to a given disorder. For example, variations in the genes that encode monoamine oxidases (MAOs), which play a central role in monoaminergic balance in the brain, have been linked to personality styles that are influenced by their environmental exposures. Specifically, adult carriers of the “low-activity” MAOA alleles (MAOA-L), who were exposed to moderate maltreatment as children, have been shown to be more likely to develop conduct disorder, antisocial personality symptoms, and violent behaviors relative to either controls or maltreated carriers of the “high-activity” MAOA alleles (MAOA-H)(Caspi et al, 2002; Weder et al, 2009). Further insight into the effects of early MAO action on the brain’s architecture comes from the association between the MAO-L allele and reduced volume and function of the anterior cingulate cortex (ACC) (Meyer-Lindenberg et al, 2006), a region belonging to one of the main control networks in the brain whose function is disrupted in SUDs. On the other hand, carriers of the MAOA-H allele (in combination with the s/s version of the serotonin transporter (5HTT)) display more efficient executive control of working memory-related performance (Enge et al, 2011). Similarly, variations in other gene classes could affect their expression at critical stages in brain development, compromising the function of neural circuits regulating emotion, negative affect, and stress later in life (Meyer-Lindenberg et al, 2006). Similar mechanisms may underlie the hereditary abnormalities in fronto-striatal connectivity that compromise inhibitory control as observed in both stimulant-dependent individuals and their drug naïve siblings (Ersche et al, 2012). However, attempts at mapping the genetic contributions to addiction must take into account the obvious lack of univocal relationships between genetic inputs and behavioral outputs, which hinders the more straightforward interpretation of genetic data. For example, 5-HTT gene promoter polymorphisms have been associated not only with anxiety and dysphoria, but also with altered stress responsiveness (Oroszi and Goldman, 2004). Another good example is the BDNF gene, whose product controls maturation of neurons during childhood and adolescence, and that has also been implicated in various neuropsychiatric disorders. In fact, a low level of BDNF impedes the normal development of serotonin neurons, and could help explain the serotonin dysfunction that has been associated with some suicidal behaviors (Sher, 2011). Interestingly, preliminary results suggest that the BDNF Val(66) Met genotype, which has been associated with neurobehavioral deficits, may promote drug-seeking phenotypes in heroin-dependent individuals (Greenwald et al, 2012).
Polymorphisms in the nicotinic acetylcholine receptor (nAChR) system have also emerged as modulators of nicotine and other SUDs, likely in part partly due to their influence on the maturation of brain circuits implicated in attention and sensory processing (Heath and Picciotto, 2009). Brain nicotinic receptors appear early and reach high levels during human gestation (Hellstrom-Lindahl and Court, 2000) modulating the expression of many downstream genes, neuronal differentiation, synapse formation, and neuronal path finding. Together with the fact that chronic nicotine exposure can upregulate nicotinic receptors (Huang and Winzer-Serhan, 2006), these observations help explain why prenatal smoking can perturb brain maturation and contribute to behavioral disorders (Latimer et al, 2012). Importantly, this decade has seen a dramatic increase in our understanding of the complex links between variations in nAChR subunits (i.e., α3, α5 and β4 genes) and not only smoking behaviors (Budulac et al, 2012), but also their adverse health effects, like nicotine dependence, lung cancer and peripheral arterial disease (Thorgeirsson et al, 2008). These findings have helped uncover new targets for the development of pharmacotherapies for nicotine and other addictions (Levine et al, 2011).
Genes and the reward circuit
The vast majority of addictive substances either trigger pleasure or relieve distress, which is why some people gravitate towards them. Since dopamine (DA) is such a critical modulator of the balance between reward and aversion (anti-reward), it is hardly surprising that so many facets of the addiction phenotype are influenced by genetic variability in the DA system and in those neurotransmitters that regulate DA signaling’ (Le Foll et al, 2009). Indeed, in humans the expression of either high or low levels of DA D2 receptors (D2R) in the striatum has been shown to predict whether a non-abusing individual will find the experience of a psychostimulant aversive or pleasurable, respectively (Volkow et al, 1999b; Volkow et al, 2002). This finding was a strong indication that the initial experiential trigger of a sustained drug abuse trajectory could be under the partial control of a set of genes (e.g., those affecting DA tone including receptor levels) that can influence the type of sensation a first time user derives from the initial drug exposure.
We are only beginning to explore the likely complex landscape of gene products that can modulate the subjective response to stimulant drug administration (Hart et al, 2012). Predictably, animal and human studies continue to provide robust examples of DA’s complex involvement in reward related behaviors. For instance, enhancement of D2R signaling interferes with drug reward whereas enhancement of DA D1 receptor (D1R) signaling increases it (Lobo and Nestler, 2011). Thus, polymorphisms that may result in reduced levels of D2R expression (D2R -141C Ins/Del in the promoter region, exon 8 and D2R TaqI A and DAT 40bp VNTR), have typically been linked to an increased predisposition to severe alcoholism (Samochowiec et al, 2000). Also, animal experiments suggest that high DA D3 receptor (D3R) expression may be a protective factor against cocaine addiction (Song et al, 2012), while allelic variations in DA D4 receptor (D4R) are likely to impact SUD trajectories through their differential effects on novelty seeking behaviors (Kluger et al, 2002) and via its modulation of the sensitivity to positive and negative environmental stimuli (Grady et al, 2013). In the prefrontal cortex (PFC), which is a brain region critically involved in self-control, DA turnover is prominently regulated by catecholamine-O-methyltransferase (COMT), a common variant of which (a V(108/158)M substitution) predicts lower DA synthesis in midbrain and impaired DA signaling in PFC (Meyer-Lindenberg et al, 2005). COMT has emerged as a prime example of a general class of genetic variations that influences the way in which we process emotions and response to stress, which is a contributor to many psychiatric disorders, including SUD. For example, carriers of the COMT Met158 allele displayed increased amygdalar activation in response to an emotional stimulus (Drabant et al, 2006). This effect was further enhanced in co-carriers of the low expression short (S) allele of the serotonin transporter gene (5-HTTLPR) (Smolka et al, 2007), with co-carriers also displaying increased fear conditioning and attentional bias to threat (Caspi et al, 2010). There is also growing evidence that COMT may impact reward processing, through its modulation of cortical and striatal activation during anticipation or receipt of a reward or during the commitment of the rewarding experience to memory (Tunbridge et al, 2012).
Genetic findings are also enriching our understanding of the complex neurobiology associated with SUD. For example, whole genome-wide association studies coupled with studies in transgenic mice implicate the nicotinic acetylcholine receptor α5 and β4 subunits, which are highly expressed in the habenula, in nicotine addiction (Fowler et al, 2011a; Glick et al, 2011). Their high regional localization in this nucleus has attracted attention to the relevance of the habenula in the neurobiology of addiction (see below).
Similarly, the large and growing number of validated regulatory relationships between morphine-modulated microRNAs and various transcript targets in the opioid system have identified new aspects of the involvement of the opioid system in the brain’s reward pathways (positive and negative) (Pietrzykowski, 2012; Wood and Lipovich, 2012). Also worth mentioning in this context is the FKBP5 chaperone protein, whose variations modulate the glucocorticoid receptor’s affinity for cortisol and interact with childhood trauma to predict PTSD (Binder et al, 2008), such that high expression FKBP5 alleles in traumatized children increases risk for PTSD, probably through the association of such alleles with increased glucocorticoid sensitivity. Inasmuch as PTSD increases risk for a SUD this exemplifies an indirect mechanism through which a gene modulates the risk of SUD.
Genes and synaptic plasticity
Genes whose products influence synapse formation and strengthening can also potentially contribute to the rate at which the brain transitions from controlled to compulsive and uncontrollable drug use. Homer proteins, for example, regulate the activity and levels of glutamate receptors and help maintain extracellular glutamate levels in corticolimbic brain regions. The function of Homer proteins is consistent with preclinical studies suggesting their involvement in various neuropsychiatric disorders, including addiction (Szumlinski et al, 2006). Another example is the matrix metalloproteinase 9 (MMP-9), which plays a key role in a variety of neuronal plasticity phenomena, including learning, memory, and drug addiction and whose polymorphisms have been implicated in alcohol dependence (Samochowiec et al, 2010). Also worth mentioning is the nuclear factor-kappa B (NF-kB), a master regulator of many immune genes in several brain regions that influences the fate of neurons (Russo et al, 2009a), and whose upregulation by cocaine mediates the drug’s synaptic effects on medium spiny neurons of the nucleus accumbens (NAc) (Ang et al, 2001; Russo et al, 2009b). Interestingly, NF-kB, a likely candidate gene for nicotine dependence (Sullivan et al, 2004), may be subject to drug-induced epigenetic markings in both tobacco smokers (Sundar et al, 2012) and alcohol abusers (Yakovleva et al, 2011).
Importantly, thanks to new imaging technologies it is now increasingly feasible to evaluate and validate the strength of suspected genetic contributions, as well as their epistatic and environmental interactions. Proper implementation of this research agenda, combined with new bioinformatics tools, will allow us to identify and better characterize (intermediate) endophenotypes. This approach is bound to further deepen our understanding of the genetic mechanisms that impact SUD trajectories (Bevilacqua and Goldman, 2011).
Epigenetics
The picture emerging from genetic studies of addiction would be incomplete without a consideration of the role of epigenetic processes on abuse and addiction trajectories. This level of control, relatively underestimated for a long time, has been likened to “life at the interface between a dynamic environment and a fixed genome” (Meaney and Szyf, 2005). For epigenetic changes represent an important part of evolution’s answer to a multicellular organism’s need to come up with adaptive changes that do not rely on glacially slow genetic mutations (Colvis et al, 2005). Indeed, epigenetic processes are routinely recruited to instantiate the persistent neuroplastic changes associated with learning and memory (Levenson and Sweatt, 2006).
The environment’s ability to shape the circuits of emotion, particularly those impacted during critical periods of pre, postnatal, and adolescent brain development (Champagne and Curley, 2005), is likely to tap heavily on epigenetic mechanisms (Holmes et al, 2005). The epigenetic impact of maternal smoking provides a telling example. Recent animal studies have shown that prenatal exposure to cigarette smoking is associated with specific brain changes and behaviors later in adolescence, some of which could be traced back to methylation events along specific genes such as BDNF (Toledo-Rodriguez et al, 2010). Similarly, chronic exposure to drugs (e.g., cocaine, alcohol) has been shown to perturb the chromatin structure around many genes, including FosB, Cdk5, and BDNF, which could set up the stage for the emergence and maintenance of an addictive state (Bilinski et al, 2012).
Not surprisingly, investigators are working to identify how drugs remodel chromatin structure to affect the recruitment of transcription factors onto specific gene promoters and how this influences behavior (Carouge et al, 2010; Kumar et al, 2005; Levine et al, 2005; Maze et al, 2010). A better understanding of the epigenetic mechanisms mediating the long lasting effects of drugs is bound to impact the efficacy of our prevention and therapeutic interventions. Consider, for example, the implications of a recent study that showed that adolescent mice pre-exposed to nicotine undergo widespread histone acetylation (a class of epigenetic mark that promotes gene transcription) in striatum and transcriptional upregulation of FosB in NAc concomitant with a parallel increase in cocaine’s reinforcing effects (Levine et al, 2011). This particular finding hints at a possible molecular mechanism -related to the still evolving gateway theory of drug abuse- that could help explain how pre-exposure to nicotine (but also perhaps to alcohol or marihuana) might increase the risk for subsequent addiction to other drugs.
Though the task of identifying and cataloguing the most relevant epigenetic alterations from amongst the millions of cell-type specific epigenomic events that are constantly taking place on many genes constitutes a massive undertaking, the potential of epigenomic intervention is huge. For once we understand the epigenetic events related to SUD, we might be able to devise ways to manipulate the genetic output of an otherwise “fixed” genome via a discriminating use of epigenome-targeting medications and/or key environmental variables. For example, recent evidence from experiments in non-human primates suggests that CpG islands throughout the 5-HTT gene may constitute accessible targets for the manipulation of gene x environment interaction with the goal of enhancing overall resilience (Kinnally et al, 2010).
Other notable examples include the ability of histone deacetylase 5 (a subtype of histone deacetylases that is highly expressed in the brain) to modify discrete epigenetic marks and control behavioral adaptations to chronic emotional stimuli (Renthal et al, 2007) and the ability of the dimethyltransferase enzyme G9a (Ehmt2) to dimethylate histone H3 at lysine 9 (H3K9me2), which is implicated in the effects of chronic cocaine (Maze et al, 2010) and morphine (Sun et al, 2012) on neural plasticity. These observations may lead one day to the design of more precise epigenetic-based therapies. For example, injection of the histone deacetylase inhibitor trichostatin A reduced the reinforcing properties of cocaine in rats (Host et al, 2009) and blocked the conditioned-cue driven reinstatement of cocaine taking (Romieu et al, 2011) without affecting the rewarding properties of sucrose, a natural reinforcer (Romieu et al, 2008). Similarly, selective inhibition of HDAC3 (a histone deacetylase also expressed in the brain) facilitated extinction of cocaine-induced conditioned place preference (CPP) in mice. Interestingly, this effect was associated with increased acetylation of genes implicated in long-lasting behavioral changes (i.e., c-fos, Nr4a2) in brain regions involved with drug reward and conditioning (Malvaez et al, 2013). Finally, animal models of cross-fostering (Roma et al, 2007), environmental enrichment (Neugebauer et al, 2004), maternal separation (Moffett et al, 2007), and early life adversity (Roth et al, 2009) provide tantalizing examples of epigenetic modulation that can impact drug’s effects and drug abuse behaviors.
Molecular
There is virtually unanimous consensus in the field of addiction research that the rewarding effects of drugs are related to their ability to increase DA, particularly in the NAc (Di Chiara and Imperato, 1988). Interestingly, DA’s role in reward does not seem to equate with hedonic pleasure, but with its ability to encode prediction of reward, imprinting incentive value to reinforcers and to facilitate learning of reward associations through its modulation of subcortical and cortical brain regions. We’ve also come to appreciate the crucial role of other neurotransmitters (e.g., glutamate, GABA, cannabinoids, opioids, serotonin) in reward processing and in the neuroadaptations associated with addiction ((Kalivas, 2009; Koob, 1992).
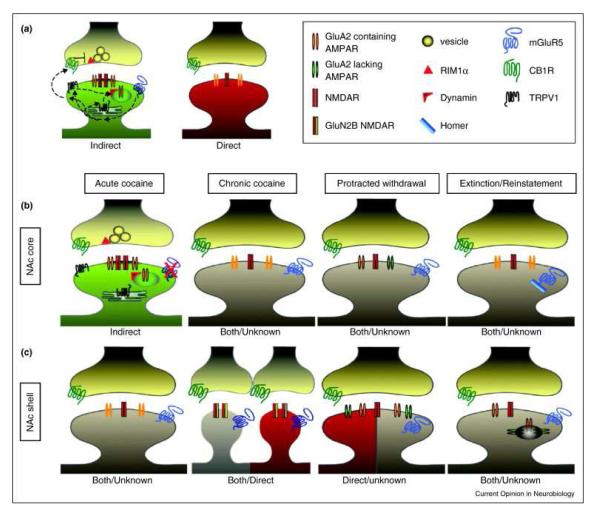
In particular, there have been major advances in our understanding of the role of excitatory synapses in the neuroplastic changes associated with repeated drug exposure. For example, It is now recognized that drugs of abuse disrupt (either increasing or decreasing) the strength of excitatory synapses by tapping into traditional mechanisms of plasticity, including long-term potentiation (LTP) and long-term depression (LTD) (Grueter et al, 2013). Synaptic plasticity is controlled pre-synaptically through the regulation of glutamate release and post-synaptically through the insertion or removal of AMPA or NMDA glutamate receptors (Bowers et al, 2013; Malenka and Bear, 2004); and drugs of abuse interfere with these processes. The observation, by several groups (Brown et al, 2011b; Huang et al, 2009; Koya et al, 2012; Lee and Dong, 2011), that cocaine administration can stimulate the creation of silent glutamatergic synapses – displaying strong NMDAR- but virtually no AMPAR-mediated responses- in the Nac is poised to further our understanding of the particularly robust nature of addiction-related learning and memory. Figure 2 summarizes the effects of drugs on excitatory synapses in NAc. For example, insertion of high calcium permeable AMPA receptors (lacking GluR2 subunit) increases after cocaine withdrawal contributing to the LTP-like increase in the AMPA:NMDA ratio (Boudreau et al, 2007; Conrad et al, 2008; Kourrich et al, 2007). Similarly, NMDA receptors containing the NR2B subunit increase after chronic cocaine or heroin (Huang et al, 2009; Shen et al, 2011).
Figure 2.
Schematic diagram of the effects of drugs on excitatory synapses in Nac MSNs. (A) Schematic representation of the differences between excitatory synapses on indirect (green) and direct (red) pathway NAc MSNs. In naïve animals, synapses on indirect pathway MSNs express TRPV1 and CB1R-dependent LTD downstream of mGluR5 activation. Synapses on direct pathway MSNs have a lesser relative amount of NMDARs and do not normally express mGluR5-dependent LTD. The properties of these two types of excitatory synapses will undergo significant changes that will be different as a function of time (along a cocaine use trajectory) and location (NAc core (b) vs shell (c)). For example, in the Nac core, acute (but not chronic) cocaine blocks mGluR5-dependent LTD. By contrast, protracted withdrawal results in synaptic incorporation of GluA2-lacking AMPARs, while extinction training also abolishes mGluR5-dependent LTD owing to changes in Homer levels. In contrast, acute cocaine does not alter synaptic function in the Nac shell. However, chronic cocaine causes a transient increase in GluN2B-containing ‘silent’ synapses. In this region, protracted withdrawal leads to an increase in AMPARs that includes incorporation of GluA2-lacking AMPARs. Following experiences known to cause reinstatement including drug re-exposure and stress, AMPARs are endocytosed. Reprinted with permission from (Grueter et al, 2013).
Gilal cells, which are activated by many drugs of abuse (Cooper et al, 2012) also modulate synaptic plasticity by regulating extracellular glutamate and setting the glutamate tone (through uptake via glutamate transporters and release via the cysteine-glutamate exchanger) (Fellin, 2009; Moussawi et al, 2009). Thus, research into glial-derived neuromodulators could also spur critical advances towards the development of addiction pharmacotherapies. A recent study, for example, provides preliminary but promising evidence that ibudilast and minocycline (two compounds that modulate glial function) can ameliorate opioid withdrawal symptoms in mice (Hutchinson et al, 2009).
Also, the combined advances of the recent past have revealed a highly interactive system of molecular communication. One prominent and extensively studied example is the interaction between the stress and reward systems (Schank et al, 2012). On one hand, animal and human studies have shown that opioids can influence the development (Lesage et al, 1998) and function (Zhou et al, 2003; Zis et al, 1985) of the HPA axis. On the other hand, active heroin addicts display abnormal basal and induced levels of ACTH and cortisol, which become normalized during methadone maintenance treatment (Kreek et al, 1984). Another example of molecular interactions and overlap is that between the homeostatic and reward pathways that modulate eating behaviors (Volkow et al, 2012b). Indeed, neurons in VTA and/or NAc express receptors for several peptides and hormones that regulate homeostatic food signals, such as glucagon-like peptide or GLP-1 (Alhadeff AL et al, 2012; Rinaman, 2010), ghrelin (Abizaid A et al, 2006; Jerlhag et al, 2007), leptin (Figlewicz et al, 2003; Leshan et al, 2010), insulin (Figlewicz et al, 2008), orexin (Fadel and AY, 2002), and melanocortin (Davis et al, 2011). Consequently, it is not surprising that these hormone/peptides can influence the rewarding responses to drugs of abuse. Such interactions could explain the findings of attenuated responses to drug reward in animal models of obesity (Davis et al, 2008).
Similarly, human studies found an inverse relationship between BMI and illicit drug use (Bluml et al, 2012) and a lower risk for SUD in obese individuals (Simon et al, 2006); including nicotine (Blendy et al, 2005) and marijuana (Warren et al, 2005). On the other hand, interventions that decrease BMI and reduce plasma levels of insulin and leptin have been shown to enhance the sensitivity to psychostimulant drugs (Davis et al, 2010); and bariatric surgery for obesity has been associated with an increased risk for relapse to alcohol abuse (Suzuki et al, 2012). These observations suggest that the homeostatic circuitry evolved to take advantage of the reward circuitry imbuing feeding behaviors not only with the conditioning-rewarding properties subsumed initially by the NAc but also with the subsequent utilization of dorsal striatal outputs to cortical structures that are central to the orchestration of goal-directed behaviors (Everitt et al, 2008). This intimate relationship between the networks governing energy and reward homeostasis has profound implications for the prevention and treatment of various eating disorders, including some forms of obesity, but also for addiction (Volkow et al, 2012b).
To recap, from the perspective of the triangular space model, we are discovering a growing number of genes whose products can influence vulnerability to SUDs by modulating the balanced function of brain regions involved with cognitive system 2. For example, carriers of the Val 158 Met polymorphism in the COMT gene, the already mentioned regulator of frontocortical DA levels, and through it of working memory and cognitive control (Cools and D’Esposito, 2011)), are at higher risk of developing alcohol dependence, especially if they have also been exposed to adverse childhood experiences (Schellekens et al, 2012). On the other side of the equation, there are gene products that can impact the sensitivity of brain regions involved with hot visceral responses. A good example of this group is the also mentioned serotonin transporter whose short allele variant can reduce serotonergic neurotransmission and facilitate impulsive behaviors (Walderhaug et al, 2010), an association that is also modulated by life experiences (Paaver et al, 2008; Wagner et al, 2009). Another example impacting the architecture of hot visceral responses involves the priming of abnormal adult stress reactivity by early maternal deprivation, partly via reversible epigenetic modification of the glucocorticoid receptor gene promoter in the hippocampus (Weaver et al, 2004).
Circuitry
An individual’s inability to escape a maladaptive behavior like addiction stems from disruptions in many different functional circuits (Redish et al, 2008) whose integrated computations (largely in Frankl’s space) and coordinated output enables goal-directed behaviors. Some of the most pernicious features of addiction are the overwhelming craving to take drugs that can reemerge even after years of abstinence, the severely compromised ability of addicted individuals to inhibit drug-seeking once the craving erupts, and the enhanced sensitivity to stress. These features combine to present one of the most formidable obstacles to successful treatment.
Based to a large degree on the imaging evidence, we and others (Koob and Le Moal, 2001; Volkow et al, 2003) have proposed that the ability to resist the urge to use a drug requires the proper functioning of neuronal circuits in charge of top-down control to oppose the conditioned responses that predict the reward and drive the motivation to maladaptive consummatory behaviors. Our model emphasizes six interacting circuits/networks: 1) reward/saliency (with key nodes in NAc and ventral pallidum); 2) memory/learning-conditioning/habits (with key nodes in amygdala and hippocampus); 3) inhibitory control/executive function (with key nodes in DLMPFC, inferior frontal cortex, OFC and ACC); 4) motivation/drive (with key nodes in OFC, subcallosal cortex, dorsal striatum and motor cortex); 5) interoception (with key nodes in insula and ACC); and 6) aversion avoidance/stress reactivity (with key nodes in habenula and amygdala)(Figure 3)(Volkow et al, 2011).
Figure 3.
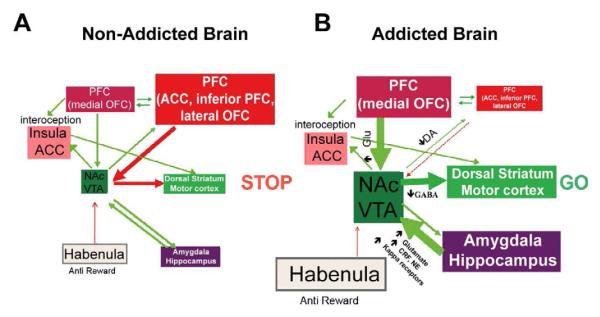
Model proposing a network of interacting circuits, disruptions in which contribute to the complex set of stereotypic behaviors underlying drug addiction and chronic overeating: reward (nucleus accumbens, VTA, and ventral pallidum), conditioning/memory (amygdala, medial OFC for attribution of saliency, hippocampus, and dorsal striatum for habits), executive control (DLPFC, ACC, inferior frontal cortex, and lateral OFC), motivation/drive (medial OFC for attribution of saliency, ventral ACC, VTA, SN, dorsal striatum, and motor cortex). Nac, nucleus accumbens, interoception (Insula and ACC), and aversion/avoidance (Habenula). (A) When these circuits are balanced, this results in proper inhibitory control and decision making. (B) During addiction, when the enhanced expectation value of the drug in the reward, motivation, and memory circuits overcomes the control circuit, favoring a positive-feedback loop initiated by the consumption of the drug and perpetuated by the enhanced activation of the motivation/drive and memory circuits. These circuits also interact with circuits involved in mood regulation, including stress reactivity (which involves the amygdala, hypothalamus, habenula) and interoception (which involves the insula and ACC and contributes to awareness of craving). Several neurotransmitters are implicated in these neuroadaptations, including glutamate, GABA, norepinephrine, corticotropin-releasing factor, and opioid receptors. CRF, corticotropin-releasing factor; NE, norepinephrine. Modified with permission (Volkow et al, 2011).
These circuits/networks receive direct innervations from DA neurons but are also connected with one another through direct or indirect projections (mostly glutamatergic and GABAergic) and modulated by other monoaminergic inputs (serotonin and norepinephrine). Their concerted operations impact a broad spectrum of behaviors, which helps explains the multidimensional nature of addiction and its extensive overlaps (symptoms and comorbidities) with other psychiatric conditions. Some of the recent advances pertaining to addiction circuitry are outlined below.
Predicting reward, assessing saliency
The mesolimbic DA pathway [DA cells in VTA that project to NAc] seems to be crucial for drug reward (Wise, 2009). However, other DA pathways [mesostriatal (DA cells in substantia nigra projecting into dorsal striatum) and mesocortical (DA cells in VTA projecting into PFC)] also contribute to drug reward and addiction (Wise, 2009). It appears that the rewarding and conditioning effects of drugs are predominantly driven by phasic DA cell firing, which leads to large and transient DA increases. In contrast, the downstream changes in executive function seen in addicted individuals are linked to changes in tonic DA cell firing and result in lower but more stable DA levels (Grace, 2000; Wanat et al, 2009). This mechanism implicates the lower affinity D1R, which stimulates cyclic AMP signaling, as being involved both in acute drug reward as well as in conditioning, since these are associated with the high DA concentrations necessary to stimulate D1R. In contrast, D2Rs, which inhibit cyclic AMP signaling, are stimulated by both phasic and tonic DA. Most clinical studies on the effects of drugs of abuse and addiction in the human brain have focused on D2Rs due to the lack of specific radiotracers for the PET imaging of D1R (D1R radioligands exist but are not sufficiently specific), D3R (PHNO is a ligand being used to assess D3R though it also binds to D2R), D4R and D5R.
In humans, PET studies have shown that several drugs [stimulants (Drevets et al, 2001; Volkow et al, 1999c; Volkow et al, 1994), nicotine (Brody et al, 2009), alcohol (Boileau et al, 2003), and marijuana (Bossong et al, 2009)] increase DA in dorsal and ventral striatum (where the NAc is located). These studies, which take advantage of several radiotracers (e.g., [11C]raclopride) that bind to D2R/D3Rs but only when they are not occupied by endogenous DA, have shown that drug-induced DA increases in striatum are proportional to the intensity of the subjective experience of euphoria or “high” [see review (Volkow et al, 2009a)]. PET studies have also revealed a clear, direct relationship between a drug’s pharmacokinetic profile (i.e., the speed with which it enters and leaves the brain) and its reinforcing effects. Specifically, the faster a drug reaches peak levels in the brain the more intense the “high” (Volkow et al, 2009a). Interestingly, there is now increasing evidence that comparable DA responses are linked with food reward and that these mechanisms are also likely to play a role in excessive food consumption and obesity (Volkow et al, 2012b).
Conditioning circuitry
The finding that stimuli that induce fast and large DA increases also trigger conditioned responses and elicit incentive motivation to procure them (Owesson-White et al, 2009) turned out to have profound implications for our understanding of addiction. Through the process of conditioning, neutral stimuli that are linked to the reinforcer (either natural or a drug) acquire the ability to increase DA in the striatum (including NAc) by themselves in anticipation of the reward, thus engendering a strong motivation to seek the drug (Owesson-White et al, 2009).
Brain imaging studies comparing the DA increases induced by stimulant drugs (methylphenidate (MP) or amphetamine (AMPH)) in cocaine addicted vs. control subjects showed a marked attenuation of drug-induced DA increases in striatum (50% lower in detoxified abusers and 80% lower in active abusers) and lower self-reports of the drug’s rewarding effects relative to non–drug-abusing controls (Martinez et al, 2007; Volkow et al, 1997; Volkow et al, 2011). This observation is somewhat puzzling since MP and AMPH are pharmacologically similar to cocaine and methamphetamine, respectively, and drug abusers cannot distinguish between them when they are administered intravenously. Since the marked reductions in the drug-induced DA increases were unlikely to be the result of withdrawal symptoms (Volkow et al, 2011), we’ve interpreted these and related results (Volkow et al, 2009a) as consistent with the hypothesis that the rewarding response becomes deficient in drug addicted individuals. They may also provide another argument in favor of the notion that the acute pharmacological DA-enhancing effects of the drug in NAc (or in dorsal striatum) cannot explain by themselves the increased motivation to consume them.
The response of VTA DA neurons to rewarding stimuli changes with repeated exposure. While DA cells fire upon the first exposure to a novel reward, repeated exposure to DA causes the neurons to stop firing upon reward consumption and to fire instead when they are exposed to stimuli that are predictive of the reward (Schultz et al, 1997). This gradual shift is likely to underlie DA’s role in learning and conditioning. Indeed, drug-induced phasic DA signaling can eventually trigger neuroadaptations in ancillary circuits that are related to habit formation and behavioral conditioning. These changes are predominantly induced by D1R signaling and synaptic changes in glutamate-modulated NMDA and AMPA receptors (Luscher and Malenka, 2011; Zweifel et al, 2009). Recruitment of these circuits is significant for disease progression because the ensuing conditioned responses help explain the intense desire for the drug (craving) and the compulsive use when addicted subjects are exposed to drug cues. This hypothesis is consistent with other observations (Volkow et al, 2006c; Wong et al, 2006) that show the power of cocaine-associated cue exposure to raise DA levels in the dorsal striatum and trigger a concomitant increase in the subjective experience of craving in detoxified cocaine abusers. Since the dorsal striatum plays a role in habit learning (Belin et al, 2009; Yin et al, 2004), the association is likely to reflect the strengthening of habits as chronicity of addiction progresses. This suggests that a basic disruption in addiction might relate to the DA-triggered conditioned responses that result in habits leading to intense craving and compulsive drug consumption. Interestingly, in actively using cocaine addicted subjects, the DA increases triggered by conditioned cues appear to be even larger than those produced by the stimulant drug itself as assessed in two separate groups of subjects (Volkow et al, 2006a; Volkow et al, 2011). This suggests that conditioned responses may drive the DA signaling that maintains the motivation to take the drug even when its pharmacological effects appear attenuated. Thus, although drugs may initially induce feelings of immediate reward through DA release in the ventral striatum, with repeated use, and as habit develops, there appears to be a shift from the drug to the conditioned stimulus. Preclinical studies suggest that these conditioned responses are mediated by glutamatergic projections from PFC and amygdala into VTA/SN and NAc (Kalivas, 2009). In this manner, the mere prediction of a reward may eventually become the reinforcer that motivates the stereotypic consummatory behavior.
Natural reinforcers are also likely to induce an equivalent and gradual shift in DA increases (from ventral to more dorsal regions of the striatum) during the transition from a novel stimulus that is inherently rewarding to that of the associated cues that predict it. The extensive glutamatergic afferents to DA neurons from regions involved in the processing of sensory (insula or primary gustatory cortex), homeostatic (hypothalamus), reward (NAc), emotional (amygdala and hippocampus), and multimodal (OFC for salience attribution) information, modulate their activity in response to rewards and to conditioned cues (Geisler and Wise, 2008). One example are the projections from the amygdala and the OFC to DA neurons and to NAc that are involved in conditioned responses to food (Petrovich, 2010). Indeed, imaging studies showed that when non-obese male subjects were asked to inhibit their craving for food -while being exposed to food cues-, they exhibited decreased metabolic activity in amygdala and OFC (as well as in hippocampus), insula and striatum, and that the decreases in OFC were associated with reductions in food craving (Wang et al, 2009). A similar inhibition of the metabolic activity in the OFC (and also in NAc) has been observed in cocaine abusers when they were asked to inhibit their drug craving upon exposure to cocaine-cues (Volkow et al, 2009b). Interestingly, craving for video-game playing, in response to video game cues has also been reported to increase the activity of the OFC, ACC, and the left inferior frontal gyrus (Han et al, 2011; Han et al, 2010).
Impaired inhibitory control in addiction
The capacity to inhibit prepotent responses is a major contributor to an individual’s vulnerability to addiction because it modulates his or her ability to avoid inappropriate behaviors (Volkow and Fowler, 2000; Volkow et al, 2008). PET studies have uncovered significant reductions in D2R availability in the striatum of addicted subjects that persist for months after protracted detoxification [reviewed in (Volkow et al, 2009a)]. Similarly, preclinical studies in rodent and non-human primates have shown that repeated drug exposure is associated with reductions in striatal D2R levels (Nader et al, 2006; Thanos et al, 2007; Volkow et al, 2001). In the striatum, D2Rs mediate signaling in the striatal indirect pathway that modulates PFC regions; and its downregulation has been shown to enhance sensitization to the effects of drugs in animal models (Ferguson et al, 2011). In humans addicted to drugs, the reduction in striatal D2R is associated with decreased activity in PFC as evidenced by decreases in baseline glucose metabolism (a marker of brain function) in OFC, ACC, and dorsolateral (dl) PFC (Volkow et al, 2001; Volkow et al, 1993; Volkow et al, 2007). Inasmuch as OFC, ACC, and dlPFC are involved with salience attribution, inhibitory control/emotion regulation, and decision making, respectively, it has been postulated that their improper regulation by D2R-mediated DA signaling in addicted subjects could underlie the enhanced motivational value of drugs in their behavior and the loss of control over drug intake (Volkow et al, 2000). In addition, because impairments in OFC and ACC are associated with compulsive behaviors and impulsivity (Fineberg et al, 2009), DA’s impaired modulation of these regions is likely to contribute to the compulsive and impulsive drug intake seen in addiction (Volkow et al, 2000). Indeed, low striatal D2R has been associated with impulsivity in methamphetamine abusers (Lee et al, 2009), and predicted compulsive cocaine administration in rodents (Everitt et al, 2008).
Although not mutually exclusive, there is also the possibility of a reverse scenario, in which a preexisting vulnerability for drug abuse in PFC becomes exacerbated by repeated drug use through further decreases in striatal D2R. Consistent with this scenario, a study done in subjects who, despite having a high risk for alcoholism (positive family history of alcoholism) were not alcoholics themselves, revealed a higher than normal striatal D2R availability that was associated with normal metabolism in OFC, ACC, and dorsolateral PFC (Volkow et al, 2006b). This suggests that, in these subjects at risk for alcoholism, the normal PFC function was linked to enhanced striatal D2R signaling, which in turn may have protected them from alcohol abuse.
Since the PFC plays a crucial role in executive function, including inhibitory control (Miller and Cohen, 2001), impairments in its operations, which are modulated by D1R and D2R (presumably also D4R), are likely to contribute to the poor control and high compulsivity seen in addiction. A better understanding of the mechanisms that lead to impaired PFC function in addiction should spur the development of better strategies to ameliorate, or perhaps even reverse, specific deficits in crucial cognitive domains. For example, delay discounting, which is the tendency to devalue a reward as a function of the temporal delay of its delivery, is one of the most extensively investigated cognitive operations pertaining to disorders associated with impulsivity and compulsivity. Delay discounting has been exhaustively investigated in drug abusers who exhibit an exaggerated preference of small-but-immediate over large-but-delayed rewards (Bickel et al, 2007). Not surprisingly, delay discounting seems to depend on the function of the NAc (Gregorios-Pippas et al, 2009) and the PFC, including lateral OFC (Bjork et al, 2009), and is sensitive to DA manipulations (Pine et al, 2010). Interestingly, abnormally high rates of discounting may reflect a trans-disease process (Bickel et al, 2012) that underlies or occurs across a wide range of disorders, including ADHD (Costa Dias et al, 2012), bipolar disorder and schizophrenia (Ahn et al, 2011), and pathological gambling (Miedl et al, 2012). Interestingly, and harking back to the genetic level of analysis, the results of a recent study suggest that the 7R allele of the D4R increases delayed discounting rate, and that this influence is modulated by childhood socioeconomic status (Sweitzer et al, 2012). When taken together, these observations seem to suggest that the complex circuitry underlying delayed discounting could be an early biomarker of broad spectrum risk for psychiatric disorders.
The Motivation substrate
The circuitry that enables the investing of continued effort toward achieving a goal is heavily modulated by DA acting through several brain regions, including NAc, ACC, OFC, dlPFC, amygdala, dorsal striatum, and ventral pallidum (Salamone et al, 2007). This helps explain why dysregulated DA signaling is associated with abnormal (enhanced) motivation to procure drugs, a hallmark of addiction, and why drug addicted individuals often engage in extreme behaviors to obtain drugs, even when they entail known severe and adverse consequences (Volkow and Li, 2005a). Because drug-taking becomes the primary motivational drive in drug addiction (Volkow et al, 2003), addicted subjects are aroused and motivated by the process of obtaining the drug but tend to become withdrawn and apathetic when exposed to non–drug-related activities. This shift has been studied by comparing the brain activation patterns occurring during exposure to conditioned cues with those occurring in the absence of such cues. In contrast to the decreases in PFC activity reported in detoxified cocaine abusers when not stimulated with drug or drug cues [see review (Volkow et al, 2009a)], PFC regions become activated when cocaine abusers are exposed to craving-inducing stimuli (either drugs or cues)(Grant et al, 1996; Volkow et al, 1999a; Wang et al, 1999). This result is reminiscent of the observation that cocaine abusers, studied shortly after an episode of cocaine binging, showed an increase in metabolic activity in OFC and ACC (also dorsal striatum) that was associated with the intensity of their craving (Volkow et al, 1991).
Moreover, when the responses to iv MP were compared between cocaine addicted and non-addicted individuals, the former responded with heightened metabolic activity in ventral ACC and medial OFC (an effect associated with craving), while the latter showed the opposite response, namely decreased metabolism in these regions (Volkow et al, 2005b). This suggests that the activation of these PFC regions with drug exposure may be specific to addiction and associated with the enhanced desire for the drug. In addition, a study that prompted cocaine-addicted subjects to purposefully inhibit craving when exposed to drug cues showed that those subjects who were successful at inhibiting craving displayed decreased metabolism in medial OFC (which processes motivational value of a reinforcer) and NAc (which predicts reward) (Volkow et al, 2009b).
Perception of internal states
Neuroimaging studies have revealed that the middle insula plays a critical role in cravings for food, cocaine and cigarettes (Bonson et al, 2002; Pelchat et al, 2004; Wang et al, 2007). The importance of the insula in the context of addiction was first noticed in a study that found that smokers with damage to this region (but not control smokers who had suffered extra-insular lesions) were able to stop smoking easily and without experiencing cravings or relapse (Naqvi et al, 2007). The insula, particularly its more anterior regions, is reciprocally connected to several limbic regions (e.g., ventromedial (vm) PFC, amygdala, and ventral striatum) and appears to have an interoceptive function that integrates the autonomic and visceral information with emotion and motivation, thus providing conscious awareness of these urges (Naqvi and Bechara, 2009). Indeed, brain lesion studies suggest that the vmPFC and insula are necessary components of the distributed circuits that support emotional (Clark et al, 2008) as well as moral (Verdejo-Garcia et al, 2012) decision-making. Consistent with this hypothesis, imaging studies show differential activation of the insula during craving (Brody et al, 2009; Goudriaan et al, 2010; Naqvi et al, 2009; Wang et al, 1999). Accordingly, the reactivity of this brain region has been suggested to serve as a biomarker to help predict relapse (Janes et al, 2010).
Aversion circuitry
DA cells will fire less than normal if the expected reward fails to materialize (Schultz et al, 1997). Several lines of evidence (Christoph et al, 1986; Lisoprawski et al, 1980; Matsumoto and Hikosaka, 2007; Nishikawa et al, 1986) point to the habenula as one of the regions that controls the decreases in firing of DA cells in VTA that may signal the failure to receive an expected reward (Kimura et al, 2007). It would be reasonable to hypothesize then that enhanced sensitivity in the habenula, as a result of chronic drug exposures, could underlie a greater reactivity to drug cues. This hypothesis is supported by the observation that activation of the habenula is associated with behavioral relapse to drug-taking upon cue exposure in cocaine addicted individuals (Brown et al, 2011a; Zhang et al, 2005). The hypothesis is also consistent with the finding that α5 nicotinic receptors in the habenula appear to modulate the aversive responses to large doses of nicotine (Fowler et al, 2011b), and with the evidence implicating α5 and α2 receptors in the habenula in nicotine withdrawal (Salas et al, 2009). The involvement of the habenula as an “antireward” hub within emotional networks is consistent with prior theoretical models of addiction that postulated sensitized anti-reward responses (mediated through enhanced sensitivity of the amygdala and increased signaling through the corticotropin releasing factor) as major drivers of drug intake in addiction (Koob and Le Moal, 2008).
Making connections
Perhaps one of the most important advances in our understanding of brain organization has come from the widening use of fMRI to peer into the functional connectivity patterns of the brain at rest (resting functional connectivity or RFC) and during specific activation in healthy and diseased states. A recent RFC study, for example, has shown that, compared to non-smokers, smokers displayed a stronger coupling between left fronto-parietal and mPFC networks, and its strength was in direct relationship with smoking-cue dependent reactivity in the dorsal striatum (Janes et al, 2012). In fact, nicotine dependence has been associated with several disruptions in RFC including the connectivity between the executive control and default mode networks (DMN), which ameliorates with nicotine replacement therapy (NRT) (Cole et al, 2010). Moreover, this approach is being used to study the mechanisms underlying the high vulnerability to SUD in patients with other psychiatric disorders (Moran et al, 2012).
Therapeutics
The identification of neurotransmitter receptors and transporters involved in the processes of drug reward and neuroplasticity in SUD has expanded the numbers of molecular targets that have potential for the development of medications against SUD (Volkow and Skolnick, 2012a).
At a more basic level, the expanding field of pharmacogenomics provides numerous examples of the way in which genetic discovery is being parlayed into future therapeutic benefits. Several recent advances, particularly in the fields of alcohol and nicotine addictions, point the way forward. Thus far, some of the most promising results have been obtained by looking at polymorphisms in the OPRM1 and CYP2A6 genes, which have been effective at predicting clinical response to naltrexone (NTX) in alcoholism and NRT in smoking, respectively. In terms of treating alcohol-dependent individuals, carriers of the A118G polymorphism have better clinical responses on NTX, including lower relapse rates, compared with carriers of the A allele (Anton et al, 2008; Oslin et al, 2003). Similarly, the T allele of the GABA receptor subunit alpha-6 (GABRA6)T1519C polymorphism has been associated with greater NTX efficacy while the C allele predicted improvement with acamprosate (Ooteman et al, 2009). For smoking cessation treatment with transdermal NRT, polymorphisms that result in a less active CYP2A6, were found to be associated with increased nicotine levels from NRT, fewer doses of nicotine spray per day, and better outcomes (Malaiyandi et al, 2006; Schnoll et al, 2009). Interestingly, and in contrast, rapid metabolizers of nicotine had higher quit-rates when treated with bupropion (FDA-approved antidepressant for the treatment of nicotine dependence) compared to placebo (34% versus 10%), whereas the opposite was true for slow metabolizers (Patterson et al, 2008). Other studies have shown that carriers of the Met/Met genotype of the COMT gene had higher rates of prolonged abstinence with NRT than carriers of the Met/Val + Val/Val group (Colilla et al, 2005; Johnstone et al, 2007). Taken together, these results provide evidence for the promise of pharmacogenomics as a conduit to more personalized treatment interventions. It is worth-noting that polymorphisms that impact NRT success have been identified in different classes of genes, whose products are involved in diverse functions, including cell adhesion, signal transduction, receptor function and enzymatic activity (Uhl et al, 2008)..
The identification of the molecular targets implicated in neuroplasticity has also guided the development of new medications, which have shown promising results in animal models of relapse or withdrawal. Some of these are being tested clinically, as in the case of N-acetylcysteine, which targets the cystine-glutamate exchanger and helps restore glutamate homeostasis and neuronal plasticity (Moussawi et al, 2011). Early clinical trials with N-acetylcysteine for the treatment of cocaine and nicotine addictions have shown positive results (Knackstedt et al, 2009; Mardikian et al, 2007). Other targets of interest include mGluR2/3 agonists, mGluR5 negative allosteric modulators, stimulators of glutamate transport, and inhibitors of either AMPA or NMDA receptors. As it has been shown for other clinical conditions, there is also evidence that combinations of compounds that target different proteins might increase their effectiveness and improve the outcome in the treatment of SUDs. This has been shown to be the case for preliminary studies of nicotine treatment with the combination of bupropion and sustained release (SR) varenicline (Ebbert et al, 2009), and for the treatment of cocaine addiction treatment with the combination of topiramate and SR amphetamine (Mariani et al, 2012).
Similarly, an increased understanding of the circuits that are disrupted by repeated drug administration has expanded the targets for therapeutic interventions aimed at restoring their function. Though still in its infancy, ongoing research is exploring the potential benefits of functional MRI-guided biofeedback (Horrell et al, 2010), repeated transcranial and electrical stimulation (Fraser and Rosen, 2012) and/or even deep brain stimulation (Muller et al, 2012).
At the same time, advances in vaccine technology and longer lasting monoclonal antibodies are making it possible to explore vaccines and passive immunization protocols as potential treatments for SUDs (Hicks et al, 2012; Kinsey et al, 2009; Norman et al, 2009; Rosenberg et al, 2012). These approaches hope to harness a person’s own immune system to produce antibodies that can bind to a specific drug (e.g., cocaine, nicotine, heroin) while still in the bloodstream, thus largely preventing its entry into the brain. The nicotine vaccine developed for smoking cessation, NicVAX, is one example that showed promising results in that smokers who generated high titers of anti-nicotine antibodies were three-times more likely to achieve sustained abstinence compared with those immunized receiving a control (placebo) vaccine (Hatsukami et al, 2011). Even subjects unable to achieve abstinence reduced their smoking by more than 50%. However, major challenges remain (Moreno and Janda, 2011), such as the need to significantly increase the antigenicity of the vaccine so that a greater number of those vaccinated can produce the high levels of antibody necessary for a therapeutic response. In parallel, development of modified enzymes involved with drug metabolism and catalytic antibodies also hold promise for the treatment of SUD (i.e., cholinesterases for cocaine addiction) (Schindler and Goldberg, 2012).
In conclusion
In the past few years we have markedly increased our understanding of the role of genes in the human brain and its development and of the ways in which their output is influenced by other genes and by the environment (including prenatal as well as postnatal drug exposures). In parallel, advances in epigenetics have clarified the mechanisms underlying the drug-induced neuroplastic changes and circuitry perturbations that facilitate the transition from casual, to chronic, and then to compulsive drug taking. Meanwhile, advances in the characterization of developmental changes in the human brain are helping us understand why the adolescent brain is more likely to enter an addiction trajectory after its initial exposure to drugs.
As mentioned at the outset, behavioral economists have proposed a useful heuristics of brain function based on two modes of operation (automatic vs. analytical) to explain human judgments and actions (Epstein, 1994; Kahneman et al, 2002; Loewenstein, 1996; Sloman, 1996); an admittedly over-simplistic model that, nevertheless, seems very relevant to addiction. The so called “System 1” (related to the hot or implicit “mind”) tends to coalesce around preferentially automatic, intuitive brain processes, whereas “System 2” (related to the cold or explicit “mind”) preferentially recruits more controlled, deliberative and analytical processes (Morewedge and Kahneman, 2010). As it follows from all the evidence presented in this and other (Baler and Volkow, 2011) reviews, the two systems can be expected to be strongly influenced by the dynamic interactions between genetic, epigenetic, developmental, and environmental factors that shape the structure, connectivity and function of the mental landscape.
Importantly, the hot and cold systems are presumed to place different demands on cognitive effort and show different susceptibility to interference by competing stimuli (Frederick, 2002). Based on social neuroscience advances of the past decade, it might be wise to refrain from trying to pin down this dual model onto clearly delimited brain regions, an effort that has been chastised as modern-day phrenology (Forbes and Grafman, 2013). While it is possible that some areas are preferentially involved in implicit vs. explicit processing (Figure 4) understanding “how” they interact is likely to be as, if not more important in addiction. For example, the stereotypic and automatic behaviors displayed by drug-addicted individuals may reflect a progressive dialing up of the connectivity weights that favor hot systems. Indeed, recent imaging and/or behavioral studies of the balance between “hot” and “cold” executive functions in substance abusers (Crunelle et al, 2012; Goldstein et al, 2004; Moreno-Lopez et al, 2012a, b; Quednow et al, 2007), children with ADHD responding to stimulant medication (Hale et al, 2011; Kubas et al, 2012), and patients with schizophrenia (Wing et al, 2013) are beginning to provide evidence of such “tilting” and its potentially broad heuristic value.
Figure 4.
Model of addiction as a disease of the brain characterized by perturbed interactions among the distributed networks that orchestrate balanced goal directed behaviors. From a behavioral economics perspective, addiction can be construed as the drug-induced consequence of a perturbed balance (in favor of system 1) between the proposed system 1 and system 2-centered processes that enable adaptive (efficient + flexible) decision making and goal-directed behaviors by mounting a proper situation-specific combination of automatic and cognitive responses. In this model, the location/identity of the various nodes is less important than the overall patterns of how information flows among them. The balanced (or lack thereof) output of this distributed network is established, maintained, and expressed by the emergent relationships among the morphological, architectural, connectivity and functional levels of the brain. These are, in turn, determined by the combined pressures and influences exerted by genetic (e.g., affecting temperament, drug metabolism), epigenetic (e.g., drug exposure, parental style), developmental (e.g., fetal, brain, adolescent), and environmental (e.g., economic, social, built) variables.
Regardless of the model used to describe addiction, its characterization as a chronic disease that disrupts the critical space between stimulus and response provides the basis for its prevention and treatment as a clinical disorder rather than a criminal behavior. Thus, although addiction still remains highly stigmatized the new scientific knowledge is starting to slowly impact the way society addresses SUDs.
Addiction is a clinical disorder, not a criminal behavior.
We are beginning to tackle addiction as a problem of “systems.”
Research has delivered countless new targets for addiction medications.
Large, open access datasets will transform the science of risk and resilience.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116(116):3229–3239. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn WY, Rass O, Fridberg DJ, Bishara AJ, Forsyth JK, Breier A, et al. Temporal discounting of rewards in patients with bipolar disorder and schizophrenia. J Abnorm Psychol. 2011;120(4):911–921. doi: 10.1037/a0023333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhadeff AL, Rupprecht LE, MR H. GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology. 2012;153:647–658. doi: 10.1210/en.2011-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang E, Chen J, Zagouras P, Magna H, Holland J, Schaeffer E, et al. Induction of nuclear factor-kappaB in nucleus accumbens by chronic cocaine administration. J Neurochem. 2001;79(1):221–224. doi: 10.1046/j.1471-4159.2001.00563.x. [DOI] [PubMed] [Google Scholar]
- Anton RF, Oroszi G, O’Malley S, Couper D, Swift R, Pettinati H, et al. An evaluation of mu-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence: results from the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) study. Arch Gen Psychiatry. 2008;65(2):135–144. doi: 10.1001/archpsyc.65.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baler RD, Volkow ND. Addiction as a systems failure: focus on adolescence and smoking. J Am Acad Child Adolesc Psychiatry. 2011;50(4):329–339. doi: 10.1016/j.jaac.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Jonkman S, Dickinson A, Robbins TW, Everitt BJ. Parallel and interactive learning processes within the basal ganglia: relevance for the understanding of addiction. Behav Brain Res. 2009;199(1):89–102. doi: 10.1016/j.bbr.2008.09.027. [DOI] [PubMed] [Google Scholar]
- Bevilacqua L, Goldman D. Genetics of emotion. Trends Cogn Sci. 2011;15(9):401–408. doi: 10.1016/j.tics.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Jarmolowicz DP, Mueller ET, Koffarnus MN, Gatchalian KM. Excessive discounting of delayed reinforcers as a trans-disease process contributing to addiction and other disease-related vulnerabilities: emerging evidence. Pharmacol Ther. 2012;134(3):287–297. doi: 10.1016/j.pharmthera.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Miller ML, Yi R, Kowal BP, Lindquist DM, Pitcock JA. Behavioral and neuroeconomics of drug addiction: competing neural systems and temporal discounting processes. Drug Alcohol Depend. 2007;90(Suppl 1):S85–91. doi: 10.1016/j.drugalcdep.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierut LJ, Madden PA, Breslau N, Johnson EO, Hatsukami D, Pomerleau OF, et al. Novel Genes Identified in a High Density Genome Wide Association Study for Nicotine Dependence. Hum Mol Genet. 2006 doi: 10.1093/hmg/ddl441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilinski P, Wojtyla A, Kapka-Skrzypczak L, Chwedorowicz R, Cyranka M, Studzinski T. Epigenetic regulation in drug addiction. Ann Agric Environ Med. 2012;19(3):491–496. [PubMed] [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299(11):1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Momenan R, Hommer DW. Delay discounting correlates with proportional lateral frontal cortex volumes. Biol Psychiatry. 2009;65(8):710–713. doi: 10.1016/j.biopsych.2008.11.023. [DOI] [PubMed] [Google Scholar]
- Blendy JA, Strasser A, Walters CL, Perkins KA, Patterson F, Berkowitz R, et al. Reduced nicotine reward in obesity: cross-comparison in human and mouse. Psychopharmacology (Berl) 2005;180(2):306–315. doi: 10.1007/s00213-005-2167-9. [DOI] [PubMed] [Google Scholar]
- Bluml V, Kapusta N, Vyssoki B, Kogoj D, Walter H, Lesch OM. Relationship between substance use and body mass index in young males. Am J Addict. 2012;21(1):72–77. doi: 10.1111/j.1521-0391.2011.00192.x. [DOI] [PubMed] [Google Scholar]
- Boileau I, Assaad JM, Pihl RO, Benkelfat C, Leyton M, Diksic M, et al. Alcohol promotes dopamine release in the human nucleus accumbens. Synapse. 2003;49(4):226–231. doi: 10.1002/syn.10226. [DOI] [PubMed] [Google Scholar]
- Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, et al. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26(3):376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Bossong MG, van Berckel BN, Boellaard R, Zuurman L, Schuit RC, Windhorst AD, et al. Delta 9-tetrahydrocannabinol induces dopamine release in the human striatum. Neuropsychopharmacology. 2009;34(3):759–766. doi: 10.1038/npp.2008.138. [DOI] [PubMed] [Google Scholar]
- Boudreau AC, Reimers JM, Milovanovic M, Wolf ME. Cell surface AMPA receptors in the rat nucleus accumbens increase during cocaine withdrawal but internalize after cocaine challenge in association with altered activation of mitogen-activated protein kinases. J Neurosci. 2007;27(39):10621–10635. doi: 10.1523/JNEUROSCI.2163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers MS, Chen BT, Bonci A. AMPA receptor synaptic plasticity induced by psychostimulants: the past, present, and therapeutic future. Neuron. 2013;67(1):11–24. doi: 10.1016/j.neuron.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, Allen-Martinez Z, Scheibal D, Abrams AL, et al. Ventral striatal dopamine release in response to smoking a regular vs a denicotinized cigarette. Neuropsychopharmacology. 2009;34(2):282–289. doi: 10.1038/npp.2008.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RM, Short JL, Lawrence AJ. Identification of brain nuclei implicated in cocaine-primed reinstatement of conditioned place preference: a behaviour dissociable from sensitization. PLoS One. 2011a;5(12):e15889. doi: 10.1371/journal.pone.0015889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TE, Lee BR, Mu P, Ferguson D, Dietz D, Ohnishi YN, et al. A silent synapse-based mechanism for cocaine-induced locomotor sensitization. J Neurosci. 2011b;31(22):8163–8174. doi: 10.1523/JNEUROSCI.0016-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budulac SE, Vonk JM, Postma DS, Siedlinski M, Timens W, Boezen MH. Nicotinic acetylcholine receptor variants are related to smoking habits, but not directly to COPD. PLoS One. 2012;7(3):e33386. doi: 10.1371/journal.pone.0033386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carouge D, Host L, Aunis D, Zwiller J, Anglard P. CDKL5 is a brain MeCP2 target gene regulated by DNA methylation. Neurobiol Dis. 2010;38(3):414–424. doi: 10.1016/j.nbd.2010.02.014. [DOI] [PubMed] [Google Scholar]
- Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry. 2010;167(5):509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297(5582):851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Sonuga-Barke EJ, Milham MP, Tannock R. Characterizing cognition in ADHD: beyond executive dysfunction. Trends Cogn Sci. 2006;10(3):117–123. doi: 10.1016/j.tics.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Curley JP. How social experiences influence the brain. Curr Opin Neurobiol. 2005;15(6):704–709. doi: 10.1016/j.conb.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Christoph GR, Leonzio RJ, Wilcox KS. Stimulation of the lateral habenula inhibits dopamine-containing neurons in the substantia nigra and ventral tegmental area of the rat. J Neurosci. 1986;6(3):613–619. doi: 10.1523/JNEUROSCI.06-03-00613.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Bechara A, Damasio H, Aitken MR, Sahakian BJ, Robbins TW. Differential effects of insular and ventromedial prefrontal cortex lesions on risky decision-making. Brain. 2008;131(Pt 5):1311–1322. doi: 10.1093/brain/awn066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DM, Beckmann CF, Long CJ, Matthews PM, Durcan MJ, Beaver JD. Nicotine replacement in abstinent smokers improves cognitive withdrawal symptoms with modulation of resting brain network dynamics. Neuroimage. 2010;52(2):590–599. doi: 10.1016/j.neuroimage.2010.04.251. [DOI] [PubMed] [Google Scholar]
- Colilla S, Lerman C, Shields PG, Jepson C, Rukstalis M, Berlin J, et al. Association of catechol-O-methyltransferase with smoking cessation in two independent studies of women. Pharmacogenet Genomics. 2005;15(6):393–398. doi: 10.1097/01213011-200506000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvis CM, Pollock JD, Goodman RH, Impey S, Dunn J, Mandel G, et al. Epigenetic mechanisms and gene networks in the nervous system. J Neurosci. 2005;25(45):10379–10389. doi: 10.1523/JNEUROSCI.4119-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454(7200):118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, D’Esposito M. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry. 2011;69(12):e113–125. doi: 10.1016/j.biopsych.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Jones JD, Comer SD. Glial modulators: a novel pharmacological approach to altering the behavioral effects of abused substances. Expert Opin Investig Drugs. 2012;21(2):169–178. doi: 10.1517/13543784.2012.651123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa Dias TG, Wilson VB, Bathula DR, Iyer SP, Mills KL, Thurlow BL, et al. Reward circuit connectivity relates to delay discounting in children with attention-deficit/hyperactivity disorder. Eur Neuropsychopharmacol. 2012 doi: 10.1016/j.euroneuro.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelle CL, Veltman DJ, Booij J, Emmerik-van Oortmerssen K, van den Brink W. Substrates of neuropsychological functioning in stimulant dependence: a review of functional neuroimaging research. Brain Behav. 2012;2(4):499–523. doi: 10.1002/brb3.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JF, Choi DL, Benoit SC. Insulin, leptin and reward. Trends Endocrinol Metab. 2010;21(2):68–74. doi: 10.1016/j.tem.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JF, Choi DL, Shurdak JD, Krause EG, Fitzgerald MF, Lipton JW, et al. Central melanocortins modulate mesocorticolimbic activity and food seeking behavior in the rat. Physiol Behav. 2011;102(5):491–495. doi: 10.1016/j.physbeh.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JF, Tracy AL, Schurdak JD, Tschop MH, Lipton JW, Clegg DJ, et al. Exposure to elevated levels of dietary fat attenuates psychostimulant reward and mesolimbic dopamine turnover in the rat. Behav Neurosci. 2008;122(6):1257–1263. doi: 10.1037/a0013111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85(14):5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty JL, O’Donovan MC, Owen MJ. Recent genomic advances in schizophrenia. Clin Genet. 2012;81(2):103–109. doi: 10.1111/j.1399-0004.2011.01773.x. [DOI] [PubMed] [Google Scholar]
- Drabant EM, Hariri AR, Meyer-Lindenberg A, Munoz KE, Mattay VS, Kolachana BS, et al. Catechol O-methyltransferase val158met genotype and neural mechanisms related to affective arousal and regulation. Arch Gen Psychiatry. 2006;63(12):1396–1406. doi: 10.1001/archpsyc.63.12.1396. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Gautier C, Price JC, Kupfer DJ, Kinahan PE, Grace AA, et al. Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biol Psychiatry. 2001;49(2):81–96. doi: 10.1016/s0006-3223(00)01038-6. [DOI] [PubMed] [Google Scholar]
- Drgon T, Zhang PW, Johnson C, Walther D, Hess J, Nino M, et al. Genome wide association for addiction: replicated results and comparisons of two analytic approaches. PLoS One. 2010;5(1):e8832. doi: 10.1371/journal.pone.0008832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbert JO, Croghan IT, Sood A, Schroeder DR, Hays JT, Hurt RD. Varenicline and bupropion sustained-release combination therapy for smoking cessation. Nicotine Tob Res. 2009;11(3):234–239. doi: 10.1093/ntr/ntn031. [DOI] [PubMed] [Google Scholar]
- Enge S, Fleischhauer M, Lesch KP, Reif A, Strobel A. Serotonergic modulation in executive functioning: linking genetic variations to working memory performance. Neuropsychologia. 2011;49(13):3776–3785. doi: 10.1016/j.neuropsychologia.2011.09.038. [DOI] [PubMed] [Google Scholar]
- Epstein S. Integration of the cognitive and the psychodynamic unconscious. Am Psychol. 1994;49(8):709–724. doi: 10.1037//0003-066x.49.8.709. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Jones PS, Williams GB, Turton AJ, Robbins TW, Bullmore ET. Abnormal brain structure implicated in stimulant drug addiction. Science. 2012;335(6068):601–604. doi: 10.1126/science.1214463. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel J, AY D. Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111(379):387. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Fellin T. Communication between neurons and astrocytes: relevance to the modulation of synaptic and network activity. J Neurochem. 2009;108(3):533–544. doi: 10.1111/j.1471-4159.2008.05830.x. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Eskenazi D, Ishikawa M, Wanat MJ, Phillips PE, Dong Y, et al. Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat Neurosci. 2011;14(1):22–24. doi: 10.1038/nn.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figlewicz D, Bennett JL, Aliakbari S, Zavosh A, AJ S. Insulin acts at different CNS sites to decrease acute sucrose intake and sucrose self-administration in rats. Am J Physiol Regul Integr Comp Physiol. 2008;295:R388–R394. doi: 10.1152/ajpregu.90334.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figlewicz D, Evans SB, Murphy J, Hoen M, Myers M, DG. B. Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat. Brain Res. 2003;(964):107–115. doi: 10.1016/s0006-8993(02)04087-8. [DOI] [PubMed] [Google Scholar]
- Fineberg NA, Potenza MN, Chamberlain SR, Berlin HA, Menzies L, Bechara A, et al. Probing compulsive and impulsive behaviors, from animal models to endophenotypes: a narrative review. Neuropsychopharmacology. 2009;35(3):591–604. doi: 10.1038/npp.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes C, Grafman J. Social neuroscience: The second phase. Front Hum Neurosci. 2013;(7):1–5. doi: 10.3389/fnhum.2013.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Lu Q, Johnson PM, Marks MJ, PJ. K. Habenular α5 nicotinic receptor subunit signalling controls nicotine intake. Nature. 2011a;471(7340):597–601. doi: 10.1038/nature09797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature. 2011b;471(7340):597–601. doi: 10.1038/nature09797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankl V. Man’s search for meaning. Beacon Press; Boston: 1959. [Google Scholar]
- Fraser PE, Rosen AC. Transcranial direct current stimulation and behavioral models of smoking addiction. Front Psychiatry. 2012;3:79. doi: 10.3389/fpsyt.2012.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick S. Automated choice heuristics. In: Gilovich T, et al., editors. Heuristics and Biases: the Psychology of Intuitive Judgment. Cambridge University Press; Cambridge: 2002. pp. 548–558. [Google Scholar]
- Geisler S, Wise RA. Functional implications of glutamatergic projections to the ventral tegmental area. Rev Neurosci. 2008;19(4-5):227–244. doi: 10.1515/revneuro.2008.19.4-5.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick SD, Sell EM, McCallum SE, IM. M. Brain regions mediating α3β4 nicotinic antagonist effects of 18-MC on nicotine self-administration. Eur J Pharmacol. 2011;669(1-3):71–75. doi: 10.1016/j.ejphar.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Leskovjan AC, Hoff AL, Hitzemann R, Bashan F, Khalsa SS, et al. Severity of neuropsychological impairment in cocaine and alcohol addiction: association with metabolism in the prefrontal cortex. Neuropsychologia. 2004;42(11):1447–1458. doi: 10.1016/j.neuropsychologia.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Goudriaan AE, de Ruiter MB, van den Brink W, Oosterlaan J, Veltman DJ. Brain activation patterns associated with cue reactivity and craving in abstinent problem gamblers, heavy smokers and healthy controls: an fMRI study. Addict Biol. 2010;15(4):491–503. doi: 10.1111/j.1369-1600.2010.00242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA. The tonic/phasic model of dopamine system regulation and its implications for understanding alcohol and psychostimulant craving. Addiction. 2000;95(Suppl 2):S119–128. doi: 10.1080/09652140050111690. [DOI] [PubMed] [Google Scholar]
- Grady DL, Thanos PK, Corrada MM, Barnett JC, Jr., Ciobanu V, Shustarovich D, et al. DRD4 Genotype Predicts Longevity in Mouse and Human. J Neurosci. 2013;33(1):286–291. doi: 10.1523/JNEUROSCI.3515-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, et al. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci U S A. 1996;93(21):12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald MK, Steinmiller CL, Sliwerska E, Lundahl L, Burmeister M. BDNF Val(66) Met genotype is associated with drug-seeking phenotypes in heroin-dependent individuals: a pilot study. Addict Biol. 2012 doi: 10.1111/j.1369-1600.2011.00431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorios-Pippas L, Tobler PN, Schultz W. Short-term temporal discounting of reward value in human ventral striatum. J Neurophysiol. 2009;101(3):1507–1523. doi: 10.1152/jn.90730.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueter BA, Rothwell PE, Malenka RC. Integrating synaptic plasticity and striatal circuit function in addiction. Curr Opin Neurobiol. 2013;22(3):545–551. doi: 10.1016/j.conb.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale JB, Reddy LA, Semrud-Clikeman M, Hain LA, Whitaker J, Morley J, et al. Executive impairment determines ADHD medication response: implications for academic achievement. J Learn Disabil. 2011;44(2):196–212. doi: 10.1177/0022219410391191. [DOI] [PubMed] [Google Scholar]
- Hamet P, Tremblay J. Genetics and genomics of depression. Metabolism. 2005;54(5 Suppl 1):10–15. doi: 10.1016/j.metabol.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Han DH, Bolo N, Daniels MA, Arenella L, Lyoo IK, Renshaw PF. Brain activity and desire for Internet video game play. Compr Psychiatry. 2011;52(1):88–95. doi: 10.1016/j.comppsych.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han DH, Kim YS, Lee YS, Min KJ, Renshaw PF. Changes in cue-induced, prefrontal cortex activity with video-game play. Cyberpsychol Behav Soc Netw. 2010;13(6):655–661. doi: 10.1089/cyber.2009.0327. [DOI] [PubMed] [Google Scholar]
- Hart AB, Engelhardt BE, Wardle MC, Sokoloff G, Stephens M, de Wit H, et al. Genome-wide association study of d-amphetamine response in healthy volunteers identifies putative associations, including cadherin 13 (CDH13) PLoS One. 2012;7(8):e42646. doi: 10.1371/journal.pone.0042646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Jorenby DE, Gonzales D, Rigotti NA, Glover ED, Oncken CA, et al. Immunogenicity and smoking-cessation outcomes for a novel nicotine immunotherapeutic. Clin Pharmacol Ther. 2011;89(3):392–399. doi: 10.1038/clpt.2010.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath CJ, Picciotto MR. Nicotine-induced plasticity during development: modulation of the cholinergic system and long-term consequences for circuits involved in attention and sensory processing. Neuropharmacology. 2009;56(Suppl 1):254–262. doi: 10.1016/j.neuropharm.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrom-Lindahl E, Court JA. Nicotinic acetylcholine receptors during prenatal development and brain pathology in human aging. Behav Brain Res. 2000;113(1-2):159–168. doi: 10.1016/s0166-4328(00)00210-2. [DOI] [PubMed] [Google Scholar]
- Hicks M, Rosenberg JB, De BP, Pagovich OE, Young CN, Qiu JP, et al. AAV-directed persistent expression of a gene encoding anti-nicotine antibody for smoking cessation. Sci Transl Med. 2012 Jun 27;4(140):140ra87. doi: 10.1126/scitranslmed.3003611. 2012. 4(140): 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, le Guisquet AM, Vogel E, Millstein RA, Leman S, Belzung C. Early life genetic, epigenetic and environmental factors shaping emotionality in rodents. Neurosci Biobehav Rev. 2005;29(8):1335–1346. doi: 10.1016/j.neubiorev.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Horrell T, El-Baz A, Baruth J, Tasman A, Sokhadze G, Stewart C, et al. Neurofeedback Effects on Evoked and Induced EEG Gamma Band Reactivity to Drug-related Cues in Cocaine Addiction. J Neurother. 2010;14(3):195–216. doi: 10.1080/10874208.2010.501498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Host L, Dietrich JB, Carouge D, Aunis D, Zwiller J. Cocaine self-administration alters the expression of chromatin-remodelling proteins; modulation by histone deacetylase inhibition. J Psychopharmacol. 2009 doi: 10.1177/0269881109348173. [DOI] [PubMed] [Google Scholar]
- Huang LZ, Winzer-Serhan UH. Chronic neonatal nicotine upregulates heteromeric nicotinic acetylcholine receptor binding without change in subunit mRNA expression. Brain Res. 2006;1113(1):94–109. doi: 10.1016/j.brainres.2006.06.084. [DOI] [PubMed] [Google Scholar]
- Huang YH, Lin Y, Mu P, Lee BR, Brown TE, Wayman G, et al. In vivo cocaine experience generates silent synapses. Neuron. 2009;63(1):40–47. doi: 10.1016/j.neuron.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Lewis SS, Coats BD, Skyba DA, Crysdale NY, Berkelhammer DL, et al. Reduction of opioid withdrawal and potentiation of acute opioid analgesia by systemic AV411 (ibudilast) Brain Behav Immun. 2009;23(2):240–250. doi: 10.1016/j.bbi.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Nickerson LD, Frederick Bde B, Kaufman MJ. Prefrontal and limbic resting state brain network functional connectivity differs between nicotine-dependent smokers and non-smoking controls. Drug Alcohol Depend. 2012;125(3):252–259. doi: 10.1016/j.drugalcdep.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S, de BFB, Chuzi S, Pachas G, et al. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol Psychiatry. 2010;67(8):722–729. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerlhag E, Egecioglu E, Dickson SL, Douhan A, Svensson L, JA E. Ghrelin administration into tegmental areas stimulates locomotor activity and increases extracellular concentration of dopamine in the nucleus accumbens. Addict Biol. 2007;12:6–16. doi: 10.1111/j.1369-1600.2006.00041.x. [DOI] [PubMed] [Google Scholar]
- Johnstone EC, Elliot KM, David SP, Murphy MF, Walton RT, Munafo MR. Association of COMT Val108/158Met genotype with smoking cessation in a nicotine replacement therapy randomized trial. Cancer Epidemiol Biomarkers Prev. 2007;16(6):1065–1069. doi: 10.1158/1055-9965.EPI-06-0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahneman D, Frederick S. Representativeness revisited: attribute substitution in intuitive judgment. In: Gilovich T, et al., editors. Heuristics and Biases: the Psychology of Intuitive Judgment. Cambridge University Press; 2002. pp. 49–81. [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10(8):561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kimura M, Satoh T, Matsumoto N. What does the habenula tell dopamine neurons? Nat Neurosci. 2007;10(6):677–678. doi: 10.1038/nn0607-677. [DOI] [PubMed] [Google Scholar]
- Kinnally EL, Capitanio JP, Leibel R, Deng L, LeDuc C, Haghighi F, et al. Epigenetic regulation of serotonin transporter expression and behavior in infant rhesus macaques. Genes Brain Behav. 2010;9(6):575–582. doi: 10.1111/j.1601-183X.2010.00588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey BM, Jackson DC, Orson FM. Anti-drug vaccines to treat substance abuse. Immunol Cell Biol. 2009;87(4):309–314. doi: 10.1038/icb.2009.17. [DOI] [PubMed] [Google Scholar]
- Kluger AN, Siegfried Z, Ebstein RP. A meta-analysis of the association between DRD4 polymorphism and novelty seeking. Mol Psychiatry. 2002;7(7):712–717. doi: 10.1038/sj.mp.4001082. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, LaRowe S, Mardikian P, Malcolm R, Upadhyaya H, Hedden S, et al. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol Psychiatry. 2009;65(10):841–845. doi: 10.1016/j.biopsych.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Neural mechanisms of drug reinforcement. Ann N Y Acad Sci. 1992;654:171–191. doi: 10.1111/j.1749-6632.1992.tb25966.x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24(2):97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27(30):7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koya E, Cruz FC, Ator R, Golden SA, Hoffman AF, Lupica CR, et al. Silent synapses in selectively activated nucleus accumbens neurons following cocaine sensitization. Nat Neurosci. 2012;15(11):1556–1562. doi: 10.1038/nn.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ, Ragunath J, Plevy S, Hamer D, Schneider B, Hartman N. ACTH, cortisol and beta-endorphin response to metyrapone testing during chronic methadone maintenance treatment in humans. Neuropeptides. 1984;5(1-3):277–278. doi: 10.1016/0143-4179(84)90081-7. [DOI] [PubMed] [Google Scholar]
- Kubas HA, Backenson EM, Wilcox G, Piercy JC, Hale JB. The effects of methylphenidate on cognitive function in children with attention-deficit/hyperactivity disorder. Postgrad Med. 2012;124(5):33–48. doi: 10.3810/pgm.2012.09.2592. [DOI] [PubMed] [Google Scholar]
- Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DE, Truong HT, et al. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48(2):303–314. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Latimer K, Wilson P, Kemp J, Thompson L, Sim F, Gillberg C, et al. Disruptive behaviour disorders: a systematic review of environmental antenatal and early years risk factors. Child Care Health Dev. 2012;38(5):611–628. doi: 10.1111/j.1365-2214.2012.01366.x. [DOI] [PubMed] [Google Scholar]
- Lau JY, Goldman D, Buzas B, Fromm SJ, Guyer AE, Hodgkinson C, et al. Amygdala function and 5-HTT gene variants in adolescent anxiety and major depressive disorder. Biol Psychiatry. 2009;65(4):349–355. doi: 10.1016/j.biopsych.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Gallo A, Le Strat Y, Lu L, Gorwood P. Genetics of dopamine receptors and drug addiction: a comprehensive review. Behav Pharmacol. 2009;20(1):1–17. doi: 10.1097/FBP.0b013e3283242f05. [DOI] [PubMed] [Google Scholar]
- Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, et al. Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci. 2009;29(47):14734–14740. doi: 10.1523/JNEUROSCI.3765-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BR, Dong Y. Cocaine-induced metaplasticity in the nucleus accumbens: silent synapse and beyond. Neuropharmacology. 2011;61(7):1060–1069. doi: 10.1016/j.neuropharm.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage J, Grino M, Bernet F, Dutriez-Casteloot I, Montel V, Dupouy JP. Consequences of prenatal morphine exposure on the hypothalamo-pituitary-adrenal axis in the newborn rat: effect of maternal adrenalectomy. J Neuroendocrinol. 1998;10(5):331–342. [PubMed] [Google Scholar]
- Leshan R, Opland DM, Louis GW, Leinninger GM, Patterson CM, Rhodes CJ, et al. Ventral tegmental area leptin receptor neurons specifically project to and regulate cocaine- and amphetamine-regulated transcript neurons of the extended central amygdala. J Neurosci. 2010;30:5713–5723. doi: 10.1523/JNEUROSCI.1001-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson JM, Sweatt JD. Epigenetic mechanisms: a common theme in vertebrate and invertebrate memory formation. Cell Mol Life Sci. 2006;63(9):1009–1016. doi: 10.1007/s00018-006-6026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A, Huang Y, Drisaldi B, Griffin EA, Jr., Pollak DD, Xu S, et al. Molecular mechanism for a gateway drug: epigenetic changes initiated by nicotine prime gene expression by cocaine. Sci Transl Med. 2011;3(107):107ra109. doi: 10.1126/scitranslmed.3003062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AA, Guan Z, Barco A, Xu S, Kandel ER, Schwartz JH. CREB-binding protein controls response to cocaine by acetylating histones at the fosB promoter in the mouse striatum. Proc Natl Acad Sci U S A. 2005;102(52):19186–19191. doi: 10.1073/pnas.0509735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisoprawski A, Herve D, Blanc G, Glowinski J, Tassin JP. Selective activation of the mesocortico-frontal dopaminergic neurons induced by lesion of the habenula in the rat. Brain Res. 1980;183(1):229–234. doi: 10.1016/0006-8993(80)90135-3. [DOI] [PubMed] [Google Scholar]
- Lobo MK, Nestler EJ. The striatal balancing act in drug addiction: distinct roles of direct and indirect pathway medium spiny neurons. Front Neuroanat. 2011;5:41. doi: 10.3389/fnana.2011.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein G. Out of control: Visceral influences on behavior. Organizational Behavior and Human Decision Processes. 1996;65:272–292. [Google Scholar]
- Luscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69(4):650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaiyandi V, Lerman C, Benowitz NL, Jepson C, Patterson F, Tyndale RF. Impact of CYP2A6 genotype on pretreatment smoking behaviour and nicotine levels from and usage of nicotine replacement therapy. Mol Psychiatry. 2006;11(4):400–409. doi: 10.1038/sj.mp.4001794. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44(1):5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Malvaez M, McQuown SC, Rogge GA, Astarabadi M, Jacques V, Carreiro S, et al. HDAC3-selective inhibitor enhances extinction of cocaine-seeking behavior in a persistent manner. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1213364110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardikian PN, LaRowe SD, Hedden S, Kalivas PW, Malcolm RJ. An open-label trial of N-acetylcysteine for the treatment of cocaine dependence: a pilot study. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(2):389–394. doi: 10.1016/j.pnpbp.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Mariani JJ, Pavlicova M, Bisaga A, Nunes EV, Brooks DJ, Levin FR. Extended-release mixed amphetamine salts and topiramate for cocaine dependence: a randomized controlled trial. Biol Psychiatry. 2012;72(11):950–956. doi: 10.1016/j.biopsych.2012.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A, et al. Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am J Psychiatry. 2007;164(4):622–629. doi: 10.1176/ajp.2007.164.4.622. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447(7148):1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- Maze I, Covington HE, 3rd, Dietz DM, LaPlant Q, Renthal W, Russo SJ, et al. Essential role of the histone methyltransferase G9a in cocaine-induced plasticity. Science. 2010;327(5962):213–216. doi: 10.1126/science.1179438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M. Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues Clin Neurosci. 2005;7(2):103–123. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Buckholtz JW, Kolachana B, A RH, Pezawas L, Blasi G, et al. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc Natl Acad Sci U S A. 2006;103(16):6269–6274. doi: 10.1073/pnas.0511311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Kohn PD, Kolachana B, Kippenhan S, McInerney-Leo A, Nussbaum R, et al. Midbrain dopamine and prefrontal function in humans: interaction and modulation by COMT genotype. Nat Neurosci. 2005;8(5):594–596. doi: 10.1038/nn1438. [DOI] [PubMed] [Google Scholar]
- Miedl SF, Peters J, Buchel C. Altered neural reward representations in pathological gamblers revealed by delay and probability discounting. Arch Gen Psychiatry. 2012;69(2):177–186. doi: 10.1001/archgenpsychiatry.2011.1552. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Moffett MC, Vicentic A, Kozel M, Plotsky P, Francis DD, Kuhar MJ. Maternal separation alters drug intake patterns in adulthood in rats. Biochem Pharmacol. 2007;73(3):321–330. doi: 10.1016/j.bcp.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran LV, Sampath H, Kochunov P, Hong LE. Brain Circuits That Link Schizophrenia to High Risk of Cigarette Smoking. Schizophr Bull. 2012 doi: 10.1093/schbul/sbs149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Lopez L, Stamatakis EA, Fernandez-Serrano MJ, Gomez-Rio M, Rodriguez-Fernandez A, Perez-Garcia M, et al. Neural correlates of hot and cold executive functions in polysubstance addiction: association between neuropsychological performance and resting brain metabolism as measured by positron emission tomography. Psychiatry Res. 2012a;203(2-3):214–221. doi: 10.1016/j.pscychresns.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Moreno-Lopez L, Stamatakis EA, Fernandez-Serrano MJ, Gomez-Rio M, Rodriguez-Fernandez A, Perez-Garcia M, et al. Neural correlates of the severity of cocaine, heroin, alcohol, MDMA and cannabis use in polysubstance abusers: a resting-PET brain metabolism study. PLoS One. 2012b;7(6):e39830. doi: 10.1371/journal.pone.0039830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno AY, Janda KD. Current challenges for the creation of effective vaccines against drugs of abuse. Expert Rev Vaccines. 2011;10(12):1637–1639. doi: 10.1586/erv.11.145. [DOI] [PubMed] [Google Scholar]
- Morewedge CK, Kahneman D. Associative processes in intuitive judgment. Trends Cogn Sci. 2010;14(10):435–440. doi: 10.1016/j.tics.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, et al. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci. 2009;12(2):182–189. doi: 10.1038/nn.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Zhou W, Shen H, Reichel CM, See R, Carr D, et al. Reversing cocaine-induced synaptic potentiation provides enduring protection from relapse. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1011265108. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller UJ, Voges J, Steiner J, Galazky I, Heinze HJ, Moller M, et al. Deep brain stimulation of the nucleus accumbens for the treatment of addiction. Ann N Y Acad Sci. 2012 doi: 10.1111/j.1749-6632.2012.06834.x. [DOI] [PubMed] [Google Scholar]
- Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, et al. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat Neurosci. 2006;9(8):1050–1056. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci. 2009;32(1):56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315(5811):531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer NM, Cunningham ST, Zhu J, Bryant RI, Middleton LS, Dwoskin LP. Effects of environmental enrichment on behavior and dopamine transporter function in medial prefrontal cortex in adult rats prenatally treated with cocaine. Brain Res Dev Brain Res. 2004;153(2):213–223. doi: 10.1016/j.devbrainres.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Nilsson KW, Sjoberg RL, Damberg M, Leppert J, Ohrvik J, Alm PO, et al. Role of monoamine oxidase A genotype and psychosocial factors in male adolescent criminal activity. Biol Psychiatry. 2006;59(2):121–127. doi: 10.1016/j.biopsych.2005.06.024. [DOI] [PubMed] [Google Scholar]
- Nilsson KW, Wargelius HL, Sjoberg RL, Leppert J, Oreland L. The MAO-A gene, platelet MAO-B activity and psychosocial environment in adolescent female alcohol-related problem behaviour. Drug Alcohol Depend. 2008;93(1-2):51–62. doi: 10.1016/j.drugalcdep.2007.08.022. [DOI] [PubMed] [Google Scholar]
- Nishikawa T, Fage D, Scatton B. Evidence for, and nature of, the tonic inhibitory influence of habenulointerpeduncular pathways upon cerebral dopaminergic transmission in the rat. Brain Res. 1986;373(1-2):324–336. doi: 10.1016/0006-8993(86)90347-1. [DOI] [PubMed] [Google Scholar]
- Norman AB, Norman MK, Buesing WR, Tabet MR, Tsibulsky VL, Ball WJ. The effect of a chimeric human/murine anti-cocaine monoclonal antibody on cocaine self-administration in rats. J Pharmacol Exp Ther. 2009;328(3):873–881. doi: 10.1124/jpet.108.146407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooteman W, Naassila M, Koeter MW, Verheul R, Schippers GM, Houchi H, et al. Predicting the effect of naltrexone and acamprosate in alcohol-dependent patients using genetic indicators. Addict Biol. 2009;14(3):328–337. doi: 10.1111/j.1369-1600.2009.00159.x. [DOI] [PubMed] [Google Scholar]
- Oroszi G, Goldman D. Alcoholism: genes and mechanisms. Pharmacogenomics. 2004;5(8):1037–1048. doi: 10.1517/14622416.5.8.1037. [DOI] [PubMed] [Google Scholar]
- Oslin DW, Berrettini W, Kranzler HR, Pettinati H, Gelernter J, Volpicelli JR, et al. A functional polymorphism of the mu-opioid receptor gene is associated with naltrexone response in alcohol-dependent patients. Neuropsychopharmacology. 2003;28(8):1546–1552. doi: 10.1038/sj.npp.1300219. [DOI] [PubMed] [Google Scholar]
- Owesson-White CA, Ariansen J, Stuber GD, Cleaveland NA, Cheer JF, Wightman RM, et al. Neural encoding of cocaine-seeking behavior is coincident with phasic dopamine release in the accumbens core and shell. Eur J Neurosci. 2009;30(6):1117–1127. doi: 10.1111/j.1460-9568.2009.06916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paaver M, Kurrikoff T, Nordquist N, Oreland L, Harro J. The effect of 5-HTT gene promoter polymorphism on impulsivity depends on family relations in girls. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(5):1263–1268. doi: 10.1016/j.pnpbp.2008.03.021. [DOI] [PubMed] [Google Scholar]
- Patterson F, Schnoll RA, Wileyto EP, Pinto A, Epstein LH, Shields PG, et al. Toward personalized therapy for smoking cessation: a randomized placebo-controlled trial of bupropion. Clin Pharmacol Ther. 2008;84(3):320–325. doi: 10.1038/clpt.2008.57. [DOI] [PubMed] [Google Scholar]
- Pelchat ML, Johnson A, Chan R, Valdez J, Ragland JD. Images of desire: food-craving activation during fMRI. Neuroimage. 2004;23(4):1486–1493. doi: 10.1016/j.neuroimage.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Petrovich GD. Forebrain circuits and control of feeding by learned cues. Neurobiol Learn Mem. 2010;95(2):152–158. doi: 10.1016/j.nlm.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Pietrzykowski AZ. Coinciding revolutions: how discovery of non-coding DNA and RNA can change our understanding of addiction. Front Genet. 2012;3:271. doi: 10.3389/fgene.2012.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine A, Shiner T, Seymour B, Dolan RJ. Dopamine, time, and impulsivity in humans. J Neurosci. 2010;30(26):8888–8896. doi: 10.1523/JNEUROSCI.6028-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quednow BB, Kuhn KU, Hoppe C, Westheide J, Maier W, Daum I, et al. Elevated impulsivity and impaired decision-making cognition in heavy users of MDMA (“Ecstasy”) Psychopharmacology (Berl) 2007;189(4):517–530. doi: 10.1007/s00213-005-0256-4. [DOI] [PubMed] [Google Scholar]
- Redish AD, Jensen S, Johnson A. A unified framework for addiction: vulnerabilities in the decision process. Behav Brain Sci. 2008;31(4):415–437. doi: 10.1017/S0140525X0800472X. discussion 437-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renthal W, Maze I, Krishnan V, Covington HE, 3rd, Xiao G, Kumar A, et al. Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron. 2007;56(3):517–529. doi: 10.1016/j.neuron.2007.09.032. [DOI] [PubMed] [Google Scholar]
- Rinaman L. Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain Res. 2010;1350:18–34. doi: 10.1016/j.brainres.2010.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roma PG, Davis CM, Riley AL. Effects of cross-fostering on cocaine-induced conditioned taste aversions in Fischer and Lewis rats. Dev Psychobiol. 2007;49(2):172–179. doi: 10.1002/dev.20168. [DOI] [PubMed] [Google Scholar]
- Romieu P, Deschatrettes E, Host L, Gobaille S, Sandner G, Zwiller J. The inhibition of histone deacetylases reduces the reinstatement of cocaine-seeking behavior in rats. Curr Neuropharmacol. 2011;9(1):21–25. doi: 10.2174/157015911795017317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romieu P, Host L, Gobaille S, Sandner G, Aunis D, Zwiller J. Histone deacetylase inhibitors decrease cocaine but not sucrose self-administration in rats. J Neurosci. 2008;28(38):9342–9348. doi: 10.1523/JNEUROSCI.0379-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg J, Hicks MJ, De BP, Pagovich O, Frenk E, Janda KD, et al. AAVrh.10-mediated expression of an anti-cocaine antibody mediates persistent passive immunization that suppresses cocaine-induced behavior. Hum Gene Ther. 2012;23(5):451–459. doi: 10.1089/hum.2011.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol Psychiatry. 2009;65(9):760–769. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Mazei-Robison MS, Ables JL, Nestler EJ. Neurotrophic factors and structural plasticity in addiction. Neuropharmacology. 2009a;56(Suppl 1):73–82. doi: 10.1016/j.neuropharm.2008.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Wilkinson MB, Mazei-Robison MS, Dietz DM, Maze I, Krishnan V, et al. Nuclear factor kappa B signaling regulates neuronal morphology and cocaine reward. J Neurosci. 2009b;29(11):3529–3537. doi: 10.1523/JNEUROSCI.6173-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter JL. Symbiotic relationship of pharmacogenetics and drugs of abuse. Aaps J. 2006;8(1):E174–184. doi: 10.1208/aapsj080121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology (Berl) 2007;191(3):461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- Salas R, Sturm R, Boulter J, De Biasi M. Nicotinic receptors in the habenulo-interpeduncular system are necessary for nicotine withdrawal in mice. J Neurosci. 2009;29(10):3014–3018. doi: 10.1523/JNEUROSCI.4934-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samochowiec A, Grzywacz A, Kaczmarek L, Bienkowski P, Samochowiec J, Mierzejewski P, et al. Functional polymorphism of matrix metalloproteinase-9 (MMP-9) gene in alcohol dependence: family and case control study. Brain Res. 2010;1327:103–106. doi: 10.1016/j.brainres.2010.02.072. [DOI] [PubMed] [Google Scholar]
- Samochowiec J, Ladehoff M, Pelz J, Smolka M, Schmidt LG, Rommelspacher H, et al. Predominant influence of the 3′-region of dopamine D2 receptor gene (DRD2) on the clinical phenotype in German alcoholics. Pharmacogenetics. 2000;10(5):471–475. doi: 10.1097/00008571-200007000-00010. [DOI] [PubMed] [Google Scholar]
- Schank JR, Ryabinin AE, Giardino WJ, Ciccocioppo R, Heilig M. Stress-related neuropeptides and addictive behaviors: beyond the usual suspects. Neuron. 2012;76(1):192–208. doi: 10.1016/j.neuron.2012.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellekens AF, Franke B, Ellenbroek B, Cools A, de Jong CA, Buitelaar JK, et al. COMT Val158Met modulates the effect of childhood adverse experiences on the risk of alcohol dependence. Addict Biol. 2012;18(2):344–356. doi: 10.1111/j.1369-1600.2012.00438.x. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Goldberg SR. Accelerating cocaine metabolism as an approach to the treatment of cocaine abuse and toxicity. Future Med Chem. 2012;4(2):163–175. doi: 10.4155/fmc.11.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoll RA, Patterson F, Wileyto EP, Tyndale RF, Benowitz N, Lerman C. Nicotine metabolic rate predicts successful smoking cessation with transdermal nicotine: a validation study. Pharmacol Biochem Behav. 2009;92(1):6–11. doi: 10.1016/j.pbb.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275(5306):1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Shen H, Moussawi K, Zhou W, Toda S, Kalivas P. Heroin relapse requires LTP-like plasticity mediated by NR2B-containing receptors. Nat Neurosci. 2011 doi: 10.1073/pnas.1112052108. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher L. The role of brain-derived neurotrophic factor in the pathophysiology of adolescent suicidal behavior. Int J Adolesc Med Health. 2011;23(3):181–185. doi: 10.1515/ijamh.2011.041. [DOI] [PubMed] [Google Scholar]
- Simon G, Von Korff M, Saunders K, Miglioretti DL, Crane PK, van Belle G, et al. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry. 2006;63:824–830. doi: 10.1001/archpsyc.63.7.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloman SA. The empirical case for two systems of reasoning. Psychol Bull. 1996;119:3–22. [Google Scholar]
- Smolka MN, Buhler M, Schumann G, Klein S, Hu XZ, Moayer M, et al. Gene-gene effects on central processing of aversive stimuli. Mol Psychiatry. 2007;12(3):307–317. doi: 10.1038/sj.mp.4001946. [DOI] [PubMed] [Google Scholar]
- Song R, Zhang HY, Li X, Bi GH, Gardner EL, Xi ZX. Increased vulnerability to cocaine in mice lacking dopamine D3 receptors. Proc Natl Acad Sci U S A. 2012;109(43):17675–17680. doi: 10.1073/pnas.1205297109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Neale BM, van den Oord E, Miles MF, Neale MC, Bulik CM, et al. Candidate genes for nicotine dependence via linkage, epistasis, and bioinformatics. Am J Med Genet B Neuropsychiatr Genet. 2004;126B(1):23–36. doi: 10.1002/ajmg.b.20138. [DOI] [PubMed] [Google Scholar]
- Sun H, Maze I, Dietz DM, Scobie KN, Kennedy PJ, Damez-Werno D, et al. Morphine Epigenomically Regulates Behavior through Alterations in Histone H3 Lysine 9 Dimethylation in the Nucleus Accumbens. J Neurosci. 2012;32(48):17454–17464. doi: 10.1523/JNEUROSCI.1357-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundar IK, Chung S, Hwang JW, Lapek JD, Jr., Bulger M, Friedman AE, et al. Mitogen- and stress-activated kinase 1 (MSK1) regulates cigarette smoke-induced histone modifications on NF-kappaB-dependent genes. PLoS One. 2012;7(2):e31378. doi: 10.1371/journal.pone.0031378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki J, Haimovici F, Chang G. Alcohol use disorders after bariatric surgery. Obes Surg. 2012;22(2):201–207. doi: 10.1007/s11695-010-0346-1. [DOI] [PubMed] [Google Scholar]
- Sweitzer MM, Halder I, Flory JD, Craig AE, Gianaros PJ, Ferrell RE, et al. Polymorphic variation in the dopamine D4 receptor predicts delay discounting as a function of childhood socioeconomic status: evidence for differential susceptibility. Soc Cogn Affect Neurosci. 2012 doi: 10.1093/scan/nss020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Kalivas PW, Worley PF. Homer proteins: implications for neuropsychiatric disorders. Curr Opin Neurobiol. 2006;16(3):251–257. doi: 10.1016/j.conb.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Michaelides M, Benveniste H, Wang GJ, Volkow ND. Effects of chronic oral methylphenidate on cocaine self-administration and striatal dopamine D2 receptors in rodents. Pharmacol Biochem Behav. 2007;87(4):426–433. doi: 10.1016/j.pbb.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Thorgeirsson TE, Geller F, Sulem P, Rafnar T, Wiste A, Magnusson KP, et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452(7187):638–642. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo-Rodriguez M, Lotfipour S, Leonard G, Perron M, Richer L, Veillette S, et al. Maternal smoking during pregnancy is associated with epigenetic modifications of the brain-derived neurotrophic factor-6 exon in adolescent offspring. Am J Med Genet B Neuropsychiatr Genet. 2010 doi: 10.1002/ajmg.b.31109. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Huber A, Farrell SM, Stumpenhorst K, Harrison PJ, Walton ME. The role of catechol-O-methyltransferase in reward processing and addiction. CNS Neurol Disord Drug Targets. 2012;11(3):306–323. doi: 10.2174/187152712800672409. [DOI] [PubMed] [Google Scholar]
- Tyndale RF, Sellers EM. Genetic variation in CYP2A6-mediated nicotine metabolism alters smoking behavior. Ther Drug Monit. 2002;24(1):163–171. doi: 10.1097/00007691-200202000-00026. [DOI] [PubMed] [Google Scholar]
- Uhl GR, Liu QR, Drgon T, Johnson C, Walther D, Rose JE, et al. Molecular genetics of successful smoking cessation: convergent genome-wide association study results. Arch Gen Psychiatry. 2008;65(6):683–693. doi: 10.1001/archpsyc.65.6.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Contreras-Rodriguez O, Fonseca F, Cuenca A, Soriano-Mas C, Rodriguez J, et al. Functional alteration in frontolimbic systems relevant to moral judgment in cocaine-dependent subjects. Addict Biol. 2012 doi: 10.1111/j.1369-1600.2012.00472.x. [DOI] [PubMed] [Google Scholar]
- Volkow N, Li TK. The neuroscience of addiction. Nat Neurosci. 2005a;8(11):1429–1430. doi: 10.1038/nn1105-1429. [DOI] [PubMed] [Google Scholar]
- Volkow N, Wang GJ, Telang F, Fowler J, Logan J, Childress A, et al. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006a;26(24):6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, et al. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158(12):2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10(3):318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain: insights from imaging studies. J Clin Invest. 2003;111(10):1444–1451. doi: 10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F. Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology. 2009a;56(Suppl 1):3–8. doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, et al. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14(2):169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Telang F, Logan J, Jayne M, et al. Cognitive control of drug craving inhibits brain reward regions in cocaine abusers. Neuroimage. 2009b;49(3):2536–2543. doi: 10.1016/j.neuroimage.2009.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wolf AP, Hitzemann R, Dewey S, Bendriem B, et al. Changes in brain glucose metabolism in cocaine dependence and withdrawal. Am J Psychiatry. 1991;148(5):621–626. doi: 10.1176/ajp.148.5.621. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Skolnick P. New medications for substance use disorders: challenges and opportunities. Neuropsychopharmacology. 2012a;37(1):290–292. doi: 10.1038/npp.2011.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Begleiter H, Porjesz B, Fowler JS, Telang F, et al. High levels of dopamine D2 receptors in unaffected members of alcoholic families: possible protective factors. Arch Gen Psychiatry. 2006b;63(9):999–1008. doi: 10.1001/archpsyc.63.9.999. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Hitzemann R, Angrist B, Gatley SJ, et al. Association of methylphenidate-induced craving with changes in right striato-orbitofrontal metabolism in cocaine abusers: implications in addiction. Am J Psychiatry. 1999a;156(1):19–26. doi: 10.1176/ajp.156.1.19. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Gifford A, et al. Prediction of reinforcing responses to psychostimulants in humans by brain dopamine D2 receptor levels. Am J Psychiatry. 1999b;156(9):1440–1443. doi: 10.1176/ajp.156.9.1440. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, et al. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386(6627):830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Wong C, et al. Reinforcing effects of psychostimulants in humans are associated with increases in brain dopamine and occupancy of D(2) receptors. J Pharmacol Exp Ther. 1999c;291(1):409–415. [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Schlyer D, Hitzemann R, et al. Imaging endogenous dopamine competition with [11C]raclopride in the human brain. Synapse. 1994;16(4):255–262. doi: 10.1002/syn.890160402. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3191–3200. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Thanos PP, Logan J, Gatley SJ, et al. Brain DA D2 receptors predict reinforcing effects of stimulants in humans: replication study. Synapse. 2002;46(2):79–82. doi: 10.1002/syn.10137. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F. Addiction: beyond dopamine reward circuitry. Proc Natl Acad Sci U S A. 2011;108(37):15037–15042. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Ma Y, Fowler JS, Wong C, Ding YS, et al. Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine-addicted subjects but not in controls: relevance to addiction. J Neurosci. 2005b;25(15):3932–3939. doi: 10.1523/JNEUROSCI.0433-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, et al. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006c;26(24):6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Jayne M, et al. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci. 2007;27(46):12700–12706. doi: 10.1523/JNEUROSCI.3371-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Tomasi D, Baler RD. Obesity and addiction: neurobiological overlaps. Obes Rev. 2012b;14(1):2–18. doi: 10.1111/j.1467-789X.2012.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S, Baskaya O, Lieb K, Dahmen N, Tadic A. The 5-HTTLPR polymorphism modulates the association of serious life events (SLE) and impulsivity in patients with Borderline Personality Disorder. J Psychiatr Res. 2009;43(13):1067–1072. doi: 10.1016/j.jpsychires.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Walderhaug E, Herman AI, Magnusson A, Morgan MJ, Landro NI. The short (S) allele of the serotonin transporter polymorphism and acute tryptophan depletion both increase impulsivity in men. Neurosci Lett. 2010;473(3):208–211. doi: 10.1016/j.neulet.2010.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanat MJ, Willuhn I, Clark JJ, Phillips PE. Phasic dopamine release in appetitive behaviors and drug addiction. Curr Drug Abuse Rev. 2009;2(2):195–213. doi: 10.2174/1874473710902020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS, Cervany P, Hitzemann RJ, Pappas NR, et al. Regional brain metabolic activation during craving elicited by recall of previous drug experiences. Life Sci. 1999;64(9):775–784. doi: 10.1016/s0024-3205(98)00619-5. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Telang F, Jayne M, Ma Y, Pradhan K, et al. Evidence of gender differences in the ability to inhibit brain activation elicited by food stimulation. Proc Natl Acad Sci U S A. 2009;106(4):1249–1254. doi: 10.1073/pnas.0807423106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Faith M, Patterson F, Tang K, Kerrin K, Wileyto EP, et al. Neural substrates of abstinence-induced cigarette cravings in chronic smokers. J Neurosci. 2007;27(51):14035–14040. doi: 10.1523/JNEUROSCI.2966-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren M, Frost-Pineda K, Gold M. Body mass index and marijuana use. J Addict Dis. 2005;24(3):95–100. doi: 10.1300/J069v24n03_08. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7(8):847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Weder N, Yang BZ, Douglas-Palumberi H, Massey J, Krystal JH, Gelernter J, et al. MAOA genotype, maltreatment, and aggressive behavior: the changing impact of genotype at varying levels of trauma. Biol Psychiatry. 2009;65(5):417–424. doi: 10.1016/j.biopsych.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing VC, Rabin RA, Wass CE, George TP. Correlations between executive function, decision-making and impulsivity are disrupted in schizophrenia versus controls. Psychiatry Res. 2013;205(1-2):168–171. doi: 10.1016/j.psychres.2012.08.022. [DOI] [PubMed] [Google Scholar]
- Wise RA. Roles for nigrostriatal--not just mesocorticolimbic--dopamine in reward and addiction. Trends Neurosci. 2009;32(10):517–524. doi: 10.1016/j.tins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DF, Kuwabara H, Schretlen DJ, Bonson KR, Zhou Y, Nandi A, et al. Increased occupancy of dopamine receptors in human striatum during cue-elicited cocaine craving. Neuropsychopharmacology. 2006;31(12):2716–2727. doi: 10.1038/sj.npp.1301194. [DOI] [PubMed] [Google Scholar]
- Wood EJ, Lipovich L. MicroRNAs in opioid addiction: elucidating evolution. Front Genet. 2012;3:241. doi: 10.3389/fgene.2012.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovleva T, Bazov I, Watanabe H, Hauser KF, Bakalkin G. Transcriptional control of maladaptive and protective responses in alcoholics: a role of the NF-kappaB system. Brain Behav Immun. 2011;25(Suppl 1):S29–38. doi: 10.1016/j.bbi.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Carmon Z, Kahn B, Malani A, Schwartz J, Volpp K, et al. The Hot-Cold Decision Triangle: A Framework for Healthier Choices. Marketing Letters: 2012;1:16. [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur J Neurosci. 2004;19(1):181–189. doi: 10.1111/j.1460-9568.2004.03095.x. [DOI] [PubMed] [Google Scholar]
- Zhang F, Zhou W, Liu H, Zhu H, Tang S, Lai M, et al. Increased c-Fos expression in the medial part of the lateral habenula during cue-evoked heroin-seeking in rats. Neurosci Lett. 2005;386(2):133–137. doi: 10.1016/j.neulet.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Spangler R, Schlussman SD, Ho A, Kreek MJ. Alterations in hypothalamic-pituitary-adrenal axis activity and in levels of proopiomelanocortin and corticotropin-releasing hormone-receptor 1 mRNAs in the pituitary and hypothalamus of the rat during chronic ‘binge’ cocaine and withdrawal. Brain Res. 2003;964(2):187–199. doi: 10.1016/s0006-8993(02)03929-x. [DOI] [PubMed] [Google Scholar]
- Zis AP, Haskett RF, Albala AA, Carroll BJ, Lohr NE. Opioid regulation of hypothalamic-pituitary-adrenal function in depression. Arch Gen Psychiatry. 1985;42(4):383–386. doi: 10.1001/archpsyc.1985.01790270073008. [DOI] [PubMed] [Google Scholar]
- Zweifel LS, Parker JG, Lobb CJ, Rainwater A, Wall VZ, Fadok JP, et al. Disruption of NMDAR-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior. Proc Natl Acad Sci U S A. 2009;106(18):7281–7288. doi: 10.1073/pnas.0813415106. [DOI] [PMC free article] [PubMed] [Google Scholar]