Abstract
We describe the development of a two-way text messaging intervention tool for substance users who are non-adherent with HIV medications, and examine message flow data for feasibility and acceptability. The assessment and intervention tool, TxText, is fully automated, sending participants mood, substance use, and medication adherence queries by text message. Participants respond, the tool recognizes the category of response, and sends the personalized intervention message that participants designed in return. In 10 months, the tool sent 16,547 messages (half initial, half follow-up) to 31 participants assigned to the TxText condition, who sent 6711 messages in response to the initial messages. Response rates to substance use (n=2370), medication (n=2918) and mood (n=4639) queries were 67%, 69%, and 64%, respectively. Responses indicating medication adherence, abstinence from substances, and good moods were more common than negative responses. The TxText tool can send messages daily over a 3 month period, receive responses, and decode them to deliver personalized affirming or intervention messages. While we await the outcomes of a pilot randomized trial, the process analysis shows that TxText is acceptable and feasible for substance abusers with HIV, and may serve as a complement to HIV medical care.
Lifesaving medication regimens for HIV disease can result in increased life expectancy with lower rates of illness for patients living with HIV. The Achilles’ heel of antiretroviral therapy (ART) is the requirement of very high medication adherence to avoid viral mutation, medication resistance, and failure of treatment (Chesney, 2003). While adherence to HIV regimens is a well-researched challenge, active retention in adequate care has been more recently identified as a source of morbidity and mortality (Kempf et al., 2010; Mugavero et al., 2009). It is now clear that both regular attendance at clinic appointments and regular, as-directed dosing of HIV medications are required to optimize health.
Some groups are at particularly high risk for non-adherence and disengagement from care. These include those newly diagnosed with HIV, but also those who experience barriers to care. In the U.S., this second group is disproportionately filled with people of color, from rural areas, from the South, with fewer financial resources, lacking transportation, and residing in communities where stigma about HIV disease is strong (Kempf et al., 2010; Konkle-Parker, Erlen, & Dubbert, 2008; Reif et al., 2011; Sandelowski, Voils, Chang, & Lee, 2009; Sandelowski, Voils, Chang, & Lee, 2009; Sayles, Wong, Kinsler, Martins, & Cunningham, 2009; Yannessa, Reece, & Basta, 2008; Young & Bendavid, 2010). All of these social factors undermine access to care and ART adherence. Additionally, mental disorders and substance use can reduce adherence; both depression and active alcohol and drug use can be related to periods of ART nonadherence (Berg, Cooperman, Newville, & Arnsten, 2009; González-Guarda et al., 2011; González-Guarda, McCabe, Florom-Smith, Cianelli, & Peragallo, 2011; Malta, Strathdee, Magnanini, & Bastos, 2008; Tucker, Burnam, Sherbourne, Kung, & Gifford, 2003).
Nonadherence to ART regimens is closely related to treatment failure (Flandre et al., 2002; Paterson et al., 2000), and in response, researchers have developed interventions to improve adherence. Unfortunately, these interventions tend to come too late, after a period of nonadherence has already resulted in viral replication and reduced immune health. Additionally, most tested HIV medication adherence interventions have modest and ephemeral effects (Simoni, Amico, Pearson, & Malow, 2008; Simoni, Pearson, Pantalone, Marks, & Crepaz, 2006). There is a clear need to develop interventions that can identify nonadherence and other behaviors that threaten adherence as they occur, in real time, and deliver an appropriate intervention immediately.
Mobile phone technology, commonly called mHealth, has the potential to address many of the concerns outlined above. Mobile phones have been spreading rapidly in the U.S. market, with near complete saturation expected by 2014 (Lenhart, Ling, Campbell, & Purcell, 2010). More African-Americans (93%) have mobile phones than other groups, and this subset of the population uses more mobile phone minutes per month (Brenner, June 6, 2013). Rural areas in the U.S. generally have some access to cellular signals as coverage extends into remote sections of the country. Therefore the reach of the technology, and its familiarity to many people, are potential advantages. Additionally, an mHealth intervention may fit the preferences of non-urban people living with HIV. Many people living with HIV express preferences for technology with which they are already familiar and may already use, such as text messaging, rather than Internet materials accessed from a computer; these options are seen as more private and more accessible by non-urban HIV patients living in the U.S. South (Farrell-Carnahan, Fabbri, & Ingersoll, 2011). Thus, a simple mobile-phone-based intervention might be acceptable to this patient subpopulation, and could potentially overcome some of the health disparities they experience. Because of variable cellular signal strength, a text messaging (SMS) mHealth tool might be particularly appropriate because text messages can often be received and sent even in areas where voice telephone calls have inadequate signal strength.
Studies of HIV-related mHealth interventions range from descriptions to observational studies to randomized trials. Most studies focus on medication adherence as the sole intervention target, with interventions delivered by SMS/text messaging, smart phones, or computers (Pellowski & Kalichman, 2012). A recent Cochrane review covering studies published through 2011 found only two good quality mHealth intervention studies (Horvath, Azman, Kennedy, & Rutherford, 2012). These studies examined weekly SMS adherence interventions conducted in Kenya, and found that weekly text messaging enhanced ART adherence and improved suppression of viral load. In March 2013, we found 26 studies published after that review with the keywords text messag* and HIV abstracted on PubMed. Most of these were focused on HIV prevention (n=7), or assessed the feasibility of an mHealth intervention to target varied HIV risk behaviors in a particular population (n=8) such as Latinos (Leite et al., 2013), African American teens (Cornelius et al., 2012), Chinese men who have sex with men (MSM) (Nehl et al., 2012), or US MSM (Reback et al., 2012), while 2 were secondary or process analyses of other studies, and 4 were reviews of various aspects of mHealth in the HIV field.
There were 5 intervention studies among people living with HIV. Three studies tested variations of one-way standardized text messaging. In a pilot RCT, text message medication dosing reminders sent to 8 Brazilian women were related to higher ART adherence by self-report, pill-count, and MEMS than in the control group (n=13) (da Costa et al., 2012). In an RCT of weekly standardized motivational one-way text messaging vs. usual care in Cameroon, there was no impact on ART adherence among 200 patients (Mbuagbaw et al., 2012). In contrast, in a single-arm cohort study in Bangalore India, weekly picture text messaging paired with weekly interactive voice messaging for 6 months among 150 patients increased optimal ART adherence over 6 months (Rodrigues et al., 2012).
Two studies tested variations of two-way and tailored text messaging. In a proof-of-concept study of tailored text messaging for ART adherence among MSM adults, investigators sent dynamically tailored text messages, primarily medication dosing reminders, over a 3 month period, with weekly adherence queries that the participants answered by two-way messaging (Lewis et al., 2013). Participants liked the text messaging intervention, and ART adherence, viral load, and CD4 count improved among those who were nonadherent at study entry. Among youth living with HIV, investigators pilot tested text messages as medication reminders and queries about dosing in a two-way text messaging intervention (Dowshen, Kuhns, Johnson, Holoyda, & Garofalo, 2012). They found that mean Visual Analog Scale adherence scores and AIDS Clinical Trial Group questionnaire 4-day recall increased at 12 and 24 weeks compared to baseline, with a trend towards improved CD4 and viral load markers. They reported good retention and acceptability to the adolescent sample living with HIV. While preliminary, these studies show that both one-way and two-way text messaging are promising for improving ART adherence.
Importantly, none of these text messaging intervention studies assessed nor intervened upon other phenomena that undermine ART adherence. In the U.S., symptoms of depression and active substance use often correlate with periods of ART nonadherence (Carrico et al., 2011; González-Guarda et al., 2011; González-Guarda et al., 2011; Malta et al., 2008; Tucker et al., 2003). In response to this pattern of related behaviors, a few studies have tested face to face ART adherence interventions among substance abusers, and have found that addressing nonadherence and substance abuse together is a promising approach that results in both improved adherence and reduced drinking and drug use (Ingersoll et al., 2011; Parsons, Golub, Rosof, & Holder, 2007; Parsons, Rosof, Punzalan, & Di Maria, 2005). Similarly, a few investigators have developed dual target interventions addressing both depression and adherence, with promising results (Daughters, Magidson, Schuster, & Safren, 2010; Safren et al., 2012). While these face to face interventions targeting ART adherence and depression or substance use had good outcomes, their reach is limited. It seems logical to extend a text messaging adherence intervention to target active substance use and poor moods that threaten adherence.
The purpose of this paper is to describe the formative, iterative development process used to create a two-way text messaging intervention tool that assesses ART adherence, substance use, and mood daily, and delivers self-created personalized intervention messages for each targeted behavior nearly immediately. Additionally, we will assess the tool’s feasibility and acceptability to participants. Then, we will examine the process data available to date. Last, we will summarize the feasibility of the text messaging tool to address ART adherence, depressed mood, and active substance use.
Methods
The overall procedures used to develop the personalized bidirectional text messaging tool follow. First, the study team reviewed data from a small preliminary study we had conducted of a one-way SMS system. This data included participant perceptions of receiving self-designed, personalized messages encouraging ART adherence as highly motivating (Delgado et al., 2009). A subset of the preliminary study team identified desired features of a new tool. These included capability to assess nonadherence, substance use, and poor mood in real time, and to send an intervention message in real time. Additionally, the team wanted to make the tool scalable, sustainable, and cost effective, and determined that it should be as automated as possible, with an easy interface for administration and data retrieval.
We sought input from the target population of patients through a series of individual and small group interviews. Nineteen participants completed questionnaires on their demographic, substance use, adherence, and mobile phone characteristics. After showing a short presentation of the goals of the study and many possible options for a text messaging tool, we elicited their feedback about potential draft components, utility, and appeal. We also interviewed health care providers who were HIV clinicians, pharmacists, or health department AIDS Drug Assistance Program (ADAP) coordinators. Fourteen professionals provided information about their HIV treatment experience and participated in a 20minute interview about their perceptions of barriers to HIV care engagement and medication adherence, misconceptions patients have about medications and treatment, major reasons patients report for missing appointments or failing to pick up medications, and the strategies patients used to overcome the barriers. After these interviews, we developed a detailed description of the text messaging tool including coded substance use queries. We reviewed our draft tool with the IRB and the University’s legal counsel (related to querying illegal behaviors by text messaging) to ensure that we had considered ethical and legal issues and had adequate participant protections in place. Finally, we incorporated patient, provider, and ethical/legal feedback into the functional specifications for the tool and built the system. The last preparatory step was a usability trial with 10 participants similar to the target population. Participants received a phone programmed with our tool and learned how to use it. They used the tool for 4 days and we monitored their responses, patterns of response, and problems using the system. Data were used to finalize the text messaging tool.
Finalized text messaging tool
The finalized assessment and intervention tool was nicknamed Treatment Extension by Text, or TxText. The tool is fully automated. It sends participants 3 types of daily queries. It inquires about mood twice per day at random times, substance use over the past 24 hours once per day at a random time, and medication adherence at the time of scheduled dosing time(s) of ART. For most participants, this translates to 4–5 queries per day. These queries ask the participant to respond with a keyword, so that the tool can recognize the category of response, and send the appropriate personalized message in return. Additionally, staff members can send one-time messages, such as reminders about upcoming appointments or to schedule transportation for clinical care appointments, ad hoc. Patients can initiate messages to the system, which are logged into an archive and checked by a staff member every 24 hours. The system responds to any unrecognizable text from participants with “Your message has been received. Thanks!”
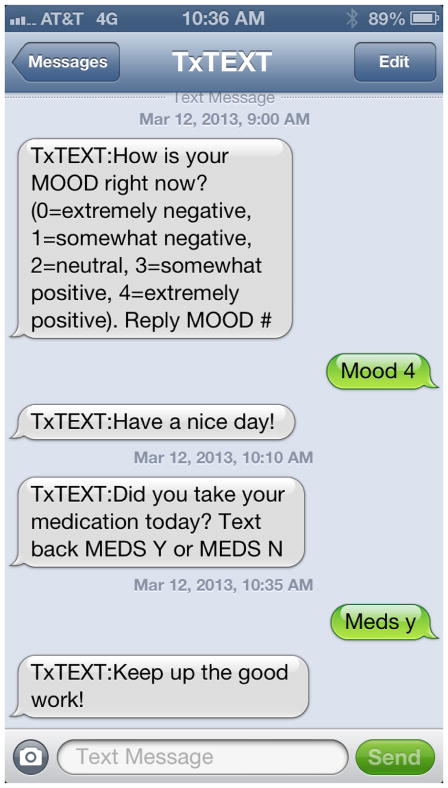
TxText relies upon a web interface for staff and patients to design personalized messages, set timing for medication reminders/queries, and set overall boundaries for when the system will send messages, to avoid the typical sleeping hours of the participant. Using the web interface, staff members prompt the participant to develop several types of personalized message that they would like to receive back depending on their initial response to medication, mood, and substance use queries. They develop both an affirming response to be sent after they reply with a positive behavior (e.g., took medications as directed, good mood, or no substance use in 24 hours) and an encouraging response to be sent after they reply with a negative behavior (e.g., did not take medications, in a neutral to poor mood, or reported drinking or drug use in past 24 hours). By design, the tool does not prioritize one particular target (adherence, mood, substance use) over the others, given that both the literature and the participants in formative interviews indicate that these are typically intertwined, and that affecting one may affect the others. Therefore, participants design their intervention messages for each targeted issue (nonadherence, poor mood, substance use), and their chosen message may be generic across problems or specific, based on the participant’s preference. A sample message flow between the system and a participant is shown in Figure 1.
Figure 1.
Sample Message Flow between TxText and Participant
While medication and mood queries are transparent and face-valid, as shown in Figure 1, the system uses a coded query for substance use that is in the form of a weather question. Specifically, participants are asked “How were the skies in the past 24 hours? Respond SKIES clear, cloudy, rainy, snowy, or other” Participants respond SKIES clear if they are reporting no drinking or drug use in the past 24 hours, SKIES cloudy if they smoked marijuana, rainy if they drank alcohol, snowy if they used crack or cocaine, and other if they used other illicit drugs. They can report multiple answers to the SKIES query if they used multiple substances. This method of coding substance use queries and responses was designed by our team and selected by a majority of formative patient participants as easy to recall and sufficiently protective of privacy.
The web-based system tracks all messages flowing from the TxText tool automatically or by one-time messages initiated by staff members, and to the TxText tool sent by participants. These data can be used to monitor for any emergency messages sent by participants, to determine gaps in responses or repeated response errors that trigger staff intervention, to provide prospective data to compare to self-report data collected at the 3-month follow-up, and provide the data to generate process observations.
Participants created a variety of affirming and encouraging messages tailored to each specific situation: taking medication, not taking medication, avoiding drinking and drug use, engaging in drinking and/or drug use, positive mood, and negative mood. Participants were given complete choice over the type of message they wanted to receive for each case. However, if participants created self-critical messages, staff asked if they thought that particular message would be helpful. Sometimes the participant said yes and kept that critical message, and others changed it to a more positive or encouraging message. Thus, while intervention developers elicited participants’ preferred messages, they provided minimal guidance into the content of personalized messages. Many participants chose song lyrics, religious messages, reminders, or encouraging phrases. Some examples of the personalized messages designed by participants were: Keep your head up, God loves you, Don’t forget your grandchildren, Great job!, You need to clear it up, Stay smiling, Be more responsible, Keep up the good work, and Yay!
Trial enrollment began in May 2012. The trial seeks to enroll 70 drug-using adult patients with HIV who report poor adherence, have basic reading ability, and are able to get mobile phone service at home. The trial will compare those randomized to receive the 3 month TxText intervention to those assigned to receive usual care at three month and six month follow-ups. The primary outcomes include engagement measured by Missed Visit Percentage at 6 months, and adherence measured by Pharmacy Refill Rate and supplemented by unannounced telephone pill counts. The trial is generating a large volume of continuous prospective data from the text messaging participants. Among those randomized to the text messaging condition, we observed the message flow from the tool to participants and back, and conducted descriptive analyses of these processes. Specifically, we calculated the number of all queries sent by the system, the overall and query-specific response rates, noted any idiosyncratic and unintelligible responses, and checked the data for each participant to explore any notable patterns of use over time during the 3 month active intervention period.
Results
Patient formative interviews
Nineteen patients living with HIV with histories of substance use and ART nonadherence participated in formative interviews, including focus groups or individual interviews. Our team is completing a formal qualitative and thematic analysis, and in the current report, we focus only on the basic participant characteristics, behaviors, and preferences reported. Formative interview participants were mostly single (52.6%), female (57.9%), African-American (52.6%), in their 40s (mean age 47.8, SD=7.8), with 36.8% reporting less than a high school education, 26.3% reporting a high school or GED education, and 36.8% reporting some college or more. A significant proportion (42.1%) reported they are disabled, and 52.6% report living in a rural area. Table 1 shows the behavioral and phone characteristics of this sample. Most (10 of 19) screened positive for a potential or definite substance use disorder on the Drug Abuse Screening Test (Gavin, Ross, & Skinner, 1989), and a quarter screened positive for harmful drinking on the AUDIT (Babor, de la Fuente, Saunders, & Grant, 2001). Additionally, most smoked cigarettes, and screened positive for low to medium levels of nicotine dependence on the Fagerstrom Test of Nicotine Dependence (Fagerstrom, 2011). Most commonly, they reported missing a dose of ART in the past week or 2–4 weeks ago on the AIDS Clinical Trial Group (ACTG) Adherence Questionnaire (Reynolds et al., 2007). Reasons for missing medications endorsed by over a quarter of participants were: falling asleep, being away from home, feeling medications were toxic, having problems taking medications at specified times, or being busy. Most participants had a mobile phone with a contract or pay as you go plan, and 84.2% used text messaging, with 52.6% having an unlimited texting plan. 21.1% had Internet access with their phones. On average, they preferred to receive 4–5 text messages per day from the study tool, with considerable variability in this preference. They reported that their own nonadherence was often associated with both depressed mood or stress and periods of drug or alcohol use. They reported that they would not feel comfortable responding to a query about substance use or drug/alcohol craving unless it were coded for privacy.
Table 1.
Demographic, Behavioral, and Phone Characteristics of Patients Completing Questionnaires and Formative Interviews
| Characteristics (n = 19) | Mean (SD) | n (%) |
|---|---|---|
| Drug Abuse Screening Test (DAST) | ||
| Mean Total Score | 7.4 (5.9) | |
| Potential substance use disorder | 10 (52.6) | |
| Definite substance abuse | 4 (21.1) | |
| Alcohol Use Disorders Identification Test (AUDIT) | ||
| Mean Total Score | 5.7 (7.1) | |
| Harmful drinking | 5 (26.3) | |
| Likely alcohol dependence | 3 (15.8) | |
| Fagerstrom Test of Nicotine Dependence | ||
| Mean Total Score | 4.0 (2.0) | |
| Not applicable | 3 (15.8) | |
| Low Dependency | 6 (31.6) | |
| Medium Dependency | 9 (47.4) | |
| High Dependency | 1 (5.3) | |
| ACTG Adherence Questionnaire: Last Dose Missed | ||
| Within the past week | 7 (36.8) | |
| 1–2 weeks ago | 3 (15.8) | |
| 2–4 weeks ago | 7 (36.8) | |
| 1–3 months ago | 1 (5.3) | |
| More than 3 months ago | 1 (5.3) | |
| Reasons for Missing Medication Doses (“sometimes or often”) | ||
| Fell asleep | 8 (42.1) | |
| Away from home | 6 (31.6) | |
| Felt it was toxic | 6 (31.6) | |
| Had problems taking at specified times | 5 (26.3) | |
| Busy | 5 (26.3) | |
| Forgot | 4 (21.0) | |
| Side effects | 4 (21.0) | |
| Did not want others noticing | 4 (21.0) | |
| Felt depressed or overwhelmed | 4 (21.0) | |
| Too many pills to take | 3 (15.8) | |
| Felt sick | 3 (15.8) | |
| Felt good | 3 (15.8) | |
| Routine change | 2 (10.5) | |
| Ran out of pills | 2 (10.5) | |
| Type of Phone Plan | ||
| Contract | 11 (57.9) | |
| Pay as you go (no contract) | 7 (36.8) | |
| None | 1 (5.3) | |
| Phone with text messaging* | ||
| Yes | 16 (84.2) | |
| No | 1 (5.3) | |
| Not applicable | 1 (5.3) | |
| Text Messaging Charges * | ||
| Unlimited | 10 (52.6) | |
| Per text | 2 (10.5) | |
| Capped amount | 2 (10.5) | |
| Not sure | 2 (10.5) | |
| Not applicable | 2 (10.5) | |
| Phone Internet Plan * | ||
| Yes | 4 (21.1) | |
| No | 13 (68.4) | |
| Not applicable | 1 (5.3) | |
| How often do you carry your cell phone? | ||
| Frequently | 12 (63.2) | |
| All the time | 6 (31.6) | |
| Not applicable | 1 (5.3) | |
| Text messages sent per day | 10.6 (23.8) ** | |
| Text messages received per day | 15.9 (29.0) ** | |
| How comfortable are you with your phone’s features, especially text messaging? | ||
| Not comfortable at all | 4 (21.1) | |
| Somewhat comfortable | 1 (5.3) | |
| Comfortable | 1 (5.3) | |
| Very comfortable | 4 (21.1) | |
| Completely comfortable | 8 (42.1) | |
| Not applicable | 1 (5.3) | |
| Any concerns about using a cell phone to participate in this study? * | ||
| None | 15 (78.9) | |
| Do not know how to text | 2 (10.5) | |
| Cost | 1 (5.3) | |
| How many text messages are you willing to receive from the study team daily? * | ||
| 1 | 1 (5.3) | |
| 2–3 | 3 (15.8) | |
| 4–5 | 5 (26.3) | |
| 10–12 | 2 (10.5) | |
| 20 | 1 (5.3) | |
| It doesn’t matter to me | 2 (10.5) | |
| I don’t know | 2 (10.5) | |
| As many as needed | 2 (10.5) | |
indicates n=18; data from one participant missing
indicates n=17; data from two participants missing
Heath care provider formative interviews
Fourteen health care providers (9 women, 5 men) completed in-person or telephone interviews about the needs of rural patients living with HIV and their barriers to adherence and engagement in care. These providers were 47 years old on average (SD=12.7), and had worked on average with rural HIV+ patients for 10.4 years (SD=9.2). They were primarily clinicians (n=8), AIDS drug assistance program coordinators (n=5), and pharmacists (n=1) working in government agencies (n=6), hospital and group practices (n=7), and academic health centers (n=1). They reported that barriers to adherence for rural patients living with HIV included lack of transportation, financial burden of medications and repeated trips to pharmacies, low income, and low employment, social stigma and related confidentiality concerns, and multiple life stressors including mental and substance use disorders, abusive relationships, and negative beliefs and feelings such as self-hatred and shame. They reported that patients overcome these obstacles by persevering, using social services, federal, and state aid programs, getting help from family and friends, and using a network of helpers including social workers, case managers, ADAP coordinators, and clinicians. They report that patients have misconceptions about HIV medications including the belief that when the viral load is undetectable, they are “cured” or no longer need medications, if they feel good they can stop taking medications, or in contrast, that medications will not be helpful, are too expensive, have horrible side effects, or should not be trusted. Several providers mentioned that patients think they can skip doses with no understanding of medication resistance. Providers described the main reasons that patients may miss HIV care appointments or medication pickups as transportation issues, embarrassment about nonadherence, fear of receiving bad news, forgetting, cognitive impairment, having no penalty for missing appointments, fearing that others will learn their status if they attend clinic, or feeling well.
Usability trial results
Ten patients with a history of ART nonadherence and recent substance use participated in usability testing. They were mostly male (n=6), African-American (n=6), disabled (n=6), single (n=7), and reported residing in rural or semi-rural areas (n=6). Half of them had mobile phones with pay as you go plans, and half with contracts, and all had text messaging. Most reported unlimited text messaging plans and no internet plan for their phones, and nearly all reported carrying their mobile phone all of the time. They reported sending 12 and receiving about 15 text messages per day on average, and most reported being completely comfortable with their phone’s features. One expressed privacy concerns about using a mobile phone for the study while the others reported they had no concerns. Most reported preferring to receive either 4–5 (30%) or 10–12 (30%) messages per day from the study.
Prior to usability testing with participants, we tested the draft system among 6 team members for several weeks and identified some programming issues to correct in the final tool. For example, on a few occasions, messages repeatedly occurred during the user’s preferred sleep time. While these issues were being corrected, we conducted usability testing by sending messages manually to participants, who were given a study phone for 4 days of usability testing. Table 2 presents the results of usability testing. The team sent 186 messages and received 155 replies (response rate = 83.3%). The highest response rate was to medication queries, followed by substance use queries, followed by mood queries. Fewer than 2% (1.3%) of responses had misspellings, all of which could be interpreted by the study staff member who was sending the queries and receiving the responses. While the modal time to respond to each type of query was 1 minute or less, the mean was large as some participants took many hours to respond to queries. The typical responses indicated that participants had taken their medications, were in a very positive mood, and had not used substances in the past 24 hours.
Table 2.
Patient 4-day Usability Testing Results (n = 10)
| Response Rate by Query Type | |
| All Queries | 83.3% |
| Mood | 77.2% |
| Medication | 92.0% |
| Substance Use | 86.4% |
| Misspelled Response Rate | 1.29% |
| Minutes to Respond to Query | |
| All Queries | |
| Mean (SD) | 53.8 (138.5) |
| Median | 5.0 |
| Range | 0–883 |
| Mode | 1.0 |
| Mood Queries | |
| Mean (SD) | 47.4 (106.0) |
| Median | 4.00 |
| Range | 0–769 |
| Mode | 1.0 |
| Medication Queries | |
| Mean (SD) | 36.9 (118.3) |
| Median | 3.5 |
| Range | 0–751 |
| Mode | 0.0 |
| Substance Use Queries | |
| Mean (SD) | 86.2 (200.1) |
| Median | 8.5 |
| Range | 0–883 |
| Mode | 1.0 |
| Content of Responses | |
| Mood Query | |
| Mood Rating Mean (SD) | 3.4 (0.9) |
| Median | 4.0 |
| Range | 1–4 |
| Mode | 4.0 |
| Mood Frequency (note: n =64) | |
| 0=very negative | 0 (0.0) |
| 1=somewhat negative | 3 (4.7) |
| 2=neutral | 9 (14.1) |
| 3=somewhat positive | 11 (17.2) |
| 4=very positive | 41 (64.1) |
| Medication Query | |
| ”yes” | 46 (100.0) |
| Coded Substance Use Query | |
| Clear (no substance use) | 31 (81.6) |
| Cloudy (marijuana use) | 4 (10.5) |
| Rainy (alcohol use) | 1 (2.6) |
| Snowy (crack/cocaine use) | 1 (2.6) |
| Cloudy and rainy (marijuana and alcohol use) | 1 (2.6) |
Process observations
As of this report, 57 participants have enrolled in the RCT. While outcomes data are not yet available and are beyond the scope of this report, we provide a description of participants to place process data in context. Most participants (59.6%) are men, report a high school or equivalent education (66%), are African-American (61.4%), and have a mean age of 42.1 (SD=10.0) All had used illicit drugs or reported an alcohol binge in the past 30 days on their eligibility screen. Further, half screened positive for harmful drinking on the AUDIT, while 42% screened positive for potential Substance Use on the DAST. On the MINI (Sheehan et al., 1998), the most common positive screens were for current Major Depression (54.4%), current Generalized Anxiety Disorder (43.9%), current Drug Dependence (36.8%), and current Alcohol Dependence (31.6%). The rate of missed visits during the 6 months prior to baseline was 26.5% (SD=.30). Medication adherence by 14-day Timeline Follow-back using procedures refined by Ingersoll et al., (2011) was 62% (SD=.31). Thus, this sample included substance abusers with significant nonadherence to medication who were at risk of disengaging from care. Thirty-one participants were randomized to the TxText condition. They were given a study phone even if they already had a mobile phone.
The following process data comes from the 23,258 messages sent to and from the system from May 22, 2012 to March 31, 2013 among the 31 TxText participants. The system sent 16,547 messages (half initial queries, and half personalized messages in response to participant messages) to participants representing 71% of all messages, and participants sent 6711 messages in response to initial queries, representing 29% of all messages. This proportion of outbound and inbound messages is expected because for each query sent by the system (outbound) and answered by a participant (inbound), an additional outbound personalized affirming or encouraging message is sent by the system. On average, across participants with different lengths of stay in the study, each participant sent 224 messages to the system and received 551 messages from it. Across the 3 month intervention period, message flow ranged from 373 outbound and 182 inbound messages at the minimum, to 823 outbound and 368 inbound messages at the maximum.
We examined messages by query type. Two thousand three hundred and seventy substance use queries were sent by the tool, and there were 1585 responses to these queries, for a response rate of 67%. Nine hundred sixty four of these responses (60.8%) were “clear,” indicating no substance use in the past 24 hours, while 354 included rainy (drinking), 297 cloudy (marijuana), 72 snowy (cocaine or crack use) and 3 included “other” (other drugs). A total of 2918 medication queries was sent, including 2385 first or single dose medication queries and 533 second medication dose queries, and there were 2018 responses to these queries for a response rate of 69.2%. Of the responses to medication queries, 1759 (87.2%) responded “Yes” and 241 (11.9%) responded “No.” A total of 4639 mood queries were sent by the system, with 2319 first and 2320 second mood queries (averaging 2 per day). Participants replied to these mood queries with 2978 responses, a response rate of 64.2%. Responses included 1906 (64.0%) reflecting a positive mood and 1060 (35.6%) reflecting a neutral to negative mood.
We examined charts of responses by day for each participant to identify patterns of responding. In general, participants engaged with the tool consistently over the 3-month intervention period. Participants were not routinely seen in person during the trial, but in some cases, did meet with study staff to address problems. We had planned to call non-responders after 2 consecutive days of non-response, but this was not done in some cases due to human error. We terminated phone service for two participants because both had relocated, had not answered the text messages for many days, and did not respond to multiple attempts at contact. One of these participants later returned to the study, reporting that their phone had been lost or stolen, and that they had had to move out of the area temporarily. Among 10 of the 29 other TxText participants, there were 19 instances of non-response periods of 7 days or more. These periods ranged from 7 days to 23 days, and were 13.9 days long on average (SD= 5.8). Nine of these 19 instances were due to reasons out of the participant’s control, such as hospitalization, incarceration, lost, stolen, or damaged phones, and lost chargers. Phones and chargers were replaced in these cases, and then participants resumed responding. These periods of non-response were not included in our calculations of response rates.
After the intervention, we interviewed participants about what was happening in their lives during periods of non-response to determine whether they represented periods of poor mood, drug use, or nonadherence. The four participants with lengthy non-response periods reported that these lapses were related to busyness, the phone dying, falling asleep and consequently missing the message, forgetting about the phone after a long time away, and not bringing the phone while travelling. Some of these were also mentioned as reasons for shorter periods of non-response among these four participants and other participants. One reported drug use during travel away, but none reported that their non-response episode was related to substance use.
While we designed the system to be able to decode most participant messages, especially those sent with a keyword, there were some cases where user error resulted in an inbound message that could not be decoded by the system, including leaving out keywords, misspelling keywords or responses, or typing characters that did not compose a word. In most of these cases, using the message log, a research assistant could decode these. However, we could not make sense of 1.6% of participant messages. Unrecognized messages prompted the system to send a thank you.
Discussion
Our aim was to develop a personalized, bidirectional text messaging assessment and intervention tool for non-urban substance users living with HIV who are non-adherent to ART. In this report, we described our formative research methods used to supplement expert specification of functionalities to create the text messaging tool. After conducting usability testing to finalize the draft Treatment Extension by Text (TxText) tool, we began testing it in a pilot RCT for its impact on objective markers of adherence and treatment engagement as well as days of drinking and drug use.
In this report, we used data collected in the ongoing RCT to analyze process data from the messaging stream of the tool. These process data show that the system can send the messages daily over a 3 month period, can receive responses, and decode them properly to deliver personalized affirming or intervention messages. These findings are important because most mHealth adherence interventions have tested one-way text messaging, using SMS to send simple reminders to take medications. Only two previous non-US studies have used bidirectional messages, and both of them improved adherence outcomes. No previous study has investigated using text messaging to reduce the impact of other phenomena that undermine adherence (e.g., mood disruption and substance abuse).
Moreover, data show that participants enrolled in a text messaging study responded daily about their mood, adherence, and substance use, and that response rates did not vary by the type of query sent by the system. We are unable to compare these observed response rates to those in other studies because those did not report these type of process data. The system received very few (less than 1%) user-initiated messages that were not sent in response to a query. Most responses reflect positive behaviors, but users also report negative behaviors and feelings. In the case of medication adherence, there may be a tendency to respond that medications were taken. It is possible that non-responses to this query may indicate nonadherence.
Our tool is unique because it targets adherence along with two factors that may undermine it, active substance use, and poor mood/depression. In fact, in our population, medication adherence is not the only problem, with significant substance use disorders and mental disorders complicating the clinical picture. Our tool can evaluate medication adherence behavior, mood, and substance use behavior in real time, and respond with a just-in-time personalized message selected by the participant.
Importantly, the participants create the messages to send themselves when reporting substance use, poor mood, or nonadherence. This approach is consistent with Motivational Interviewing, which posits that evoking a person’s own perspective on a problem and their own language about change is effective (Miller & Rollnick, 2013; Moyers, Martin, Houck, Christopher, & Tonigan, 2009). While data collection is not complete, the process analysis already shows that TxText is feasible and may serve as a complement to routine HIV medical care.
Participants who completed formative interviews, usability testing, and post-trial interviews spontaneously reported their acceptance and liking of this two-way text messaging intervention. They reported feeling cared for and connected to the clinic, despite understanding that the tool was automated. This finding is consistent with results from an SMS trial in Kenya. In a post-hoc process analysis, van der Kop and colleagues found that a minority of participant responses indicated a problem, and that most of these were related to health issues (van der Kop et al., 2012). Moreover, 62% of a subset of patients interviewed after the trial reported they had experienced no barriers to the intervention, but that they perceived benefits from the reminders to take medications, and that the weekly messages had made them believe that “someone cares.”
In a subsequent report, we will present the primary outcomes of the RCT. If TxText is found efficacious, it could be easily scaled up to serve the needs of other patients living with HIV, substance use disorders, and mental disorders, given its automation and simple web interface. A necessary next step will be to enable installation on user-owned phones across service provider platforms, while enabling study and clinical sites to capture critical message flow data. Our process data show that a bidirectional, personalized text messaging tool that assesses and intervenes on nonadherence, substance use, and poor mood engages substance-using patients living with HIV and elicits their responses over time.
Acknowledgments
This study was funded by R34 DA031640 from the National Institute on Drug Abuse of the National Institutes of Health. We thank Dr. Shoshana Kahana of NIDA for her tireless leadership in this area of research. Dr. Dillingham’s participation was partially supported by grant K23 AI077339. We thank the staff and patients of the University of Virginia Ryan White Clinic, of the Medical Associates of Central Virginia, and of the Virginia Department of Health’s AIDS Drug Assistance Program for their participation in the study, for their referral of appropriate participants, and for their facilitation of data collection. We thank William Hervey, Brian Au, Daniel Young, and Erin Wispelwey for their help with screening and enrolling participants. We thank Lee Ann Brooks of nTelos for supplying free study phones and discounted monthly service plans for the study. Preliminary and partial versions of this report were presented to the 2012 Annual Scientific Meeting of the Society of Behavioral Medicine in New Orleans, LA and the 2013 Meeting of the International Society for Research on Internet Interventions in Chicago, IL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Babor TF, de la Fuente JR, Saunders J, Grant M. AUDIT. the alcohol use disorders identification test. guidelines for use in primary health care. 2 2001. [Google Scholar]
- Berg KM, Cooperman NA, Newville H, Arnsten JH. Self-efficacy and depression as mediators of the relationship between pain and antiretroviral adherence. AIDS Care. 2009;21(2):244–248. doi: 10.1080/09540120802001697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner J. Pew internet: Mobile in the Pew Internet and American life project. 2013 Jun 6; Retrieved July 4, 2013, from http://www.pewinternet.org/Commentary/2012/February/Pew-Internet-Mobile.aspx.
- Carrico AW, Riley ED, Johnson MO, Charlebois ED, Neilands TB, Remien RH, Chesney MA. Psychiatric risk factors for HIV disease progression: The role of inconsistent patterns of antiretroviral therapy utilization. Journal of Acquired Immune Deficiency Syndromes (1999) 2011;56(2):146–150. doi: 10.1097/QAI.0b013e318201df63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesney M. Adherence to HAART regimens. AIDS Patient Care and STDs. 2003;17(4):169–177. doi: 10.1089/108729103321619773. [DOI] [PubMed] [Google Scholar]
- Cornelius JB, Dmochowski J, Boyer C, St Lawrence J, Lightfoot M, Moore M. Text-messaging-enhanced HIV intervention for african american adolescents: A feasibility study. The Journal of the Association of Nurses in AIDS Care: JANAC. 2012 doi: 10.1016/j.jana.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Costa TM, Barbosa BJ, Gomes e Costa DA, Sigulem D, de Fatima Marin H, Filho AC, Pisa IT. Results of a randomized controlled trial to assess the effects of a mobile SMS-based intervention on treatment adherence in HIV/AIDS-infected brazilian women and impressions and satisfaction with respect to incoming messages. International Journal of Medical Informatics. 2012;81(4):257–269. doi: 10.1016/j.ijmedinf.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughters SB, Magidson JF, Schuster RM, Safren SA. ACT HEALTHY: A combined cognitive-behavioral depression and medication adherence treatment for HIV-infected substance users. Cognitive and Behavioral Practice. 2010;17(3):309–321. doi: 10.1016/j.cbpra.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado S, Brant J, Rafaly M, White J, Freeman J, Sinha T, Dillingham R. Promoting adherence to HIV care in rural virginia through the development of an individualized short message service (SMS) delivery application. 4th International Conference on HIV Treatment and Adherence” International Association of Physicians in AIDS Care; Miami, FL. 2009. [Google Scholar]
- Dowshen N, Kuhns LM, Johnson A, Holoyda BJ, Garofalo R. Improving adherence to antiretroviral therapy for youth living with HIV/AIDS: A pilot study using personalized, interactive, daily text message reminders. Journal of Medical Internet Research. 2012;14(2):e51. doi: 10.2196/jmir.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerstrom K. Determinants of tobacco use and renaming the FTND to the fagerstrom test for cigarette dependence. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco. 2011 doi: 10.1093/ntr/ntr137. [DOI] [PubMed] [Google Scholar]
- Farrell Carnahan L, Fabbri S, Ingersoll K. Technicalities: Getting and staying connected to people living with HIV/AIDS in the southern united states. Patient Education and Counseling. 2011;83(1):139–40. doi: 10.1016/j.pec.2010.06.036. author reply 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flandre P, Peytavin G, Meiffredy V, Saidi Y, Descamps D, Delagnes M Trilege (Agence Nationale de Recherches sur le SIDA 072) Study Team. Adherence to antiretroviral therapy and outcomes in HIV-infected patients enrolled in an induction/maintenance randomized trial. Antiviral Therapy. 2002;7(2):113–121. [PubMed] [Google Scholar]
- Gavin DR, Ross HE, Skinner HA. Diagnostic validity of the drug abuse screening test in the assessment of DSM-III drug disorders. British Journal of Addiction. 1989;84(3):301–307. doi: 10.1111/j.1360-0443.1989.tb03463.x. [DOI] [PubMed] [Google Scholar]
- González-Guarda RM, McCabe BE, Florom-Smith A, Cianelli R, Peragallo N. Substance abuse, violence, HIV, and depression: An underlying syndemic factor among latinas. Nursing Research. 2011;60(3):182. doi: 10.1097/NNR.0b013e318216d5f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath T, Azman H, Kennedy GE, Rutherford GW. Mobile phone text messaging for promoting adherence to antiretroviral therapy in patients with HIV infection. Cochrane Database of Systematic Reviews (Online) 2012;3:CD009756. doi: 10.1002/14651858.CD009756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingersoll KS, Farrell-Carnahan L, Cohen-Filipic J, Heckman CJ, Ceperich SD, Hettema J, Marzani-Nissen G. A pilot randomized clinical trial of two medication adherence and drug use interventions for HIV+ crack cocaine users. Drug and Alcohol Dependence. 2011;116(1–3):177–187. doi: 10.1016/j.drugalcdep.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempf MC, McLeod J, Boehme AK, Walcott MW, Wright L, Seal P, Moneyham L. A qualitative study of the barriers and facilitators to retention-in-care among HIV-positive women in the rural southeastern united states: Implications for targeted interventions. AIDS Patient Care and STDs. 2010 doi: 10.1089/apc.2010.0065. [DOI] [PubMed] [Google Scholar]
- Leite L, Buresh M, Rios N, Conley A, Flys T, Page KR. Cell phone utilization among foreign-born latinos: A promising tool for dissemination of health and HIV information. Journal of Immigrant and Minority Health/Center for Minority Public Health. 2013 doi: 10.1007/s10903-013-9792-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenhart A, Ling R, Campbell S, Purcell K. Teens and mobile phones. Washington, D.C: Pew Research Center; 2010. Retrieved from http://pewinternet.org/~/media//Files/Reports/2010/PIP-Teens-and-Mobile-2010-with-topline.pdf. [Google Scholar]
- Lewis MA, Uhrig JD, Bann CM, Harris JL, Furberg RD, Coomes C, Kuhns LM. Tailored text messaging intervention for HIV adherence: A proof-of-concept study. Health Psychology: Official Journal of the Division of Health Psychology, American Psychological Association. 2013;32(3):248–253. doi: 10.1037/a0028109. [DOI] [PubMed] [Google Scholar]
- Malta M, Strathdee SA, Magnanini MM, Bastos FI. Adherence to antiretroviral therapy for human immunodeficiency virus/acquired immune deficiency syndrome among drug users: A systematic review. Addiction (Abingdon, England) 2008;103(8):1242–1257. doi: 10.1111/j.1360-0443.2008.02269.x. [DOI] [PubMed] [Google Scholar]
- Mbuagbaw L, Thabane L, Ongolo-Zogo P, Lester RT, Mills EJ, Smieja M, Kouanfack C. The cameroon mobile phone SMS (CAMPS) trial: A randomized trial of text messaging versus usual care for adherence to antiretroviral therapy. PloS One. 2012;7(12):e46909. doi: 10.1371/journal.pone.0046909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WR, Rollnick S. Motivational interviewing. New York: Guilford Press; 2013. [Google Scholar]
- Moyers TB, Martin T, Houck JM, Christopher PJ, Tonigan JS. From in-session behaviors to drinking outcomes: A causal chain for motivational interviewing. Journal of Consulting and Clinical Psychology. 2009;77(6):1113–1124. doi: 10.1037/a0017189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugavero MJ, Lin HY, Allison JJ, Giordano TP, Willig JH, Raper JL, Saag MS. Racial disparities in HIV virologic failure: Do missed visits matter? Journal of Acquired Immune Deficiency Syndromes (1999) 2009;50(1):100–108. doi: 10.1097/QAI.0b013e31818d5c37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehl EJ, He N, Wang X, Lin L, Wong FY, Yu F. Feasibility and willingness of using e-technologies for HIV prevention and research targeting chinese MSM. AIDS Care. 2012 doi: 10.1080/09540121.2012.726344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JT, Golub SA, Rosof E, Holder C. Motivational interviewing and cognitive-behavioral intervention to improve HIV medication adherence among hazardous drinkers: A randomized controlled trial. Journal of Acquired Immune Deficiency Syndromes (1999) 2007;46(4):443. doi: 10.1097/qai.0b013e318158a461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JT, Rosof E, Punzalan JC, Di Maria L. Integration of motivational interviewing and cognitive behavioral therapy to improve HIV medication adherence and reduce substance use among HIV-positive men and women: Results of a pilot project. AIDS Patient Care and STDs. 2005;19(1):31–39. doi: 10.1089/apc.2005.19.31. [DOI] [PubMed] [Google Scholar]
- Paterson DL, Swindells S, Mohr J, Brester M, Vergis EN, Squier C, Singh N. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Annals of Internal Medicine. 2000;133(1):21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- Pellowski JA, Kalichman SC. Recent advances (2011–2012) in technology-delivered interventions for people living with HIV. Current HIV/AIDS Reports. 2012;9(4):326–334. doi: 10.1007/s11904-012-0133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reback CJ, Grant DL, Fletcher JB, Branson CM, Shoptaw S, Bowers JR, Mansergh G. Text messaging reduces HIV risk behaviors among methamphetamine-using men who have sex with men. AIDS and Behavior. 2012;16(7):1993–2002. doi: 10.1007/s10461-012-0200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds NR, Sun J, Nagaraja HN, Gifford AL, Wu AW, Chesney MA. Optimizing measurement of self-reported adherence with the ACTG adherence questionnaire: A cross-protocol analysis. Journal of Acquired Immune Deficiency Syndromes (1999) 2007;46(4):402–409. doi: 10.1097/qai.0b013e318158a44f. [DOI] [PubMed] [Google Scholar]
- Rodrigues R, Shet A, Antony J, Sidney K, Arumugam K, Krishnamurthy S, DeCosta A. Supporting adherence to antiretroviral therapy with mobile phone reminders: Results from a cohort in south india. PloS One. 2012;7(8):e40723. doi: 10.1371/journal.pone.0040723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safren SA, O’Cleirigh CM, Bullis JR, Otto MW, Stein MD, Pollack MH. Cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected injection drug users: A randomized controlled trial. Journal of Consulting and Clinical Psychology. 2012;80(3):404–415. doi: 10.1037/a0028208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Dunbar GC. The mini-international neuropsychiatric interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of Clinical Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- Simoni JM, Amico KR, Pearson CR, Malow R. Strategies for promoting adherence to antiretroviral therapy: A review of the literature. Current Infectious Disease Reports. 2008;10(6):515–521. doi: 10.1007/s11908-008-0083-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni JM, Pearson CR, Pantalone DW, Marks G, Crepaz N. Efficacy of interventions in improving highly active antiretroviral therapy adherence and HIV-1 RNA viral load. A meta-analytic review of randomized controlled trials. Journal of Acquired Immune Deficiency Syndromes 1999. 2006;43(Suppl 1):S23–35. doi: 10.1097/01.qai.0000248342.05438.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker JS, Burnam MA, Sherbourne CD, Kung FY, Gifford AL. Substance use and mental health correlates of nonadherence to antiretroviral medications in a sample of patients with human immunodeficiency virus infection. The American Journal of Medicine. 2003;114(7):573–580. doi: 10.1016/s0002-9343(03)00093-7. [DOI] [PubMed] [Google Scholar]
- van der Kop ML, Karanja S, Thabane L, Marra C, Chung MH, Gelmon L, Lester RT. In-depth analysis of patient-clinician cell phone communication during the WelTel Kenya1 antiretroviral adherence trial. PloS One. 2012;7(9):e46033. doi: 10.1371/journal.pone.0046033. [DOI] [PMC free article] [PubMed] [Google Scholar]