Abstract
The serine/threonine kinase Akt has been shown to mediate the anti-apoptotic activity through hexokinase (HK)-mitochondria interaction. We previously reported that Akt activation in retinal rod photoreceptor cells is mediated through light-dependent insulin receptor (IR)/PI3K pathway. Our data indicate that light-induced activation of IR/PI3K/Akt results in the translocation of HK-II to mitochondria. We also found that PHLPPL, a serine/threonine phosphatase, enhanced the binding of HK-II to mitochondria. We found a mitochondrial targeting signal in PHLPPL and our study suggests that Akt translocation to mitochondria could be mediated through PHLPPL. Our results suggest that light-dependent IR/PI3K/Akt pathway regulates hexokinase-mitochondria interaction in photoreceptors. Down-regulation of IR signaling has been associated with ocular diseases of retinitis pigmentosa, diabetic retinopathy, and Leber Congenital Amaurosis-type 2, and agents that enhance the binding interaction between hexokinase and mitochondria may have therapeutic potential against these ocular diseases.
Keywords: Insulin receptor, Phosphoinositdie 3-kinase, Glycogen synthase kinase, Insulin, Neuroprotection, Mitochondria, Hexokinase, Akt, Retina, Retinal degenerative diseases, Photoreceptors
1. Introduction
Emerging evidence suggests that energy metabolism and cell survival are interrelated processes that are mainly coordinated by the serine/threonine kinase Akt (Robey and Hay, 2005; Whiteman et al., 2002). Several studies suggest mitochondrial hexokinase (mtHK) is a major downstream effector of Akt- and growth factor-mediated cell survival (Gottlob et al., 2001; Majewski et al., 2004; Bryson et al., 2002; Majewski et al., 2004). Activated Akt has been shown to inhibit HK dissociation from mitochondria, which is an early event following induction of apoptosis (Gottlob et al., 2001). It has also been shown that growth factors increase mitochondrial-HK association in normal cells, an effect that is markedly deregulated in Akt deficient cells (Majewski et al., 2004). In mammalian cells, the HK activity exists both in soluble and membrane fractions (CRANE and SOLS, 1953; Katzen et al., 1970). Membrane activity can be largely accounted for by the specific binding of high affinity HKI and HKII isoforms to the outer mitochondrial membrane (OMM) at mitochondrial contact sites (Wilson, 1995). The binding of HK to OMM is mediated, at least in part, by specific interactions between OMM voltage-dependent anion channel (VDAC) and hydrophobic N-terminal mitochondrial binding domains found in HKI and HKII, but not the corresponding HKIII and HKIV isoforms (Polakis and Wilson, 1985; Gelb et al., 1992; Pastorino et al., 2002; Majewski et al., 2004). The binding of HK-II to VDAC has been extensively studied and further reduced binding of HK-II to mitochondria lacking VDAC has also been reported (Anflous-Pharayra et al., 2007). HK associates with mitochondria and catalyzes the first committed step in glucose metabolism, the ATP-dependent phosphorylation of glucose to generate glucose-6-phosphate (Glu-6-P) (Wilson, 1995). The dynamic movement of HK between mitochondrial and cytosolic compartments is greatly influenced by physiological concentrations of Glu-6-P, ATP, and inorganic phosphate, and by intracellular pH (Miccoli et al., 1996; Miccoli et al., 1998; Robey and Hay, 2005).
Akt activation inhibits opening of the mitochondrial permeability transition pore through the inactivation of glycogen synthase kinase-3β (GSK-3β), whereby it induces the translocation of HK to mitochondria and binding to VDAC (Feng et al., 2005; Pastorino et al., 2005). Phosphorylation of serine-9 of GSK-3β is well known to inhibit its activity (Cross et al., 1995). Thus, one consequence of the action of Akt in mitochondria is to inhibit GSK-3β (Feng et al., 2005; Pastorino et al., 2005). In the absence of Akt activation, GSK-3β becomes active and phosphorylates VDAC on threonine 51, which disrupts the binding of HK to VDAC (Pastorino et al., 2005). In this manner, activated Akt inhibits the mitochondrial cytochrome c release and apoptosis (Majewski et al., 2004; Robey and Hay, 2006).
We previously reported that Akt activation is neuroprotective as down regulation of this pathway leads to photoreceptor degeneration (Li et al., 2007; Li et al., 2008). In photoreceptor cells, Akt activation is light-dependent and regulated through G-protein coupled receptor activated IR/PI3K pathway (Rajala et al., 2007; Li et al., 2008). In this study, we examined the role of light-dependent IR/PI3K/Akt signaling on HK-mitochondria interaction. Our results indicate that light-induced activation of IR/PI3K/Akt leads to the translocation of HK-II to mitochondria, and this light-dependent translocation of HK-II is significantly reduced in rod photoreceptors conditionally depleted of the insulin receptor gene. Our studies also reveal that GSK-3β inhibitor enhanced the binding of HK-II to mitochondria, whereas PI3K inhibitor reversed this effect. We also made a novel observation that PHLPPL, a serine/threonine phosphatase (Brognard et al., 2007), potentiates the effect of Akt and thereby enhances the binding of HK-II to mitochondria. Dissociation of hexokinase from mitochondria has been shown to induce apoptosis (Galluzzi et al., 2008; Chiara et al., 2008) and our study suggests a mechanism whereby light activation of the IR regulates mitochondrial hexokinase in photoreceptors, which provides retinal neuroprotection.
2. Materials and Methods
2.1. Materials
Polyclonal anti-hexokinase II, anti-VDAC, anti-cytochrome c, anti-HSP60, anti-PHB1 (prohibitin-1), anti-pAkt (S473), anti-Akt, anti-pGSK-3α/β, anti-GSK-3β, anti-Flag, and monoclonal anti-Myc antibodies, and PI3K inhibitor LY294002 were obtained from Cell Signaling (Danvers, MA). Actin antibody was obtained from Affinity BioReagents (Golden, CO). Polyclonal PHLPPL antibody was obtained from Novus Biologicals (Littleton, CO). GSK-3β inhibitor N-(4-Methoxybenzyl)-N’-(5-nitro-1,3-thiazol-2-yl)urea was obtained from Calbiochem (San Diego, CA). Mitochondria isolation kit for tissue (Zhang et al., 2010) was obtained from Thermo Fisher Scientific Inc (Rockford, IL). Human insulin R (rDNA origin) was obtained from Eli Lilly & Company (Indianapolis, IN). All other chemicals were purchased from Sigma (St. Louis, MO).
2.2. Animals
All animal work was performed in strict accordance with the Association for Research in Vision and Ophthalmology statement on the “Use of Animals in Ophthalmic and Vision Research.” All protocols were approved by the Institutional Animal Care and Use Committee of the University of Oklahoma Health Sciences Center and the Dean A. McGee Eye Institute. A breeding colony of albino Sprague-Dawley (SD) rats is maintained in our vivarium in cyclic light (5 lux; 12 h on/12 h off). Experiments were carried out on both male and female rats (150-200 g). Photoreceptor specific conditional insulin receptor knockout mice (Rajala et al., 2008) and p85α knockout mice (Ivanovic et al., 2011a) on BALB/c background were born and raised in 60-lux cyclic light (12h on/off) in our animal facility. Mitochondria were prepared from two independent sets of light- and dark-adapted retinas (either ex vivo or in vivo). C57BL/6J Ins2Akita heterozygote mice (Jackson Laboratory, Bar Harbor, ME) were bred in the Dean A. McGee Eye Institute Animal vivarium. Diabetic phenotype and genotype was confirmed 4.5 weeks after birth by blood glucose >250 mg/dL (TrueTrack Smart System; AR-MED Ltd, Egham, UK) in a drop of blood from a tail puncture. The disease is 100% penetrant in mice with the Ins2 mutation (Wang et al., 1999). Each set of mitochondrial preparations had at least 20 rats or mice (10 from light- and 10 for dark-adapted animals).
2.3. Ex vivo retinal cultures
For insulin treatment of retinal in vivo explants, rats were dark-adapted overnight, killed the next day, and retinas removed and placed in Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA). Insulin (10-1000 nM) or an equal volume of PBS was added and the retinas were incubated at 27 °C for 5 minutes. To inhibit PI3K activity or GSK-3β activity, retinal in vivo explants were incubated in 100 μM LY294002 or 100 μM GSK-3β inhibitor, or an equal volume of DMSO at 27 °C for 30 min, after which half of the retinal in vivo explants were exposed to 300 lux light for 30 min prior to the isolation of mitochondria.
2.4. Plasmids and vectors
Flag-tagged PHLPPL construct has been reported earlier (Kanan et al., 2010). We amplified a fragment of PHLPPL that contains the PP2C domain with (amino acids 130 to 1028) and without (amino acids 148-1028) a mitochondrial targeting signal (MT) using sense (+MT: AGA TCT ATG ATT CGA TTT TAT GGT GGA AAA CC; -MT: AGA TCT CGA ATC CTA CTG TCT GGC ATC) and antisense (GTC GAC TCA AAC CAC CAT TGC CCC CAC GTTG) primers and cloned into Myc-tagged pcDNA3 as a BglII/SalI fragment. We thank Dr. Morris Birnbaum (University of Pennsylvania) for his generous gift of mammalian expression constructs of Akt. The dominant negative Akt1 (K179M) (Zhou et al., 2000) construct was kindly provided by Dr. Mein-Chie Hung, M. D. Anderson Cancer Center, Houston, Texas. The dominant negative Akt1 (K179M) (Addgene plasmid 16243) was obtained from Addgene Inc, Cambridge, MA (http://www.addgene.org/pgvec1).
2.3. Cell Lines and Culture Condition
HEK-293T cells were maintained in DMEM medium containing 10% (v/v) FBS at 37 °C. Approximately 2.5 × 105 cells were seeded in each culture dish 12-18 h before transfection. Calcium phosphate-mediated DNA transfection was performed and cells were harvested for experiments ~48 h post-transfection.
2.4. Phosphatase assay
The in vitro phosphatase activity assay was conducted based on a protocol previously described (Kanan et al., 2010). Proteins expressed in HEK-293T cells were immunoprecipitated overnight at 4 °C with anti-Flag (PHLPPL) or anti-Myc (PP2C domain) antibodies. The immunocomplexes were precipitated with protein A-Sepharose at 4 °C for an additional 2 h. Immunoprecipitates were washed in phosphatase assay buffer [100 mM HEPES (pH 7.6), 2 mM EDTA, 1 mM dithiothreitol, 150 mM NaCl, and 0.5 mg/ml bovine serum albumin]. The peptide substrate RRLIEDAEPYAARG was added to a final concentration of 200 μM in total reaction volume of 60 μl in phosphatase assay buffer, and the reaction was allowed to proceed for 1 h at 30 °C. At the end of the reaction, 40-μl aliquots were placed in 96-well plate, 100 μl of Malachite green phosphatase reagent was added, and absorbance was measured at 630 nm.
2.5. Exposure of Animals to Light Stress
Sprague-Dawley rats were born and raised in dim cyclic (5 lux) light. Albino rats were exposed to constant light for 3 h at an illuminance level of 5000 lux (Kanan et al., 2010). During light exposure, animals were maintained in transparent polycarbonate cages with stainless-steel wire bar covers. Drinking water was supplied by a bottle attached to the side of the cage, so that there was no obstruction between the light and the animal, and food was placed on bedding in the bottom of the cage.
2.6. Statistical analysis
Student “t” test was used to determine statistical significance (p<0.05).
3. RESULTS
3.1. Localization of Hexokinase II (HK-II) in the retina
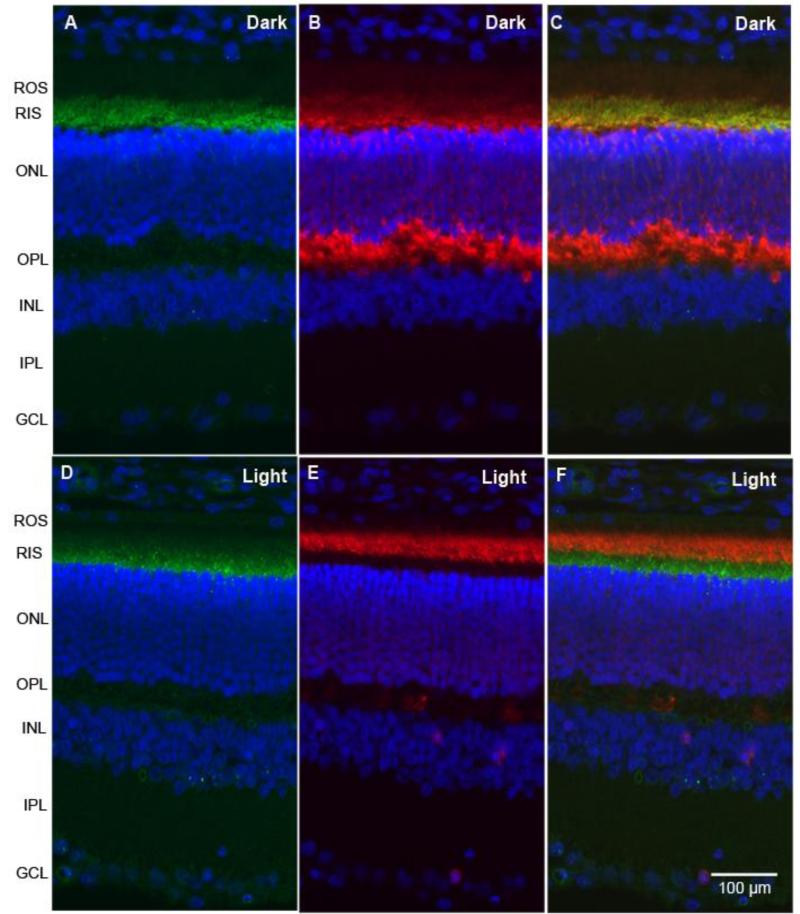
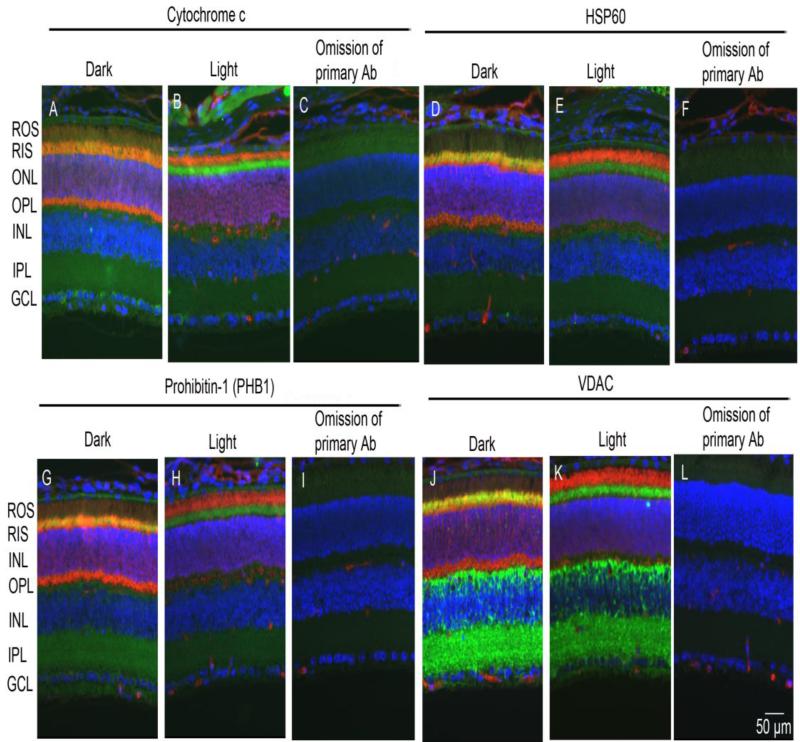
Retinal sections from dark- and light-adapted (300 lux for 30 min) rats were subjected to immunohistochemistry with hexokinase-II (HK-II) and arrestin antibodies. Immunolocalization studies suggest that HK-II is exclusively localized to rod inner segments (Fig. 1 A and D) and co-localizes with arrestin in dark-adapted retinas (Fig. 1C). The adaptability of animals to dark and light conditions is examined with arrestin immunolocalization. In dark-adapted retinas, arrestin is localized to the rod inner segments and the outer plexiform layer (Fig. 1B), and upon light illumination it translocates to photoreceptor outer segments (Fig. 1E). Our immunohistochemical data suggest that HK-II is predominantly localized to rod inner segments irrespective of dark or light adaptation. Co-labeling of retinal sections with mitochondrial markers (cytochrome c, HSP60, PHB1, and VDAC) and a photoreceptor specific marker arrestin indicates that except for VDAC, all mitochondrial markers localized to rod inner segments and co-localizes with arrestin in dark-adapted retinas (Fig. 2). The localization of mitochondrial markers did not change in response to light-adaptation (Fig. 2). In addition to photoreceptor inner segments, VDAC is also expressed in outer and inner plexiform layers of the retina (Fig. 2). These observations suggest that HK-II co-localizes with mitochondrial markers in photoreceptor inner segments.
Figure 1. Immunofluorescence analysis of HK-II in rat retina.
Prefer-fixed sections of dark-(A-C) and light-adapted (D-F) rat retinas were stained for HK-II (A, D), arrestin (B, E), and DAPI (A-F), and the immunofluorescence was analyzed by epifluorescence. Panels C and F represent the merge images of HK-II and arrestin. ROS, rod outer segments; RIS, rod inner segments; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer.
Figure 2. Immunofluorescence analysis of mitochondrial markers in retina.
Prefer-fixed sections of dark- and light-adapted retinas were co-stained for arrestin (red) and mitochondrial markers (green) of cytochrome c (A, B), HSP60 (D, E), PHB1 (G, H), and VDAC (J, K). The immunofluorescence was analyzed by epifluorescence. Panel C, F, I and L represent omission of primary antibodies on retinal section (light-adapted). ROS, rod outer segments; RIS, rod inner segments; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer.
3.2. Light- and insulin-induced association of HK with mitochondria
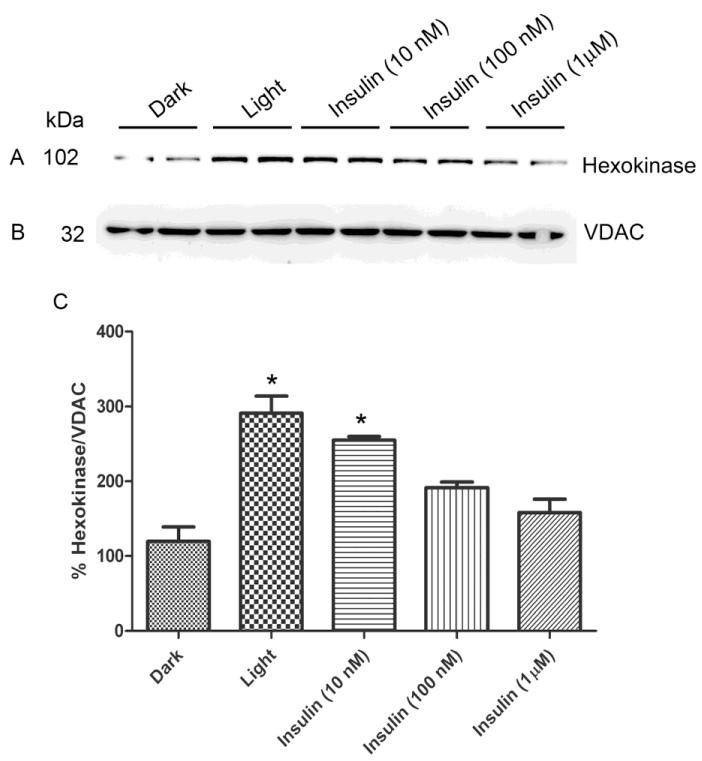
We previously reported that physiological light induced the activation of retinal IR and its downstream effectors, PI3K and Akt (Rajala et al., 2002; Rajala et al., 2007; Li et al., 2008). In this experiment, we examined the effect of light and insulin on the association of HK with mitochondria. Rats were dark-adapted overnight and half of the animals were light-adapted for 30 min at normal room light (300 lux). Dark-adapted retinal ex vivo explants in culture was stimulated with varying concentrations of insulin or PBS (vehicle control). Mitochondria were prepared from insulin-stimulated and dark- and light-adapted rat retinas, and the proteins were immunoblotted with anti-HK (Fig. 3A) and anti-VDAC (Fig. 3B) antibodies. The results indicate that the binding of HK to mitochondria was significantly higher in light-adapted conditions compared to dark-adapted conditions (Fig. 3C). We found increased binding of HK to VDAC when the retinas were stimulated with 10 nM insulin and this binding is comparable to the light-adapted conditions, although the binding gradually decreased as the concentration of insulin increased in the ex vivo explants (Fig. 3C). These experiments suggest that physiological light and/or insulin can cause the increased binding of HK to mitochondria.
Figure 3. Light- and insulin-induced association of HK-II with mitochondria.
Rats were dark-adapted overnight and half of the animals were light-adapted for 30 min at normal room light. Dark-adapted retinal ex vivo explants in cultures were stimulated with varying concentrations of insulin. Mitochondria were prepared from insulin-stimulated and dark- and light-adapted rat retinas (duplicate) and the proteins were immunoblotted with anti-HK-II (A) and anti-VDAC (B). Densitometric analysis of HK-II normalized to VDAC (C). Values are mean + SD, n=2. The dark control was set as 100 percent. *p<0.001 compared to dark-adapted control. Mitochondria were prepared from two independent sets of light- and dark-adapted retinas (either ex vivo or in vivo). Each set had 20 rats (10 animals for light- and 10 animals for dark-adaptation).
3.3. IR-dependent association of HK with mitochondria
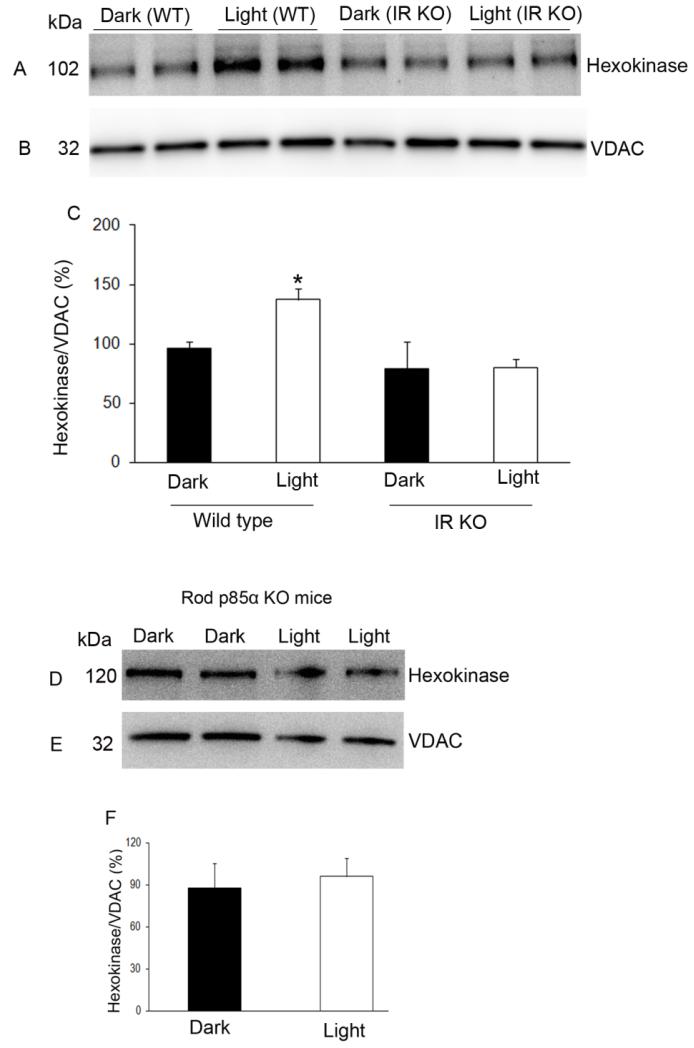
Mitochondria were prepared from dark- and light-adapted wild type and conditional rod-specific IR knockout mice (Rajala et al., 2008) and proteins were immunoblotted with anti-HK and anti-VDAC antibodies. The results indicate that light-induced activation of the IR resulted in the translocation of HK to mitochondria, and this light-dependent translocation of HK was significantly reduced in IR−/− mouse retinas (Fig. 4 A-C).
Figure 4. Association of HK-II with mitochondria in rod-specific IR and p85α KO mouse retinas.
Mitochondria were prepared from either dark- and light-adapted wild type and conditional rod-specific IR KO (A-C) or light-adapted p85α KO (D-F) mouse retinas. Mitochondrial proteins were immunoblotted with anti-HK-II (A, D) and anti-VDAC (B, E) antibodies. Densitometric analysis of HK-II normalized to VDAC (C, F). The dark control was set as 100 percent. Values are mean + SD, n=2. *p<0.001. Mitochondria were prepared from two independent sets of light- and dark-adapted retinas. Each set had at least 20 mice (10 animals for light- and 10 animals for dark-adaptation).
3.4. PI3K-dependent association of HK to mitochondria
Mitochondria were prepared from dark- and light-adapted wild type and conditional rod-specific p85α knockout mice and proteins were immunoblotted with anti-HK and anti-VDAC antibodies. The results indicate that there was no difference in the binding of HK to mitochondria between wild type and p85α knockout mice (Fig. 4D-F). These results suggest that other PI3K isoforms (Rajala, 2010) may be complementing binding of HK to mitochondria.
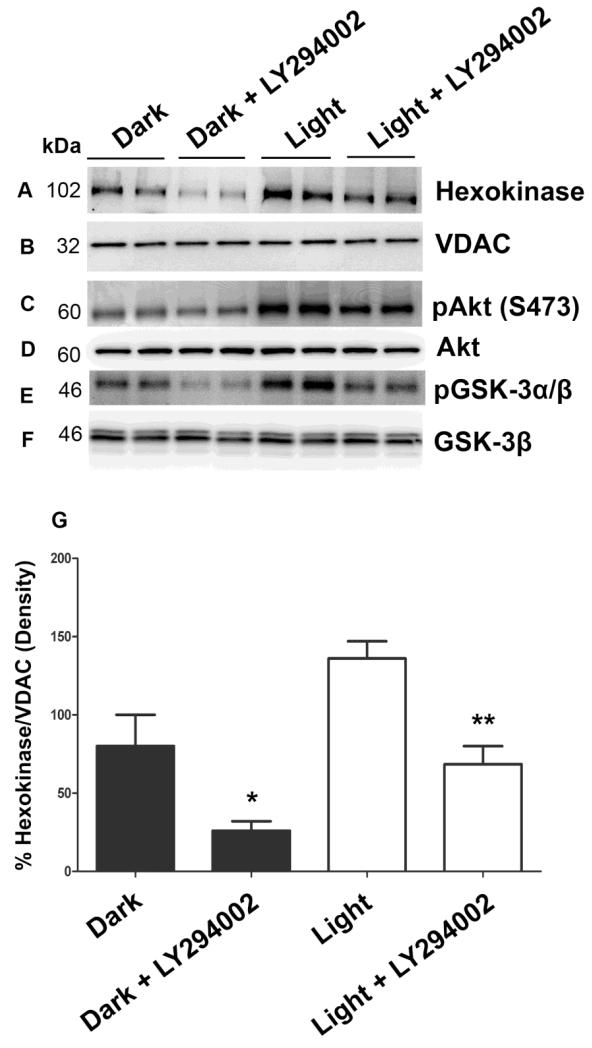
To determine whether other PI3K isoforms are complementing the binding of HK to mitochondria, retinal ex vivo explants from dark-adapted rats were incubated with and without PI3K inhibitor, LY294002, and were either exposed to light or kept in dark for 30 min. Mitochondria were prepared and the proteins were immunoblotted with anti-HK, anti-VDAC, anti-pAkt, anti-Akt, anti-pGSK-3α/β, and anti-GSK-3β antibodies. As reported previously (Li et al., 2008), we observed increased Akt phosphorylation in light-adapted conditions compared to dark-adapted conditions (Fig. 5C) and this phosphorylation was inhibited by LY294002 (Fig. 5C). It is interesting to note in this experiment that there is light-independent activation of Akt in dark and this activity was also PI3K-dependent as LY294002 inhibited this activity as well (Fig. 5C). GSK-3β is a direct substrate of Akt, and PI3K inhibitor decreased the phosphorylation of GSK-3β in both dark- and light-adapted conditions (Fig. 5E). LY294002 treatment did not affect the total Akt and GSK-3β levels (Fig. 5D and F). The binding of HK to mitochondria was significantly affected by PI3K inhibition in both dark- and light-adapted conditions (Fig. 5A and G). These experiments suggest that binding of HK to mitochondria in both dark- and light- adapted conditions is PI3K-dependent. This experiment also indicates two important aspects: 1) the existence of a PI3K-independent association of HK with mitochondria (Fig. 5A and G) and 2) involvement of other PI3K isoform(s) in enhancing the binding of HK to mitochondria.
Figure 5. PI3K-dependent association of HK-II with mitochondria.
Retinal explants from dark-adapted rats were incubated with and without PI3K inhibitor LY294002 and exposed to light or kept in the dark for 30 min. Mitochondria were prepared and the proteins were immunoblotted with anti-HK-II (A), anti-VDAC (B), anti-pAkt (C), anti-Akt (D), anti-pGSK-3α/β (E), and anti-GSK-3β (F) antibodies. Densitometric analysis of HK-II normalized to VDAC (G). Values are mean + SD, n=2. The dark control was set as 100 percent. *p<0.01 compared to dark-adapted control. **p<0.001 compared to light-adapted control. Mitochondria were prepared from two independent sets of light- and dark-adapted retinas. Each set had at least 20 rats (10 animals for light- and 10 animals for dark-adaptation).
3.5. GSK-3β regulation of the binding of HK to mitochondria
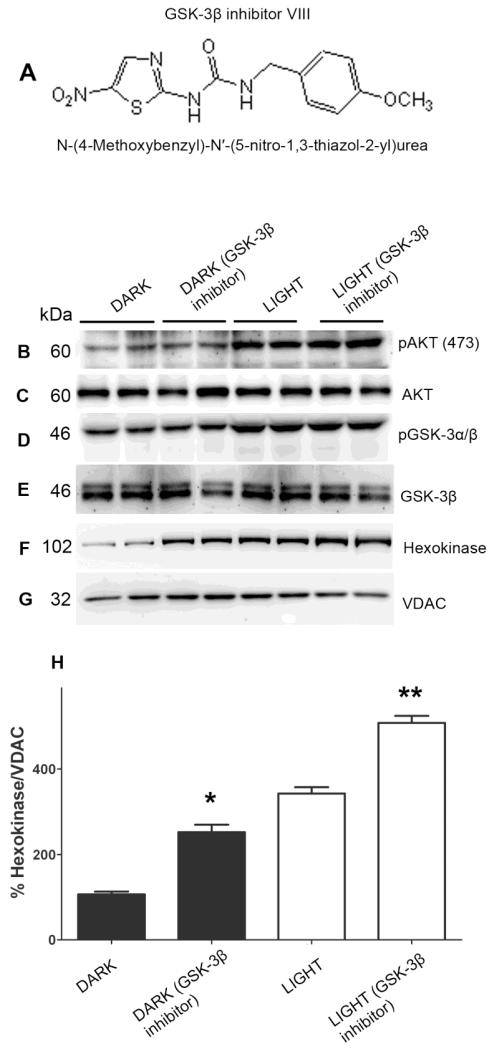
Akt activation induces translocation of HK to mitochondria, where it binds to the VDAC and prevents GSK-3β-mediated phosphorylation of VDAC and mitochondrial permeability transition (MPT) (Feng et al., 2005; Pastorino et al., 2005). It has been reported previously that in the absence of Akt activation, GSK-3β phosphorylates VDAC and thereby blocks the interaction of HK with VDAC (Feng et al., 2005; Pastorino et al., 2005). To determine if reduced binding of HK to mitochondria in the dark is due to phosphorylation of VDAC by GSK-3β, retinal ex vivo explants from dark-adapted rats were incubated with and without GSK-3β inhibitor (Fig. 6A). The ex vivo explants were then exposed to light or kept in dark for 30 min. Mitochondria were prepared and the proteins were immunoblotted with anti-HK, anti-VDAC, anti-pAkt, anti-Akt, anti-pGSK3α/β and anti-GSK3β antibodies. GSK-3β is a downstream effector of Akt and addition of GSK-3β inhibitor to ex vivo explants did not affect light-induced Akt or GSK-3β phosphorylation (Fig. 5B and D). The level of Akt and GSK-3β phosphorylation was higher in light and light plus GSK-3β inhibitor group compared to dark and dark-plus GSK-3β inhibitor group (Fig. 6B and D). The GSK-3β inhibitor did not affect the levels of total Akt and GSK-3β (Fig. 6C and E). The binding of HK to mitochondria was significantly higher in dark plus GSK-3β inhibitor compared to dark-adapted ex vivo cultures (Fig. 6F and H). Under light-adapted conditions, the presence of inhibitor significantly enhanced the binding of HK to mitochondria compared to light-adapted ex vivo cultures (Fig. 6F and H). These experiments suggest that GSK-3β negatively regulates the HK association with mitochondria and light-induced activation of Akt enhances the binding of HK to mitochondria through the inactivation of GSK-3β. We could not directly measure the effect of GSK-3β-mediated phosphorylation on VDAC as there are no commercially available anti-VDAC antibodies that immunoprecipitate the protein.
Figure 6. GSK-3β regulation of binding of HK-II to mitochondria.
Chemical structure of GSK-3β inhibitor N-(4-Methoxybenzyl)-N’-(5-nitro-1,3-thiazol-2-yl)urea (A). Retinal explants from dark-adapted rats were incubated with and without GSK-3β inhibitor. The ex vivo explants were exposed to light or kept in the dark for 30 min. Mitochondria were prepared and the proteins were immunoblotted with anti-pAkt (B), anti-Akt (C), anti-pGSK-3α/β (D), anti-GSK3β (E), anti-HK-II (F), and anti-VDAC (G) antibodies. Densitometric analysis of HK-II normalized to VDAC (H). The dark control was set as 100 percent. Values are mean + SD, n=2. *p<0.01 compared to dark-adapted control. **p<0.001 compared to light-adapted control. Mitochondria were prepared from two independent sets of light- and dark-adapted retinas. Each set had at least 20 rats (10 animals for light- and 10 animals for dark-adaptation).
3.6. Increased association of PHLPPL with mitochondria in vivo
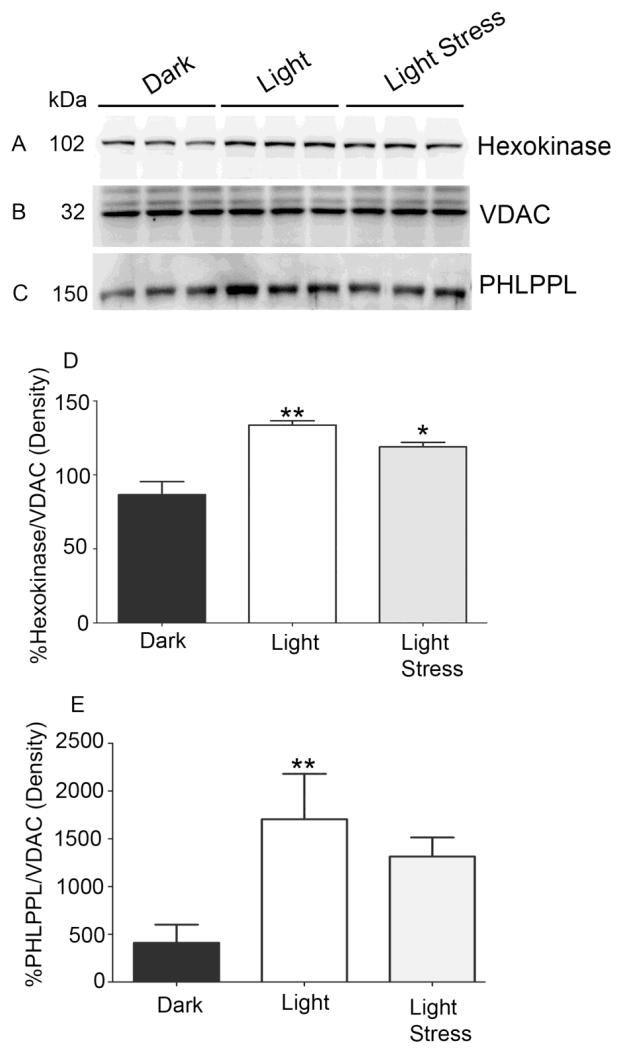
PHLPP and PHLPPL are serine/threonine protein phosphatases that are known to dephosphorylate Akt (Brognard et al., 2007; Gao et al., 2005). In our previous publication, we showed that PHLPPL, but not PHLPP, is expressed in retinal rod inner segments (Kanan et al., 2010), however, the precise role of PHLPPL in photoreceptor functions is not known. To determine if the light-dependent enhanced binding of HK to mitochondria is mediated through PHLPPL, we examined the association of PHLPPL with mitochondria under dark- and light-adapted conditions. Mitochondrial proteins were prepared and immunoblotted with anti-HK (Fig. 7A), anti-VDAC (Fig. 7B), and anti-PHLPPL (Fig. 7C) antibodies. There was a significant increase in association of PHLPPL to mitochondria in light-adapted compared to dark-adapted retinas (Fig. 7C and E). Consistent with this result, we also found enhanced binding of HK to mitochondria (Fig. 7A and 7D). These results collectively suggest that enhanced binding of HK to mitochondria under light-adapted conditions may be PHLPPL-dependent.
Figure 7. Binding of HK-II and PHLPPL with mitochondria under light-adapted and light-stressed conditions.
Mitochondria were prepared from dark-, light-adapted (300 lux) and light-stressed (5000 lux for 3 h) rats and immunoblotted with anti-HK-II (A), anti-VDAC (B), and anti-PHLPPL (C) antibodies. Densitometric analysis of HK-II was normalized to VDAC (D) and PHLPPL was normalized to VDAC (E). The dark control was set as 100 percent. Values are mean + SD, n=3. **p<0.001, * p<0.05.
3.7. Light stress results in decreased association of HK-II and PHLPPL with mitochondria
To determine whether light stress effects the association of HK-II and PHLPPL with mitochondria, we have subjected albino rats to light stress for 3h at 5000 lux. At the end of light exposure, retinas were removed, and mitochondria prepared. Mitochondrial proteins were immunoblotted with anti-HK (Fig. 7A), anti-VDAC (Fig. 7B), and anti-PHLPPL (Fig. 7C) antibodies. There was a significant decrease in the association of HK-II to mitochondria in light-stress compared to light-adapted retinas (Fig. 7D). Consistent with this result, we also found a decrease in the binding of PHLPPL to mitochondria (Fig. 7E). These results suggest that stress response may cause dysfunction of mitochondria due to decreased binding of HK-II and PHLPPL.
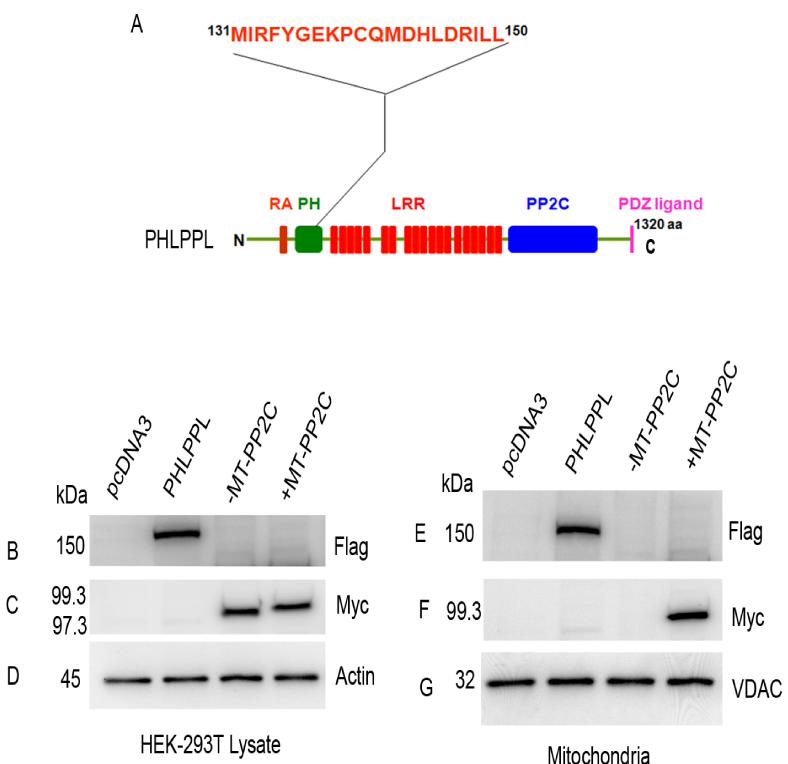
3.8. PHLPPL protein contains a functional mitochondrial targeting signal
The bio-informatic tools Predotar (Small et al., 2004), iPSORT and PSORTII (Bannai et al., 2002), MitoPred (Guda et al., 2004), and SignalP (Nielsen et al., 1997) for the prediction of PHLPPL targeting to mitochondria were used. These programs revealed a putative mitochondrial targeting signal present between amino acids 131 and 150 (MIRFYGEKPCQMDHLDRILL) located in the PH domain of PHLPPL (Fig. 8A). This putative mitochondrial targeting signal was absent in PHLPP. To determine if this targeting signal is functional in PHLPPL, we expressed Myc-tagged phosphatase domain (PP2C) of PHLPPL, with and without mitochondrial (MT) targeting signal, in HEK-293T cells. The MT targeting signal was added to the N-terminal end of the PP2C domain. The full length Flag-tagged PHLPPL and pcDNA3 vectors were also expressed as positive and negative controls. Expressed proteins were immunoblotted with anti-Flag, anti-Myc, and anti-actin antibodies, which indicated the presence of all three in HEK-293T cells (Fig. 8B and C). The results indicate that neither the presence nor absence of MT targeting signal affected the expression levels of the PP2C domain (Fig. 8C). However, the PP2C domain lacking the MT targeting signal failed to associate with the mitochondrial membranes (Fig. 8F), whereas the full length PHLPPL and PP2C domain containing the MT targeting signal did (Fig. 8E and F). These experiments suggest that PHLPPL protein may contains a functional mitochondrial targeting signal.
Figure 8. Identification of a functional mitochondrial targeting signal PHLPPL.
A putative mitochondrial targeting signal was present between amino acids 131 and 150 in the PH domain of PHLPPL (A). RA, Ras association domain; PH, pleckstrin homology domain; LRR, leucine-rich repeats; PP2C, phosphatase domain; PDZ, P=post-synaptic density protein; D= Drosophila disc large tumor suppressor, and Z= zonula occludens-1 protein [zo-1]) binding motif. HEK-293T cells were transiently transfected with either pCDNA3 or wild type FLAG-tagged PHLPPL or Myc-tagged PP2C-phosphatase domain with (99.3 kDa) or without (97.3 kDa) a mitochondrial targeting signal (MT). Lysates were immunoblotted with anti-Flag (B), anti-Myc (C), and anti-actin (D) antibodies. Mitochondrial fractions were immunoblotted with anti-Flag (E), anti-Myc (F), and anti-VDAC (G) antibodies.
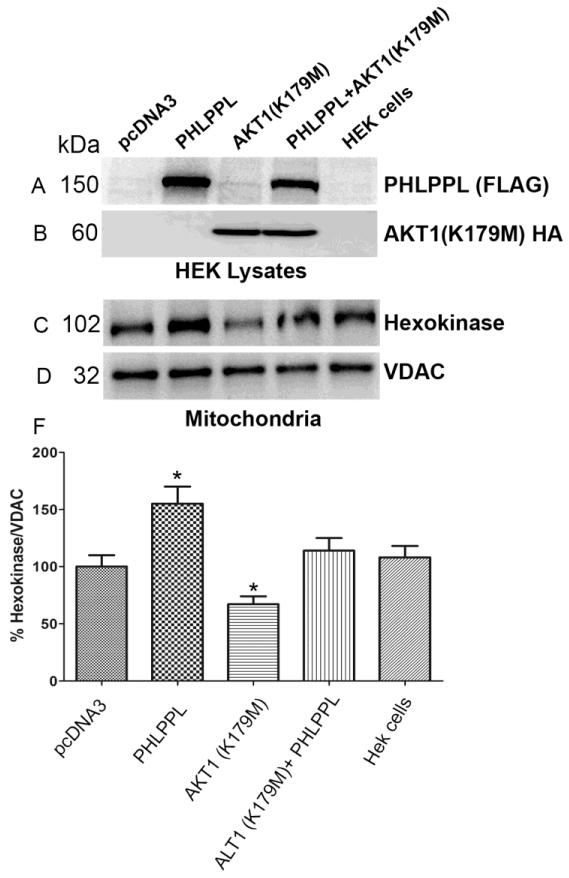
3.9. Role of Akt1 in PHLPP-induced enhanced binding of HK-II to mitochondria
We previously reported that light induced the activation of IR/PI3K pathway in retinal rod photoreceptor cells, which results in the activation of Akt1 (Li et al., 2008). Further, PHLPPL has been shown to selectively bind Akt1 and Akt3, but not Akt2 (Brognard et al., 2007). In this experiment, we examined whether Akt1 is required for the PHLPPL-induced enhanced binding of HK-II to mitochondria. HEK-293T cells were transiently transfected with either pcDNA3, FLAG-tagged PHLPPL, or dominant negative HA-tagged Akt1 (K179M), or co-expressed Flag-tagged PHLPPL with either pcDNA3 or dominant negative HA-tagged Akt1. Cell lysates were immunoblotted with anti-FLAG (PHLPPL) and anti-HA (dominant negative Akt1) antibodies to confirm protein expression (Fig. 9A and B). Mitochondrial fractions were prepared and proteins were immunoblotted with anti-HK-II and anti-VDAC antibodies. We found that PHLPPL enhanced the binding of HK-II to mitochondria compared to either vector control or HEK-293T cells (Fig. 9C and F). A significantly reduced binding of HK-II to mitochondria was observed when the cells were transfected with dominant negative Akt1, compared to either vector control or HEK-293 cells (Fig. 9C and F). Dominant negative Akt1 significantly reduced the PHLPPL-induced HK-II binding to mitochondria and the binding was similar to vector control or HEK-293T cells (Fig. 9C and F). These results suggest that PHLPPL induced binding of HK-II to mitochondria is Akt1-dependent.
Figure 9. Role of Akt1 in PHLPPL-induced enhanced binding of HK-II to mitochondria.
HEK-293T cells were transiently transfected with either pCDNA3, Flag-tagged PHLPPL, or HA-tagged dominant negative Akt1 (K179M). Lysates were immunoblotted with anti-Flag (A) and anti-HA (B) antibodies. Mitochondrial fractions were immunoblotted with anti-HK-II (C) and anti-VDAC (D) antibodies. Densitometric analysis of HK-II was normalized to VDAC (E). The vector control was set as 100%. Values are mean + SD, n=2. *p<0.001.
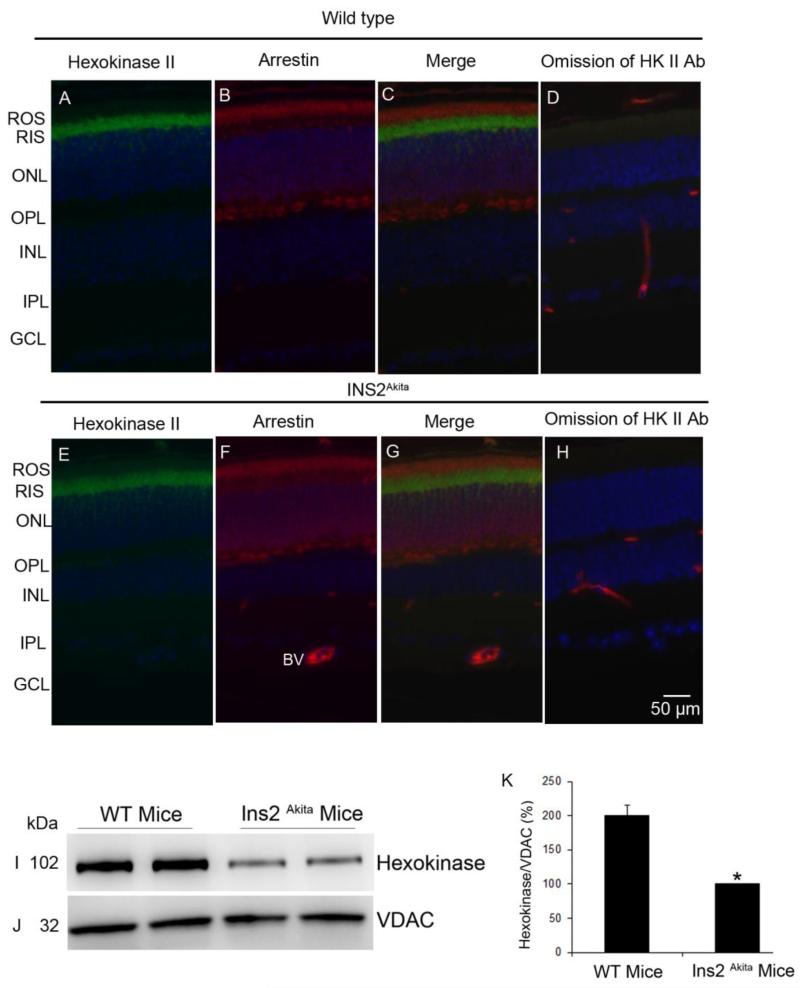
3.10. The association of HK-II with mitochondria is reduced in type-1 diabetic mice
Down regulation of IR/PI3K/Akt activities and activation of GSK-3β have been observed in diabetic retinopathy (Reiter et al., 2006). In this study we have examined the HK-II binding to mitochondria in Ins2Akita mice. The Ins2Akita mutation results in a single amino acid substitution in the insulin 2 gene that causes misfolding of the insulin protein (Haseyama et al., 2002). Male mice heterozygous for this mutation have progressive loss of beta-cell function, decreased pancreatic beta-cell density, and significant hyperglycemia, as early as 4 weeks of age (Haseyama et al., 2002). To test the hypothesis that HK-II binding to mitochondria is decreased in diabetes, we prepared mitochondria from wild type and type-1 diabetic Ins2Akita mouse retinas. Retinal proteins were immunoblotted with anti-HK-II and anti-VDAC antibodies. The results indicate a decreased binding HK-II to mitochondria in diabetic Ins2Akita mouse retina compared to wild type (Fig. 10 I-K). To determine if the decrease in HK-II binding to mitochondria is due to a decrease in HK-II protein expression in the Ins2Akita retina, we carried out immunohistochemistry of HK-II on wild type and Ins2Akita mouse retinas. Our results indicate that there was no difference in the expression of HK-II between wild type and Ins2Akita mouse retina (Fig. 10 A-H).
Figure 10. The expression and the association of HK-II with mitochondria in diabetic Ins2Akita mouse retina.
Prefer-fixed sections of wild type (C57BL/6J) and Ins2Akita mouse retinas were stained for HK-II (A, E), arrestin (B, F), and DAPI (A, B, E, F) and the immunofluorescence was analyzed by epifluorescence. Panels C and G represent the merge images of HK-II and arrestin. Panels D and H represent the omission of primary antibody (HK-II). ROS, rod outer segments; RIS, rod inner segments; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. BV, blood vessel. Mitochondria were prepared from wild type and diabetic mice and immuno blotted with anti-HK-II (I) and anti-VDAC (J) antibodies. Densitometric analysis of HK-II normalized to VDAC (K). Data are mean + SD, n=2. The WT control was set as 100 percent. *p<0.01.
4. DISCUSSION
We have previously reported that light induces the activation of the IR, PI3K and Akt in photoreceptor neurons (Rajala et al., 2002; Rajala et al., 2007; Li et al., 2008). The goal of this study was to understand the functional role of light-dependent activation of IR/PI3K/Akt pathway in photoreceptor neuron survival. Insulin receptor activation has been shown to rescue retinal neurons from apoptosis through a PI3K cascade (Barber et al., 2001; Barber et al., 1998) and we reported previously that ablation of the IR in rod photoreceptors resulted in stress-induced photoreceptor degeneration (Rajala et al., 2008). We also demonstrated that Akt2, another downstream target of the IR, is essential for photoreceptor survival as ablation of this protein in the retina resulted in stress-induced retinal degeneration (Li et al., 2007). Specific deletion of bcl-xl (downstream effector of Akt) from rod photoreceptor cells also resulted in stress-induced photoreceptor degeneration (Zheng et al., 2006). Studies from our laboratory suggest an important role of IR/PI3K/Akt in retinal neuroprotection, although the mechanism of this neuroprotection is not known. In this study, we found that light activation of the IR regulates the association of hexokinase with mitochondria, and dissociation of this interaction has previously been shown to induce cell death (Galluzzi et al., 2008; Chiara et al., 2008).
Studies from our laboratory show that physiological light (300 lux) activates the IR signaling pathway and we propose that it provides neuroprotection to retina (Rajala et al., 2002). Induction of apoptosis is in response to chronic or acute light stress (2700 lux in the case of albino and 14,000 lux in the case of pigmented animals) has been well established. Previously published studies suggest that oxidant stress is a major factor in light stress-induced in cell death (Tanito et al., 2007). Administration of a number of antioxidants has been shown to protect photoreceptors from light-induced degeneration (Ranchon et al., 2003). Products of non-enzymatic peroxidation of polyunsaturated fatty acids increased in the nuclei of light-stressed albino rat photoreceptor cells, prior to cell death (Tanito et al., 2006). Oxidative stress-induced retinal damage up-regulates mitochondrial DNA repair enzymes (DNA polymerase gamma and 8-oxoguanine-DNA-glycosylase) in photoreceptor synaptic mitochondria has previously been reported (Cortina et al., 2005). However, there are no studies available on the translocation of HK-II to mitochondria in response to light-stress dependent induction of apoptosis.
Mammalian retinal neurons have a remarkable ability to survive in a hostile environment, being subjected constantly to high levels of oxygen and bombardment by photons. We propose that IR signaling is an endogenous neuroprotective pathway that acts as molecular sunglasses against stress-induced degenerations. Our study also suggests a decreased association of HK-II with mitochondria in acute light stress (5000 lux) compared to light-adaptation (300 lux). We have previously reported that loss of IR expression in rod photoreceptors significantly impaired the retinal function and loss of photoreceptors in mice exposed to bright light stress. Rod cells undergo apoptosis, detected by the activation of caspase-3 (Rajala et al., 2008). It has previously been shown that mitochondrial cytochrome c-mediated caspase-3 activation is followed by apoptosis in diabetic mouse myocardium (Cai et al., 2002). These studies suggest a signaling communication between the IR and mitochondria in light-stress induced retinal degenerations.
In this study mitochondria were isolated from retina using Mitochondria Isolation Kit (Zhang et al., 2010). The isolation procedure also yields mitochondria from non-photoreceptor cells. The immunoblots with VDAC will include mitochondria from both photoreceptor and non-photoreceptor cells. However, the light effect is localized to photoreceptor neurons and also the HK-II is exclusively localized to photoreceptor inner segments. Furthermore, the association of HK-II with mitochondria is decreased in IR KO mice compared to wild type mice, suggesting that the observed association of HK with mitochondria is from photoreceptors even though the denominator (VDAC) represents mitochondria from total retina.
The direct connection between mitogenic signaling and mitochondrial function was discovered when it was found that Akt promotes binding to HK-II to the mitochondrial VDAC (Gottlob et al., 2001); disruption of this association allows cytochrome c release and subsequent apoptosis (Majewski et al., 2004). Interesting to note is that in retinal photoreceptor cells, Akt activation is through light-induced activation of the IR/PI3K pathway (Rajala et al., 2002; Rajala et al., 2007; Li et al., 2008). Therefore, we examined whether light-dependent IR/PI3K/Akt pathway promotes the binding of HK-II to mitochondria. Our studies suggest that light induced the binding of HK-II to mitochondria and this binding is IR-dependent, as conditional deletion of IR in rods resulted in the loss of light-dependent binding of HK-II to mitochondria. In the retina, HK-II binding to mitochondria is PI3K-dependent. Akt phosphorylates GSK-3β on serine-9 and this phosphorylation is well known to inhibit its activity (Cross et al., 1995). We observed a light-dependent Akt activation that correlated with increased serine-9 phosphorylation on GSK-3β compared to dark-adapted conditions. The GSK-3β has been shown to be highly active in mitochondria (Bijur and Jope, 2003a) and growth factor withdrawal induces neuronal apoptosis due to its activation (Hetman et al., 2000). The reduced binding of HK-II to mitochondria in dark-adapted conditions could be due to phosphorylation of VDAC by GSK-3β, which is consistent with idea that addition of GSK-3β inhibitor to dark adapted ex vivo explants significantly enhanced the binding of HK-II to mitochondria. These studies clearly suggest that light-dependent IR/PI3K/Akt signaling regulates mitochondrial integrity by inhibiting the activity of GSK-3β.
It is interesting to note in this study that we found a decreased association of HK-II with mitochondria in IR−/− mouse retinas, but not in p85α−/− mouse retinas. This discrepancy may be due to the complementation of p85β isoform for loss of p85α in rod photoreceptors (Ivanovic et al., 2011a). Consistent with this hypothesis, conditional deletion of the p85α in rod surprisingly gave no phenotype, but cones die (Ivanovic et al., 2011b). We subsequently determined that rods, but not cones, also express p85β (which complements p85α), which accounts for rod survival (Ivanovic et al., 2011a). On the other hand, the PI3K inhibitor LY294002 completely inhibited the activation of PI3K, which led to decreased binding of HK-II with mitochondria. LY294002 inhibits all PI3K catalytic isoforms, but not regulatory subunits (p85α and p85β). However, the PH-domain of Akt binds to class I PI3K-generated phosphoinositides (PI-3,4,-P2 and PI-3,4,5-P3, but not PI-3-P) for activation. Class III PI3K-generated PI-3-P does not support Akt activation. These studies suggest that class I PI3K isoform affects HK-mitochondria association.
Hexokinase catalyzes the first step of glycolysis, the phosphorylation of glucose to glucose-6-phosphate, and this action of HK sequesters glucose inside the cell. This reaction is the rate limiting step in glycolysis (Middleton, 1990). Of the four HKs (I, II, III and IV), HK I and II are found to directly associate with the mitochondria (Robey and Hay, 2006). The HK that we examined in our study is HK-II. Unlike the majority of neurons rich in HK I, but similar to other highly metabolically active cells, photoreceptors express HK II (Reidel et al., 2011). Hexokinase II has a very high catalytic activity when associated with mitochondria, and in the current study we found that it localized to the inner segments of photoreceptors where the mitochondrial machinery is available. The mitochondrial localized HKs have direct access to high concentrations of ATP, the major product of mitochondrial function. The ability of mitochondrial HKs to use mitochondrial ATP allows them to couple glycolysis with oxidative phosphorylation when cytosolic ATP levels are low (Robey and Hay, 2005; Stiles, 2009). The ability of Akt to maintain mitochondrial integrity is dependent on glucose availability through increased mtHK activity (Majewski et al., 2004). Consistent with these observations, it has been shown recently that administration of glucose and insulin delayed cone photoreceptor death in retinitis pigmentosa animal models (Punzo et al., 2009).
It is interesting to note that PHLPPL enhances the binding of HK-II to mitochondria. It has been shown recently that PHLPP negatively regulates mitochondrial Akt activity in cardiac cell survival (Miyamoto et al., 2010). Our studies suggest that this enhancement is not due to its effect on VDAC, as we failed to observe any HK-II enhanced binding when we transfected with the PP2C-phosphatase domain (data not shown). Our current study suggests that PHLPPL-induced enhanced binding of HK-II to mitochondria is Akt-dependent, as dominant negative Akt1 abolished the PHLPPL effect. Rapid accumulation of Akt in mitochondria following growth factor activation has been reported, even though Akt lacks a mitochondrial targeting signal (Bijur and Jope, 2003b). Mitoport database predicted that Akt has a 0.25% chance of being translocated to mitochondria (Mookherjee et al., 2007). PHLPPL has previously been shown to physically associate with Akt1 (Brognard et al., 2007). Our current study, along with the existing literature, suggests that Akt may use PHLPPL for its translocation to mitochondria. The other potential mechanism of HK-II translocation to mitochondria is the direct association of HK-II with mitochondria, as it has a mitochondrial binding motif in its N-terminus (Majewski et al., 2004). It has recently been shown that Akt phosphorylates HK-II at Thr473 and increases mitochondrial HK-II association to protect cardiomyocytes (Roberts et al., 2013).
Mice heterozygous for the Akita spontaneous mutation (Ins2Akita) are viable and fertile (Haseyama et al., 2002). Symptoms in the heterozygous mutant mice include hyperglycemia, hypoinsulinemia, polydipsia, and polyuria, beginning around 3-4 weeks of age (Haseyama et al., 2002). Ins2Akita is a naturally occurring mouse model for type-1 diabetes (Haseyama et al., 2002). Down regulation of PI3K/Akt activities and activation of GSK-3β have been observed in diabetic retinopathy (Reiter et al., 2006). Further, retinal IR activation has been shown to be down regulated in Ins2Akita mice (Barber et al., 2005). In this study we found a decreased binding of HK-II to mitochondria in Ins2Akita mouse retains, but the decrease is not due to a decrease in the expression of HK-II in these mice. Further studies are required to examine the effect of GSK-3β inhibitors on the progression of diabetic retinopathy.
Of all of the tissues in the body, the retina is the most susceptible to oxidant stress, owing to the high levels of polyunsaturated fatty acids in the outer segments (primarily docosahexaenoic acid DHA, 22:6n3), the high concentration of oxygen that passes through these membranes from the pigment epithelium to the mitochondria in the inner segments, and the production by light of free radicals in outer segments (Fliesler SJ and Anderson RE, 1983). In addition, the huge oxygen consumption by the mitochondria in light and dark generates abundant free radicals and reactive oxygen species (ROS). To survive these daily challenges, the retina has developed remarkable endogenous neuroprotective pathways. The Akt activation through IR we described in this study may neutralize the toxic effects of light during the daylight portion of the day. The light-dependent IR/PI3K/Akt pathway regulates retinal mitochondrial integrity through facilitating the binding of HK-II to mitochondria, thus preventing apoptosis. Our studies suggest that blocking the GSK-3β could be neuroprotective. Down-regulation of insulin receptor-PI3K signaling occurs in retinitis pigmentosa (Punzo et al., 2009; Rajala et al., 2013) diabetic retinopathy (Reiter et al., 2006), and Leber Congenital Amaurosis-type 2 (Rajala et al., 2007; Rajala et al., 2010). We suggest that enhancing the binding interaction between HK-II and mitochondria may have therapeutic value for these ocular diseases. Pharmacological agents that enhance the binding of HK-II to mitochondria could preserve mitochondrial function and thereby protect dying retinal cells in retinal degenerations.
Highlights.
Akt mediates anti-apoptotic activity through hexokinase-mitochondria interaction.
Insulin and light regulates the binding of hexokinase to mitochondria.
Decreased binding of hexokinase to mitochondria in diabetic mouse retinas.
A Therapeutic value for agents enhances the binding of hexokinase to mitochondria.
ACKNOWLEDGEMENTS
This study was supported by grants from the National Institutes of Health (EY016507, EY00871, RR17703, and NEI Core grant EY021725). This work was also supported by an unrestricted departmental grant from Research to Prevent Blindness, Inc.
Abbreviations
- IR
insulin receptor
- PI3K
phosphoinositide 3-kinase
- ROS
rod outer segments
- HK-II
hexokinase
- VDAC
voltage-dependent anion channel
- GSK-3β
glycogen synthase kinase-3β
- PHLPPL
PH domain and leucine-rich repeat protein phosphatase-like
- PHB1
prohibitin-1
- HSP60
heat shock protein 60
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Anflous-Pharayra K, Cai ZJ, Craigen WJ. VDAC1 serves as a mitochondrial binding site for hexokinase in oxidative muscles. Biochim. Biophys. Acta. 2007;1767:136–142. doi: 10.1016/j.bbabio.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Bannai H, Tamada Y, Maruyama O, Nakai K, Miyano S. Extensive feature detection of N-terminal protein sorting signals. Bioinformatics. 2002;18:298–305. doi: 10.1093/bioinformatics/18.2.298. [DOI] [PubMed] [Google Scholar]
- Barber AJ, Antonetti DA, Kern TS, Reiter CE, Soans RS, Krady JK, Levison SW, Gardner TW, Bronson SK. The Ins2Akita mouse as a model of early retinal complications in diabetes. Invest Ophthalmol Vis Sci. 2005;46:2210–2218. doi: 10.1167/iovs.04-1340. [DOI] [PubMed] [Google Scholar]
- Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J. Clin. Invest. 1998;102:783–791. doi: 10.1172/JCI2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber AJ, Nakamura M, Wolpert EB, Reiter CE, Seigel GM, Antonetti DA, Gardner TW. Insulin rescues retinal neurons from apoptosis by a phosphatidylinositol 3-kinase/Akt-mediated mechanism that reduces the activation of caspase-3. J. Biol. Chem. 2001;276:32814–32821. doi: 10.1074/jbc.M104738200. [DOI] [PubMed] [Google Scholar]
- Bijur GN, Jope RS. Glycogen synthase kinase-3 beta is highly activated in nuclei and mitochondria. Neuroreport. 2003a;14:2415–2419. doi: 10.1097/00001756-200312190-00025. [DOI] [PubMed] [Google Scholar]
- Bijur GN, Jope RS. Rapid accumulation of Akt in mitochondria following phosphatidylinositol 3-kinase activation. J. Neurochem. 2003b;87:1427–1435. doi: 10.1046/j.1471-4159.2003.02113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brognard J, Sierecki E, Gao T, Newton AC. PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Mol. Cell. 2007;25:917–931. doi: 10.1016/j.molcel.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Bryson JM, Coy PE, Gottlob K, Hay N, Robey RB. Increased hexokinase activity, of either ectopic or endogenous origin, protects renal epithelial cells against acute oxidant-induced cell death. J. Biol. Chem. 2002;277:11392–11400. doi: 10.1074/jbc.M110927200. [DOI] [PubMed] [Google Scholar]
- Cai L, Li W, Wang G, Guo L, Jiang Y, Kang YJ. Hyperglycemia-induced apoptosis in mouse myocardium: mitochondrial cytochrome C-mediated caspase-3 activation pathway. Diabetes. 2002;51:1938–1948. doi: 10.2337/diabetes.51.6.1938. [DOI] [PubMed] [Google Scholar]
- Chiara F, Castellaro D, Marin O, Petronilli V, Brusilow WS, Juhaszova M, Sollott SJ, Forte M, Bernardi P, Rasola A. Hexokinase II detachment from mitochondria triggers apoptosis through the permeability transition pore independent of voltage-dependent anion channels. PLoS. One. 2008;3:e1852. doi: 10.1371/journal.pone.0001852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortina MS, Gordon WC, Lukiw WJ, Bazan NG. Oxidative stress-induced retinal damage up-regulates DNA polymerase gamma and 8-oxoguanine-DNA-glycosylase in photoreceptor synaptic mitochondria. Exp. Eye Res. 2005;81:742–750. doi: 10.1016/j.exer.2005.04.017. [DOI] [PubMed] [Google Scholar]
- CRANE RK, SOLS A. The association of hexokinase with particulate fractions of brain and other tissue homogenates. J. Biol. Chem. 1953;203:273–292. [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- Feng J, Lucchinetti E, Ahuja P, Pasch T, Perriard JC, Zaugg M. Isoflurane postconditioning prevents opening of the mitochondrial permeability transition pore through inhibition of glycogen synthase kinase 3beta. Anesthesiology. 2005;103:987–995. doi: 10.1097/00000542-200511000-00013. [DOI] [PubMed] [Google Scholar]
- Fliesler SJ, Anderson RE. Chemistry and metabolism of lipids in the vertibrate retina. In: Holman RT, editor. Progress in Lipid Research. Pergamon Press; Elmsford, NY: 1983. pp. 79–131. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Kepp O, Tajeddine N, Kroemer G. Disruption of the hexokinase-VDAC complex for tumor therapy. Oncogene. 2008;27:4633–4635. doi: 10.1038/onc.2008.114. [DOI] [PubMed] [Google Scholar]
- Gao T, Furnari F, Newton AC. PHLPP: a phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Mol. Cell. 2005;18:13–24. doi: 10.1016/j.molcel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Gelb BD, Adams V, Jones SN, Griffin LD, MacGregor GR, McCabe ER. Targeting of hexokinase 1 to liver and hepatoma mitochondria. Proc. Natl. Acad. Sci. U. S. A. 1992;89:202–206. doi: 10.1073/pnas.89.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlob K, Majewski N, Kennedy S, Kandel E, Robey RB, Hay N. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev. 2001;15:1406–1418. doi: 10.1101/gad.889901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guda C, Fahy E, Subramaniam S. MITOPRED: a genome-scale method for prediction of nucleus-encoded mitochondrial proteins. Bioinformatics. 2004;20:1785–1794. doi: 10.1093/bioinformatics/bth171. [DOI] [PubMed] [Google Scholar]
- Haseyama T, Fujita T, Hirasawa F, Tsukada M, Wakui H, Komatsuda A, Ohtani H, Miura AB, Imai H, Koizumi A. Complications of IgA nephropathy in a non-insulin-dependent diabetes model, the Akita mouse. Tohoku J. Exp. Med. 2002;198:233–244. doi: 10.1620/tjem.198.233. [DOI] [PubMed] [Google Scholar]
- Hetman M, Cavanaugh JE, Kimelman D, Xia Z. Role of glycogen synthase kinase-3beta in neuronal apoptosis induced by trophic withdrawal. J. Neurosci. 2000;20:2567–2574. doi: 10.1523/JNEUROSCI.20-07-02567.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanovic I, Allen DT, Dighe R, Le YZ, Anderson RE, Rajala RV. Phosphoinositide 3-kinase signaling in retinal rod photoreceptors. Invest Ophthalmol Vis Sci. 2011a;52:6355–6362. doi: 10.1167/iovs.10-7138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanovic I, Anderson RE, Le YZ, Fliesler SJ, Sherry DM, Rajala RV. Deletion of the p85alpha regulatory subunit of phosphoinositide 3-kinase in cone photoreceptor cells results in cone photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2011b;52:3775–3783. doi: 10.1167/iovs.10-7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanan Y, Matsumoto H, Song H, Sokolov M, Anderson RE, Rajala RV. Serine/threonine kinase akt activation regulates the activity of retinal serine/threonine phosphatases, PHLPP and PHLPPL. J. Neurochem. 2010;113:477–488. doi: 10.1111/j.1471-4159.2010.06609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzen HM, Soderman DD, Wiley CE. Multiple forms of hexokinase. Activities associated with subcellular particulate and soluble fractions of normal and streptozotocin diabetic rat tissues. J. Biol. Chem. 1970;245:4081–4096. [PubMed] [Google Scholar]
- Li G, Anderson RE, Tomita H, Adler R, Liu X, Zack DJ, Rajala RV. Nonredundant role of Akt2 for neuroprotection of rod photoreceptor cells from light-induced cell death. J. Neurosci. 2007;27:203–211. doi: 10.1523/JNEUROSCI.0445-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Rajala A, Wiechmann AF, Anderson RE, Rajala RV. Activation and membrane binding of retinal protein kinase Balpha/Akt1 is regulated through light-dependent generation of phosphoinositides. J. Neurochem. 2008;107:1382–1397. doi: 10.1111/j.1471-4159.2008.05707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewski N, Nogueira V, Bhaskar P, Coy PE, Skeen JE, Gottlob K, Chandel NS, Thompson CB, Robey RB, Hay N. Hexokinase-mitochondria interaction mediated by Akt is required to inhibit apoptosis in the presence or absence of Bax and Bak. Mol. Cell. 2004;16:819–830. doi: 10.1016/j.molcel.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Miccoli L, Beurdeley-Thomas A, De PG, Sureau F, Oudard S, Dutrillaux B, Poupon MF. Light-induced photoactivation of hypericin affects the energy metabolism of human glioma cells by inhibiting hexokinase bound to mitochondria. Cancer Res. 1998;58:5777–5786. [PubMed] [Google Scholar]
- Miccoli L, Oudard S, Sureau F, Poirson F, Dutrillaux B, Poupon MF. Intracellular pH governs the subcellular distribution of hexokinase in a glioma cell line. Biochem. J. 1996;313(Pt 3):957–962. doi: 10.1042/bj3130957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton RJ. Hexokinases and glucokinases. Biochem. Soc. Trans. 1990;18:180–183. doi: 10.1042/bst0180180. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Purcell NH, Smith JM, Gao T, Whittaker R, Huang K, Castillo R, Glembotski CC, Sussman MA, Newton AC, Brown JH. PHLPP-1 negatively regulates Akt activity and survival in the heart. Circ. Res. 2010;107:476–484. doi: 10.1161/CIRCRESAHA.109.215020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mookherjee P, Quintanilla R, Roh MS, Zmijewska AA, Jope RS, Johnson GV. Mitochondrial-targeted active Akt protects SH-SY5Y neuroblastoma cells from staurosporine-induced apoptotic cell death. J. Cell Biochem. 2007;102:196–210. doi: 10.1002/jcb.21287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen H, Engelbrecht J, Brunak S, von HG. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- Pastorino JG, Hoek JB, Shulga N. Activation of glycogen synthase kinase 3beta disrupts the binding of hexokinase II to mitochondria by phosphorylating voltage-dependent anion channel and potentiates chemotherapy-induced cytotoxicity. Cancer Res. 2005;65:10545–10554. doi: 10.1158/0008-5472.CAN-05-1925. [DOI] [PubMed] [Google Scholar]
- Pastorino JG, Shulga N, Hoek JB. Mitochondrial binding of hexokinase II inhibits Bax-induced cytochrome c release and apoptosis. J. Biol. Chem. 2002;277:7610–7618. doi: 10.1074/jbc.M109950200. [DOI] [PubMed] [Google Scholar]
- Polakis PG, Wilson JE. An intact hydrophobic N-terminal sequence is critical for binding of rat brain hexokinase to mitochondria. Arch. Biochem. Biophys. 1985;236:328–337. doi: 10.1016/0003-9861(85)90633-2. [DOI] [PubMed] [Google Scholar]
- Punzo C, Kornacker K, Cepko CL. Stimulation of the insulin/mTOR pathway delays cone death in a mouse model of retinitis pigmentosa. Nat. Neurosci. 2009;12:44–52. doi: 10.1038/nn.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajala A, Anderson RE, Ma JX, Lem J, Al Ubaidi MR, Rajala RV. G-protein-coupled Receptor Rhodopsin Regulates the Phosphorylation of Retinal Insulin Receptor. J. Biol. Chem. 2007;282:9865–9873. doi: 10.1074/jbc.M608845200. [DOI] [PubMed] [Google Scholar]
- Rajala A, Dighe R, Agbaga MP, Anderson RE, Rajala RV. Insulin Receptor Signaling in Cones. J. Biol Chem. 2013;288:19503–19515. doi: 10.1074/jbc.M113.469064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajala A, Tanito M, Le YZ, Kahn CR, Rajala RV. Loss of neuroprotective survival signal in mice lacking insulin receptor gene in rod photoreceptor cells. J. Biol. Chem. 2008;283:19781–19792. doi: 10.1074/jbc.M802374200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajala RV. Phosphoinositide 3-kinase signaling in the vertebrate retina. J. Lipid Res. 2010;51:4–22. doi: 10.1194/jlr.R000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajala RV, McClellan ME, Ash JD, Anderson RE. In vivo regulation of phosphoinositide 3-kinase in retina through light-induced tyrosine phosphorylation of the insulin receptor beta-subunit. J. Biol. Chem. 2002;277:43319–43326. doi: 10.1074/jbc.M206355200. [DOI] [PubMed] [Google Scholar]
- Rajala RV, Tanito M, Neel BG, Rajala A. Enhanced retinal insulin receptor-activated neuroprotective survival signal in mice lacking the protein-tyrosine phosphatase-1B gene. J. Biol. Chem. 2010;285:8894–8904. doi: 10.1074/jbc.M109.070854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranchon I, LaVail MM, Kotake Y, Anderson RE. Free radical trap phenyl-N-tert-butylnitrone protects against light damage but does not rescue P23H and S334ter rhodopsin transgenic rats from inherited retinal degeneration. J. Neurosci. 2003;23:6050–6057. doi: 10.1523/JNEUROSCI.23-14-06050.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidel B, Thompson JW, Farsiu S, Moseley MA, Skiba NP, Arshavsky VY. Proteomic profiling of a layered tissue reveals unique glycolytic specializations of photoreceptor cells. Mol. Cell Proteomics. 2011;10:M110. doi: 10.1074/mcp.M110.002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter CE, Wu X, Sandirasegarane L, Nakamura M, Gilbert KA, Singh RS, Fort PE, Antonetti DA, Gardner TW. Diabetes reduces basal retinal insulin receptor signaling: reversal with systemic and local insulin. Diabetes. 2006;55:1148–1156. doi: 10.2337/diabetes.55.04.06.db05-0744. [DOI] [PubMed] [Google Scholar]
- Roberts DJ, Tan-Sah VP, Smith JM, Miyamoto S. Akt phosphorylates HK-II at Thr473 and increases mitochondrial HK-II association to protect cardiomyocytes. J. Biol Chem. 2013 doi: 10.1074/jbc.M113.482026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey RB, Hay N. Mitochondrial hexokinases: guardians of the mitochondria. Cell Cycle. 2005;4:654–658. doi: 10.4161/cc.4.5.1678. [DOI] [PubMed] [Google Scholar]
- Robey RB, Hay N. Mitochondrial hexokinases, novel mediators of the antiapoptotic effects of growth factors and Akt. Oncogene. 2006;25:4683–4696. doi: 10.1038/sj.onc.1209595. [DOI] [PubMed] [Google Scholar]
- Small I, Peeters N, Legeai F, Lurin C. Predotar: A tool for rapidly screening proteomes for N-terminal targeting sequences. Proteomics. 2004;4:1581–1590. doi: 10.1002/pmic.200300776. [DOI] [PubMed] [Google Scholar]
- Stiles BL. PI-3-K and AKT: Onto the mitochondria. Adv. Drug Deliv. Rev. 2009;61:1276–1282. doi: 10.1016/j.addr.2009.07.017. [DOI] [PubMed] [Google Scholar]
- Tanito M, Agbaga MP, Anderson RE. Upregulation of thioredoxin system via Nrf2-antioxidant responsive element pathway in adaptive-retinal neuroprotection in vivo and in vitro. Free Radic. Biol. Med. 2007;42:1838–1850. doi: 10.1016/j.freeradbiomed.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Tanito M, Haniu H, Elliott MH, Singh AK, Matsumoto H, Anderson RE. Identification of 4-hydroxynonenal-modified retinal proteins induced by photooxidative stress prior to retinal degeneration. Free Radic. Biol. Med. 2006;41:1847–1859. doi: 10.1016/j.freeradbiomed.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Wang J, Takeuchi T, Tanaka S, Kubo SK, Kayo T, Lu D, Takata K, Koizumi A, Izumi T. A mutation in the insulin 2 gene induces diabetes with severe pancreatic beta-cell dysfunction in the Mody mouse. J. Clin. Invest. 1999;103:27–37. doi: 10.1172/JCI4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteman EL, Cho H, Birnbaum MJ. Role of Akt/protein kinase B in metabolism. Trends Endocrinol. Metab. 2002;13:444–451. doi: 10.1016/s1043-2760(02)00662-8. [DOI] [PubMed] [Google Scholar]
- Wilson JE. Hexokinases. Rev. Physiol Biochem. Pharmacol. 1995;126:65–198. doi: 10.1007/BFb0049776. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Anderson RE, Agbaga MP, Rucker EB, III, Le YZ. Loss of BCL-XL in Rod Photoreceptors: Increased Susceptibility to Bright Light Stress. Invest Ophthalmol. Vis. Sci. 2006;47:5583–5589. doi: 10.1167/iovs.06-0163. [DOI] [PubMed] [Google Scholar]
- Zhou BP, Hu MC, Miller SA, Yu Z, Xia W, Lin SY, Hung MC. HER-2/neu blocks tumor necrosis factor-induced apoptosis via the Akt/NF-kappaB pathway. J. Biol. Chem. 2000;275:8027–8031. doi: 10.1074/jbc.275.11.8027. [DOI] [PubMed] [Google Scholar]