Abstract
Children diagnosed with Disruptive Behavior Disorders (DBD), especially those with psychopathic traits, are at risk of developing persistent and severe antisocial behavior. Deficient fear conditioning may be a key mechanism underlying persistence, and has been associated with altered regional brain function in adult antisocial populations. In this study, we investigated the associations between the neural correlates of fear conditioning, persistence of childhood-onset DBD during adolescence and psychopathic traits. From a cohort of children arrested before the age of 12 years, participants who were diagnosed with Oppositional Defiant Disorder or Conduct Disorder in previous waves (mean age of onset 6.5 years, s.d. 3.2) were reassessed at mean age 17.6 years (s.d. 1.4) and categorized as persistent (n=25) or desistent (n=25) DBD. Using the Youth Psychopathic Traits Inventory and functional magnetic resonance imaging during a fear conditioning task, these subgroups were compared with 26 matched healthy controls from the same cohort. Both persistent and desistent DBD subgroups were found to show higher activation in fear processing-related brain areas during fear conditioning compared with healthy controls. In addition, regression analyses revealed that impulsive-irresponsible and grandiose-manipulative psychopathic traits were associated with higher activation, whereas callous-unemotional psychopathic traits were related to lower activation in fear-related areas. Finally, the association between neural activation and DBD subgroup membership was mediated by impulsive-irresponsible psychopathic traits. These results provide evidence for heterogeneity in the neurobiological mechanisms underlying psychopathic traits and antisocial behavior and, as such, underscore the need to develop personalized interventions.
Keywords: antisocial, callous-unemotional, disruptive behavior disorders, fear conditioning, fMRI, psychopathy
Introduction
Juvenile antisocial behavior causes major personal harm and economic losses.1 In child and adolescent psychiatry, pervasive patterns of antisocial behavior are diagnosed as either Oppositional Defiant Disorder (ODD) or Conduct Disorder (CD), which are often grouped as Disruptive Behavior Disorders (DBD). Early onset of DBD is an important risk factor for persistence of antisocial behavior into adulthood.2 However, much remains unknown about which DBD children will persist or desist.
In this respect, one influential neurobiological theory posits that fear conditioning, the association of a neutral cue with an aversive outcome, is a prerequisite for typical moral development, 3 whereas deficits in this basic learning process may put one at risk for persistent antisocial development.4 Indeed, deficient autonomic fear conditioning has been reported in antisocial adults (for a review, see Raine5) and juveniles,6, 7 and has been linked to reduced reactivity in limbic brain circuits in adult psychopathic offenders.8 Furthermore, although adults with criminal records show lower levels of autonomic fear conditioning as early as 3 years of age,9 higher levels of autonomic fear conditioning have been shown to characterize antisocial adolescents desisting from crime 14 years later.10 Therefore, neural hyporesponsiveness during fear conditioning may be a biomarker for persistent antisocial behavior. However, studies investigating this specific hypothesis are currently lacking.
Moreover, although recent reviews have emphasized heterogeneity within antisocial samples,11, 12 most previous fear conditioning studies lack dimensional specification of findings. Consequently, it remains unclear whether fear conditioning deficits are a characteristic of all antisocial individuals or pertain to specific clinical characteristics within this group. In this respect, psychopathic personality traits (In recent years, the traits within the psychopathy construct have been increasingly used in the study of childhood and adolescent antisocial behavior (for a review, see Frick and White13). Although the authors acknowledge the concerns of some scholars with regard to their measurement14 and interpretation15 in minors, these traits will be referred to as psychopathic traits throughout this article for reasons of brevity.), specifically callous-unemotional (CU) traits, have been proposed as the most important specifiers within antisocial populations16 and have been included as such in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V). This is not only because of their clinical relevance for defining a severe and stable subgroup of antisocial youths17 but also for their associated genetic risk and neurobiological deficits, including aberrant reactivity in limbic and prefrontal brain regions (Marsh et al.18and Finger et al.,19 for a review see Viding and McCrory20). The only study to date investigating the relation between psychopathic traits and fear conditioning in antisocial juveniles7 found no association between fear conditioning and overall psychopathic traits scores in conduct-disordered girls. However, in this study the correlated but distinct dimensions of the psychopathy construct were not analyzed separately. This is of relevance because psychopathic traits dimensions (i.e. CU, grandiose-manipulative (GM) and impulsive-irresponsible (II) traits) show distinctive correlations with criterion variables related to emotional reactivity,21 whereas most studies focus on the CU traits dimension as a proxy for the general construct of psychopathy. Specifically, impulsive-conduct problems (similar to II traits) are positively correlated with measures of emotional reactivity, whereas CU traits are inversely correlated with such measures, when corrected for each other (suppressor effect; e.g. Frick et al.,22 Patrick23 and Sebastian et al.24). These findings indicate the importance of accounting not only for the multidimensional nature of psychopathic personality traits but also for their mutually suppressing effects.
To gain further insight into the role of fear conditioning in the persistence of childhood-onset DBD during adolescence, the current study investigates the neural correlates of fear conditioning in persistent and desistent DBD subgroups in a cohort of early-onset offenders.25 In addition, the study relates fear conditioning deficits to specific psychopathic traits. We hypothesize that youths showing persistent patterns of DBD exhibit reduced levels of limbic reactivity during fear conditioning. Furthermore, we hypothesize that desisters are characterized by higher levels of limbic reactivity to fear conditioning, when compared with persisters and healthy controls (HCs). In addition, we hypothesize that CU personality traits are related to limbic hyporesponsiveness, whereas the opposite is expected for II personality traits, with both these effects being stronger when correcting for suppressor effects of the other psychopathy dimensions.
Materials and methods
Participants
Participants were recruited from a cohort25, 26 of 364 adolescents who were first arrested by the police before the age of 12 years and had participated in three previous waves of this longitudinal study: mean age at study entrance 10.9 (s.d. 1.4) and mean age at wave three 13.1 (s.d. 1.5). Children meeting the criteria for either ODD or CD (DBD) on the parent version of the NIMH Diagnostic Interview Schedule for Children version IV (DISC-IV; Shaffer et al.27; see below) during at least one of the previous waves were selected for the current study (mean age of DBD onset 6.5 years, s.d. 3.2 (retrospective estimate); current mean age 17.6 years, s.d. 1.4). Of these 80 adolescents, 30 (37.5%) did not participate in the current study because of refusal (n=23), exclusion for the magnetic resonance imaging (MRI) session (n=1) or low-quality functional MRI data, including incomplete analysis masks compromising the regions of interest (n=6), leaving 50 participants for analysis. The study group (n=50) did not differ from the non-participating group (n=30) on data from previous waves, including gender, IQ (block design and vocabulary subtests from the Wechsler Intelligence Scale for Children28), age, socioeconomic status, ethnicity, aggression scores (Reactive and Proactive aggression Questionnaire (RPQ)29) or psychopathic traits scores (Youth Psychopathic Traits Inventory (YPI)30) (all P>0.10).
For this report, the 50 DBD participants were divided into two subgroups: (1) those meeting the criteria of DBD in any of the previous waves but without a current DBD diagnosis at wave four (desisters; DBD-d, n=25), and (2) those meeting the criteria of DBD in any of the previous waves and with a current DBD diagnosis at wave four (persisters, DBD-p, n=25). Of the persisters, 13 (52%) met the criteria for CD and 12 for ODD in the current wave.
In addition, 26 matched HCs were selected from the same cohort of children (as minor offenses occur in typically developing children as well, see Moffitt et al.31 for a review). Inclusion criteria for this group were: (1) no diagnosis of DBD on the Diagnostic Interview Schedule for Children (DISC-IV) during any of the previous assessments, (2) below median aggression scores on the RPQ in the current fourth wave, (3) below median current wave psychopathic traits scores on the YPI and (4) no history of any other psychiatric condition (DSM-IV axis 1 or axis 2).
Procedure
This study was approved by the IRB of the VU University Medical Center Amsterdam and was conducted in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki Principles). All participants (and their parents/custodians, if age of the participant was below 18 years) signed informed consent and were visited at home for a structured psychiatric interview (DISC-IV) and questionnaires, including the YPI, RPQ, Child-Behavior Checklist and Youth Self-Report32 (see below). On a second occasion, participants were scanned in a Philips 3 T Intera MRI scanner (Philips Medical Systems, Best, The Netherlands) at the Academic Medical Center Amsterdam (AMC). All participants were instructed to refrain from using alcohol, cannabis or psychostimulant medication for at least 24 h before the MRI scan.
Assessment
Both the parent and youth version of the National Institute of Mental Health DISC-IV27 (Dutch translation: Ferdinand and Ende33) were used to assess the criteria for DSM-IV ODD and CD. Test–retest reliability of the DISC-IV is in the moderate to good range.27 As previous versions of this instrument showed moderate to good diagnostic sensitivity only when information from both parent and youth was taken into account,34 and similar to previous studies,35 a diagnosis of ODD and/or CD was made only if children met the diagnostic criteria according to either the parent or youth report, taking into account time periods mentioned in DSM-IV for each disorder (i.e. ODD: four symptoms in the last half year; CD: three symptoms in the last year, including one symptom during the last half year). Conversely, we defined desistence as not meeting DSM-IV criteria during the past given time period for each disorder. For descriptive purposes, the criteria for Attention Deficit/Hyperactivity Disorder and Post-Traumatic Stress Disorder were also assessed.
The YPI30 is a 50-item self-report instrument, which was developed to study personality traits associated with adult psychopathy in juvenile community samples. To ensure that all participants would understand the questions, the Dutch child version of the YPI was used,36 which has been shown to exhibit good 6-month test–retest reliability (intraclass correlation coefficient range: 0.61–0.76). This version uses the same questions as the adolescent YPI in simplified wordings. Two age-specific items in the II scale of the child version were not appropriate for our age group and therefore we excluded these items, leaving a total of 48 items (range: 1–4). In the current study, internal consistency (Cronbach's α) of the total score and its constituting dimensions were good to excellent: CU α 0.88; GM α 0.93; II α 0.88; and YPI Total score α 0.95. Between-dimension Pearson's correlation coefficients (r) were 0.62 (CU-II), 0.67 (CU-GM) and 0.68 (GM-II).
Other clinical characteristics of the previously mentioned subgroups were assessed using self- and parent-report questionnaires. First, aggression was investigated using the Reactive–Proactive Aggression Questionnaire, a reliable and valid 23-item self-report instrument (RPQ29; Dutch translation: Domburgh and Popma37). In the current study, internal consistency was excellent (Cronbach's α 0.93). Two subtests (vocabulary and block design) of the Wechsler Intelligence Scale for Children—version III (WISC-III; Wechsler28) were used to estimate intelligence. Finally, total externalizing and internalizing problem scores were reported by both parent (Child Behavior Checklist32) and child (Youth Self-Report32).
Experimental task
During a classical differential delay fear conditioning task (adapted from Birbaumer and co-workers8), participants viewed pictures of two neutral male faces (as used in Birbaumer et al.8) projected on a blank screen. These faces served as conditioned stimuli (CS). The task consisted of three phases: a habituation phase (four trials of both CS, each lasting 3.5 s), an acquisition phase (eight trials of both CS, each lasting 10 s) and an extinction phase (four trials of both CS, each lasting 7 s). During the acquisition phase, only one of the two CS (the CS+) was paired with a 20 ms aversive electric unconditioned stimulus (US) at the end of the CS in 100% of the trials, whereas the other CS (the CS−) was never paired with an electric stimulus. CS were presented in pseudorandom order. The US was an electric stimulus applied to the right lower leg, and was calibrated to a level that was rated as ‘aversive, but not painful' by the participant (mean intensity: 36.2 mA (s.d. 12.4), no group differences: F2,73=1.4, P=0.26). Participants were told that they would view pictures of two male faces, and would occasionally feel a shock at the right lower leg.
To assess whether fear conditioning induction was successful, skin conductance levels (SCLs) were collected using MRI-compatible Ag/AgCl electrodes and BIOPAC recording hardware and software (AcqKnowledge 4.1). SCLs were extracted from the raw signal with the Versatile Stimulus Response Registration Program.38 To assess fear conditioning and to compare groups with respect to psychophysiological measures of conditioning, skin conductance responses (SCRs) were computed, based on z-standardized SCLs. SCRs were defined as the difference between baseline (i.e. the mean SCL of the 2 s before CS onset) and the maximum SCL during the CS. Furthermore, after each phase of the experiment, participants rated arousal and anxiety levels on a 9-point Likert scale and expectancy levels of the US on a 5-point Likert scale for each CS face separately.
Functional MRI protocol
First, T1-weighted anatomical scans, consisting of 180 sagittal 1 mm thickness slices, with an in-plane resolution of 1 × 1 mm2 (field of view 256 × 256 mm2, repetition time 9.0 ms, echo time 3.5 ms), were acquired using an 8-channel SENSE head coil. Furthermore, 400 T2*-weighted echo-planar images were acquired during fear conditioning, each volume consisting of 38 ascending slices of 3 mm thickness and 2.29 × 2.29 in-plane resolution, parallel to the anterior commissure–posterior commissure line (field of view 220 × 220 mm2, repetition time 2300, echo time 30 ms).
Statistical analysis
Differences in sociodemographics and clinical characteristics between groups were analyzed using analysis of variances and Tukey's post hoc tests for dimensional measures with equal variances (and Welch's robust test of equality of means and Games–Howell post hoc tests for variables with unequal variance across groups) and Fisher's exact tests for categorical variables.
Repeated-measures general linear models were used to assess differential SCRs to both CS across acquisition (eight trials). CS type and time were used as within-subject measures and diagnostic group (DBD-p vs DBD-d vs HC) as between-subject measure. Wilks' λ multivariate tests of significance were used to account for violations of the compound symmetry and sphericity assumptions. Similar repeated-measures general linear models were performed with psychopathic traits (CU, GM, II and total) as dimensional between-subject measures. Finally, we used paired t-tests to compare self-reported conditioning indices (fear, arousal and contingency awareness) to CS+ vs CS−, and analysis of variance to compare self-reported conditioning across groups.
Functional MRI data were processed using SPM8. Preprocessing included realignment, unwarping, slice-time correction to the middle slice, normalization to MNI space based on the segmented anatomical scan and 8 mm full-width at half-maximum smoothing. First-level models included separate regressors for CS+ and CS− during habituation, acquisition and extinction, US and rating blocks. Similar to previous studies showing fast within-trial habituation of salience processing neurocircuitry,39 inspection of our data revealed that activation returned to baseline 3.3 s after CS onset. To account for these within-trial habituation effects and to reduce collinearity between the convolved hemodynamic response function- and US-related movement artifacts, acquisition trials were subdivided into three 3.3 s duration subepochs, which were modeled as separate regressors, similar to previous studies with long trial durations aiming to assess amygdala reactivity.40 Furthermore, a first-order across-trial time-modulation regressor was incorporated for each of these three regressors to model across-trial habituation. Next, contrast images were computed for CS+ minus CS− (first 3.3 s of each trial) and entered into a one-way analysis of variance to assess between-group (DBD-p vs DBD-d vs HC) differences. In addition, we performed three separate regression analyses to evaluate the relations between dimensional measures of psychopathic traits (CU, GM and II scores) and CS+ minus CS− responses. Finally, we performed a multiple regression analysis incorporating all three psychopathic traits dimensions to evaluate the unique association for each dimensional measure of psychopathic traits while controlling for any suppressor effects from the remaining predictor dimensions.
To reduce the effects of movement in inhomogeneous magnetic fields during the scanning session, we included unwarping as implemented in SPM8 in the preprocessing procedure of the functional MRI data. In a post hoc analysis, we additionally added the six realignment parameters (translation and rotation in three dimensions) as separate regressors to the first-level models to control for any residual movement effects.
Analyses were conducted at a whole-brain level, as well as in a priori regions of interest. On the basis of a large body of literature that provides evidence for their role in fear conditioning, and consistent activation in neuroimaging studies of the acquisition of conditioned fear responses (for a review, see Sehlmeyer et al.41), we selected amygdala, anterior cingulate cortex (ACC) and insula as our regions of interest. Amygdala and insula were anatomically defined using the Automated Anatomic Labeling atlas.42 Similar to previous neuroimaging studies on fear conditioning,43 we assessed ACC effects using a 16 mm radius sphere centered on the peak coordinates (x=−2, y=14, z=40) from a recent meta-analysis of 15 classical fear conditioning studies,44 to account for the functional specialization of different ACC subregions and evidence for selective recruitment of a specific subregion during fear conditioning.44 Whole-brain analyses were corrected for multiple comparisons at family-wise error (FWE) P<0.05. To correct for multiple comparisons in our regions of interest, we used small volume correction at an adjusted threshold of FWE P<0.029 (=α/k^(1−r)=0.05/5^(1−0.66), where k is the number of outcome variables and r their mean correlation, that is, Bonferroni correction, taking into account the correlation between these outcome variables.45)
Finally, mediation analyses were performed using both classical Sobel tests with standardized coefficients for use with dichotomous outcomes, and bootstrapping procedures with 5000 resamples using the SPSS macro INDIRECT provided by Andrew Hayes (http://www.afhayes.com; Preacher and Hayes46) were performed, thresholded at one-sided α 0.05. Mean neuronal activation for each regions of interest was extracted using the MarsBaR toolbox for SPM (http://marsbar.sourceforge.net; Brett et al.47).
Results
Clinical characteristics
Groups were similar with respect to sociodemographic variables (see Table 1; all P⩾0.28). In addition, all groups showed a similarly low IQ (P=0.40). However, whereas DBD-d scored higher on aggression than HCs, DBD-p reported higher aggression scores compared with both DBD-d and HCs. Both DBD groups reported more internalizing and externalizing problems than HC, whereas according to parent reports the DBD-p group was currently more troubled than both other groups. Attention Deficit/Hyperactivity Disorder comorbidity was higher in both DBD groups than in HC. DBD-p did not differ from DBD-d on psychopathic personality traits, but both groups reported higher GM, II and total psychopathic traits scores compared with HC. DBD-p also showed higher CU traits than HC.
Table 1. Characteristics of DBD subgroups and controls.
| HC (n=26) | DBD-d (n=25) | DBD-p (n=25) | Group difference | |
|---|---|---|---|---|
| Male gender, no. (%) | 23 (89%) | 20 (80%) | 18 (72%) | Fisher's exact1 P>0.31 |
| Low SES neighborhood, no. (%) | 18 (69%) | 15 (60%) | 13 (54%) | Fisher's exact1 P>0.55 |
| Non-Western ethnicity, no. (%) | 11 (42%) | 4 (16%) | 9 (36%) | Fisher's exact4 P>0.28 |
| Age, mean (s.d.) (years) | 17.8 (1.2) | 17.6 (1.7) | 17.3 (1.4) | F2,69=0.7, P>0.52 |
| DBD age of onset, mean (s.d.) (years) | — | 6.6 (3.5) | 6.5 (3.0) | T47=0.15, P>0.87 |
| IQ, mean (s.d.) | 91.9 (12.3) | 91.2 (15.6) | 86.7 (13.2) | F2,66=0.9, P>0.39 |
| RPQ aggression, mean (s.d.) | 4.5 (2.6) | 12.2 (7.0) | 18.0 (8.8) | Welch2;35.2=34.8, P<0.001a,b,c |
| CBCL internalizing, mean (s.d.) | 47.9 (10.6) | 53.4 (12.0) | 60.9 (6.3) | Welch2;42.7=14.3, P<0.001b,c |
| YSR internalizing, mean (s.d.) | 41.8 (8.1) | 48.4 (10.0) | 53.2 (10.5) | F2,72=9.0, P<0.001a,b |
| CBCL externalizing, mean (s.d.) | 44.2 (6.5) | 57.0 (8.9) | 66.6 (6.1) | Welch2;43.8=74.8, P<0.001a,b,c |
| YSR externalizing, mean (s.d.) | 43.7 (5.6) | 55.3 (8.5) | 60.1 (11.4) | Welch2;43.2=29.7, P<0.001a,b |
| ADHD (%) | 5 (19%) | 12 (48%) | 16 (64%) | Fisher's exact1 P=0.004a,b |
| PTSD (%) | 0 (0%) | 0 (0%) | 2 (9%) | Fisher's exact1 P=0.09 |
| YPI callous-unemotional, mean (s.d.) | 20.2 (3.9) | 24.2 (7.5) | 26.8 (9.0) | Welch2;39.9=7.09, P=0.002b |
| YPI grandiose-manipulative, mean (s.d.) | 23.1 (3.4) | 32.8 (10.1) | 33.5 (9.4) | Welch2;35.7=16.7, P<0.001a,b |
| YPI impulsive-irresponsible, mean (s.d.) | 24.1 (5.3) | 33.5 (9.4) | 36.4 (8.4) | Welch2;43.2=24.5, P<0.001a,b |
| YPI total psychopathic traits, mean (s.d.) | 67.5 (8.5) | 90.5 (23.3) | 95.9 (24.6) | Welch2;36.6=22.8, P<0.001a,b |
| Mean translation, mean (s.d.), mm | 0.08 (0.02) | 0.11 (0.06) | 0.13 (0.07) | Welch2;38.7=7.4, P=0.002b |
| Mean rotation, mean (s.d.), (deg.) | 0.08 (0.02) | 0.11 (0.06) | 0.13 (0.09) | Welch2;38.0=7.4, P=0.002b |
Abbreviations: ADHD, Attention Deficit/Hyperactivity Disorder; CBCL, Child Behavior Checklist; DBD, Disruptive Behavior Disorders; DBD-d, desistent DBD subgroup; DBD-p, persistent DBD subgroup; HC, healthy control; IQ, intelligence quotient; PTSD, post-traumatic stress disorder; RPQ, Reactive Proactive aggression Questionnaire, SES, socioeconomic status; YPI, Youth Psychopathic Traits Inventory; YSR, Youth Self-Report.
Significant difference between HC and desisters.
HC vs persisters.
Desisters vs persisters.
Conditioning indices
With respect to SCRs, significant fear conditioning effects were found for all three groups together (CS-type effects: F=21.3, d.f.=1;75, P<0.001, partial η2=0.22; time effects: F=4.6, d.f.=7;69, P<0.001, partial h2=0.32; and CS-type × time effect: F=4.2, d.f.=7;69, P=0.001, partial η2=0.30). Post hoc analyses showed that fear conditioning induction was successful, with higher SCRs related to CS+ than to CS− during both the first (t=2.62, d.f.=75, P=0.011, d=0.30) and second half (t=5.1, d.f.=75, P<0.001, d=0.58) of the acquisition phase. Marginally significant CS-type × time × group interaction tests indicated group differences in CS differentiation time courses across conditioning (Wilks' λ F=1.7, d.f.=14;134, P=0.064, partial η2=0.15). Although the direction of the effects implied that both DBD-d and DBD-p showed stronger differential conditioning than HCs on several trials, post hoc between-group differences were not significant. In fact, each group showed successful skin conductance conditioning, as evidenced by significant CS and CS × time effects across acquisition trials (all P⩽0.027).
When the three psychopathic dimensions were entered into separate repeated-measures general linear models, CU traits showed a trend toward significant CS × time × CU effects (Wilks' λ F=2.0, d.f.=7;66, P=0.07, partial η2=0.17), whereas GM and II traits did not interact with CS, time or CS × time effects. There were no significant post hoc tests, but the direction of parameter estimates implied that higher CU trait scores were related to higher differential conditioning on several trials. When all three dimensions were simultaneously entered into a single repeated-measures general linear models, none of the psychopathic traits showed a significant interaction with CS, time or CS × time.
There were no differences between groups on self-reported fear, arousal or contingency awareness. Subjects' self-reports indicated a trend toward significant differential conditioning with respect to arousal (CS+ vs CS−: t=1.8, d.f.=73, P=0.078, Cohen's d=0.21), but not contingency (t=1.0, d.f.=73, P=0.32, Cohen's d=0.12) or fear (t=1.0, d.f.=73, P=0.32, d=0.12), although all observed differences were in the expected direction (higher US expectation and higher fear for CS+ than for CS−). Self-reported contingency awareness was not related to psychopathic trait scores.
During the fear conditioning experiment, DBD-p showed higher mean scan-to-scan translation and rotation compared with HCs.
Functional MRI
Whole-brain analysis for the contrast ‘CS+ more than CS−' during acquisition showed significant (PFWE−SVC <0.029) bilateral fear conditioning effects in the ACC and the insula. At a lower statistical threshold (P⩽0.001 uncorrected), fear conditioning effects were found throughout the fear learning neurocircuitry, including the amygdala, brainstem, caudate nucleus, hippocampus, thalamus and dorsolateral prefrontal cortex.
Between-group analyses revealed significantly higher activation during the acquisition of conditioned responses in a priori defined regions of interest in the DBD-d and the DBD-p subgroups compared with HCs (see Table 2). Although statistically significant results were only found in the left ACC for DBD-d vs HC, and in the right insula, left amygdala and left ACC for DBD-p vs HC, inspection of these results at a lower statistical threshold revealed that both DBD groups showed higher activation throughout the regions previously mentioned as showing task main effects. DBD-p and DBD-d did not differ with respect to differential neural responses during fear conditioning. Groups did not differ with respect to neural activation in response to the US.
Table 2. Significant between-group differences in activity for conditioned responses to CS+ vs CS− (n=76).
| Group comparison | Brain region | PFWE−SVC | Z-score | x | y | z |
|---|---|---|---|---|---|---|
| HC<DBD-d | Left insula | 0.046 | 3.71 | −38 | −20 | 24 |
| Right insula | 0.043 | 3.72 | 42 | −4 | 6 | |
| 0.048 | 3.68 | 36 | −18 | 14 | ||
| Left ACC | 0.010 | 4.11a | −14 | 10 | 40 | |
| 0.029 | 3.80 | −12 | 16 | 40 | ||
| HC<DBD-p | Right insula | 0.013 | 4.04a | 36 | −10 | 4 |
| 0.037 | 3.74 | 40 | −6 | 2 | ||
| 0.038 | 3.73 | 34 | −16 | 10 | ||
| Left amygdala | 0.028 | 3.23a | −30 | −6 | −12 | |
| 0.043 | 3.08 | −24 | −6 | −10 | ||
| Left ACC | 0.007 | 4.21a | −16 | 18 | 38 | |
| 0.030 | 3.76 | −16 | 10 | 38 | ||
| 0.035 | 3.72 | −14 | 6 | 38 |
Abbreviations: ACC, anterior cingulate cortex; CS+, conditioned stimulus followed by unconditioned stimulus; CS−, conditioned stimulus never followed by unconditioned stimulus; DBD, Disruptive Behavior Disorders; DBD-d, desisters; DBD-P, persisters; HC, healthy controls; PFWE−SVC, family-wise error small volume correction for multiple comparison.
Statistically significant at adjusted α PFWE−SVC <0.029.
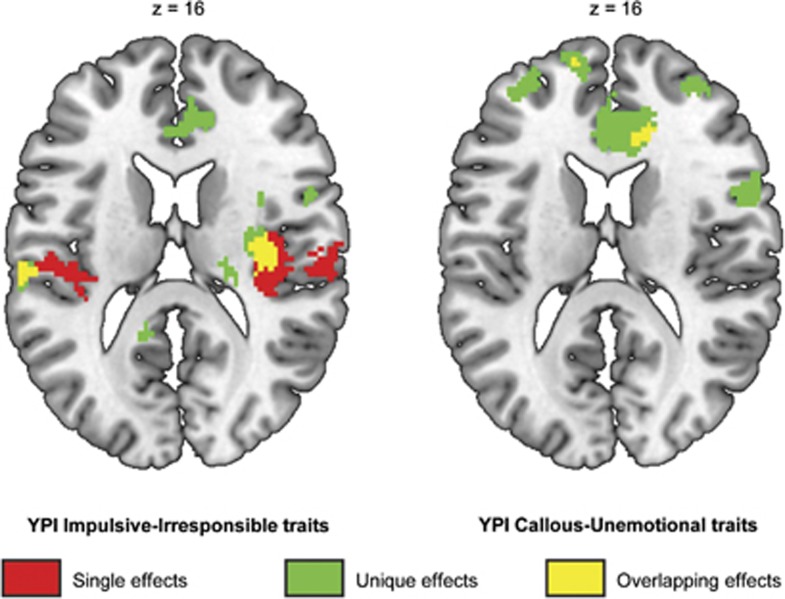
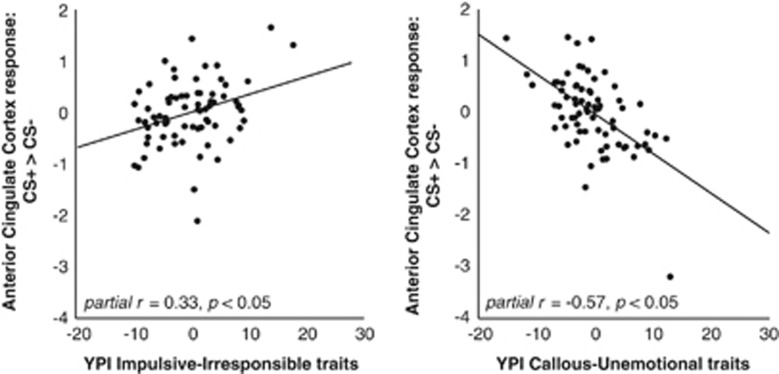
Regression analyses showed that higher levels of II traits were associated with higher activation in the right insula as well as in the left and right ACC. When all dimensions were entered into a multiple regression model, both GM and II traits were associated with higher activation. In contrast, CU traits were associated with lower levels of activation. This effect was significant at P<0.05 (whole-brain FWE-corrected) in the ACC, and was present at a lower statistical threshold throughout the fear conditioning network (see Table 3 and Figures 1 and 2). Post hoc exclusion of an outlier with respect to differential activation in the ACC (visible in Figure 2) slightly reduced the effect size of the latter CU traits result (from peak voxel partial r=0.57 to partial r=0.50, uncorrected P<0.001, PFWE=0.095) and increased the effect of the II trait result (from peak voxel partial r=0.33 to partial r=0.38, uncorrected P<0.001, PFWE=0.005). All between-group effects remained significant after exclusion of this outlier. Psychopathic traits' dimensions were unrelated to neural activation to the US.
Table 3. Psychopathic trait dimensions' relation with regional BOLD response for CS+ vs CS− (n=74)a.
| Psychopathic traits | Brain region | PFWE−SVC | Z-score | x | y | z |
|---|---|---|---|---|---|---|
| II (positive association) | Right insula | 0.003 (whole-brain) | 5.32b | 36 | −18 | 14 |
| 0.003 (whole-brain) | 5.27b | 34 | −14 | 10 | ||
| 0.016 (whole-brain) | 4.93b | 50 | −2 | 8 | ||
| 0.002 | 4.51c | 34 | −8 | 12 | ||
| 0.003 | 4.44c | 38 | −8 | 4 | ||
| Left ACC | 0.025 | 3.79c | −8 | 0 | 42 | |
| 0.03 | 3.73 | −6 | 0 | 46 | ||
| Right ACC | 0.01 | 4.05c | 8 | 6 | 36 | |
| Statistically corrected for the effects of both other dimensions | ||||||
| CU (negative association) | Right ACC | 0.005 (whole brain) | 5.19b | 12 | 32 | 18 |
| GM (positive association) | Right amygdala | 0.015 | 3.48c | 20 | 0 | −10 |
| II (positive association) | Left insula | 0.019 | 3.93c | −36 | 4 | −12 |
| 0.023 | 3.88c | −34 | 8 | −12 | ||
| Right insula | 0.005 (whole brain) | 5.18b | 32 | −12 | 12 | |
| 0.001 | 4.69c | 32 | −16 | 14 | ||
| 0.004 | 4.36c | 34 | −8 | 12 | ||
| Left amygdala | 0.038 | 3.11 | −30 | 4 | −16 | |
| Right ACC | 0.021 | 3.84c | 6 | 6 | 36 | |
Abbreviations: ACC, anterior cingulate cortex; BOLD, blood oxygen level dependent; CS+, conditioned stimulus followed by unconditioned stimulus; CS−, conditioned stimulus never followed by unconditioned stimulus; CU, callous-unemotional; FEW, family-wise error; GM, grandiose-manipulative; II, impulsive-irresponsible; PFWE−SVC=family-wise error small volume correction for multiple comparison.
Youth Psychopathic Trait Inventories were missing for two participants.
Statistically significant at PFWE−whole brain corrected <0.05.
Statistically significant at adjusted α PFWE−SVC <0.029.
Figure 1.
Association between differential neural fear conditioning responses (CS+>CS− contrast) and Youth Psychopathic traits Inventory (YPI) impulsive-irresponsible traits (left; positive association), and callous-unemotional traits (right; negative association). Single dimension regression analysis effects (red), unique effects (green; i.e. corrected for the other psychopathy dimensions in a multiple regression analysis) and their overlap (yellow) are displayed at P<0.001, k⩾10 uncorrected for display purposes, and are overlaid on an anatomical template. CS, conditioned stimuli.
Figure 2.
Partial regression plots displaying the association between neural differential fear conditioning responses (CS+>CS− contrast) in the anterior cingulate cortex peak voxel (MNI coordinates 12, 32, 18) and Youth Psychopathic Traits Inventory (YPI) impulsive-irresponsible (left), and callous-unemotional traits (right). CS, conditioned stimuli.
As DBD-p and DBD-d as well as II traits were characterized by higher rather than lower fear conditioning in the right insula and anterior cingulate cortex, we performed post hoc analyses, assessing whether II traits mediated the relation between neural responses during fear conditioning in these regions and DBD-d and DBD-p group membership (tested dichtomously vs HC). We found that the paths from right insula responsiveness to both DBD-d and DBD-p were significantly—and nearly fully (88% for DBD-d, P=0.016, 90% confidence interval (CI): 0.57–2.5; and 82% for DBD-p, P=0.008, 90% CI: 0.58–4.1)—mediated by II traits. Similarly, ACC effects on DBD-p (95%, P=0.032, 90% CI: 0.5–3.0) were significantly mediated by II traits, whereas for DBD-d the evidence for mediation was mixed (65%, P=0.036, 90% CI: −0.05 to 1.4).
Post hoc analyses
Several post hoc analyses were performed. It was found that neither restricting second-level analyses to the group with CD (compared with HCs), excluding all girls, adding self-reported anxiety to the model nor adding realignment parameters to the first-level models to control for the effects of head movement significantly altered their results.
Discussion
This study investigated fear conditioning and its neural correlates in youngsters with persistent vs desistent patterns of childhood-onset DBD during adolescence, as compared with HCs. To this end, we recruited a large sample of early-onset offenders and focused on phenotypical characterization of differential fear conditioning. Unexpectedly, functional MRI data showed higher instead of lower levels of activation during acquisition of conditioned fear responses in both persisters and desisters as compared with HCs. Enhanced fear conditioning was observed throughout the fear processing neurocircuitry but most prominently in the insula and ACC. Furthermore, we found that II psychopathic personality trait scores were most strongly associated with higher limbic activation during fear conditioning. Correcting for the effects of the other psychopathic traits dimensions in a multiple regression analysis showed a suppressor effect, as limbic activation correlated positively with II and GM traits, but negatively with CU traits. Finally, there were indications that the association between neural responsiveness and DBD subgroup membership was mediated by II traits.
This is the first neuroimaging study investigating fear conditioning in a sample of very young first offenders followed up during adolescence. The observed increased neural responsiveness during fear conditioning in desisters relative to HC is consistent with a previous study showing higher autonomic fear conditioning in antisocial adolescents desisting from crime during adulthood compared with HCs. 10 Our finding of similar hyper-reactivity of the fear circuitry in DBD-p, on the other hand, is in contrast with most other studies reporting reduced fear conditioning in antisocial subjects. However, it should be noted that while there is strong empirical evidence for reduced fear conditioning in adult psychopaths, results in antisocial youths are less consistent. For instance, Raine and co-workers10 (mentioned earlier) did not find significantly reduced fear conditioning in persisters vs controls, although the observed effect was in the predicted direction. Similarly, in another study, Raine and co-workers48 found evidence for reduced fear conditioning only in high socioeconomic class ‘undersocialized' juveniles, whereas the effect for those from lower socioeconomic environments was in the opposite direction.
As fear conditioning deficits in the current study varied as a function of dimensional measures of psychopathic traits, and were mediated by such traits, we propose that inconsistent results of previous studies comparing antisocial groups with HCs may be explained at least in part by heterogeneity in study samples with respect to these clinical characteristics and their underlying neurobiological mechanisms.11, 12 Recent reviews have emphasized the relevance of subtyping ‘cool' fearless and ‘hot' impulsive subsamples of antisocial youths,11, 12 and have hypothesized that even in those showing persistent patterns of antisocial behavior, multiple etiologically diverse subgroups may exist.49 In this study, we aimed to investigate a study group highly representative of the general antisocial population by recruiting all subjects from a high-risk young offender sample, including those with lower IQ scores50 and not restricting our analyses to those with a diagnosis of conduct disorder (for a review, see Vermeiren51). Our results suggest that, compared to previous studies that excluded low IQ participants, or selected specific subpopulations (e.g. Fairchild et al.6,7 and Birbaumer et al.8), adolescents in this clinically relevant and unselected offender cohort showed higher rates of ‘hot' than of ‘cool' features. Consistent with this notion, conduct problems in our sample were accompanied by higher rates of internalizing problems. As such, the current study does not falsify the hypothesis that reduced fear conditioning may put some children at risk of persistent antisocial development, but rather extends the fear conditioning literature to the overall adolescent antisocial population, confirming theories regarding a subgroup of persistent antisocial youths that is characterized by below average IQ, impulsivity and emotional hyper-responsiveness on a clinical and neurobiological level.49
As mentioned earlier, the desister group in the current study was also characterized by higher fear conditioning. Although it may be argued that this represents a protective mechanism (as in Raine et al.10), this explanation is at odds with our finding that fear conditioning effects on desister group membership are mediated by II traits. Although desisters did not meet the criteria for DBD any more, they still showed higher levels of II traits, as well as internalizing and externalizing symptoms, compared with controls. As such, higher neural responses during fear conditioning in this group may actually reflect emotional—and as a consequence, behavioral—dysregulation, similar to the persister group. It could be hypothesized that the buffering effects of other protective factors or absence of additional risk factors protect the desister group from a full DBD diagnosis.
Consistent with theories about ‘cool' and ‘hot' antisocial subtypes, the current findings suggest that differential neural responses during fear conditioning are positively associated with II psychopathic traits (‘hot'), but negatively associated with CU traits (‘cool'). However, our results also show that suppressor effects between these correlated domains of the psychopathy construct may obscure their distinct neurobiological correlates. This finding is consistent with a recent study by Sebastian and co-workers24 investigating the independent roles of conduct problems and CU traits in empathic neural responses in adolescents, as well as with the body of literature on suppressor effects between dimensions of the psychopathy construct with respect to criterion variables in behavior and psychophysiology23, 52 in adults. Clinically, these findings stress the need to consider psychopathic traits in adolescents as a multidimensional concept with differing underlying mechanisms.
Limitations and directions for future research
There are several limitations to our study. First, although our study investigated prospective longitudinal patterns of DBD, neural correlates were studied cross-sectionally in late adolescence. Prospective studies with functional MRI assessments in young offenders are needed to address predictive value and causality. Second, although HCs for the current study were intentionally recruited from the same early-onset offender cohort as DBD participants, to ensure absence of behavior problems by means of longitudinal data, it would be interesting to additionally recruit HCs who have never offended. Third, our non-selective antisocial population was highly heterogeneous. As such, one may hypothesize that the direction of our results was either due to the high proportion of ODD or due to comorbid anxiety symptoms. Importantly, post hoc analyses showed that CD diagnoses were also associated with increased rather than reduced differential activation during fear conditioning (i.e. this finding was not solely attributable to ODD). Similarly, adding self-reported anxiety to the model did not significantly influence our results. Taken together, these results suggest that our persister group is marked by general emotional hyper-responsiveness. However, our results cannot be generalized to all antisocial populations, but rather warrant replication in other samples. Finally, we used neutral faces as CS. Previous studies showed increased limbic responses to neutral faces in conduct-disordered youths.53 Therefore, one may speculate that higher differential activation in the DBD groups compared with controls in the current study reflect potentiation of conditioning to the CS+ by higher ecological validity of CS54 in our experimental design. However, as Birbaumer and co-workers8 found reduced conditioning in adult psychopaths with the same experimental stimuli, our results are likely to be explained by sample differences. Indeed, contrasting the CS− with the low-level baseline for exploratory purposes provided no evidence for heightened reactivity to the neutral face in DBD subgroups.
As our data suggest that persistence of DBD, as compared with desistent DBD, is not explained by abnormal fear conditioning, we suggest that future studies should investigate alternative explanations for persistent dysfunction in other neural circuits (such as reward circuitry), environmental factors (e.g. parenting, peer delinquency) or biosocial interactions (e.g. fearfulness and parenting; Kochanska et al.55) Furthermore, it should be noted that categorical DBD diagnoses are rather crude measures of persistence, and it would be interesting to assess alternative outcome measures, such as reoffending, as well.
Notwithstanding these caveats, the divergent correlations of several relevant dimensions within this population with such a basic physiological mechanism as fear conditioning do suggest possibilities for the development of future interventions, as well as subtyping antisocial youths for personalized treatment. To this end, future studies should address the plasticity of fear conditioning to intervention as well as its relation with treatment success.
Conclusion
This is the first neuroimaging study to investigate fear conditioning in a representative sample of persistent and desistent childhood-onset DBD adolescents from an early-onset offender cohort. Our results indicate that both persistent and desistent subgroups showed enhanced rather than reduced neural responses during fear conditioning. Although differential neural reactivity in the limbic circuitry was positively associated with II and GM psychopathic traits, the CU traits were negatively associated with neural reactivity. These results stress the need to consider antisocial youths as a heterogeneous population and to utilize psychopathic traits as a concept with multiple dimensions, each with distinct neurobiological profiles.
Acknowledgments
We thank PFC Groot for technical assistance. This study was funded by a Netherlands Organization for Scientific Research (NWO) Mosaic Grant (017.007.022) and by a NWO Brain and Cognition Grant (056-23-010). Funders were not involved in any phase of the study.
The authors declare no conflict of interest.
References
- Cohen MA, Piquero AR. New evidence on the monetary value of saving a high risk youth. J Quant Criminol. 2009;25:25–49. [Google Scholar]
- Moffitt TE, Caspi A, Harrington H, Milne BJ. Males on the life-course-persistent and adolescence-limited antisocial pathways: follow-up at age 26 years. Dev Psychopathol. 2002;14:179–207. doi: 10.1017/s0954579402001104. [DOI] [PubMed] [Google Scholar]
- Mowrer OH. Learning Theory and Behavior. Wiley: New York, NY, USA; 1960. [Google Scholar]
- Eysenck HJ. Crime and Personality. St Albans, Paladin: Hertfordshire, UK; 1977. [Google Scholar]
- Raine A. The Psychopathology of Crime: Criminal Behaviour as a Clinical Disorder. Academic Press: San Diego, CA, USA; 1993. [Google Scholar]
- Fairchild G, van Goozen SH, Stollery SJ, Goodyer IM. Fear conditioning and affective modulation of the startle reflex in male adolescents with early-onset or adolescence-onset conduct disorder and healthy control subjects. Biol Psychiatry. 2008;63:279–285. doi: 10.1016/j.biopsych.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Fairchild G, Stobbe Y, van Goozen SH, Calder AJ, Goodyer IM. Facial expression recognition, fear conditioning, and startle modulation in female subjects with conduct disorder. Biol Psychiatry. 2010;68:272–279. doi: 10.1016/j.biopsych.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbaumer N, Veit R, Lotze M, Erb M, Hermann C, Grodd W, et al. Deficient fear conditioning in psychopathy: a functional magnetic resonance imaging study. Arch Gen Psychiatry. 2005;62:799–805. doi: 10.1001/archpsyc.62.7.799. [DOI] [PubMed] [Google Scholar]
- Gao Y, Raine A, Venables PH, Dawson ME, Mednick SA. Association of poor childhood fear conditioning and adult crime. Am J Psychiatry. 2010;167:56–60. doi: 10.1176/appi.ajp.2009.09040499. [DOI] [PubMed] [Google Scholar]
- Raine A, Venables PH, Williams M. Better autonomic conditioning and faster electrodermal half-recovery time at age 15 years as possible protective factors against crime at age 29 years. Dev Psychol. 1996;32:624–630. [Google Scholar]
- Hodgins S, De Brito SA, Simonoff E, Vloet T, Viding E. Getting the phenotypes right: an essential ingredient for understanding aetiological mechanisms underlying persistent violence and developing effective treatments. Front Behav Neurosci. 2009;3:44. doi: 10.3389/neuro.08.044.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler C, Poustka F, Sterzer P. The heterogeneity of disruptive behavior disorders—implications for neurobiological research and treatment. Front Psychiatry. 2010;1:21. doi: 10.3389/fpsyt.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick PJ, White SF. Research review: the importance of callous-unemotional traits for developmental models of aggressive and antisocial behavior. J Child Psychol Psychiatry. 2008;49:359–375. doi: 10.1111/j.1469-7610.2007.01862.x. [DOI] [PubMed] [Google Scholar]
- Cauffman E, Kimonis ER, Dmitrieva J, Monahan KC. A multimethod assessment of juvenile psychopathy: comparing the predictive utility of the PCL:YV, YPI, and NEO PRI. Psychol Assess. 2009;21:528–542. doi: 10.1037/a0017367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seagrave D, Grisso T. Adolescent development and the measurement of juvenile psychopathy. Law Hum Behav. 2002;26:219–239. doi: 10.1023/a:1014696110850. [DOI] [PubMed] [Google Scholar]
- Frick PJ, Moffitt TE.A Proposal to the DSM-V Childhood Disorders and the ADHD and Disruptive Behavior Disorders Work Groups to Include a Specifier to the Diagnosis of Conduct Disorder based on the Presence of Callous-Unemotional Traits . http://www.dsm5.org/Proposed%20Revision%20Attachments/Proposal%20for%20Callous%20and%20Unemotional%20Specifier%20of%20Conduct%20Disorder.pdf 2010American Psychiatric Association [Google Scholar]
- Frick PJ, Stickle TR, Dandreaux DM, Farrell JM, Kimonis ER. Callous-unemotional traits in predicting the severity and stability of conduct problems and delinquency. J Abnorm Child Psychol. 2005;33:471–487. doi: 10.1007/s10648-005-5728-9. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Mitchell DG, Reid ME, Sims C, Kosson DS, et al. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. Am J Psychiatry. 2008;165:712–720. doi: 10.1176/appi.ajp.2007.07071145. [DOI] [PubMed] [Google Scholar]
- Finger EC, Marsh AA, Mitchell DG, Reid ME, Sims C, Budhani S, et al. Abnormal ventromedial prefrontal cortex function in children with psychopathic traits during reversal learning. Arch Gen Psychiatry. 2008;65:586–594. doi: 10.1001/archpsyc.65.5.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viding E, McCrory EJ. Genetic and neurocognitive contributions to the development of psychopathy. Dev Psychopathol. 2012;24:969–983. doi: 10.1017/S095457941200048X. [DOI] [PubMed] [Google Scholar]
- Carre JM, Hyde LW, Neumann CS, Viding E, Hariri AR. The neural signatures of distinct psychopathic traits. Soc Neurosci. 2013;8:122–135. doi: 10.1080/17470919.2012.703623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick PJ, Lilienfeld SO, Ellis M, Loney B, Silverthorn P. The association between anxiety and psychopathy dimensions in children. J Abnorm Child Psychol. 1999;27:383–392. doi: 10.1023/a:1021928018403. [DOI] [PubMed] [Google Scholar]
- Patrick CJ. Emotion and psychopathy: startling new insights. Psychophysiology. 1994;31:319–330. doi: 10.1111/j.1469-8986.1994.tb02440.x. [DOI] [PubMed] [Google Scholar]
- Sebastian CL, McCrory EJ, Cecil CA, Lockwood PL, De Brito SA, Fontaine NM, et al. Neural responses to affective and cognitive theory of mind in children with conduct problems and varying levels of callous-unemotional traits. Arch Gen Psychiatry. 2012;69:814–822. doi: 10.1001/archgenpsychiatry.2011.2070. [DOI] [PubMed] [Google Scholar]
- Domburgh Lv VermeirenR, Blokland AA, Doreleijers TA. Delinquent development in Dutch childhood arrestees: developmental trajectories, risk factors and co-morbidity with adverse outcomes during adolescence. J Abnorm Child Psychol. 2009;37:93–105. doi: 10.1007/s10802-008-9260-6. [DOI] [PubMed] [Google Scholar]
- Cohn M, van DL, Vermeiren R, Geluk C, Doreleijers T. Externalizing psychopathology and persistence of offending in childhood first-time arrestees. Eur Child Adolesc Psychiatry. 2012;21:243–251. doi: 10.1007/s00787-012-0257-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Intelligence Scale for Children—Revised. Psychological Corporation: New York, NY, USA; 1974. [Google Scholar]
- Raine A, Dodge K, Loeber R, Gatzke-Kopp L, Lynam D, Reynolds C, et al. The Reactive-Proactive Aggression Questionnaire: differential correlates of reactive and proactive aggression in adolescent boys. Aggress Behav. 2006;32:159–171. doi: 10.1002/ab.20115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andershed H, Hodgins S, Tengstrom A. Convergent validity of the Youth Psychopathic Traits Inventory (YPI): association with the Psychopathy Checklist: Youth Version (PCL:YV) Assessment. 2007;14:144–154. doi: 10.1177/1073191106298286. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Arseneault L, Jaffee SR, Kim-Cohen J, Koenen KC, Odgers CL, et al. Research review: DSM-V conduct disorder: research needs for an evidence base. J Child Psychol Psychiatry. 2008;49:3–33. doi: 10.1111/j.1469-7610.2007.01823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achenbach TM. Manual for the Child Behavior Checklist/4-18 and 1991 Profile. University of Vermont: Burlingtion, VT, USA; 1991. [Google Scholar]
- Ferdinand RF, Ende Jvd. Dutch translation of the DISC-IV; Diagnostic Interview Schedule for Children. Department of Child and Adolescent Psychiatry Sophia's Children Hospital: Rotterdam, The Netherlands; 2002. [Google Scholar]
- Schwab-Stone ME, Shaffer D, Dulcan MK, Jensen PS, Fisher P, Bird HR, et al. Criterion validity of the NIMH Diagnostic Interview Schedule for Children Version 2.3 (DISC-2.3) J Am Acad Child Adolesc Psychiatry. 1996;35:878–888. doi: 10.1097/00004583-199607000-00013. [DOI] [PubMed] [Google Scholar]
- Pajer K, Gardner W, Rubin RT, Perel J, Neal S. Decreased cortisol levels in adolescent girls with conduct disorder. Arch Gen Psychiatry. 2001;58:297–302. doi: 10.1001/archpsyc.58.3.297. [DOI] [PubMed] [Google Scholar]
- Baardewijk Yv SteggeH, Andershed H, Thomaes S, Scholte E, Vermeiren R. Measuring psychopathic traits in children through self-report. The development of the Youth Psychopathic Traits Inventory-Child Version. Int J Law Psychiatry. 2008;31:199–209. doi: 10.1016/j.ijlp.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Domburgh Lv, Popma A. Reactive and Proactive Questionnaire [Dutch translation] Duivendrecht, The Netherlands; 2003. [Google Scholar]
- Molenkamp E.Versatile Stimulus Response Registration Program (VSRRP98) www.test.uva.nl/ozi_psychology/index.php?Page0Software. University of Amsterdam: Amsterdam, The Netherlands; 1998 [Google Scholar]
- Quirk GJ, Armony JL, LeDoux JE. Fear conditioning enhances different temporal components of tone-evoked spike trains in auditory cortex and lateral amygdala. Neuron. 1997;19:613–624. doi: 10.1016/s0896-6273(00)80375-x. [DOI] [PubMed] [Google Scholar]
- Phelps EA, O'Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M. Activation of the left amygdala to a cognitive representation of fear. Nat Neurosci. 2001;4:437–441. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- Sehlmeyer C, Schoning S, Zwitserlood P, Pfleiderer B, Kircher T, Arolt V, et al. Human fear conditioning and extinction in neuroimaging: a systematic review. PLoS One. 2009;4:e5865. doi: 10.1371/journal.pone.0005865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Well Sv VisserRM, Scholte HS, Kindt M. Neural substrates of individual differences in human fear learning: evidence from concurrent fMRI, fear-potentiated startle, and US-expectancy data. Cogn Affect Behav Neurosci. 2012;12:499–512. doi: 10.3758/s13415-012-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechias ML, Etkin A, Kalisch R. A meta-analysis of instructed fear studies: implications for conscious appraisal of threat. NeuroImage. 2010;49:1760–1768. doi: 10.1016/j.neuroimage.2009.09.040. [DOI] [PubMed] [Google Scholar]
- Li W, van Tol MJ, Li M, Miao W, Jiao Y, Heinze HJ, et al. Regional specificity of sex effects on subcortical volumes across the lifespan in healthy aging Hum Brain Mappadvance online publication, 21 September 2012; doi: : 10.1002/hbm.22168 (e-pub ahead of print). [DOI] [PMC free article] [PubMed]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline J-B. Region of interest analysis using an SPM toolbox (Presented at the 8th International Conference on Functional Mapping of the Human Brain, 2–6 June 2002, Sendai, Japan) NeuroImage. 2002;16:1140–1141. [Google Scholar]
- Raine A, Venables PH. Socialization and classical conditioning—a biosocial interaction. Person Individ Differ. 1981;2:273–283. [Google Scholar]
- Hodgins S. Persistent violent offending: what do we know. Br J Psychiatry Suppl. 2007;49:s12–s14. doi: 10.1192/bjp.190.5.s12. [DOI] [PubMed] [Google Scholar]
- Chitsabesan P, Kroll L, Bailey S, Kenning C, Sneider S, Mac Donald W, et al. Mental health needs of young offenders in custody and in the community. Br J Psychiatry. 2006;188:534–540. doi: 10.1192/bjp.bp.105.010116. [DOI] [PubMed] [Google Scholar]
- Vermeiren R. Psychopathology and delinquency in adolescents: a descriptive and developmental perspective. Clin Psychol Rev. 2003;23:277–318. doi: 10.1016/s0272-7358(02)00227-1. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Hicks BM, Krueger RF, Lang AR. Relations between psychopathy facets and externalizing in a criminal offender sample. J Pers Disord. 2005;19:339–356. doi: 10.1521/pedi.2005.19.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passamonti L, Fairchild G, Goodyer IM, Hurford G, Hagan CC, Rowe JB, et al. Neural abnormalities in early-onset and adolescence-onset conduct disorder. Arch Gen Psychiatry. 2010;67:729–738. doi: 10.1001/archgenpsychiatry.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohman A. Face the beast and fear the face: animal and social fears as prototypes for evolutionary analyses of emotion. Psychophysiology. 1986;23:123–145. doi: 10.1111/j.1469-8986.1986.tb00608.x. [DOI] [PubMed] [Google Scholar]
- Kochanska G, Aksan N, Joy ME. Children's fearfulness as a moderator of parenting in early socialization: two longitudinal studies. Dev Psychol. 2007;43:222–237. doi: 10.1037/0012-1649.43.1.222. [DOI] [PubMed] [Google Scholar]