Abstract
Background & objectives:
Survival pattern among children infected with the human immune deficiency virus (HIV) follows a bimodel distribution. Some children survive beyond 9 years age and are known as long term survivers (LTS) while others had a more rapid course to death during the first few years of life. In the LTS group of children, two sub-populations have emerged, the long term non-progressors (LTNP) who have remained asymptomatic over a period of years and those who have survived despite clinical and laboratory evidence of disease progression, the long term progressors (LTP). The aim of the present study was to determine the factors influencing the conversion of LTNPs to LTPs in a group of perinatally HIV infected children who were followed up for five years.
Methods:
A total of 26 HIV seropositive paediatric patients were monitored from 2006 to 2011 with CD4 cell counts, onset of clinical manifestations, body weight, biochemical, haematological and immunological parameters. Statistical analyses, both qualitative and quantitative, were used to determine the degree of conversion of non-progressors to progressors.
Results:
All 26 (13 female and 13 male) perinatally HIV infected children, born during1991-1996 were healthy until 2006. But by 2011, 18 were placed in progressors group with antiretroviral therapy (ART), while six remained in non progressors group and two died. As per the Kaplan-Meier survival analysis, AIDS free median survival period (years) in LTP group (CD4 count) of the cohort was 10±0.66 (<200; P=<0.05); 11±0.61 (200-350, P=<0.05), 12±0.18 (>350, P=<0.05). Intercurrent and opportunistic infections (OIs) were observed in LTPs only. The incidence of OI in LTPs was higher when compared to general paediatric population.
Interpretation & conclusions:
Our findings show that CD4 counts and OIs play an important role in influencing the survival chances of perinatally HIV infected children.
Keywords: AIDS, HIV, LTNPs, LTP, paediatric, progressors
The total number of people living with human immunodeficiency virus (HIV) infection in India is very high (as much as 2.5 millions), including an estimated 0.09 million paediatric HIV patients1. Mean duration of survival after diagnosis of HIV infection in India is 92 months1. Perinatal transmission is the most common mode of acquiring HIV in the paediatric age group and is responsible for about 67 to 87 per cent of paediatric HIV infection2. The clinical features of HIV infection in children are different from those in adults3. One of the most striking characteristics of HIV-1 infection is its individual variability in terms of the time required to progress towards AIDS. A small percentage (5%) of HIV-1 infected children remain free from AIDS-defining illnesses for longer than 9-10 yr in the absence of therapy and are termed as long term survivors (LTS). The LTS group of children is differentiated into two subgroups, the long term non progressors (LTNPs) and long term progressors (LTP).
LTNPs are HIV-infected individuals who have remained asymptomatic over a period of years and have maintained CD4-positive lymphocyte counts greater than 500 cells/μl (in Indian populations normal range 304-1864 cells/μl)4 without receiving therapy. LTPs represent the individuals who have survived despite clinical and laboratory evidence of diseases progression. LTNPs represent an important group of HIV infected individuals to study because of their potentially unique resistance to virus infection due to innate immune responses. An understanding of the clinical profile of LTNPs would provide an insight into the parameters that may influence the survival probability and the extent of LTNP conversion to LTPs, requiring antiretroviral treatment (ART). The present study was undertaken on a group of 26 perinatally HIV infected children who were followed up for five years to determine the factors influencing the conversion of LTNPs to LTPs.
Material & Methods
This is a descriptive study based on the data collected from a group of children infected with HIV from perinatal transmission being followed up prospectively from birth.
Study subjects: This study initiated in August 2006 was conducted on 26 HIV perinatally infected children born between 1991-1996 in the LODI multipurpose voluntary organization hospital, Warangal, Andhra Pradesh, India. These children were followed up at MGM Hospital affiliated to Kakatiya Medical College, Warangal. HIV infection was diagnosed by rapid tests and was finally confirmed with the Western blot and PCR. The study proposal was approved by the following two institutes’ ethics committees, Kakatiya Medical College, Warangal and University of Hyderabad, Hyderabad.
Inclusion criteria: Perinatally HIV infected children, who were >9 yr age (as on August 2006), had >300 CD4 cells/μl, no opportunistic infections, WHO-Stage 1 (Normal and asymptomatic) and were not receiving ART5,6.
Exclusion criteria: Children who were not perinatally infected and were <9 yr of age (as on August 2006), had <300 CD4 cells/μl, HIV-opportunistic infections, WHO-Stages II, III, IV and under ART.
Case definitions: In this study group of 26 children, baseline CD4 counts, BMI, onset of opportunistic infections, biochemical parameters [bilirubin, serum albumin, serum glutamic oxaloacetic transaminase (SGOT), serum glutamic-pyruvic transaminase (SGPT), alkaline phosphatase, gamma-glutamyl transferase (GGT), serum amylase, blood urea nitrogen, serum creatinine and haematology parameters complete blood picture (CBP), total lymphocyte count (TLC), Hb, erythrocyte sedimentation rate (ESR)] were monitored from 2006 to 2011. No disease progression to AIDS was monitored with the above parameters at every six month intervals over a period of five years.
CD4 absolute counts and percentage were estimated at two places by flow cytometry (FACSCalibur, MultiSETV2.2, USA) at the Kakatiya Medical College, Warangal as well as at the University of Hyderabad, for the purpose of validation and to minimize bias.
Clinical evaluation: The baseline health status of the patients was reviewed every six months. Relevant investigations like chest X-ray, Mantoux test, aspiration cytology, ultrasonography, neuroimaging, were conducted and other clinical and laboratory parameters (anaemia, complete blood picture, cervical lymphadenopathy, bronchitis, otitis media, cardiomegaly, dermatitis, hepatomegaly, pneumonitis, granulomatous disease of brain, scabies, oral candidiasis, meningitis, glaucoma, non-Hodgkin's lymphoma of Burkitt type, toxoplasmosis of brain) were recorded. Patients were treated symptomatically based on their clinical presentation. Opportunistic infections were treated with a course of antibiotics.
Statistical analysis: Statistical analysis was performed using the statistical software package SPSS Inc., Chicago, USA (Windows, version 17.0). Kaplan-Meier survival analysis was used to estimate median probabilities and cumulative probabilities with 95% confidence intervals. The non parametric Chi-square test and t-test were also applied in comparative analysis results between different groups.
Results
A total of 26 HIV perinatally infected paediatric patients fulfilling all relevant criteria were enrolled as long term non progressors in August 2006. Subsequently, at the end of the study, (by August 2011), 20 (76%) of 26 patients converted to LTPs and six (24%) remained LTNPs (Table I). All six LTNPs were above nine years of age and showed absolute CD4 counts >300 cells/μl. As per the WHO criteria they were asymptomatic, showed normal activity, did not have any WHO defined AIDS related illness and were not on ART. When classified according to WHO staging system, five children were in stage I and one in stage II. In contrast, the 20 patients in the LTP group, exhibited AIDS associated opportunistic infections and were on antiretroviral treatment. When classified according to WHO staging system, 12 (60%) were stage I, three (10%) II, one (5%) stage III, four (20%) stage IV.
Table I.
Characteristics of long-term non-progressors (LTNP) and long-term progressors (LTP) groups
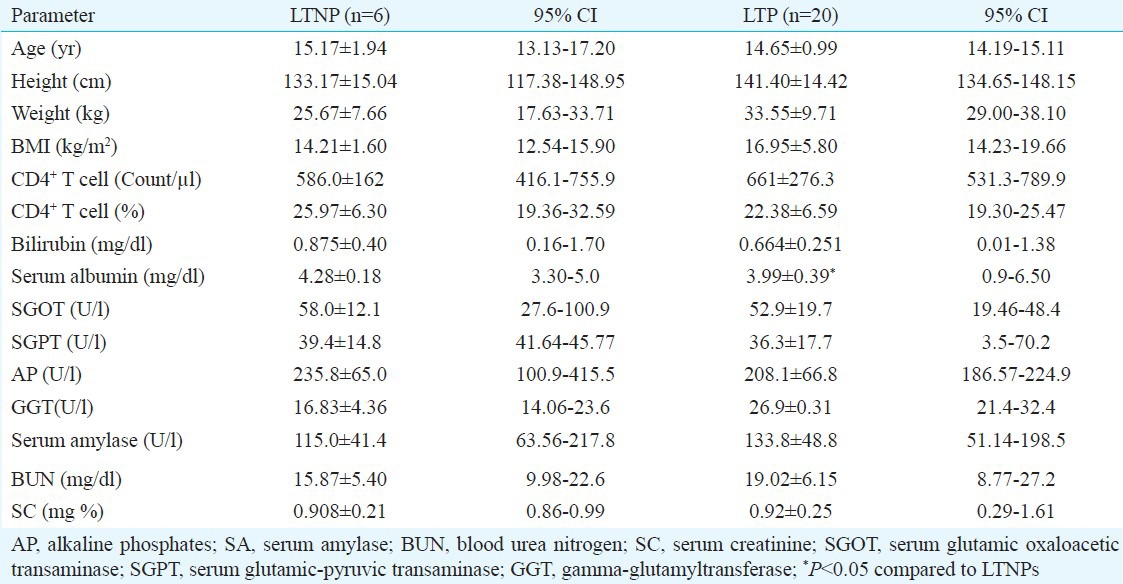
The CD4 count of the study group ranged from 17 to 2214/μl with CD4 mean count of 586±162 cells/μl for LTNPs and at 661±276 cells/μl for LTPs (Table I). There was no significant difference in any parameter between the two groups except for serum albumin (P<0.05). Haematological abnormalities like anisocytosis, macro-ovalocytes, poikilocytosis, microcytosis, elliptocytosis, rouleaux formation, tear drop cells, transformed lymphocytes and hypersegmented WBC were seen both in LTNPs and LTPs.
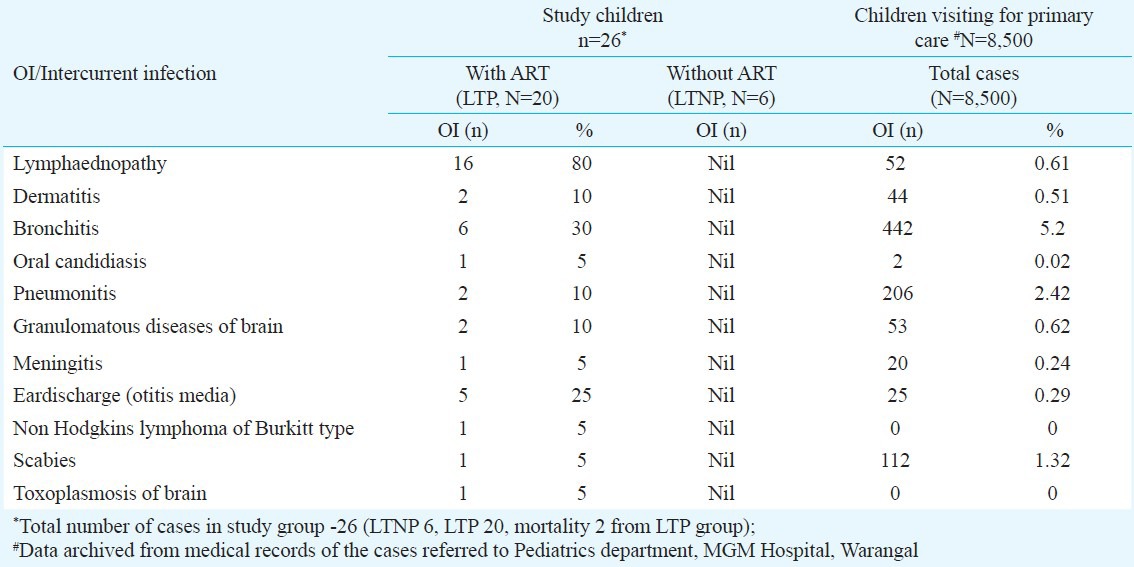
Children in LTP group experienced several OIs including cervical lymphaednopathy, bronchitis, otitis media, dermatitis, pneumonitis, granulomatous disease of brain, scabies, oral candidiasis, meningitis, toxoplasmosis of brain and HIV associated malignancy like non-Hodgkin's lymphoma of Burkitt type. No opportunistic infections were observed in six children in LTNP group.
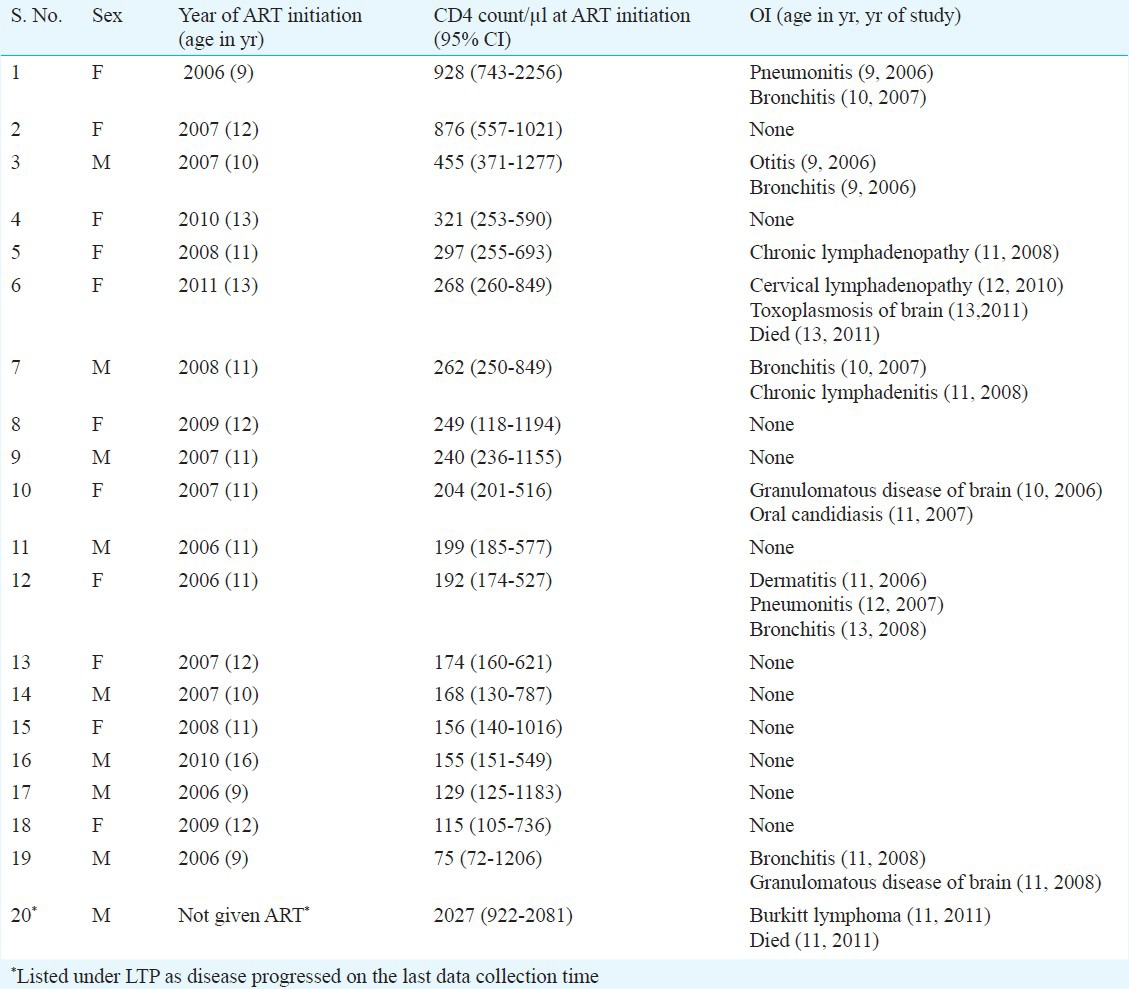
No mortality was noticed in the LTNP group but in the LTP group two patients died (one with non-Hodgkin's Lymphoma of Burkitt type and another with toxoplasmosis of brain) (Table II).
Table II.
Profile of antiretroviral therapy (ART) initiation and opportunistic infections (OI)/clinical status in long term progressors
During the study period (2006-2011), the progression of LTNP to LTP occurred due to intercurrent and opportunistic infections and warranted ART. Table III shows a comparison of occurrence of OIs in HIV patients and the general paediatric population visiting the same hospital during the same period.
Table III.
Incidence of opportunistic infections (OIs) in total children compared to paediatric cases referred to the hospital during 2006-2011
Survival analysis of LTNPs and LTPs: All non-progressors were alive until the last data collection point. As per the Kaplan-Meier survival analysis the incubation period in LTP patients ranged from 9 to 16 yr. The survival period in LTPs as estimated from the analysis and its dependence on CD4 in terms of median survival time (MST) in years was 10±0.66 (CI:8.69-11.30) at CD4 count <200 cells/μl; 11±0.61 yr (CI: 9.8-12.2) with CD4 count between 200 and 350 cells/μl and 12±0.18 (CI 11.63-12.37) for CD4 count >350 cells/μl. The association between CD4 counts and survival time was found to be significant (P<0.05). The median survival time after ART was 13±0.262 yr (CI: 12.49-13.51) and the CD4 count was found to >350 cells/μl for all the patients in this group.
Discussion
The progression of the disease and the abnormalities associated with HIV/AIDS are influenced by several factors such as baseline health and nutritional status, environment, endemic diseases and access to therapy. The median survival time of perinatally HIV infected children is approximately 8 to 9 yr7. Mean survival time of Indian patients after diagnosis of HIV is 92 months or 8 years7. Median time for progression from HIV infection to AIDS was reported to be about 7.9 yr8.
The mean age of the LTP children was 13 ± 2.29 yr and that of LTNP was 12.83 ± 3.06 yr. Incubation period and AIDS free survival period was 9-16 yr in LTPs and in LTNPs it ranged from 11 to 19 yr. Median age of AIDS progression in perinatally infected HIV children in US was reported to be 4.1 yr and that of AIDS free children up to 13 yr9. In a European cohort, 36 per cent of the perinatally infected children developed AIDS by 6 years10. In UK, the reported median age of AIDS progression is 5 years and survival time 8 years11. In Pediatric Spectrum of Disease project, the estimated mean time from birth to progression to AIDS was 4.8 years and the mean survival time from birth was 9.4 yr12. In Rwanda, a 5 year prospective study suggested that the mother to child transmission to AIDS progression was 2 to 5 years and the estimated median survival time was 12.4 months13. According to a study from south India1 the mean survival time for adults from serodiagnosis was 92 months or 7.6 years1. Studies on various perinatally infected paediatric cohorts suggest that the onset time of disease progression ranges from 2 months to 13 years while the incidence time of mortality ranges from 3 months to 5 years and by 3 years as many as 89 per cent of the infected children die as reported in an African study14.
Results from the present study showed that the median survival time was about 12 years for the LTP group. CD4 counts play a significant role in influencing the survival time. An earlier study4 showed that persons with a CD4 lymphocyte count <200 cells/μl were 19 times more likely to have shorter survival time than those with CD4 cell count of >350 cells/μl. Results from the present study showed that one child with high CD4count >700 cells/μl was diagnosed with Burkitt's lymphoma stage IV and died subsequently. Kaplan-Meier survival analysis showed that with the progression of age the AIDS free survival time has decreased thus suggesting a dependence on CD4 count.
The nature of OIs associated with perinatally HIV infected children differ for cohorts drawn from different regions and from different countries. In various Indian studies the commonly reported OIs are otitis media, oral candidiasis, dermatological manifestations, hepatomegaly, and lymphadenopathy15. In studies from South Africa, Ethiopia, Rwanda, Italy, the OIs reported include otitis media, dermatological manifestation, hepatomegaly, lymphadenopathy, with deaths occurring at age 5-13 yr12,14,15.
In our study 11 per cent of LTP group developed mild cardiomegaly. In one study cardiac dysfunction was reported in 18 to 39 per cent of HIV-infected children and this was associated with an increased risk of death16. Extra-pulmonary tubercular manifestations occurred in 46 to 79 per cent of patients in another study4. In the present group, only one patient exhibited granulomatous disease of brain which presented as tuberculosis in the brain. One patient among LTPs developed pneumonia. Bacterial pneumonitis has been reported as an opportunistic infection in 1.8 per cent of a large southern Indian cohort of HIV-positive patients1.
An increase in the incidence (5 to 10%) of non-Hodgkin lymphoma (NHLs) in HIV-infected population is reported17. In the present study, one LTP developed Burkitt's lymphoma and died within 6 months. Burkitt's lymphoma is a high-grade aggressive subgroup of non-Hodgkin's lymphoma and is manifested as small, non-cleaved, diffuse, undifferentiated malignant cells of lymphoid origin18. Its association with Epstein-Barr virus (EBV) varies from 25 to 80 per cent16. As per the CDC AIDS case definition for surveillance, patients with CD4 mean count < 200 cell/μl may develop Burkitt's lymphoma. But in the present study, the lone patient who developed Burkitt's lymphoma had a high CD4 count amounting to >700 cell/μl. Another death occurred due to toxoplasmosis of brain at CD4 count above >700 cell/μl. CNS toxoplasmosis has been the most common OI in HIV patients1. Although with differing frequencies chronic diarrhoea, pyrexia of unknown origin, chronic parotitis, pruritic popular eruption (PPE), have been reported in Indian HIV paediatric patients with differing frequencies2.
Haematologic abnormalities are among the most common manifestations of HIV/AIDS infection in children. A variety of such abnormalities associated with HIV infection was reported in different studies19,20. In the present study, both the groups showed hematological abnormalities, HIV infected children showed 68 per cent RBC abnormal morphology14 and the microcytosis with hypochromasia being the most frequently reported abnormality21. Clinically significant haematological abnormalities are common in HIV patients and are mainly due to impaired haematopoiesis, immune mediated cytopenia and altered coagulation mechanisms. Decline of CD4 counts below 200 cells/μl increases the risk of anaemia21.
In the present small group of perinatally HIV infected children, approximately one-fourth survived beyond 14 yr of age in the absence of ART therapy, and three-fourth survived beyond 9 yr of age with ART therapy. The present findings suggest that persistent low level viral replication is not necessarily associated with disease progression if it is efficiently controlled over time. Long term survivors of HIV provide a ray of hope indicating that it is possible to live with HIV without progressing to AIDS. Further longitudinal studies with a large cohort need to be done to confirm these findings.
Acknowledgment
This study and the first author was supported by the UGC- Dr D.S. Kothari Post doctoral fellowship, Government of India. The authors acknowledge the collaboration of the LODI multipurpose voluntary organization, Kazipet and Kakatiya Medical College, Warangal, Andhra Pradesh, India, and thank Director Father Leeno and staff especially Sister Mary, Balashowry of LODI, Smt. A.N.R. Laxmi, Principal, Kakatiya Medical College, Warangal, and all HIV infected children and their family for their cooperation. Authors also acknowledge Dr Vijay Kumar, Department of Pediatrics, MGM, Hospital, Warangal, for providing archived medical records.
References
- 1.Kumarasamy N, Solomon S, Flanigan TP, Hemalatha R, Thyagarajan SP, Mayer KH, et al. Natural history of human immunodeficiency virus disease in southern India. Clin Infect Dis. 2003;36:79–85. doi: 10.1086/344756. [DOI] [PubMed] [Google Scholar]
- 2.Shilpa RS, Milind ST, Jaishree RK. Clinical profile of pediatric HIV infection from India. Arch Med Res. 2005;36:24–31. doi: 10.1016/j.arcmed.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 3.d’ ArminioMonfote A, Vago L, Lazarin A, Boldorini R, Bini T, Guzzetti S, et al. AIDS defining illnesses in 250 HIV-infected patients : a comparitive study of clinical autopsy diagnosis. AIDS. 1992;6:467–74. doi: 10.1097/00002030-199210000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Grabar S, Selinger-Leneman H, Abgrall S, Pialoux G, Weiss L, Costagliola D. Prevalence and comparative characteristics of long-term nonprogressors and HIV controller patients in the French Hospital Database on HIV. AIDS. 2009;23:1163–9. doi: 10.1097/QAD.0b013e32832b44c8. [DOI] [PubMed] [Google Scholar]
- 5.Klein MR, Miedema F. Long-term survivors of HIV-1 infection. Trends Microbiol. 1995;3:386–91. doi: 10.1016/s0966-842x(00)88984-2. [DOI] [PubMed] [Google Scholar]
- 6.Pantaleo G, Menzo S, Vaccarezza M, Graziosi C, Cohen OJ, Demarest JF, et al. Studies in subjects with long-term nonprogressive human immunodeficiency virus infection. N Engl J Med. 1995;332:209–16. doi: 10.1056/NEJM199501263320402. [DOI] [PubMed] [Google Scholar]
- 7.Frederick T, Mascola L, Eller A. Progression of human immunodeficiency virus disease among infants and children infected perinatally with human immunodeficiency virus or through neonatal blood transfusion. Pediatr Infect Dis J. 1994;13:1091–7. doi: 10.1097/00006454-199412000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Hira SK, Shroff HJ, Lanjewar DN, Dholkia YN, Bhatia VP, Dupont HL. The natural history of human immunodeficiency virus infection among adults in Mumbai. Natl Med J India. 2003;16:126–31. [PubMed] [Google Scholar]
- 9.Pliner V, Weedon J, Thomas PA, Steketee RW, Abrams EJ, Lambert G, et al. New York city perinatal HIV transmission collaborative study group. Incubation period of HIV-1 in perinatally infected children. AIDS. 1998;12:759–66. doi: 10.1097/00002030-199807000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Blanche S, Newell ML, Mayaux MJ, Dunn DT, Teglas JP, Rouzioux C, et al. The French Pediatric HIV Infection Study Group and European Collaborative Study. Morbidity and mortality in European children vertically infected by HIV-1. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;14:442–50. doi: 10.1097/00042560-199704150-00008. [DOI] [PubMed] [Google Scholar]
- 11.Tovo PA, Gabiano C, Palomb E, Martino de M, Galli L, Cappello N, et al. Prognostic factors and survival in children with perinatal HIV-1. Lancet. 1992;339:1249–53. doi: 10.1016/0140-6736(92)91592-v. [DOI] [PubMed] [Google Scholar]
- 12.Huiman XB, Caldwell MB, Thomas P, Mascola L, Ortiz I, Hsu HW, et al. Pediatric spectrum of disease clinical consortium natural history of human immunodeficiency virus disease in perinatally infected children: An analysis from the Pediatric Spectrum of Disease Project. Pediatrics. 1996;97:710–6. [PubMed] [Google Scholar]
- 13.Spira R, Lepage P, Msellati P, Perre PV, Leroy V, Simonon A, et al. Mother-to-Child HIV- Transmission Study Group natural history of human immunodeficiency virus Type 1 infection in children: A five-year prospective study in Rwanda. Pediatrics. 1999;104:e56. doi: 10.1542/peds.104.5.e56. [DOI] [PubMed] [Google Scholar]
- 14.Eley BS, Sive AA, Shuttleworth M, Hussey GD. A prospective, cross-sectional study of anaemia and peripheral iron status in antiretroviral naïve, HIV-1 infected children in Cape Town, South Africa. BMC Infec Dis. 2002;2:3. doi: 10.1186/1471-2334-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madhivanan P, Mothi SN, Kumarasamy N, Yepthomi T, Venkatesan C, Lambert JS, et al. Clinical manifestations of HIV infected children. Indian J Pediatr. 2001;70:615–20. doi: 10.1007/BF02724249. [DOI] [PubMed] [Google Scholar]
- 16.Starc TJ, Lipshultz SE, Easley KA, Kaplan S, Bricker JT, Colan SD, et al. Incidence of cardiac abnormalities in children with human immunodeficiency virus infection: The prospective P2C2 HIV study. J Pediatr. 2002;141:327–34. doi: 10.1067/mpd.2002.126301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsimberidou AM, Sarris AH, Medeiros LJ, Mesina O, Rodriguez MA, Hagemeister FB, et al. Hodgkin's disease in patients infected with human immunodeficiency virus: frequency, presentation and clinical outcome. Leuk Lymphoma. 2001;41:535–44. doi: 10.3109/10428190109060344. [DOI] [PubMed] [Google Scholar]
- 18.Ugar DA, Bozkaya S, Karaca I, Tokman B, Pinarli FG. Childhood craniofacial Burkitt's lymphoma presenting as maxillary swelling: Report of a case and review of literature. J Dent Child (Chic) 2006;73:45–50. [PubMed] [Google Scholar]
- 19.Zon LI, Arkin C, Groopman JE. Haematologic manifestations of human immune deficiency virus (HIV) Br J Haematol. 1987;66:251–6. doi: 10.1111/j.1365-2141.1987.tb01307.x. [DOI] [PubMed] [Google Scholar]
- 20.Davis BR, Zauli G. Effect of human immunodeficiency virus infection on haematopoiesis. Baillieres Clin Haematol. 1995;8:113–30. doi: 10.1016/s0950-3536(05)80234-3. [DOI] [PubMed] [Google Scholar]
- 21.Jam S, Ramezani A, Sabzvari D, Moradmand-Badie B, Seyedalinaghi S, Jabbari H, et al. A cross-sectional study of anemia in human immunodeficiency virus-infected patients in Iran. Arch Iran Med. 2009;12:145–50. [PubMed] [Google Scholar]


