Abstract
Background & objectives:
Environmental factors including weather variables may play a significant role in the transmission of dengue. This study investigated the effect of seasonal variation on the abundance of Aedes aegypti and Ae. albopictus larvae and explored the impact of weather variability on dengue transmission in Sisaket, Thailand.
Methods:
The monthly mosquito larval surveys were carried out in urban and rural areas in Sisaket, Thailand from January to December 2010. Data on monthly-reported cases of dengue fever over the period 2004-2010 were obtained from the Ministry of Public Health. Weather data over the same period were obtained from the Thai Meteorological Department. Chi-square test was used to find the differences relating to seasonal variability, areas of study, and mosquito species factors using entomological survey data. Time series Poisson regression analysis was performed using data on monthly weather variables and dengue cases.
Results:
There were more Ae. aegypti larvae per household than Ae. albopictus larvae in the winter and rainy seasons. More Aedes larvae per household were found in the rainy season than in the winter and summer seasons. Relative humidity at a lag of one month and rainy days in the current month were significant predictors of dengue incidence in Sisaket.
Interpretation & conclusions:
Increased rain during the current month and less humidity during the previous month might trigger a higher incidence of dengue epidemic in Sisaket. The present findings suggest that the dengue incidence corresponds with the number of Aedes larvae. The seasonal patterns of dengue outbreaks coincide with the rainy season.
Keywords: Aedes aegypti, Ae. albopictus, dengue, season, transmission prediction, weather
The incidence and distribution of dengue-related illness have grown dramatically in recent decades1. It is estimated that 50 million dengue infections occur annually1 and about 2.5 billion people live in the regions with potential risk of dengue transmission1. Aedes mosquitoes transmit dengue virus, causing both classical dengue fever (DF) and potentially fatal dengue haemorrhagic fever (DHF). The first reported epidemic of DHF occurred in Southeast Asia in 19532. Since the first dengue epidemic outbreak in Thailand in 19583, there has been an upward trend in the incidence of DHF. In 2010, Sisaket province in Thailand was classified as a dengue high risk area. In that year, the province had the highest number of dengue cases in the northeastern region with 2,618 dengue cases and dengue incidence rate of 180.25 cases per 100,000 population3.
Environmental factors including weather variables may play a significant role in the transmission of dengue which is a mosquito-borne disease with seasonal distribution4,5. Temperature, rainfall, and relative humidity are major parameters influencing the incidence of dengue fever in Thailand5,6. The prediction of global climate change and transmission of dengue and its geographic spread has been widely studied5,7,8. Since dengue transmission is highly dependent on local environmental factors, it may not be possible to predict dengue incidence outside locations. However, investigations of local weather conditions and dengue incidence in different environmental and regional contexts can improve our understanding of the linkages between weather variables and dengue transmission, and provide strong scientific evidence for predicting future transmission patterns9.
Monitoring of mosquito larvae, finding mosquito larval indices, and predicting dengue incidence can facilitate early warning and disease control and prevention. In this study, we examined the numbers of Aedes larvae and larval indices in both rural and urban areas of Sisaket province over three seasons to assess the impact of weather variables on dengue transmission.
Material & Methods
Study area: Sisaket is one of the northeastern provinces of Thailand. It has a land area of 8,839.976 km2 and a population of about 1.45 million. The province borders Cambodia to the south. The summer season in Sisaket is from March to May. The rainy season starts in June and ends in October. The winter season follows from November to February. The annual mean temperature is about 26 °C. The annual rainfall is about 1,598 mm. The mean summer and mean winter temperatures are approximately 29 and 24 °C, respectively. The average maximum and minimum temperatures are 35.5 and 18.8 °C, respectively.
Mosquito larval survey: The monthly mosquito larval surveys were carried out by examining all containers in indoor and outdoor water containers in both urban and rural areas from January to December 2010. The urban area was in Kanthararom district with population density of 141 inhabitants/km2. The rural areas covered 15 sub-districts (Epad, Tam, Buanoi, Muang-Noi, La-Tay, Hnong Wang, Hnong Kaw, Hnong Bua, Doon, Non Sang, Du, Kam Niam, Pak Paw, Yang and Hnong Hua Chang) with average population density of nine inhabitants/km2. Mosquito larvae were collected from both indoor and outdoor containers using fine-meshed fishnets. The outdoor larval surveys were conducted within 15 m of houses10,11. All containers holding water were sampled for Aedes immatures (III/IV instars). Water in very small containers was emptied into the fishnet. Larger water containers were sampled by dipping the net in the water, starting at the top of the container and continuing to the bottom in a swirling motion that sampled all edges of the container11. Immediately after collection, mosquito larvae were put on plastic bags with water-filled from the water container until processed later that same day. In the laboratory with a stereoscopic microscope, all live mosquito larvae were identified to species level using Rattanarithikul and Panthusiri's keys12. In this study, the first, second instars and pupae were discarded because immature mosquitoes at these stages could not be identified and found in such a low number (less than five percentage of total mosquito larvae collected). Immature mosquitoes at these stages were never found in water containers without the third and fourth instars. Discarding immature mosquitoes at these stages should not affect mosquito larval indices [i.e. Container Index (CI), House Index (HI) and Breteau Index (BI)]13,14.
There were a total of 21 container categories in this study. Indoor containers were all containers inside the house that held some water such as small water jars, large water jars, cement tanks, plastic tanks, ant guards, flower vases and refrigerator drainages. Outdoor containers were all containers within 15 m of the house, such as small water jars, large water jars, cement tanks, plastic tanks, areca husks, coconut shells, used cans, used tires, plastic bottles, discarded objects, animal pans, pot saucers, plant pots and bamboo clumps. For the water jar, we classified water jars into two categories: small water jars (<500 L) and large water jars (≥500 L).
Data collection for predictive model: The computerized data sets were obtained on monthly-reported dengue cases in Kanthararom district for the period of January 2004-December 2010 from the Kanthararom Health Office, Bureau of Epidemiology, Department of Disease Control, Ministry of Public Health. Weather data over the same period were obtained from the Thai Meteorological Department (TMD) consisting of monthly rainfall, the number of rainy days, daily maximum rainfall, relative humidity, min/mean/max temperatures, sunshine and evaporation.
Statistical analysis: Chi-square test was used to find the differences between seasonal, area and mosquito species factors of the number of Ae. aegypti and Ae. albopictus larvae. Mosquito larval indices - i.e. CI, HI and BI were calculated. Ae. aegypti and Ae. albopictus larval indices were developed as per standard WHO guidelines1. All tests were two-tailed.
Spearman's correlation analysis was conducted to examine the relationship between monthly dengue incidence and weather variables with a lag of zero to three months. Poisson regression adjusted for auto-correlation, secular trend and lag effects, was used to quantify the relationship between weather variables and dengue incidence. In order to control for a potential long-term trend in the number of cases over the study period, a year variable was included in the regression model.
Results
Aedes larvae, breeding habitats, season and area: A total of 2,326 Ae. aegypti larvae and 300 Ae. albopictus larvae were found during the one year sampling period. There were more Ae. aegypti larvae per household than Ae. albopictus larvae per household in the winter (P<0.05) and rainy (P<0.001) seasons but the number of Ae. aegypti and Ae. albopictus larvae did not differ in the summer season (Fig. 1). There were more Aedes larvae per household in the rainy season than in the winter (P<0.05) and summer seasons (P<0.001) in both urban and rural areas. The number of Aedes larvae per household found in the urban area did not differ from that for the rural area.
Fig. 1.
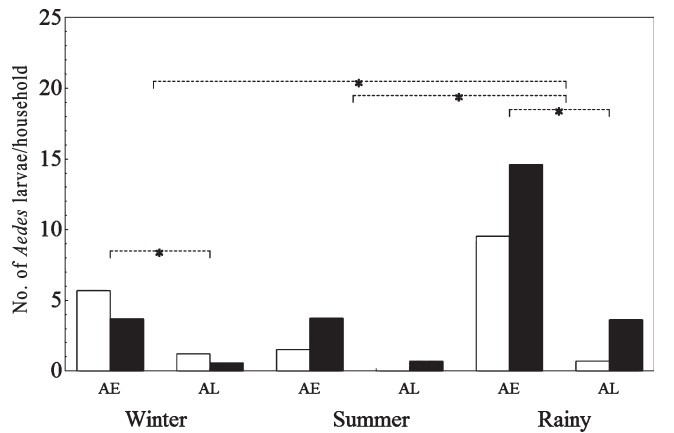
Number of Ae. aegypti (AE) and Ae. albopictus (AL) larvae per household in urban (□) and rural (◾) areas in winter, summer and rainy seasons at Sisaket, Thailand from January to December 2010. Horizontal lines indicate the statistical differences between the means.
In the winter season, Ae. aegypti larvae were found mostly in outdoor plastic tanks in the urban area whereas Ae. aegypti larvae were found mostly in used tires, pot saucers and outdoor plastic tanks in the rural area. Ae. albopictus larvae were found mostly in outdoor concrete tanks both in the urban and rural areas. In the summer season, Ae. aegypti larvae were found mostly in plastic bottles in the urban area whereas, Ae. aegypti larvae were found mostly in bamboo clumps and animal pans in the rural area. No Ae. albopictus larvae were found in the urban area. Ae. albopictus larvae were found mostly in indoor large water jars. In the rainy season, Ae. aegypti larvae were found mostly in indoor cement tanks both in the urban and rural areas. Ae. albopictus larvae were found mostly in bamboo clumps and used tires in the rural area.
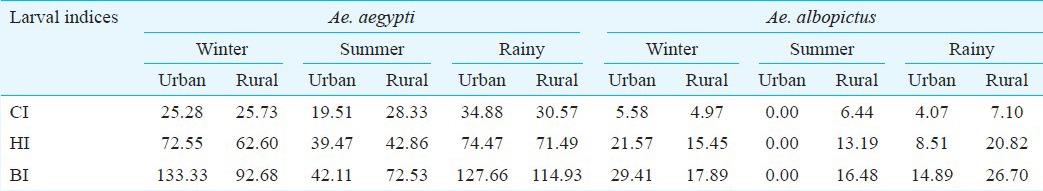
Larval indices, season and area: CI for Ae. aegypti was higher than CI for Ae. albopictus in all seasons. CI in the rainy season was higher than CI in the winter and summer seasons. HI for Ae. aegypti was higher than HI for Ae. albopictus in all seasons. The HI in the summer season was lower than HI in the winter and rainy seasons. BI for Ae. aegypti was higher than that for Ae. albopictus in all seasons. BI in the summer season was lower than BI in the winter and rainy seasons (Table I).
Table I.
Container, House and Breteau indices (CI, HI, BI) of Ae. aegypti and Ae. albopictus larvae in different seasons
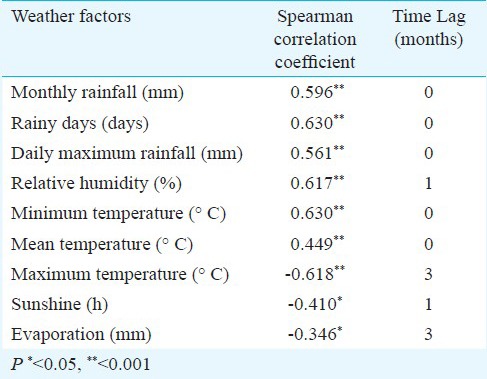
Impact of weather variability on dengue transmission: Monthly rainfall, the number of rainy days, daily maximum rainfall, relative humidity, and min/mean temperature, at a lag between zero to three months, were positively associated with dengue incidence in Sisaket over the study period (Table II). Maximum temperature, sunshine and evaporation were negatively correlated with dengue incidence at a lag of zero to three months (Table II).
Table II.
Spearman correlation coefficients between dengue incidence rate and weather factors at Sisaket, Thailand
The incidence rate was first-order autoregressive indicating that the dengue incidence rate in the current month was related to the incidence rate occurring in the previous month (β = 0.431, P<0.001, Table III). Relative humidity at a lag of one month had a negative effect on dengue incidence (β = -0.045, P<0.001, Table III). The number of rainy days had a positive effect on dengue incidence of the same month (β = 0.060, P<0.001, Table III). The year of occurrence was included in the model as an independent variable (β = 0.094, P<0.05, Table III) indicating that there was a long-term decline and increase in the number of dengue cases notified over the study period (Fig. 2).
Table III.
Poisson regression coefficients of weather data on dengue cases in Sisaket, Thailand
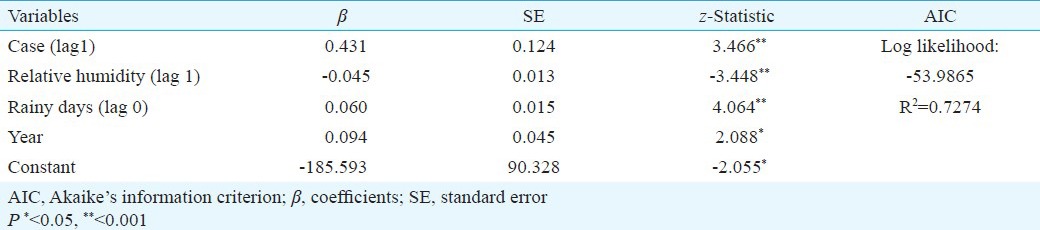
Fig. 2.
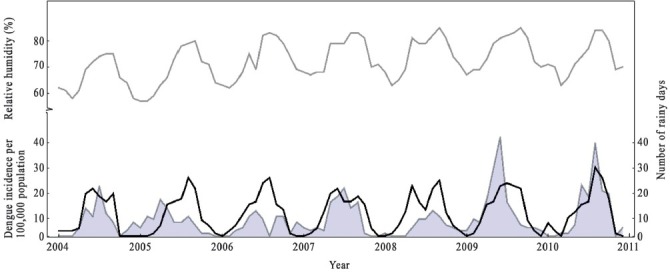
Relative humidity (%) (gray line), the number of rainy days (black line) and dengue incidence per 100,000 population (shaded area) in Sisaket, Thailand from January 2004 to December 2010.
Weather Data Source: Thai Meteorological Department.
The time series Poisson regression model was constructed with the data for the period January 2004-December 2010 (Fig. 3). The model demonstrated goodness-of-fit with a correlation between observed and predicted number of dengue incidence rate of 72.74 per cent (Table III, Fig. 3). The goodness-of-fit analyses revealed that the model fitted the data reasonably well.
Fig. 3.
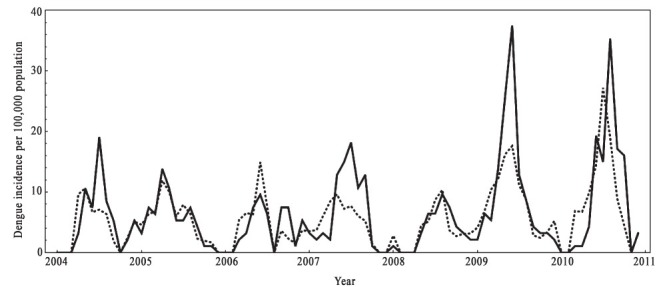
The actual dengue cases (continuous line) and the predicted dengue cases (dotted line) from January 2004 to December 2010 by the Poisson regression model in Sisaket, Thailand.
Source: Kanthararom Health Office, Bureau of Epidemiology, Department of Disease Control, Kanthararom.
Discussion
This study demonstrated that the number of Aedes larvae was higher in the rainy season than in the winter and summer seasons. Many studies have reported similar findings in many other parts of Thailand10,11,15,16 and Côte d’Ivoire17. The seasonality of Aedes larvae in Sisaket showed a similar pattern to that observed by Mogi et al15 in Chiang Mai, Thailand, that Aedes larvae remained low in summer and winter seasons, but increased in the rainy season. However, Aedes larvae in Bangkok showed a non-seasonal fluctuation pattern due to water-filled containers being present year round18. Scarcity of larval habitats seems to be a limiting factor.
Our results support previous findings that the number of Ae. aegypti larvae was higher than that of Ae. albopictus larvae in the winter and rainy seasons10,11. Although Ae. aegypti was introduced to Thailand a long time ago, possibly via rubber tires, and Ae. albopictus is native to Thailand12, Ae. aegypti is now the primary dengue vector and has greater prevalence than Ae. albopictus. This could be due to four possible reasons. Firstly, Ae. aegypti larvae are competitively superior to Ae. albopictus larvae19. Secondly, Ae. aegypti has a higher net reproductive rate than Ae. albopictus15. Thirdly, eggs of Ae. aegypti are more desiccation-resistant than those of other Aedes species17. Fourthly, the females of these two species select different ovipositing habitats. Ae. aegypti females prefer to oviposite in all types of artificial and natural indoor water containers, whereas Ae. albopictus females prefer to oviposite in outdoor habitats, especially trash containers10,11. This could be due to the fact that Ae. albopictus larvae are more tolerant of food shortage than Ae. aegypti larvae15.
Ae. aegypti is a highly domesticated mosquito and is widely found in urban and residential areas15. Aedes mosquito has shown some adaptation toward urbanization. For example, Aedes eggs in urban areas, where there is limited vegetation and low humidity, are more resistant to desiccation than conspecific strains in rural areas20. As water supply is readily available in the urban area, residents have no need to store water inside and around the house. The possible larval habitat for Aedes mosquitoes in the urban area is the concrete drainage systems. Aedes larvae require clear, but not necessarily clean water21. Our results contradict the previous findings that the number of Aedes larvae in the urban area did not differ from that in the rural area. This could be due to the fact that degree of urbanization in Sisaket province is not yet significant enough to cause the difference. In some highly urbanized areas of Southeast Asia, such as Bangkok and Manila, Ae. aegypti has replaced Ae. albopictus22. The growing urban population and the increasing population mobility with rapid development of transportation are the risk factors that may contribute to further geographic expansion and density of Aedes23.
In this study, all three Aedes larval indices (CI, HI, BI) for Ae. aegypti were higher than that for Ae. albopictus in all seasons, and significantly higher than those accepted by WHO1. Larval indices are important to vector control efforts because these provide the means to prioritize locations or categories of larval habitats and evaluate the entomologic effectiveness of control measures.
It has been proposed that understanding weather variables can increase the predictive power of dengue models24. The relationship between weather and dengue has been assessed in multiple settings using different statistical methods5,6,7,8. However, mosquito population dynamics vary in different geographic regions where dengue is transmitted, suggesting that the influence of weather on dengue may be site-specific25. Our results indicated that an increase in the number of rainy days and a decrease in relative humidity were associated with an increase of dengue incidence in Sisaket. Rainfall, daily maximum rainfall, minimum/maximum/mean temperatures, sunshine and evaporation were associated with the dengue incidence, while these failed to enter the best fitting predictive model.
Production of mosquitoes is determined by the availability of suitable and sufficient habitat for the larval stages and this is dependent on rainfall26. Rainfall has been found to correlate with dengue in many countries such as Thailand5,6 and Brazil8. This study found that rainfall, rainy days and daily maximum rainfall were positively associated with the dengue incidence. Increased rain may increase larval habitat and vector population size by creating a new habitat or increase adult survival27. However, in tropical areas in particular, extensive and continuous rainfall can delay the build-up of some mosquito species until late in the season and thus delay transmission26.
Relative humidity influences longevity, mating, dispersal, feeding behaviour, egg production, oviposition of mosquitoes and dengue virus transmission28,29. Adult survival and hatching rate are affected by the rise in temperature and lower humidity30. In the predictive model, relative humidity at a lag of one month was negatively associated with dengue incidence. This suggests that less humidity of the previous month might trigger a higher incidence of dengue epidemics. This is because relatively lower humidity in the surrounding environment could assist mosquitoes in seeking target hosts and facilitate disease transmission7.
The regression model indicates that the number of dengue cases in a current month can be estimated by observing the number of dengue cases occurring in the previous month. This may provide a warning indicator to the local communities and health authorities. The dengue incidence corresponds with the number of Aedes larvae in the study site; the number of Ae. aegypti and Ae. albopictus larvae was highest in the rainy season when dengue incidence outbreak was observed in the area. Previous studies conducted in Thailand10,11 suggest that the seasonal patterns of dengue outbreaks coincide with the rainy season. Two peaks were observed in 2009 and 2010 with 37.51 and 35.32 cases respectively per 100,000 population and might be affected by the weather conditions.
In conclusion, the number of Ae. aegypti larvae and larval indices for Ae. aegypti were higher than that for Ae. albopictus in Sisaket. An increase in the number of rainy days and a decrease in relative humidity were associated with increase in dengue incidence.
Acknowledgment
Authors thank Thana na Nagara, John Endler, and Leslie Gordon for constructive comments. Financial support from the Thailand Research Fund through the Royal Golden Jubilee Ph.D. Program (Grant No. PHD/0201/2548) to Siriwan Wongkoon and Mullica Jaroensutasinee, Walailak University Fund 06/2552 and Center of Excellence for Ecoinformatics, NECTEC/Walailak University are acknowledged. Authors also thank the Kanthararom Health Office, the Ministry of Public Health for dengue cases data, Thai Meteorological Department for weather data and Satapisat Kraisee and students from Kanthararom School for field surveys in this study.
References
- 1.France: World Health Organization; 2009. WHO. Dengue: Guidelines for diagnosis, treatment, prevention and control. [PubMed] [Google Scholar]
- 2.Gubler DJ. Dengue and dengue hemorrhagic fever; its history and resurgence as a global public health problem. In: Gubler DJ, Kuno G, editors. Dengue and dengue hemorrhagic fever. London: CAB International Press; 1997. pp. 1–22. [Google Scholar]
- 3.Dengue incidence situation. Thailand: Department of Disease Control, Ministry of Public Health; 2010. Dengue Section. [Google Scholar]
- 4.Bi P, Zhang Y, Parton KA. Weather variables and Japanese encephalitis in the metropolitan area of Jinan city, China. J Infect. 2007;55:551–6. doi: 10.1016/j.jinf.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Wongkoon S, Jaroensutasinee M, Jaroensutasinee K. Climatic variability and dengue virus transmission in Chiang Rai, Thailand. Biomedica. 2011;27:5–13. [Google Scholar]
- 6.Thammapalo S, Chongsuwiwatwong V, McNeil D, Geater A. The climatic factors influencing the occurrence of dengue hemorrhagic fever in Thailand. Southeast Asian J Trop Med Public Health. 2005;36:191–6. [PubMed] [Google Scholar]
- 7.Wu PC, Guo HR, Lung SC, Lin CY, Su HJ. Weather as an effective predictor for occurrence of dengue fever in Taiwan. Acta Trop. 2007;103:50–7. doi: 10.1016/j.actatropica.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 8.Luz PM, Mendes BVM, Codeco CT, Struchiner CJ, Galvani AP. Time series analysis of dengue incidence in Rio de Janeiro, Brazil. Am J Trop Med Hyg. 2008;79:933–9. [PubMed] [Google Scholar]
- 9.Bosello F, Roson R, Tol RSJ. Economy-wide estimates of the implications of climate change: human health. Ecol Econ. 2006;58:579–91. [Google Scholar]
- 10.Thavara U, Tawatsin A, Chansang C, Kong-ngamsuk W, Paosriwong S, Boon-Long J, et al. Larval occurrence, oviposition behavior and biting activity of potential mosquito vectors of dengue on Samui Island, Thailand. J Vector Ecol. 2001;26:172–80. [PubMed] [Google Scholar]
- 11.Wongkoon S, Jaroensutasinee M, Jaroensutasinee K, Preechaporn W. Development sites of Aedes aegypti and Ae. albopictus in Nakhon Si Thammarat, Thailand. Dengue Bull. 2007;31:141–52. [Google Scholar]
- 12.Rattanarithikul R, Panthusiri P. Illustrated keys to the medically important mosquitoes of Thailand. Southeast Asian J Trop Med Public Health. 1994;25(Suppl 1):1–66. [PubMed] [Google Scholar]
- 13.Nguyen LAP, Clements ACA, Jeffery JAL, Yen NT, Nam VS, Vaughan G, et al. Abundance and prevalence of Aedes aegypti immatures and relationships with household water storage in rural areas in southern Viet Nam. Int Health. 2011;3:115–25. doi: 10.1016/j.inhe.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Tewari SC, Thenmozhi V, Katholi CR, Manavalan R, Munirathinam A, Gajanana A. Dengue vector prevalence and virus infection in a rural area in south India. Trop Med Int Health. 2004;9:499–507. doi: 10.1111/j.1365-3156.2004.01103.x. [DOI] [PubMed] [Google Scholar]
- 15.Mogi M, Khambooruang C, Choochote W, Suwanpanitc P. Ovitrap surveys of dengue vector mosquitoes in Chiang Mai, Northern Thailand: serarsonal shifts in relative abundance of Aedes albopitus and Ae. aegypti. Med Vet Entomol. 1988;2:319–24. doi: 10.1111/j.1365-2915.1988.tb00203.x. [DOI] [PubMed] [Google Scholar]
- 16.Strickman D, Kittayapong P. Dengue and its vectors in Thailand: calculated transmission risk from total pupal counts of Aedes aegypti and association of wing-length measurements with aspects of the larval habitat. Am J Trop Med Hyg. 2003;68:209–17. [PubMed] [Google Scholar]
- 17.Guindo-Coulibaly N, Adja AM, Koudou BG, Konan YL, Diallo M, Koné AB, et al. Distribution and seasonal variation of Aedes aegypti in the Health District of Abidjan (Côte d’Ivoire) Eur J Sci Res. 2010;40:522–30. [Google Scholar]
- 18.Tonn RJ, Sheppard PM, Macdonald WW, Bang YH. Replicate surveys of larval habitats of Aedes aegypti in relation to Dengue Hemorrhagic fever in Bangkok, Thailand. Bull World Health Organ. 1969;40:819–29. [PMC free article] [PubMed] [Google Scholar]
- 19.Moore CG, Fisher BR. Competition in mosquitoes. Density and species ratio effects on growth, mortality, fecundity and production of growth retardant. Ann Entomol Soc Am. 1969;62:1325–31. doi: 10.1093/aesa/62.6.1325. [DOI] [PubMed] [Google Scholar]
- 20.Mogi M, Miyagi I, Abadi K, Syafruddin Inter- and intraspecific variation in resistance to desiccation by adult Aedes (Stegomyia) spp.(Diptera: Culicidae) from Indonesia. J Med Entomol. 1996;33:53–7. doi: 10.1093/jmedent/33.1.53. [DOI] [PubMed] [Google Scholar]
- 21.Lee HL. Breeding habitats and factor affecting breeding of Aedes larvae in urban towns of Peninsular Malaysia. J Biosci. 1990;1:107–12. [Google Scholar]
- 22.Hammon WM, Rudnick A, Sather GE. Viruses associated with epidemic hemorrhagic fever of the Philippines and Thailand. Science. 1960;131:1102–3. doi: 10.1126/science.131.3407.1102. [DOI] [PubMed] [Google Scholar]
- 23.El-Badry AA, Al-Ali KH. Prevalence and seasonal distribution of dengue mosquito, Aedes aegypti (Diptera: Culicidae) in Al-Madinah Al-Munawwarah, Saudi Arabia. J Entomol. 2010;7:80–8. [Google Scholar]
- 24.Geneva: World Health Organization; 2004. WHO. Using climate to predict infectious disease outbreaks: A review. [Google Scholar]
- 25.Scott TW, Morrison AC. Aedes aegypti and the risk of dengue virus transmission. In: Takken W, Scott TW, editors. Ecological aspects for application of genetically modified mosquitoes. Dordretch The Natherlands: FRONTIS; 2003. pp. 187–206. [Google Scholar]
- 26.Russell RC. Mosquito-borne arboviruses in Australia: the current scene and implications of climate change for human health. Int J Parasitol. 1998;28:955–69. doi: 10.1016/s0020-7519(98)00053-8. [DOI] [PubMed] [Google Scholar]
- 27.Gubler DJ, Reiter P, Ebi KL, Yap W, Nasci R, Patz J. Climate variability and change in the United States: potential impacts on vector- and rodent-borne diseases. Environ Health Persp. 2001;109(Suppl 2):223–33. doi: 10.1289/ehp.109-1240669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McMichael AJ, Haines A, Kovats RS, Slooff R. Geneva: World Health Organization; 1996. Climate changes and human health. [Google Scholar]
- 29.Lu L, Lin H, Tian L, Yang W, Sun J, Liu Q. Time series analysis of dengue fever and weather in Guangzhou, China. BMC Public Health. 2009;9:395–9. doi: 10.1186/1471-2458-9-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Costa EAPA, Santos EMM, Correia JC, Albuquerque CMR. Impact of small variation in temperature and humidity on the reproductive activity and survival of Aedes aegypti (Diptera, Culicidae) Rev Bras Entomol. 2010;54:488–93. [Google Scholar]


