Abstract
The National Prophylaxis Programme against Nutritional Blindness due to vitamin A deficiency (NPPNB due to VAD) was started in 1970 with the specific aim of preventing nutritional blindness due to keratomalacia. The Programme was launched as an urgent remedial measure to combat the unacceptably high magnitude of xerophthalmic blindness in the country seen in the 1950s and 1960s. Clinical VAD has declined drastically during the last 40 years. Also, indicators of child health have shown substantial gains in different States in the country. The prevalence of severe undernutrition has come down significantly. Immunization coverage for measles and other vaccine preventable diseases has improved from 5-7 per cent in early seventies to currently 60-90 per cent, in different States. Similarly, there has been a significant improvement in the overall dietary intake of young children. There has been virtual disappearance of keratomalacia, and a sharp decline in the prevalence of Bitot spots. Prophylactic mega dose administration of vitamin A is primarily advocated because of the claim of 23 per cent reduction in childhood mortality. However, benefits on this scale have been found only in areas with rudimentary health care facilities where clinical deficiency is common, and there is substantial heterogeneity, especially with inclusion of all trials. There is an urgent need for adopting a targeted rather than universal prophylactic mega dose vitamin A supplementation in preschool children. This approach is justified on the basis of currently available evidence documenting a substantial decline in VAD prevalence, substantial heterogeneity and uncertainty about mortality effects in present era with improved health care, and resource constraints with competing priorities.
Keywords: Massive dose, serum retinol, supplementation, VAD, vitamin A
Introduction
Vitamin A (VA) is an essential nutrient needed in small amounts for the normal functioning of the visual system, growth and development, maintenance of epithelial cellular integrity, immune function and reproduction. Severe deficiency of VA is known to produce corneal xerophthalmia, keratomalacia and blindness in children. Vitamin A deficiency (VAD) is mainly seen amongst the young children as they have high requirements due to increased physical growth and have low dietary intake. Further, episodes of illnesses such as acute respiratory tract infection and measles, which deplete VA reserves from the body, are common in this age group1.
This article focuses on the urgent need for adopting a targeted instead of universal approach for the massive dose vitamin A (MDVA) prophylaxis programme. This approach is justified on the basis of available scientific evidence documenting: (i) a decline in the prevalence VAD in the country, (ii) side effects of MDVA supplementation, and (iii) resource constraints with competing priorities.
Evolution of VA supplementation (VAS) programme in India
The National Prophylaxis Programme against Nutritional Blindness due to Vitamin A Deficiency (NPPNB due to VAD) was initiated in 1970 with the specific aim of preventing nutritional blindness due to keratomalacia2. The Programme was started as a 100 per cent centrally sponsored programme. It was launched as an urgent remedial measure to combat the unacceptably high magnitude of xerophthalmic blindness in the country reported in the 1950s and 1960s. To begin with, this Programme was initiated in 11 States of the country. Evaluation studies conducted by the National Institute of Nutrition (NIN), Hyderabad in 1976 in two States revealed positive results of the Programme. In subsequent years, the Programme was extended to all States in the country3.
In 1994, under the National Child Survival and Safe Motherhood (CSSM) Programme, the NPPNB due to VAD was modified keeping in view of the vulnerability of VA deficiency in young children4. The age group of eligible children for coverage was restricted to 9 to 36 months of age. Accordingly, each child was to receive five doses of VA before her/his 3rd birthday (children age 6-11 months, 1 dose of 100,000 IU of VA and in age 12- 36 months of age one dose of 200,000 IU of VA every six months). In view of operational feasibility, the administration of first dose of VA was linked to measles immunization.
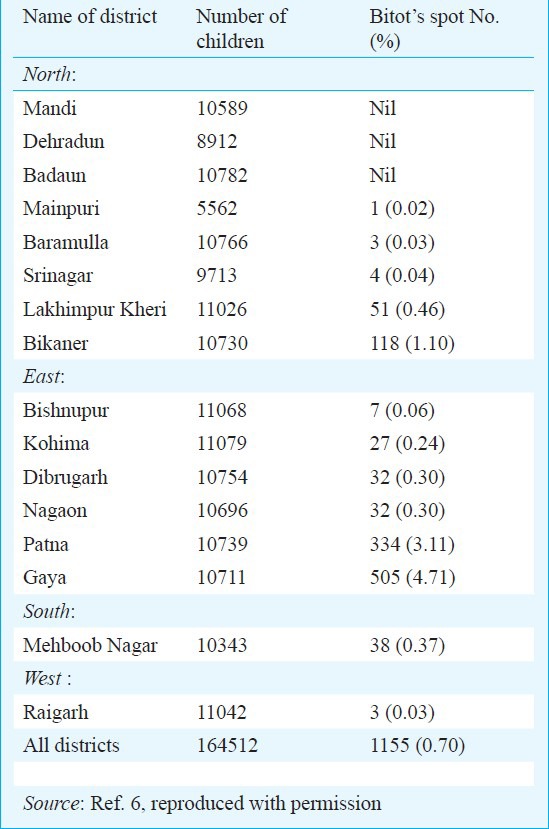
In 2006, the age group of eligible children was revised as 6-59 months. This was done after reconsidering the recommendations of WHO, UNICEF and Ministry of Women and Child Development5. This was despite the evidence that clinical VAD was limited to a few isolated geographical pockets in the country. A National survey conducted by Indian Council of Medical Research (ICMR) in 2001, covering 16 districts in all five regions of the country showed that only three out of 16 districts had prevalence of Bitot spots (BS) of 0.5 per cent and more (Table)6.
Table.
Prevalence of vitamin A deficiency disorders in children (<6 yr)
Presently, vitamin A supplementation (VAS) is implemented through the existing network of primary health centres and sub-centres. The female multipurpose worker and other paramedics at the village level sub-health centres are responsible for administering vitamin A solution. The services of Integrated Child Development Services (ICDS) functionaries are also utilized for the implementation of the Programme. Monthly intensive drives are undertaken every six months to achieve universal coverage of those children who could not be covered during the routine visits of health functionaries.
Current status of VAD
Clinical VAD has declined drastically during the last 40 years. There has been virtual disappearance of keratomalacia, and a sharp decline in the prevalence of Bitot spots7. The decline antedated an efficiently functioning VAS programme; an increase in coverage with universal vitamin A supplementation in recent years was not associated with disappearance or substantial decline of clinical deficiency. Recent surveys indicate that the prevalence of Bitot spots of 0.5 per cent and more (conventional cut-off to define public health problem) is limited to population groups which are socio-economically backward, poverty stricken and have poor health infrastructure. Also, clinical VAD is seen only in certain seasons when green leafy vegetables are in short supply8. Repeat surveys of the National Nutrition Monitoring Bureau (NNMB), ICMR conducted in the same villages have shown that prevalence of Bitot's spots had declined from 1.8 to 0.7 per cent in 1996-1997 as compared with 1975-19799. The prevalence of Bitot's spot amongst children in 6-71 months of age has been most commonly utilized as an indicator of VAD in a population. However, with disappearance of Bitot's spot, now the subclinical deficiency of VA, as assessed by serum retinol (SR) levels is being advocated and utilized as a marker of VAD10. Serum retinol may not be an operationally feasible indicator to be utilized in communities to establish the prevalence of VAD. Estimation of SR requires collection of blood samples of children and sophisticated laboratory equipment11.
Validity of SR in predicting VAD
The acceptability, technical feasibility and performance of SR in predicting VAD in communities has been debated. There are many factors that can influence SR levels and hence its validity as an indicator of VAD has been questioned12. After administration of massive dose of VA, the serum retinol remains elevated only up to 60 days and then comes back to deficiency level for serum cut-off's for VAD. Retinol transport in the blood requires two specific proteins, retinol binding protein (RBP) and transthyretin (TTR). Synthesis of RBP is dependent on child's protein, retinol, iron and zinc status. Zinc status influences several aspects of vitamin A metabolism including its absorption, transport and utilization. Zinc deficiency impairs synthesis of RBP which affects retinol transport from liver to the blood and other tissues13,14. Iron deficiency affects vitamin A metabolism leading to reduction in serum retinol and an increase in hepatic retinol and retinyl esters15. The mobilization of vitamin A from the liver is hampered in protein calorie malnutrition, dietary protein is required to mobilize liver reserves of vitamin A into the blood stream and increased protein intake results in greater vitamin A requirement16. Plasma retinol is reduced by clinical and subclinical infection17,18.
Overestimation of VAD burden with serum retinol
There have been several reports mentioning higher prevalence of VAD in different parts of the country based on serum retinol levels. A recent report documented 61 per cent prevalence of VAD in children in rural areas of West Bengal using estimation of SR. However, simultaneously serum C reactive protein (CRP) levels were not conducted to adjust for concomitant subclinical infection10. Similar studies have been reported from other parts of the country which do not provide true status of the burden of VAD in the country. These studies are based on estimation of SR levels which have not been adjusted for CRP levels11,12. Also additional factors influencing serum retinol levels were not considered. Vitamin A deficiency burden in India is, therefore, likely to be overestimated on the basis of serum retinol measurements alone.
Reasons for decline in VAD in India
During the last four decades indicators of child health have shown substantial gains in different States in the country. The prevalence of severe undernutrition has come down significantly. Immunization coverage for measles and other vaccine preventable diseases has improved from 5-7 per cent in early seventies to currently 60-90 per cent, in different States. Similarly, there has been a significant improvement in the overall dietary intake of young children19. The Government is also addressing the issue of food insecurity. The ICDS which covers 80 per cent of rural India provides nutritional supplements to children less than six years of age, nutrition education to mothers and also facilitates the distribution of vitamin A supplements. Improvements in infrastructure have led to better access to health care facilities. Food availability in India has improved in the last 30 years. All these factors have positively influenced the VA status of children and led to reduced prevalence of VAD in the country20.
VAS and under five mortality reduction in India
Child deaths can be substantially decreased in any community with the implementation of primary health care activities. Infants and children in economically poor communities die of common diseases whose detection, cure and even prevention can be done with non-expensive appropriate technology applied by a primary health care worker. The major causes of child mortality in India are diarrhoea, respiratory infections and low birth weight (prematurity)21. These causes contribute to more than 80 per cent of the child mortality beyond the first month of life. Evidence suggests that vitamin A supplementation does not have any impact on reduction of morbidity and mortality due to diarrhoea, respiratory infections and low birth weight22.
All the populations have different epidemiological characteristics. What is true in Indonesia may not be true for Nigeria and similarly for India. This is also reflected in varying results of randomized control trials for evaluating the impact of VA supplementation on under five mortality, conducted in different countries. Investigations carried out by National Institute of Nutrition (ICMR), Hyderabad did not substantiate the mortality reduction claim of VAS found in other countries of the world23. A meta-analysis of VAS concluded that the findings of these trials are not consistent and there is no scientific evidence as yet in favour or against substantive benefit of universal vitamin A supplementation to children in India24. Subsequently, one of the largest trials exploring the role of massive-dose vitamin A administration in reducing childhood mortality was conducted in 72 blocks in Uttar Pradesh between 1999 and 2004. Children from different areas were given six-monthly massive doses of vitamin A, six-monthly de-worming or both or neither. Approximately one million children were followed and mortality rates in children 1-6 yr of age were recorded. There was no significant difference in death rates between children who received the massive-dose of vitamin A and those who did not25. Thus the collated evidence from India does not support the under five mortality reduction claim of massive dose vitamin A prophylaxis.
Problems with current MDVA policy
Under the present, national government policy a total of nine massive doses of synthetic vitamin A are to be given to all children between the ages of 9 and 60 months irrespective of their socio-economic and nutritional status. In India in 1999-2000, vitamin A administration was briefly linked to pulse polio immunization to universalize coverage rapidly. This approach was discontinued after the Assam tragedy and a strong opposition by the Indian Academy of Pediatrics26.
Unjustified claims of benefit
Administration of massive doses of vitamin A is advocated on the grounds that this could bring about a 23 per cent reduction in mortality. However, benefits on this scale have been found only in areas with rudimentary health care facilities where clinical deficiency is common, and the biological mechanisms suggested as explanations are conjectural. Two recent Cochrane systematic reviews assessing the effects and safety of vitamin A supplementation in children 6-59 months of age have been published27,28. One review evaluated the effectiveness of vitamin A supplements in the prevention of morbidity and mortality in children 6-59 months of age. It showed that giving vitamin A supplements to children reduces the rates of mortality and some diseases27. A meta-analysis of 17 trials (11 in Asia, 5 in Africa and 1 in Latin America) for all-cause mortality indicated that vitamin A reduces the overall risk of death by 24 per cent [risk ratio (RR) 0.76; 95% confidence interval (CI) 0.69-0.83]. When an unpublished cluster-randomized trial involving one million children in north India (the DEVTA trial) was considered, vitamin A supplementation reduced the effect size of all-cause mortality from 24 to 12 per cent (RR 0.88; 95% CI 0.84-0.94)26. Further, when only Indian trials were considered, there was no evidence of a mortality benefit24.
Indiscriminate mega-dosing
Universal supplementation of vitamin A to Indian children is being undertaken irrespective of their family background and nutritional status. However, deficiency is now limited to isolated geographical pockets in the country. Also, there is no evidence of benefit of VA supplementation of children without clinical signs of deficiency. Vitamin A is toxic in high doses. The mega-dose of vitamin A (200, 000 IU) given to children is 500 times higher than the daily recommended dose (400 IU). Children hospitalized for acute infectious diseases with low vitamin A status on admission tend to benefit from high-dose supplements, but no benefits and even adverse effects are observed among those with adequate pre--admission vitamin A status29. It is, therefore, inappropriate to administer a pharmacological dose of vitamin A to a child whose vitamin A status is adequate. At present, the National Programme for Prophylaxis against Nutritional Blindness aims at universal synthetic vitamin A supplementation in all States without consideration of prevalence of deficiency, immunization status, under five mortality rate, dietary intake of vitamin A and access to health care. For example, despite widely varying child health and nutrition indicators, the States of Kerala and Uttar Pradesh have the same policy of vitamin A supplementation.
Possible adverse effects
The potential adverse effects of administering a pharmacological dose of vitamin A to a child who is not suffering from deficiency, have not received due attention.
Bulging fontanel: Nearly12 per cent of young children when administered 50,000 IU of VA developed bulging fontanel30. A significant proportion of brain development takes place before three years of age. In India, as per the National Family Health Survey (NFHS)-331 48 per cent of children suffer from undernutrition (below minus 2 SD). Subjecting these malnourished under three children to repeated episodes of increased intra-cranial tension could contribute to retarded brain development. There is lack of scientific evidence on the long term ill effects of these repeated episodes of raised intra-cranial tension on brain development of intrauterine growth retarded children who start their lives with psychomotor deficits32,33.
Vitamin D antagonism: Animal studies suggest that vitamin A is an antagonist of vitamin D action. Massive doses of vitamin A intensify the severity of bone demineralization and inhibit the ability of vitamin D to prevent such demineralization. Increasing amounts of retinyl acetate produce progressive and significant decrease in total bone ash and increase in epiphyseal plate width. Increasing the levels of retinyl acetate abrogates the ability of vitamin D to elevate the level of serum calcium34,35,36,37. Considering, the current epidemic prevalence of vitamin D deficiency in the country, interventions potentially detrimental for bone health (massive dose VAS) are best avoided22.
Potential zinc deficiency: There is a possibility that zinc deficiency, which is already present in these children, could be aggravated by massive doses of vitamin A. Zinc is required for growth of children. Under these circumstances, the administration of massive doses of vitamin A to children who may be deficient in multiple vitamins including vitamin D and zinc could aggravate growth retardation. The potential role of massive-dose vitamin A prophylaxis in the persistence of stunting in poor children requires serious consideration22.
Risk of acute respiratory infection: Vitamin A administration has been associated with a significant increase in rate of pneumonia in well nourished children who received 10,000 IU of supplements weekly. A meta-analysis concluded that ‘VAS has no consistent overall protective effect on the incidence of diarrhoea, however, it slightly increases the incidence of respiratory tract infections; hence, ‘high dose vitamin A supplements are not recommended on a routine basis for all pre school children and should be offered only to individuals or populations with vitamin A deficiency’38.
A recent review of nine randomized controlled trials enrolling 33,179 children with lower respiratory tract infections (31,379 in the community and 1800 in hospital) concluded that vitamin A supplementation was not helpful for preventing pneumonia, at least in normally nourished children, and might rather worsen the situation. According to the investigators, the results should force the policy makers to think twice before continuing or starting a universal vitamin A supplementation programme39.
Cost of vitamin A coverage
According to a recent evaluation, the annual cost per child dosed is 1.14 USD (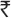 =68) which includes: (i) programme-specific costs 0.42 USD (
=68) which includes: (i) programme-specific costs 0.42 USD (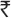 =26), (ii) personnel cost 0.55 USD (
=26), (ii) personnel cost 0.55 USD (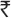 =33) and (iii) capital costs 0.17 USD (
=33) and (iii) capital costs 0.17 USD (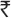 =6)40. The total number of under five children in country will be about 160 million (15% of 1200 million). A cost of
=6)40. The total number of under five children in country will be about 160 million (15% of 1200 million). A cost of 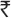 = 8,000 million (160 million x
= 8,000 million (160 million x 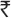 =50) is being spent and a large proportion of this expenditure is being undertaken for questionable health benefits of VAS to non deficient children. Apart from the cost of the micronutrient, the programme also consumes precious human and material resources meant for delivery of primary health care.
=50) is being spent and a large proportion of this expenditure is being undertaken for questionable health benefits of VAS to non deficient children. Apart from the cost of the micronutrient, the programme also consumes precious human and material resources meant for delivery of primary health care.
Need for targeting VAS
WHO has recently issued guidelines41, which provide global, evidence-informed recommendations on the use of vitamin A supplements for the reduction of morbidity and mortality. According to these guidelines, vitamin A supplementation in children 6-59 months of age is recommended in settings where vitamin A deficiency is a public health problem. The mechanisms by which vitamin A reduces mortality are not fully understood, and it is not clear whether its action is mediated through the correction of underlying deficiencies or through adjuvant therapeutic effects41. In the Indian context, it would, therefore, be prudent to restrict massive dose prophylaxis in isolated geographical pockets or areas where clinical vitamin A deficiency is a significant public health problem rather than continuing universal prophylaxis.
What approach should be adopted for VA supplementation?
The era of gross and rampant vitamin A deficiency leading to blindness is past in India. At this stage, we should focus our efforts on sustainable food based approaches to combat vitamin A deficiency. The solution is to increase local production and consumption of green leafy vegetables and other plant foods that are rich sources of carotenoids. Green leafy vegetables, many fruits and other plant foods are also good sources of folate, vitamin C, Fe, Ca and many other micronutrients and bioactive compounds. These contribute to improvement of the overall nutritional status of children and protection against infectious and other diseases. The micronutrient deficiencies are often the result of lack of enough habitual food in the household rather than to the poor quality of such foods. When overall food intake becomes adequate enough to provide basic energy needs, needs of other nutrients would be met to a considerable extent even with the current diets. A food-based approach to combat VAD in non-clinically deficient areas would be a sustainable and cost effective solution42. States of Bihar, Uttar Pradesh and Madhya Pradesh with poor intake of food providing all micronutrients, may have targeted approach of VAS in areas endemic for clinical VAD43.
In India, for cost-effective utilization of limited resources available to the health sector, the ‘Triple A’ (Assessment, Analysis and Action) strategy should be adopted; first, assess the problem of VAD, then undertake the detailed analysis of causes of VAD, and then decide the combination of approaches to be adopted for prevention and control in the community. Countries with limited financial resources and competing health priorities cannot afford the luxury of initiating interventions to raise serum biochemistry alone.
Conclusion
In summary, India is currently at a stage when universal vitamin A supplementation must immediately transit to a targeted supplementation programme. The primary focus should be on adopting sustainable food based approaches to combat vitamin A deficiency.
References
- 1.Geneva: WHO Press; 2002. World Health Organization (WHO). Human vitamin and mineral requirements, World Health Organization, Food and Agriculture Organization of the United Nations, Rome; pp. 22–8. [Google Scholar]
- 2.New Delhi: Government of India Press; 1970. Maternal and child health scheme for prophylaxis against nutritional blindness in children caused by vitamin A deficiency. Family Planning Programme, Fourth Five-Year Plan Technical Information: MCH No. 2; pp. 1–22. [Google Scholar]
- 3.Kapil U, Chaturvedi S, Nayar D. National nutrition supplementation programmes. Indian Pediatr. 1992;29:1601–13. [PubMed] [Google Scholar]
- 4.New Delhi: Government Press; 1994. Vitamin A prophylaxis. In: National child survival and safe motherhood programme, MCH Division, Department of Family Welfare, Ministry of Health and Family Welfare, Government of India; pp. 88–93. [Google Scholar]
- 5.Government of India, Ministry of Health and Family Welfare, Department of Family Welfare, Child Health Division, Order no. Z.28020/30/2003-CH dated 2 November 2006. [accessed on September 12, 2013]. Available from: http://www.poshan.nic.in/jspui/bitstream/DL/389/1/mcn-vitamin-a-ifa-supplementation.pdf .
- 6.Toteja GS, Singh P, Dhillon BS, Saxena BN. Vitamin A deficiency disorders in 16 districts of India. Indian J Pediatr. 2002;69:603–5. doi: 10.1007/BF02722689. [DOI] [PubMed] [Google Scholar]
- 7.Kapil U. Ethical issues in vitamin A supplementation in India. Indian J Prac Doctor. [accessed on December 2, 2012]. Available from: http://www.indmedica.com/jounals.php/12?journalid=3&issueid=132&articleid=1745&action=article .
- 8.National Consultation on benefits and safety of administration of vitamin A to pre-school children and pregnant and lactating women. Indian Pediatr. 2001;38:37–42. [PubMed] [Google Scholar]
- 9.Hyderabad, India: National Institute of Nutrition, Indian Council of Medical Research; 2006. National Nutrition Monitoring Bureau (NNMB). Prevalence of vitamin A deficiency among rural preschool children. Report No 23. [Google Scholar]
- 10.Arlappa N, Balakrishna N, Laxmaiah A, Nair KM, Brahmam GN. Prevalence of clinical and sub-clinical vitamin A deficiency among rural preschool children of West Bengal, India. Indian Pediatr. 2010;48:47–9. doi: 10.1007/s13312-011-0023-z. [DOI] [PubMed] [Google Scholar]
- 11.Arlappa N, Laxmaiah A, Balakrishna N, Harikumar K, Brahmam GNV. Clinical and sub-clinical vitamin A deficiency among rural pre-school children of Maharashtra. Ann Hum Biol. 2008;35:606–14. doi: 10.1080/03014460802380778. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization (WHO) Geneva: WHO; 1996. Indicators for assessing Vitamin A deficiency and their application in monitoring and evaluating intervention programmes. [Google Scholar]
- 13.Christian P, West PK. Interaction between zinc and vitamin A. Am J Clin Nutr. 1998;68(Suppl 2):4355–415. doi: 10.1093/ajcn/68.2.435S. [DOI] [PubMed] [Google Scholar]
- 14.Munoj EC, Rosado JL, Lopez P, Furr HC, Allen LH. Effect of iron and zinc supplementation improves indicators of vitamin A status of Mexican preschoolers. Am J Clin Nutr. 2000;71:789–94. doi: 10.1093/ajcn/71.3.789. [DOI] [PubMed] [Google Scholar]
- 15.Oliveira JM, Michelazzo FB, Stefanello J, Ronodo PH. Influence of iron on vitamin A nutritional status. Nutr Rev. 2008;66:141–7. doi: 10.1111/j.1753-4887.2008.00018.x. [DOI] [PubMed] [Google Scholar]
- 16.De Fatima C, Caninha M, Da Sebra Divinz A. Serum retinol concentrations in hospitalized severe protein energy malnourished children. J Trop Pediatr. 2008;54:248–52. doi: 10.1093/tropej/fmn018. [DOI] [PubMed] [Google Scholar]
- 17.Christian P, Schulze K, Stoltzfus RJ, West KP., Jr Hyporetinolemia, illness symptoms, and acute phase protein response in pregnant women with and without night blindness. Am J Clin Nutr. 1998;67:1237–43. doi: 10.1093/ajcn/67.6.1237. [DOI] [PubMed] [Google Scholar]
- 18.Thurnham DI, McCabe GP, Northrop-Clewes CA, Nestel P. Effect of sub-clinical infection on plasma concentrations and assessment of prevalence of vitamin A deficiency: meta analysis. Lancet. 2003;362:2052–8. doi: 10.1016/s0140-6736(03)15099-4. [DOI] [PubMed] [Google Scholar]
- 19.Hyderabad: National Institute of Nutrition, Indian Council of Medical Research; 1997. 25 Years of National Nutrition Monitoring Bureau. [Google Scholar]
- 20.Gopalan C, Tamber B. Food-based approaches to prevent and control micronutrient malnutrition: scientific evidence and policy implications. World Rev Nutr Diet. 2003;91:76–131. doi: 10.1159/000069925. [DOI] [PubMed] [Google Scholar]
- 21.Bang Abhay T, Bang Rani A, Baitule Sanjay B, Reddy M. Hanimi, Deshmukh Mahesh D. Effect of home-based neonatal; care and management of sepsis on neonatal mortality: field trial in rural India. Lancet. 1999;354:1955–61. doi: 10.1016/S0140-6736(99)03046-9. [DOI] [PubMed] [Google Scholar]
- 22.Gopalan C. Massive dose vitamin A prophylaxis should now be scrapped. World Nutr. 2010;1:79–85. [Google Scholar]
- 23.Vijayaraghavan K, Radhaiah G, Prakasam BS, Sarma KV, Reddy V. Effect of massive dose vitamin A on morbidity and mortality in Indian children. Lancet. 1990;336:1342–5. doi: 10.1016/0140-6736(90)92895-o. [DOI] [PubMed] [Google Scholar]
- 24.Gupta P, Indrayan A. Effect of vitamin A supplementation on childhood morbidity and mortality: critical review of Indian studies. Indian Pediatr. 2002;39:1099–118. [PubMed] [Google Scholar]
- 25.Awasthi S, Peto R, Read S, Bundy D. Six-monthly vitamin A from 1 to 6 years of age. DEVTA: cluster-randomised trial in 1 million children in North India. 2007. [accessed on January 1, 2011]. Available from: http://www.ctsu.ox.ac.uk/projects/devta/resolveuid/
- 26.Indian Academy of Pediatrics. Policy on linking vitamin A to the pulse polio program. Indian Pediatr. 2000;37:727. [Google Scholar]
- 27.Imdad A. Vitamin A supplementation for preventing morbidity and mortality in children from 6 months to 5 years of age. Cochrane Database Syst Rev. 2010;12 doi: 10.1002/14651858.CD008524.pub2. CD008524. [DOI] [PubMed] [Google Scholar]
- 28.Irlam JH. Micronutrient supplementation in children and adults with HIV infection. Cochrane Database Syst Rev. 2010;12 doi: 10.1002/14651858.CD003650.pub3. CD003650. [DOI] [PubMed] [Google Scholar]
- 29.Kapil U. Update on vitamin A-related deaths in Assam, India. Am J Clin Nutr. 2004;80:1083–4. doi: 10.1093/ajcn/80.4.1082. [DOI] [PubMed] [Google Scholar]
- 30.De Francisco A, Chakraborty J, Chowdhury HR, Yunus M, Baqui AH, Siddique AK. Acute toxicity of vitamin A given with vaccines in infancy. Lancet. 1993;342:526–7. doi: 10.1016/0140-6736(93)91648-6. [DOI] [PubMed] [Google Scholar]
- 31.Mumbai: International Institute of Population Sciences; 2007. IIPS, ORCMacro. National Family Health Survey (NFHS-3), 2005-06: India. [Google Scholar]
- 32.Kapil U, Tyagi M. Scientific rationale for targeted vitamin A supplementation to children in India. Indian J Community Health. 2011;23:1–3. [Google Scholar]
- 33.Gopalan C. Vitamin A deficiency and child mortality. NFI Bull. 1986;7:3–6. [Google Scholar]
- 34.Rohde CM, Manatt M, Clagett-Darne M, DeLuca HF. Vitamin A antagonizes the action of vitamin D in rats. J Nutr. 1999;129:2246–50. doi: 10.1093/jn/129.12.2246. [DOI] [PubMed] [Google Scholar]
- 35.Ragavan VV, Smith JE, Bilezikian JP. Vitamin A toxicity and hypercalcemia. Am J Med Sci. 1982;283:161–4. doi: 10.1097/00000441-198205000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Freudenheim JL, Johnson NE, Smith EL. Relationships between usual nutrient intake and bone-mineral content of women 35-65 years of age: Longitudinal and cross-sectional analysis. Am J Clin Nutr. 2005;44:863–76. doi: 10.1093/ajcn/44.6.863. [DOI] [PubMed] [Google Scholar]
- 37.Melhus H, Michaëlsson K, Kindmark A, Bergström R, Holmberg L, Mallmin H. Excessive dietary intake of vitamin A is associated with reduced bone mineral density and increased risk for hip fracture. Ann Intern Med. 1998;129:770–8. doi: 10.7326/0003-4819-129-10-199811150-00003. [DOI] [PubMed] [Google Scholar]
- 38.Shah D. Does vitamin A supplementation help in preventing pneumonia? Indian Pediatr. 2009;46:17–27. [PubMed] [Google Scholar]
- 39.Stansfield SK, Pierre-Louis M, Lerebours G, Augustin A. Vitamin A supplementation and increased prevalence of childhood diarrhoea and acute respiratory infections. Lancet. 1993;342:578–82. doi: 10.1016/0140-6736(93)91410-n. [DOI] [PubMed] [Google Scholar]
- 40.Arlington, VA: USAID; 2004. [accessed on May 20, 2011]. USAID. Cost analysis of the national vitamin A supplementation programs in Ghana, Nepal, and Zambia: a synthesis of three studies. Available from: httpf/www.mostproject.org/IVACG/GhanaNepalZambiaSythesis.pdf . [Google Scholar]
- 41.Geneva: WHO; 2011. [accessed on January 20, 2011]. World Health Organization. Guidelines for Vitamin A supplementation in Infants and Children 6-59 months of age. Available from: www.who.int/entity/nutrition/ [Google Scholar]
- 42.Gopalan C. Micronutrient malnutrition in SAARC. The need for a Food-based approach. NFI Bull. 1998;19:1–3. [Google Scholar]
- 43.Kapil U. Time to stop giving indiscriminate massive doses of synthetic vitamin A to Indian children. Public Health Nutr. 2009;12:285–6. doi: 10.1017/S1368980008004448. [DOI] [PubMed] [Google Scholar]


