Abstract
Background:
Elevated plasma homocysteine (Hcy) level has been established as a significant risk factor for venous thrombosis and cardiovascular disease. Homozygosity for the methylenetetrahydrofolate reductase (MTHFR) C677T mutation has been associated with elevated plasma Hcy concentration and may contribute to retinal vein thrombosis (RVT) development. The aim of the present study was to investigate whether the hyperhomocysteinemia and/or homozygosity for the MTHFR C677T mutation are associated with an increased risk for RVT.
Materials and Methods:
Our study population consisted of 73 consecutive patients (50-78 years old) with RVT and 73 control subjects (51-80 years old), matched for age and sex. Genotyping for the MTHFR C677T mutation was performed by polymerase chain reaction-restriction fragment length polymorphism technique and Hcy level was determined by an enzyme immunoassay kit.
Results:
The prevalence of 677TT genotype was higher in patients than control subjects, but the difference in frequency didn't reach a significant value (P = 0.07). The frequency of the 677T allele was 26% and 21.2% in patients and controls, respectively and did not differ significantly between the two groups (odds ratio = 1.3, 95% confidence interval (0.75-2.24), P = 0.33). Fasting plasma total Hcy level was significantly higher in patients than controls (P = 0.001).
Conclusion:
Our study demonstrated that hyperhomocysteinemia, but not the MTHFR C677T mutation, is associated with RVT.
Keywords: Methylenetetrahydrofolate reductase, mutation, polymerase chain reaction-restriction fragment length polymorphism, retinal vein thrombosis, thrombophilia
INTRODUCTION
Retinal vein thrombosis (RVT) is the most common retinal vascular disease after diabetic retinopathy.[1] It is a disease with multifactorial nature, which may affect small, medium, and large ocular vessels.[2] It is generally accepted that a mildly elevated homocysteine (Hcy) level is a risk factor for atherosclerosis.[3] Similarly, several studies have suggested that hyperhomocysteinemia is a risk factor for RVT,[4,5,6] but not all studies have confirmed this.[7,8] Hyperhomocysteinemia may result from dietary deficiencies (especially folate) and/or inherited defects in the controlling enzymes of Hcy metabolism.[9,10] 5, 10-methylenetetrahydrofolate reductase (MTHFR) catalyses the conversion of 5, 10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, which is the main circulating form of folate and the methyl donor for the vitamin B12 dependent remethylation of Hcy to methionine.
A common 677C->T transition in the MTHFR gene results in a thermolabile variant with specific decreased enzymatic activity. The molecular basis of this thermolability is a missense mutation in exon 4 of the MTHFR gene, a cytosine to thymine substitution at nucleotide 677, which converts an alanine to a valine codon. Homozygosity for the MTHFR C677T mutation has been associated with an increased plasma Hcy level, particularly in the presence of suboptimal folate intake.[10]
Studies investigating the role of hyperhomocysteinemia and MTHFR C677T mutation in patients with RVT have yielded conflicting results. Some studies suggest that the thermolabile variant of MTHFR C677T polymorphism is a risk for RVT,[11,12,13] whereas others do not support such an association.[7,8,14,15] Regarding this controversy, we performed a case control study to investigate the prevalence of elevated Hcy level and MTHFR C677T genotypes among patients with RVT and compared the results with age and sex matched control group.
MATERIALS AND METHODS
Study and control subjects
Our study population consisted of 78 consecutive patients with RVT referred to coagulation center of Iranian Blood Transfusion Organization for thrombophilia screening during 2008 and 2009 years in Tehran. The clinical records and the objective documentation of RVT were reviewed by an Ophthalmologist to confirm the diagnosis. Five patients were excluded from the analysis because the diagnosis was uncertain and altogether 73 patients (50-78 years old) were included in the study. The control subjects consisted of 73 individuals (51-80 years old) were matched for age and sex with patients and were selected from Iranian blood transfusion organization stuffs. In addition, some of the control subjects were selected from normal populations, which were resident around Shahid Bagheri dormitory in Tehran. In order to exclude any history of thrombosis and/or use of vitamin B supplements and folate in control subjects, we completed questionnaires regarding many items including age, family history of eye diseases, history of thrombosis, renal failure, liver disease, hypertension, malignancy and use of drugs especially vitamin B supplements, and folate. Informed consent was obtained from all participants. All steps of our research were conducted according to Ethical Committee Guidelines of Iranian Blood Transfusion Organization.
Total plasma Hcy assay
Overnight fasting (12 h) blood samples were drawn from the RVT patients and control subjects. Blood was collected into Ethylenediaminetetraacetic acid (EDTA) -containing tubes and kept on ice. Samples were centrifuged at 4°C and plasma fraction was aspirated and transferred to plastic tube and stored at − 20°C until analysis for total Hcy. Total Hcy was measured with a microplate enzyme immunoassay method using Axis Shield Hcy Enzyme Immunoassay Kit (Axis Shield Diagnostic, Ltd, UK). The assay's detection range was 2.5-50.0 μM.
Deoxyribonucleic acid (DNA) isolation and genetic analysis
DNA was extracted from blood leukocyte according to the method described by Lahiri et al.[16] MTHFR 677 C->T polymorphism was analyzed by polymerase chain reaction (PCR)-restriction fragment length polymorphism method as previously described by Frosst et al. with slight modification.[10] PCR was carried out on 40 μL reaction mixture containing 200 ng genomic DNA in 1 Χ PCR buffer, 2.2 mM MgCl2, 200 μM Deoxyribonucleotide triphosphates (dNTPs), 0.5 μM primers, and 1.5 U AmpliTaq DNA polymerase (Applied Bio systems). The sequences of the primers were: 5’-TGA AGG AGG TGT CTG CGG GA-3’ and 5’-AGG ACG GTG CGG TGA GAG TG-3.’ The PCR conditions included an initial denaturation at 94°C for 2 min, followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 62°C for 30 s, extension at 72°C for 30 s, and a final extension at 72°C for 7 min. The amplified products were digested with 5 units of Hinf I (Promega, Madison, WI, USA) at 37°C for 12 h.
The digested products were separated by electrophoresis on a 3% agarose gel and visualized using ethidium bromide. Individuals with the CC (wild-type homozygosity) genotype showed a single band of 198 bp, those with the CT (heterozygosity) genotype showed bands of 198 bp and 175 bp, and those with the TT (mutated homozygosity) genotype showed a single band of 175 bp [Figure 1].
Figure 1.
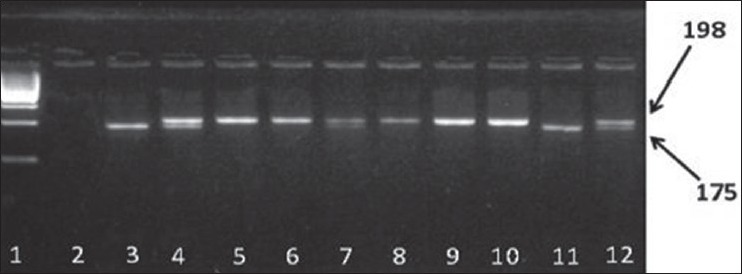
Polymorphism analysis of methylenetetrahydrofolate reductase C677T. The polymerase chain reaction products were digested by restriction enzyme Hinf I:1 (ladder 100 bp); 2 negative control; 3 positive control for homozygote (TT) genotype; 4 positive control for heterozygote (CT) genotype; 5, 6, 8, 9, 10 CC genotype (198 bp); 7, 12 CT genotype (198 bp, 175 bp); 11 TT genotype (175 bp)
Statistical analysis
Allele frequencies were calculated by gene counting in RVT patients and control subjects. Mean age and total Hcy level differences between patients and controls were tested by Student t-test. Chi-square test was used to assess the association of MTHFR C677T mutation with RVT and odds ratio (OR) along with 95% confidence interval (CI) was calculated. Chi-square test also was used to analyze the distribution of MTHFR C677T genotypes between male and female in RVT patients and controls, and to test sex differences between patients and controls. Statistical analysis was performed by statistical package for the social sciences 15 (SPSS 15) software with a statistical significance level of P < 0.05.
RESULTS
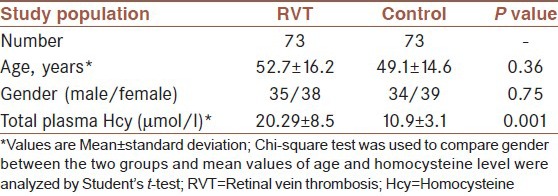
Our study population consisted of 73 patients with RVT and 73 apparently healthy subjects matched for age and sex as control. As shown in Table 1, between the RVT patients and control subjects, no significant differences were observed in mean age and gender. Mean plasma Hcy level was significantly higher in the RVT patients than in the control subjects (P = 0.001).
Table 1.
Homocysteine level and other characteristics in RVT patients and control subjects
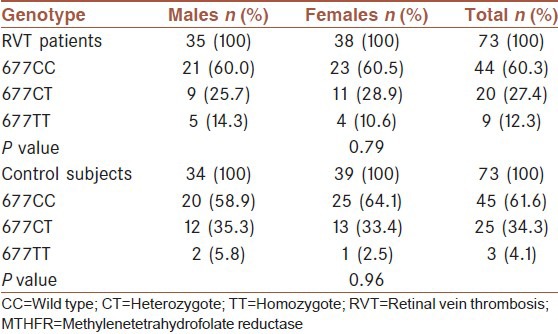
The distribution of genotypes and the relative allele frequency of the C677T polymorphism of the MTHFR gene in RVT patients and controls are given in Table 2. The frequency of the 677TT genotype in RVT patients was higher than control subjects, but the difference did not reach a significant value (P = 0.07). Moreover, given to our null hypothesis, T allele variation may be involved in the disease process. Therefore, in order to remove the effects of confounding factors such as age and sex, T allele frequency was adjusted for age and sex. The frequency of the mutant T allele in the RVT patients and control subjects after adjusting for age and sex was 26% and 21.2%, respectively and did not differ significantly between the two groups (P = 0.33). In addition, the OR for RVT related to the 677T allele was 1.3, 95% CI (0.75-2.24), confirming the absence of an association. Furthermore, there was a significant increase in mean serum Hcy level of subjects carrying TT genotype compared to subjects carrying CC and CT genotype in both patients (P = 0.001) and controls (P = 0.03) as shown in Table 3. According to Table 4, no significant difference in the distribution of genotypes of the MTHFR C677T mutation was observed between males and females in RVT patients (P = 0.79) and also control subjects (P = 0.96).
Table 2.
MTHFR C677T genotypes and allelic frequencies of RVT patients and control subjects
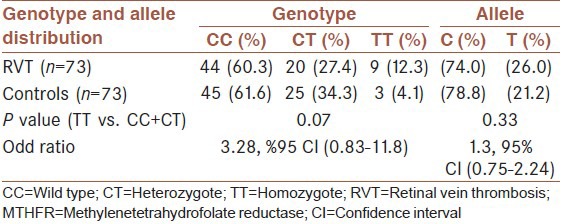
Table 3.
Effects of MTHFR C677T genotypes on total Hcy level in patients and controls
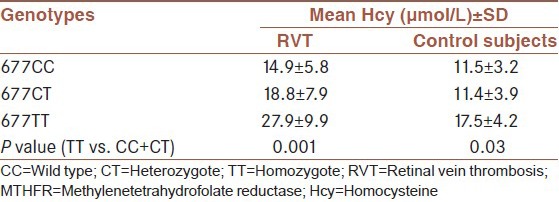
Table 4.
the prevalence rate of MTHFR C677T polymorphism according to gender in RVT patients and control subjects
DISCUSSION
RVT is a disease that is relatively common in elderly people. According to Blue Mountains Eye Study, The frequency of retinal vein occlusion is 0.7% in individuals younger than 60 years, 1.2% in individuals aged 60-69 years, and 4.6% in individuals older than 80 years.[1] The identification of risk factors may have a significant role in the prevention of this potentially warning disorder.
Hyperhomocysteinemia has been reported to be associated with venous thrombotic disorders in several studies.[10,17] Our study demonstrated that elevated plasma Hcy level is a risk factor for RVT; thus, confirming the results of other investigators reported a positive correlation between hyperhomocysteinemia and RVT.[4,5,6,15,18,19] Our result also is inconsistent with some studies reporting no association of hyperhomocysteinemia with RVT.[7,8,20]
Increased concentration of plasma Hcy level may result from acquired factors (e.g., deficiencies of vitamins such as folic acid, pyridoxine, and cobalamin),[21] and genetic factors (e.g., Mutation in MTHFR gene).[22] The C677T mutation, the best characterized mutation of MTHFR gene has been reported to result in a less active enzymatic variant that putatively leads to increased total Hcy level.[22]
Our study also demonstrates that 677TT genotype is associated with elevated Hcy level, so verifying the results of other studies.[17,22] Despite the relationship of 677TT genotype with elevated Hcy level, the association of MTHFR 677TT genotype with RVT remains controversial. Some studies have indicated MTHFR 677TT genotype as a risk factor for RVT[11,12,13,23] while other studies did not prove such an association.[7,8,14,15,24,25]
Although, in our study, a higher prevalence of 677TT genotype was seen in patients than controls, but the difference was not significant. So, our study was consistent with those studies showed no relationship of MTHFR 677 TT genotype with RVT.[7,8,14,15,24] The reasons for non-replication of association studies are numerous and many factors such as population heterogeneity, ethnic stratification, and sample size may contribute to variable association results. Population-specific linkage disequilibrium between markers and causal variants, variation in study design, confounding sampling bias, misclassification of phenotypes, and gene-gene and gene-environment interactions are other factors that influence genetic association results.
The results of the current study show a relationship between hyperhomocysteinemia and RVT, but do not confirm a causal relationship. Whether the elevated plasma Hcy level is a cause or merely a consequence of RVT cannot be confirmed by studies with a retrospective design like ours. Interventional trials will be needed to evaluate its potential causal role and the effectiveness of Hcy lowering treatment for diminishing the risk of RVT development.
Possible mechanisms by which elevated plasma total Hcy level may contribute to RVT include activation of factor V, inhibition of protein C activation, promoting endothelial tissue factor expression and suppressing endothelial cell surface heparin sulfate.[21,26] Since hyperhomocysteinemia can be safely and easily corrected by vitamin supplementation, Hcy should be routinely measured in RVT patients, and even slightly elevated plasma Hcy level should be treated to decrease the risks associated with RVT.[25]
CONCLUSION
Based on this study, we suggest that hyperhomocysteinemia, but not MTHFR C677T polymorphism is a significant risk factor for RVT development.
ACKNOWLEDGMENT
We greatly appreciate the assistance provided by Dr. Zohre Sharifi of the coagulation center of Iranian blood transfusion organization. We also thank all the participants for their cooperation in the study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Cugati S, Wang JJ, Rochtchina E, Mitchell P. Ten-year incidence of retinal vein occlusion in an older population: The Blue Mountains eye study. Arch Ophthalmol. 2006;124:726–32. doi: 10.1001/archopht.124.5.726. [DOI] [PubMed] [Google Scholar]
- 2.Cheung N, Klein R, Wang JJ, Cotch MF, Islam AF, Klein BE, et al. Traditional and novel cardiovascular risk factors for retinal vein occlusion: The multiethnic study of atherosclerosis. Invest Ophthalmol Vis Sci. 2008;49:4297–302. doi: 10.1167/iovs.08-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fegan CD. Central retinal vein occlusion and thrombophilia. Eye (Lond) 2002;16:98–106. doi: 10.1038/sj.eye.6700040. [DOI] [PubMed] [Google Scholar]
- 4.Janssen MC, den Heijer M, Cruysberg JR, Wollersheim H, Bredie SJ. Retinal vein occlusion: A form of venous thrombosis or a complication of atherosclerosis? A meta-analysis of thrombophilic factors. Thromb Haemost. 2005;93:1021–6. doi: 10.1160/TH04-11-0768. [DOI] [PubMed] [Google Scholar]
- 5.Salaun N, Delyfer MN, Rougier MB, Korobelnik JF. Assessment of risk factors for retinal vein occlusions in patients under 60 years of age. J Fr Ophtalmol. 2007;30:918–23. doi: 10.1016/s0181-5512(07)74029-9. [DOI] [PubMed] [Google Scholar]
- 6.Turello M, Pasca S, Daminato R, Dello Russo P, Giacomello R, Venturelli U, et al. Retinal vein occlusion: Evaluation of “classic” and “emerging” risk factors and treatment. J Thromb Thrombolysis. 2010;29:459–64. doi: 10.1007/s11239-009-0384-5. [DOI] [PubMed] [Google Scholar]
- 7.Larsson J, Hultberg B, Hillarp A. Hyperhomocysteinemia and the MTHFR C677T mutation in central retinal vein occlusion. Acta Ophthalmol Scand. 2000;78:340–3. doi: 10.1034/j.1600-0420.2000.078003340.x. [DOI] [PubMed] [Google Scholar]
- 8.McGimpsey SJ, Woodside JV, Bamford L, Gilchrist SE, Graydon R, McKeeman GC, et al. Retinal vein occlusion, homocysteine, and methylene tetrahydrofolate reductase genotype. Invest Ophthalmol Vis Sci. 2005;46:4712–6. doi: 10.1167/iovs.04-1229. [DOI] [PubMed] [Google Scholar]
- 9.Young IS, Woodside JV. Folate and homocysteine. Curr Opin Clin Nutr Metab Care. 2000;3:427–32. doi: 10.1097/00075197-200011000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, et al. A candidate genetic risk factor for vascular disease: A common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10:111–3. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 11.Loewenstein A, Goldstein M, Winder A, Lazar M, Eldor A. Retinal vein occlusion associated with methylenetetrahydrofolate reductase mutation. Ophthalmology. 1999;106:1817–20. doi: 10.1016/S0161-6420(99)90357-3. [DOI] [PubMed] [Google Scholar]
- 12.Salomon O, Moisseiev J, Rosenberg N, Vidne O, Yassur I, Zivelin A, et al. Analysis of genetic polymorphisms related to thrombosis and other risk factors in patients with retinal vein occlusion. Blood Coagul Fibrinolysis. 1998;9:617–22. doi: 10.1097/00001721-199810000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Marcucci R, Giusti B, Betti I, Evangelisti L, Fedi S, Sodi A, et al. Genetic determinants of fasting and post-methionine hyperhomocysteinemia in patients with retinal vein occlusion. Thromb Res. 2003;110:7–12. doi: 10.1016/s0049-3848(03)00293-7. [DOI] [PubMed] [Google Scholar]
- 14.Cahill M, Karabatzaki M, Donoghue C, Meleady R, Mynett-Johnson LA, Mooney D, et al. Thermolabile MTHFR genotype and retinal vascular occlusive disease. Br J Ophthalmol. 2001;85:88–90. doi: 10.1136/bjo.85.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cahill MT, Stinnett SS, Fekrat S. Meta-analysis of plasma homocysteine, serum folate, serum vitamin B (12), and thermolabile MTHFR genotype as risk factors for retinal vascular occlusive disease. Am J Ophthalmol. 2003;136:1136–50. doi: 10.1016/s0002-9394(03)00571-3. [DOI] [PubMed] [Google Scholar]
- 16.Lahiri DK, Nurnberger JI., Jr A rapid non-enzymatic method for the preparation of HMW DNA from blood for RFLP studies. Nucleic Acids Res. 1991;19:5444. doi: 10.1093/nar/19.19.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Den Heijer M, Lewington S, Clarke R. Homocysteine, MTHFR and risk of venous thrombosis: A meta-analysis of published epidemiological studies. J Thromb Haemost. 2005;3:292–9. doi: 10.1111/j.1538-7836.2005.01141.x. [DOI] [PubMed] [Google Scholar]
- 18.Marcucci R, Sofi F, Grifoni E, Sodi A, Prisco D. Retinal vein occlusions: A review for the internist. Intern Emerg Med. 2011;6:307–14. doi: 10.1007/s11739-010-0478-2. [DOI] [PubMed] [Google Scholar]
- 19.McGimpsey SJ, Woodside JV, Cardwell C, Cahill M, Chakravarthy U. Homocysteine, methylenetetrahydrofolate reductase C677T polymorphism, and risk of retinal vein occlusion: A meta-analysis. Ophthalmology. 2009;116:1778–1787. doi: 10.1016/j.ophtha.2009.02.033. e1. [DOI] [PubMed] [Google Scholar]
- 20.Pianka P, Almog Y, Man O, Goldstein M, Sela BA, Loewenstein A. Hyperhomocystinemia in patients with nonarteritic anterior ischemic optic neuropathy, central retinal artery occlusion, and central retinal vein occlusion. Ophthalmology. 2000;107:1588–92. doi: 10.1016/s0161-6420(00)00181-0. [DOI] [PubMed] [Google Scholar]
- 21.Brustolin S, Giugliani R, Félix TM. Genetics of homocysteine metabolism and associated disorders. Braz J Med Biol Res. 2010;43:1–7. doi: 10.1590/s0100-879x2009007500021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsai AW, Cushman M, Tsai MY, Heckbert SR, Rosamond WD, Aleksic N, et al. Serum homocysteine, thermolabile variant of methylene tetrahydrofolate reductase (MTHFR), and venous thromboembolism: Longitudinal investigation of thromboembolism etiology (LITE) Am J Hematol. 2003;72:192–200. doi: 10.1002/ajh.10287. [DOI] [PubMed] [Google Scholar]
- 23.Ferrazzi P, Di Micco P, Quaglia I, Rossi LS, Bellatorre AG, Gaspari G, et al. Homocysteine, MTHFR C677T gene polymorphism, folic acid and vitamin B 12 in patients with retinal vein occlusion. Thromb J. 2005;3:13. doi: 10.1186/1477-9560-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sottilotta G, Siboni SM, Latella C, Oriana V, Romeo E, Santoro R, et al. Hyperhomocysteinemia and C677T MTHFR genotype in patients with retinal vein thrombosis. Clin Appl Thromb Hemost. 2010;16:549–53. doi: 10.1177/1076029609348644. [DOI] [PubMed] [Google Scholar]
- 25.McCully KS. Homocysteine, vitamins, and vascular disease prevention. Am J Clin Nutr. 2007;86:1563S–8S. doi: 10.1093/ajcn/86.5.1563S. [DOI] [PubMed] [Google Scholar]
- 26.Weiss N, Keller C, Hoffmann U, Loscalzo J. Endothelial dysfunction and atherothrombosis in mild hyperhomocysteinemia. Vasc Med. 2002;7:227–39. doi: 10.1191/1358863x02vm428ra. [DOI] [PubMed] [Google Scholar]


