Abstract
Background:
Crimean Congo hemorrhagic fever (CCHF) has high morbidity and mortality. Therefore the treatment effect of Ribavirin with and without intravenous Immunoglobulin (IVIG) in viral Crimean Congo hemorrhagic fever was evaluated.
Materials and Methods:
In a clinical trial study, 40 patients with confirmatory positive serology test, 12 (30%) received Ribavirin and IVIG (case group) and 28 (70%) received only ribavirin as standards therapy (control group). The patients were followed and compared by defervescence and other clinical symptoms, and laboratory results such as white blood cell count (WBC), platelets; liver function test (LFT) and duration of hospitalization and mortality rate after eight weeks.
Results:
The mean (SD) period for defervescence and stopping bleedings was five (0.6) days in case group and five (0.5) days in control group with no significant differences (P = 0.27). The mean period for return of WBC to normal was three (0.6) days in case group and five (0.8) days in control group (P = 0.002). The mean period for return of LFT to normal was three (0.9) days in case group and seven (0.5) days in control group which showed a meaningful difference (P = 0.001) Normalization of platelets was returned within four (0.8) days in case group compared to 6 (0.6) days in control group. Mortality was observed in three cases of each group.
Conclusion:
Considering our results, using IVIG in viral hemorrhagic fever (VHF) may need further evaluations.
Keywords: Congo fever, hemorrhagic, intravenous Immunoglobulin, Ribavirin, viral
INTRODUCTION
CCHF is caused by a virus that is widely distributed in domestic and wild animals, birds and ticks in many regions of Africa, Europe and Asia.[1] CCHF was first observed in the Crimea in 1944 by Russian scientists. The virus was first isolated in Africa from the blood of a febrile patient in 1956.[2]
In 1967, Simpson DI, et al., described different type of virus from the one isolated in 1956, and that this type of Congo virus was also similar to other virus strains from central Asia, USSR an Bulgaria.[3]
The virus has been classified as a nairovirus genus from the family of bunyaviridae. The virus contains RNA and inactivated by lipid solvents and detergents.[4] In Africa the virus has been isolated from a variety of animals and from a number of ticks that parasitize them.[5]
In Iran, Hyalomma SPP probably plays the main role in transmitting the infection from animals to humans.[6]
Although not endemic in America, Crimean Congo hemorrhagic fever, and Hantaan virus cause serious and fatal acute disease with hemorrhagic manifestations.[7]
Heavy rainfall in east Africa resulted in a major recurring epidemic in 2006-2007.[8]
Mortality rate range from 20-35%.[9]
The disease, as a reemerging disease, is acute, febrile, hemorrhagic and sometimes lethal. It is transmitted by Hyalomma tick bites or close contacts with blood or secretions of infected animals or patients.[10]
After an incubation period of 3-10 days, disease begins with Influenza like disease. The major signs and symptoms are fever, severe headache, muscle pain and then severe mucocutaneous hemorrhagia, disseminated intravascular coagulation (DIC), severe recurrent bleeding and shock. Involvement of teticuloendothelial system such as hepatic cells and hepatitis is common.[11]
The definite diagnosis is based of Isolation of virus from the blood, but it can be diagnosed by epidemiologic studies and clinical presentation. The diagnosis must be confirmed in reference laboratory by detection or a rise in specific IgG or IgM titer using EliSA.[10]
Treatment in this disease mostly is supportive as transfusion of blood and platelets and protected from hemorrhagic shock and acute hepatic failure. Treatment options for CCHF are limited. There is currently no specific antiviral therapy for CCHF approved for use in humans.[12]
Intravenous or oral ribavirin has been used in several countries to treat the disease for more than 20 years. Evidence of its efficacy is limited to observational studies.[13]
There is no controlled study about Anti-viral such ribavirin treatment on CCHF[14] but clinical experiences and compare of retrograde studies showed that it can be useful.[15] The world health organization (who) currently recommended ribavirin as a potential therapeutic drug for CCHF.[16,17] Animal studies showed the efficacy of Ribavirin in CCHF and Rift vally fever.[18] Ribavirin is aguanosine analog with a broad spectrum of activity in vitro against RNA virus, and it has been shown to inhibit viral replication of the CCHF in vitro.[19]
Extracorporal membrane oxygenation may improve survival but has never been tested in a controlled trial.[20]
Adjuvant therapy with elevators of Immune levels such as Immune globulins and activator of macrophages has an important role in recovery of disease but the role of corticosterios is upsque however some scientist offer it in sever form of disease.[18,21]
Immune serum transfusion from previous infected patient in early stage of disease advised by some physician.
As a Whole, use of Ribavirin is advised in CCHF treatment, but adjuvant therapy for this disease is open to discussion. The aim of our study was evaluating the rule of IVIG beside Ribavrin in treatment of CCHF.
MATERIALS AND METHODS
In a research project with the number: 80270 approved by Vice Chancellor for Research, Isfahan University of Medical sciences as a single blinded clinical trial, 65 patients suspected to CCHF with epidemiological, clinical and paraclinical data were studied. They were examined for serology with specific IgM and IgG antibody by Elisa test in reference laboratory. From total 65 patients, 40 patients had inclusion criteria of CCHF and they entered in this study. All patients with positive serology, received 30mg/kg Ribavirin as loading dose then 16mg/kg QID for four days and continued 8mg/kg TID for six days as standard therapy, beside supportive therapy such as balance water and electrolyte, blood and platelet transfusion and so on. 12 (30%) of patients randomly selected and received 30-50g IGIV as adjuvant therapy (case group).
Other 28 (70%) patient received only ribavirin as standard therapy (control group).
Patient followed in hospital and one week after discharge.
Defervesnce and discontinue of Mucocutaneous and visceral bleeding regard as clinical response. patient with, 10000 ml < WBC < 3000 ml which became about 50000-10000/ml and also platelets > 100000ml and normalization of Serum glutamic oxaloacetic transaminase and serum glutamic pyruvic transaminase as liver function tests regard as laboratory response. The duration of clinical and laboratory response and also mortality rate in both groups were determined and compared together. Results were analyzed by IBM SPSS Statistics 20.0.
RESULTS
From total 40 patients, 26 (65%) cases were male and 14 (35%) cases were female.
In case groups were eight male and four female and in control group 18 and 10 respectively. Age ranges of patients were 21-50 years old (mean 35 years old). All cases had close contacts with infected blood and secretions of domestic animals and two cases had tick bites. The most common clinical symptoms as a frequent, were fever, muscle pain, bleeding from organs and subcutaneous hemorrhage such as petechia and purpura, Maliase and fatigue and ICter [Table 1].
Table 1.
Distribution of clinical signs and symptoms of Crimean Congo hemorrhagic fever patients
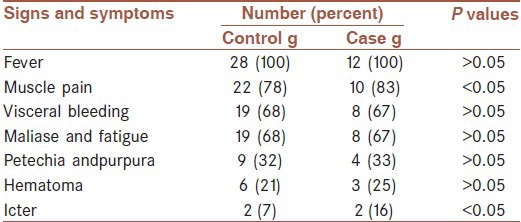
Bleeding of organs as a frequent was in gingival, Gasterointestinal and urinary tract.
In all of cases the beginning of disease was suddenly and duration of it was between three to eight days.
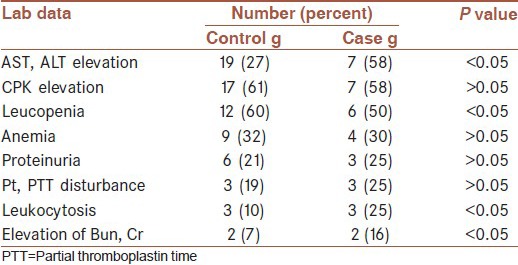
The most frequent laboratory results of patients (in both men and women) were thrombocytopenia (Normal range = 150000-350000ml), elevation of AST and ALT up to 120(Normal range = 40 unit), elevation of CPK to 36(Normal range = 12mg/dl), leucopenia (WBC < 3000/ml) (Normal range = 5000-10000/ml), Anemia (Hb < 10 g/dl) (NL = 14-16), proteinuria(>1+), elevation of protrombin time (PT) to 36 seconds (Normal range = 13 s) and elevation of partial thromboplastin time (PTT) to 72 seconds (Normal range = 36s) (>two fold), leukocytosis (WBC > 10000 ml) (Normal range = 5000-10000/ml), elevation of BUN to 50 (Normal range = 10-20 mg/dl) and creatinin to two (Normal range = 0.6-1). Laboratory results in both groups are shown in Table 2.
Table 2.
laboratory data of Crimean Congo hemorrhagic fever patients
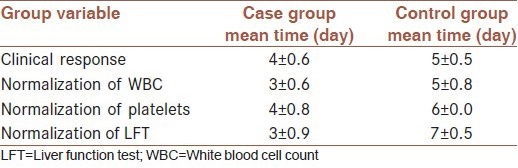
Duration to clinical response in case group was between 4-4.6 days and in control group, between 5-5.5 days, which it has not significant difference meaning (P = 0.27).
Duration of normalization of WBC in case group was between 3-3.6 days and in control group was between 5-5.8 days with a significant meaningful difference (P = 0.002).
Duration for normalization of platelets in case was 4- 4.8 days and in control group about six days, with no significant difference meaning (P = 0.49).
Duration of normalization of LFT in case group was 3- 3.9 days and in control group between 7-7.5 days with a significant meaningful difference (P < 0.001). Mortality rate in both group were 3 cases with no significant difference meaning (P = 0.24) [Table 3].
Table 3.
Clinical and laboratory response in Crimean Congo hemorrhagic fever patients
DISCUSSION
By notice that CCHF can transmitted by tick bite, almost always infect villagers, but in our study almost patients were in city that they infected by close contact with animal products and infected secretions. In fisher hoch study transmission between physician and health workers, reported.[22] Also in our study we had one physician case, that universal percussion can prophylaxy this disease.
Almost patients had a prodromal phase (unbleeding) in about 5 days which it is compatible with Swanepoel and coworkers.[23] In all patients specific IgM was detected in first week and IgG in third week by Elisa, that it is compatible with Leaman PA and Shepered.[24] Following the prodromal phase abruptly began fever, severe headache, anorexia, vomiting, skeletal pain and then bleeding (petechia and purpura and then visceral bleeding) appeared which it is compatible with before study.[25]
In the historical study conducted by Mardani et al., in Iran during 1999-2001, the case fatality rate was 11.6% (8 of 69 patients) in the group treated with oral ribavirin compared with 58.3% (7 of 12 patients) in the untreated group, which corresponded to 80% efficacy (P < 0.001).[26] This study constituted the basis for the World Health Organization (WHO) recommendations for treating CCHF patients with ribavirin. Promising results were also reported by others, and were mainly associated with early treatment.[27,28]
Animal studies was shown that horse Immune serum (Passive serum immune from infected horse) could relief the viral hemorrhage fever in monkeys but it needs specific antibody in humans.[29]
Intravenous immunoglobulin used in the treatment of a diverse variety of conditions. Despite improvements in antimicrobial therapies, there are a large number of pathogens that remain difficult to control and others for which no specific chemotherapy exist. Thus polyclonal IVIG continues to be used in the treatment of a variety of infectious diseases.[30]
In this study gammaglubulin could relief the severity of signs and symptoms. Use of combination IVIG and Interferon in monkeys before infection with Ebola can retard the death but for treatment it needs Antiviral Drugs such as ribavirin.[31] Transfusion of convalescent serum of Ebola patients, in Congo epidemy in 1995 to CCHF patients could relief the severity of clinical symptoms and reduced the mortality rate from 80% to 12.5%.[32,33]
Transfusion of Immune serum to mice could survive 100% of them and it shows Antibody can induce protection alone.[34] In bone marrow transplantation weekly IGIV administration of 500 mg/kg for three months followed by monthly for one year has reduced the number of infection and septicemia and interstitial pneumonia.[35]
CONCLUSION
In our study IVIG could relief the severity of clinical signs and symptoms and reduce the duration of disease but it had not meaning difference in mortality rates in both groups (P = 0.171).
Considering our results, using IVIG in viral hemorrhagic fever (VHF) may need further evaluations.
ACKNOWLEDGMENT
Authors thank Vice chancellor for research for funding this research.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Fisher-Hoch SP, McCormick JB, Swanepoel R, Van Middlekoop A, Harvey S, Kustner HG. Risk of human infection with Crimean-Congo hemorrhage fever virus in a South African rural community. Am J Trop Med Hyg. 1992;47:337–45. doi: 10.4269/ajtmh.1992.47.337. [DOI] [PubMed] [Google Scholar]
- 2.Gear JH, Thomson PD, Hopp M, Andronikou S, Cohn RJ, Ledger J, et al. Crimean-congo haemorrhagic fever in South Africa. Report of a fatal case in the Transvaal. S Afr Med J. 1982;62:576–80. [PubMed] [Google Scholar]
- 3.Simpson DI, Knight EM, Courtois G, Williams MC, Weinbren MP, Kibukamusoke JW. Congo virus: a hitherto undescribed virus occurring in Africa. I. Human isolations – clinical notes. East Afr Med J. 1967;44:86–92. [PubMed] [Google Scholar]
- 4.Papa A, Ma B, Kouidou S, Antoniadis A. Genetic characterization of the mRNA segment of Crimean – Congo hemorrhagea fever virus strains, china. Emerg Infect Dis. 2002;8:50–3. doi: 10.3201/eid0801.010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burt FJ, Spencer DC, Leman PA, Patterson B, Swanepoel R. Investigation of tick-borne viruses as pathogens of human in South Africa and evidence of Dugbe virus infection in a patient with prolonged thrombocytopenia. Epidemiol Infect. 1996;116:353–61. doi: 10.1017/s0950268800052687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chinikar S. The specific serological investigation of suspected human and animals to have Crimean-congo hemorrhagic fever in various parts of Iran using ELISA. Hakim. 2002;4:294–300. [Google Scholar]
- 7.Peters CJ, Mills JN, Spiroponto C. 2nd ed. Philadelphia: Elsevier Churchill Livingstone; 2006. Hanta virus infections. In: Tropical infectious diseases-principles, pathogens and practice; pp. 762–80. [Google Scholar]
- 8.Bird BH, Githinji JW, Macharia JM. Multiple viruses lineages sharing recurrent common ancestry were associated with a large hemorrhagic fever in Kenya. 2006-2007. J Virol. 2008;82:11152–66. doi: 10.1128/JVI.01519-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deyede VM, Khristova ML, Rollin PE. Crimean congo hemorrhagic fever. J Virol. 2006;80:8834–42. doi: 10.1128/JVI.00752-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World health organization. Fact sheet No, 208. [Last accessed on 2001]. Available from: http://www.who.int/inf-fs/en/fact208Htm/
- 11.Joubert JR, King JB, Rossouw DJ, Cooper R. A nosocomial outbreak of Crimean-Congo hemorrhagic fever at Tygerberg hospital. Part III. Clinical pathology and pathogenesis. S Afr Med J. 1985;68:722–8. [PubMed] [Google Scholar]
- 12.Whitehouse CA. Crimean Congo hemorrhagic fever. Antivir Res. 2004;64:145–60. doi: 10.1016/j.antiviral.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Ergonul O. Treatment of Crimean Congo hemorrhagic fever. Antiviral Res. 2008;78:125–31. doi: 10.1016/j.antiviral.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 14.CDC update: Management of patient with suspected viral hemorrhagic fever USA. MMWR. 1995;44:475. [PubMed] [Google Scholar]
- 15.Peters CJ. Management of patients with high-hazard viruses. Arch virol. 2002;8:141. doi: 10.1007/978-3-7091-7482-1_13. [DOI] [PubMed] [Google Scholar]
- 16.Crimean Congo hemorrhagic fever. Geneva: World Health Organization; 2001. [Last accessed 2008 Jul 20]. World health organization. Available from: http://www.who.int/mediacentre/factsheets/fs 208/en/ [Google Scholar]
- 17.Geneva: WHO; 2008. [Last accessed on 2008 Jul 20]. Who Ribavirin. Available from: http://www.who.int/emlib/medicinedisplay.aspx . [Google Scholar]
- 18.Peters CJ, Rcynold JA. Prophylaxis of rift valley fever and CCHF with antiviral Drugs, immuno cerum, and interferon inducer and a macrophage activator antiviral. Rev Infect Dis. 1986;6:285. doi: 10.1016/0166-3542(86)90024-0. [DOI] [PubMed] [Google Scholar]
- 19.Bray M. Highly pathogenic RNA viral infection. Challenges for antiviral research. Antiviral Res. 2008;78:1–8. doi: 10.1016/j.antiviral.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Dielt CA, Wernly JA, Pett SB. Extra corporal membrane oxygenation support improves survival of patients with severe Hantavirus cardiopulmonary syndrome. J Thorac Cardiovasc Surg. 2008;135:579–84. doi: 10.1016/j.jtcvs.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 21.Robert B. Tcsh: Crimcan congo hemorrhagic fever. Feigin and Cherry's Textbook of Pediatric Infectious Diseases: Expert Consult - Online and Print, 2-Volume. In: Ralph D, Feigin MD, James Cherry MD, Gail J, Demmler-Harrison MD, Sheldon L, et al., editors. chapter 191. Vol. 1. London: Saunders; 2009. pp. 2160–1. [Google Scholar]
- 22.Fisher Hoch SP, khan JA. Crimean congo hemorrhagic fever treated with oral Ribaverin. Lancet. 1995;346:427–5. doi: 10.1016/s0140-6736(95)91323-8. [DOI] [PubMed] [Google Scholar]
- 23.Burt FJ, Swanepoel R, Shieh WJ, Smith JF, Leman PA, Greer PW, et al. Immunohistochemical and in situlocalization of Crimean-Congo hemorrhagic fever (CCHF) virus in human tissues and implications for CCHF pathogenesis. Arch Pathol Lab Med. 1997 Aug;121:839–46. [PubMed] [Google Scholar]
- 24.Shepred AJ, Swanpoel R. Antibody response in Crimean - Congo hemorrhagic fever. Rev infect Dis. 1989;11(Suppl 4):S801–6. doi: 10.1093/clinids/11.supplement_4.s801. [DOI] [PubMed] [Google Scholar]
- 25.Salehi H, Mostafavizadeh K, Karimi I, Rostami M, Emami A, Javadi AA, et al. Primitive reports of crimean Congo hemorrhagic fever in Isfahan, Iran. Res Med. 1381;1:78–9. [Google Scholar]
- 26.Mardani M, Jahromi MK, Naeni KH, Zeinali M. The efficacy of oral ribavirin in the treatment of Crimean-Congo hemorrhagic fever. Clin Infect Dis. 2003;36:1613–8. doi: 10.1086/375058. [DOI] [PubMed] [Google Scholar]
- 27.Fisgin NT, Ergonul O, Doganci L, Tulek N. The role of ribavirin in the therapy of Crimean-Congo hemorrhagic fever: Early use is promising. Eur J Clin Microbiol Infect Dis. 2009;28:929–33. doi: 10.1007/s10096-009-0728-2. [DOI] [PubMed] [Google Scholar]
- 28.Izadi S, Salehi M. Evaluation of the efficacy of ribavirin therapy on survival of Crimean-Congo hemorrhagic fever patients: A case-control study. Jpn J Infect Dis. 2009;62:11–5. [PubMed] [Google Scholar]
- 29.Jahrling PB, Geisber J. Passive immunization of Ebola virus – infected monkeys, with immunoglobulin from immune horse. Arch Virol Suppl. 1996;11:135–40. doi: 10.1007/978-3-7091-7482-1_12. [DOI] [PubMed] [Google Scholar]
- 30.Aydin K, Koksal I, Aksoy F, Yilmaz G, Sozen EE, Iskender S. Chicago, IL, USA: 2007. Sep, The Patients with Crimean-Congo Hemorrhagic Fever Treated With Intravenosus Immunoglobulin. 47th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC). 47th ICAAC abstracts, V-1908. [Google Scholar]
- 31.Jahrling PB, Geisbert TW, Geisbert JB, Swearengen JR, Bray M, Jaax NK, et al. Evaluation of immune globulin and recombinant interferon-α2b for treatment of experimental Ebola virus infections. J Infect Dis. 1999;179(Suppl 1):S224–S234. doi: 10.1086/514310. [DOI] [PubMed] [Google Scholar]
- 32.Mupapa K, Massambe M, Kibadi K, Kuvula K, Bwaka A, Kipasa M, et al. Treatment of Ebola hemorrhagic fever with blood transfusion from convalescent patients. J Infect Dis. 1999;179(Suppl 1):S18–23. doi: 10.1086/514298. [DOI] [PubMed] [Google Scholar]
- 33.Sadek RF, khan AS. Ebola hemorrhagic fever determination of survival. J Infect Dis. 1999;79:24–77. doi: 10.1086/514311. [DOI] [PubMed] [Google Scholar]
- 34.Gupta M, Mahanty S, Bray M, Ahmed R, Rollin PE. Passive transfer of antibodies protects immunocompetent and imunodeficient mice against lethal Ebola virus infection without complete inhibition of viral replication. J Virol. 2001 May;75:4649–54. doi: 10.1128/JVI.75.10.4649-4654.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sullivan KM, Kopecky KJ, Jocom J, Fisher L, Buckner CD, Meyers JD, et al. Immunimodulatory and antimicrobial efficacy of Intravenous Immunoglubulin in bore narrow transplantation. N Engl J Med. 1990;323:705–12. doi: 10.1056/NEJM199009133231103. [DOI] [PubMed] [Google Scholar]


