Abstract
Background:
Protection of the catheter site by antimicrobial agents is one of the most important factors in the prevention of infection. Povidone iodine and chlorhexidine gluconate are the most common used agents for dressing. The purpose of this study was to compare the effects of povidone iodine, chlorhexidine gluconate and octenidine hydrochloride in preventing catheter related infections.
Materials and Methods:
Patients were randomized to receive; 4% chlorhexidine gluconate, 10% povidone iodine or octenidine hydrochlorodine for cutaneous antisepsis. Cultures were taken at the site surrounding catheter insertion and at the catheter hub after removal to help identify the source of microorganisms.
Results:
Catheter related sepsis was 10.5% in the povidone iodine and octenidine hydrochlorodine groups. Catheter related colonization was 26.3% in povidone iodine group and 21.5% in octenidine hydrochlorodine group.
Conclusion:
4% chlorhexidine or octenidine hydrochlorodine for cutaneous disinfection before insertion of an intravascular device and for post-insertion site care can reduce the catheter related colonization.
Keywords: Catheter infection, chlorhexidine, cutaneous, octenidine hydrochlorodine, povidone iodine
INTRODUCTION
As progress in critical care has advanced, medical technology has led to the creation of many intravascular devices for the purpose of fluid and drug administration, hemodynamic monitoring, hemodialysis and other functions that have greatly improved our ability to deliver care to critically ill patients. Although such catheters provide necessary vascular access, the use of these catheters puts patients at risk for complications such as local site infection, catheter-related bloodstream infection, septic trombophlebitis, endocarditis and metastatic infections (e.g., lung abscess, brain abscess, osteomyelitis and endophthalmitis).[1] Furthermore, these conditions contribute to increased morbidity, mortality, length of stay and excessive cost of care.[2] Because nosocomial infection rates become more frequently included in the criteria used for assessing the quality of patient care, implementation of prevention strategies becomes more important.
Protection of the catheter site by antimicrobial agents is one of the most important factors in the prevention of infection. Appropriate sterile dressings should cover the insertion site and be replaced on a regular basis.[3] Povidone iodine, chlorhexidine gluconate and octenidine hydrochloride are the most common used agents for dressing. The purpose of this study is to compare the effects of povidone iodine, chlorhexidine gluconate and octenidine hydrochloride in preventing catheter related infections.
MATERIALS AND METHODS
The study was performed in anesthesiology and reanimation intensive care unit (ICU) of Eskisehir Osmangazi University Medical Faculty. After approval from the hospital ethics and research committee, written permissions were obtained either from the patients themselves or from the legal surrogate. Fifty seven patients requiring intravascular catheterization were included in this trial. Completed data could be evaluated for 109 catheters from these patients (57 arterial catheters and 52 central venous catheters). By use of a blinded randomization schedule, we assigned to each patient to 1 of 3 groups according to the antiseptic solution used for initial and subsequent cutaneous antisepsis. The catheter data collected included location, insertion date, duration and site inspection. Additional patient data collected included age, sex, duration in ICU, underlying medical problems, treatment with steroids or antibiotics, temperature and clinical outcome.
Patients were randomized to receive 4% chlorhexidine gluconate (Group I, n = 19), 10% povidone iodine (Group II, n = 19) or octenidine hydrochlorodine (Group III, n = 19) for cutaneous antisepsis. The skin was cleaned by designed antiseptic solution before the insertion of the catheter and the same antiseptic solution used for the following days. The insertion site and dressings were inspected daily by patient's physician to search for signs of infection, inflammation or cutaneous allergic events to the disinfectants. Cultures were taken at the site surrounding catheter insertion daily and at the catheter hub after removal to help identify the source of microorganisms. Standard microbiologic methods and criteria identified recovered microorganisms. The laboratory technicians were unaware of the antiseptic solution used for skin preparation. Colonization of the catheter was related to the skin when the same bacterial isolate yielded from the catheter and the skin surrounding the insertion site.
Statistical analysis
All statistical analyses were performed using the SPSS for windows 13.0. Data are expressed as medians for characteristics of the patients and catheters or percentages for categorical variables. Data were analyzed for differences between the study groups by one-way anova and Bonferroni's post-hoc test. A P value of <0.05 was considered statistically significant.
RESULTS
A total of 57 patients requiring intravascular catheterization were included in this trial. Completed data could be evaluated for 109 catheters from these patients (57 arterial catheters and 52 central venous catheters). There were no significant differences between groups in demographic characteristics [Table 1]. The clinical characteristics of patients and the risk factors for infection were similar in the two groups. Neither local nor systemic hypersensitivity reactions were observed with the use of either antiseptic solution. Catheters were kept in place for 7.2 days in Group I, 7.8 days in Group II and 7.5 days in Group III.
Table 1.
Characteristics of the patients in the groups
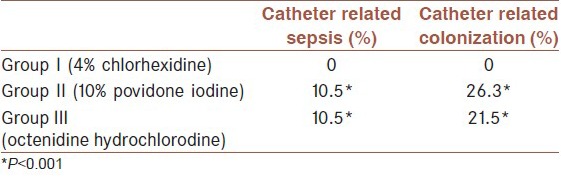
There was a statistically significant difference between groups in catheter related sepsis and colonization (P < 0.001). Documented catheter related sepsis rate was 10.5% in the povidone iodine and octenidine hydrochlorodine groups. Catheter related colonization rate was 26.3% in povidone iodine group and 21.5% in octenidine hydrochlorodine group. In chlorhexidine group, there was no catheter related sepsis or colonization [Table 2].
Table 2.
Catheter related sepsis and colonization rates of the groups
The only bacterial species isolated was Acinetobacter calcoaceticus (100%) in catheter related sepsis A. calcoaceticus (80%) and Enterococcus fecalis (20%) were the colonizing microorganisms. The skin surrounding the catheter insertion site was the origin of the colonizing microorganisms.
DISCUSSION
Regardless of the type of catheter inserted, the major risk factor for the development of catheter related infection is the breach of a major host defense against infection, the skin.[4] Migration of skin organisms at the insertion site in to the cutaneous catheter tract with colonization is the most common route of infection. Skin flora introduced either at the time of puncture or a result of bacterial migration along a catheter or needle tract has been implicated as a potential source of infection. Aseptic technique at the time of insertion and during access of the intravascular device is important in reducing the risk of infection. Many different methods have been used in an attempt to reduce microbial contamination at the insertion site of catheter.
Povidone iodine and chlorhexidine gluconate are the most common used agents for dressing. Octenidine hydrocloride is a byspyridine skin and wound disinfectant. It is a compound that is active on cell walls due to its cationic properties.[5] Our study demonstrates that use of 4% chlorhexidine rather than 10% Povidone iodine or octenidine hydrochlorodine for cutaneous disinfection before insertion of an intravascular device and for post-insertion site care can substantially reduce the incidence of device-related infection. This finding is consistent previous reports.[6,7,8,9,10,11] Maki et al. concluded that use of 2% chlorhexidine, rather than 10% Povidone-iodine or 70% alcohol for cutaneous disinfection before insertion of an intravascular device and for post-insertion site care can reduce incidence of infection.[6] Likewise, Mimoz et al. demonstrated that 4% alcohol-based solution of 0.25% chlorhexidine gluconate was more effective than 10% Povidone iodine for insertion site care of central venous and arterial catheters.[12] Chlorhexidine gluconate is a potent broad-spectrum germicide and it is effective against nearly all nosocomial bacteria and yeasts.[13] Superior performance of chlorhexidine is explained by its more potent bactericidal activity and its high permeability in to the hair follicules.[14] And the success of this agent might also be attributable to antimicrobial activity that persists longer than that for the other agents.[4] Our study supports these findings.
But also there are some studies, which are not in accordance with these conclusions. Kasuda et al. demonstrated that the effect of 0.5% chlorhexidine ethanol is not different from that of 10% Povidone iodine in reducing catheter colonization.[15] In a randomized trial comparing Povidone-iodine to a chlorhexidine gluconate impregnated dressing for prevention of central venous catheter infections in neonates that there is no difference in the efficiency of two antiseptic solutions.[16] In a prospective randomized trial of 10% Povidone-iodine versus 0.5% tincture of chlorhexidine as a cutaneous antisepsis, Humar et al. concluded that there was no difference between two antiseptic solutions.[17]
Coagulase-negative staphylococci (including Staphylococcus epidermidis and other species) are most commonly implicated in catheter related infections, followed by Staphylococcus aureus, a variety of aerobic gram-negative bacilli, other gram-positive cocci and bacilli and Candida and other yeasts.[4,18,19] In our study, A. calcoaceticus was the most common microorganism in catheter related sepsis and colonization. The superiority may be explained by the flora of our ICU and hospital during the study period. Catheter colonization can arise from clinicians’ and nurses’ handling of syringes and solutions.
There were some limitations of our study. The first one was the number of patients. The larger number of patients may give more details about the effect of agents on different microorganisms. The second one was that the physicians were not blinded because of the difference in the color of the antiseptic solutions. The third limitation was the different duration of the patients in the ICU; although, there was no significant difference between the durations of the groups. Longer duration will increase the incidence of catheter related infections. And the last one was that it was not the same clinician who is responsible of catheter care.
CONCLUSION
Chlorhexidine is an effective disinfectant agent in adult ICU. The use of 4% chlorhexidine rather than 10% povidone iodine or octenidine hydrochlorodine for cutaneous disinfection before insertion of an intravascular device and for post-insertion site care can substantially reduce the incidence of catheter related infection.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.O’Grady NP. Applying the science to the prevention of catheter-related infections. J Crit Care. 2002;17:114–21. doi: 10.1053/jcrc.2002.34366. [DOI] [PubMed] [Google Scholar]
- 2.Dixon JM, Carver RL. Daily chlorohexidine gluconate bathing with impregnated cloths results in statistically significant reduction in central line-associated bloodstream infections. Am J Infect Control. 2010;38:817–21. doi: 10.1016/j.ajic.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Sanders WM. Infectious complications of intravascular access devices. In: Hall JB, Schmidt GA, Wood LD, editors. Critical Care. United States of America: McGraw-Hill; 1996. pp. 483–8. [Google Scholar]
- 4.Bradley SF, Kaufmann CA. Infections associated with vascular catheters. In: Rippe JM, Irwin RS, Fink MP, Cerra FB, editors. Intensive Care Medicine. 3rd ed. United States of America: Little, Brown and Company; 1996. pp. 1141–52. [Google Scholar]
- 5.Harke HP. Octenidine dihydrochloride, properties of a new antimicrobial agent. Zentralbl Hyg Umweltmed. 1989;188:188–93. [PubMed] [Google Scholar]
- 6.Maki DG, Ringer M, Alvarado CJ. Prospective randomised trial of povidone-iodine, alcohol, and chlorhexidine for prevention of infection associated with central venous and arterial catheters. Lancet. 1991;338:339–43. doi: 10.1016/0140-6736(91)90479-9. [DOI] [PubMed] [Google Scholar]
- 7.Chaiyakunapruk N, Veenstra DL, Lipsky BA, Sullivan SD, Saint S. Vascular catheter site care: The clinical and economic benefits of chlorhexidine gluconate compared with povidone iodine. Clin Infect Dis. 2003;37:764–71. doi: 10.1086/377265. [DOI] [PubMed] [Google Scholar]
- 8.Krau SD. Review: Chlorhexidine gluconate is more effective than povidone-iodine for preventing vascular catheter related bloodstream infection. Evid Based Nurs. 2003;6:18. doi: 10.1136/ebn.6.1.18. [DOI] [PubMed] [Google Scholar]
- 9.Chaiyakunapruk N, Veenstra DL, Lipsky BA, Saint S. Chlorhexidine compared with povidone-iodine solution for vascular catheter-site care: A meta-analysis. Ann Intern Med. 2002;136:792–801. doi: 10.7326/0003-4819-136-11-200206040-00007. [DOI] [PubMed] [Google Scholar]
- 10.Mimoz O, Karim A, Mercat A, Cosseron M, Falissard B, Parker F, et al. Chlorhexidine compared with povidone-iodine as skin preparation before blood culture. A randomized, controlled trial. Ann Intern Med. 1999;131:834–7. doi: 10.7326/0003-4819-131-11-199912070-00006. [DOI] [PubMed] [Google Scholar]
- 11.Carson SM. Chlorhexidine versus povidone-iodine for central venous catheter site care in children. J Pediatr Nurs. 2004;19:74–80. doi: 10.1016/j.pedn.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Mimoz O, Pieroni L, Lawrence C, Edouard A, Costa Y, Samii K, et al. Prospective, randomized trial of two antiseptic solutions for prevention of central venous or arterial catheter colonization and infection in intensive care unit patients. Crit Care Med. 1996;24:1818–23. doi: 10.1097/00003246-199611000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Rubin C, Louthan RB, Wessels E, McGowan MB, Downer S, Maiden J. Chlorhexidine gluconate: To bathe or not to bathe? Crit Care Nurs Q. 2013;36:233–6. doi: 10.1097/CNQ.0b013e31828404d1. [DOI] [PubMed] [Google Scholar]
- 14.Kinirons B, Mimoz O, Lafendi L, Naas T, Meunier J, Nordmann P. Chlorhexidine versus povidone iodine in preventing colonization of continuous epidural catheters in children: A randomized, controlled trial. Anesthesiology. 2001;94:239–44. doi: 10.1097/00000542-200102000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Kasuda H, Fukuda H, Togashi H, Hotta K, Hirai Y, Hayashi M. Skin disinfection before epidural catheterization: Comparative study of povidone-iodine versus chlorhexidine ethanol. Dermatology. 2002;204(Suppl 1):42–6. doi: 10.1159/000057724. [DOI] [PubMed] [Google Scholar]
- 16.Garland JS, Alex CP, Mueller CD, Otten D, Shivpuri C, Harris MC, et al. A randomized trial comparing povidone-iodine to a chlorhexidine gluconate-impregnated dressing for prevention of central venous catheter infections in neonates. Pediatrics. 2001;107:1431–6. doi: 10.1542/peds.107.6.1431. [DOI] [PubMed] [Google Scholar]
- 17.Humar A, Ostromecki A, Direnfeld J, Marshall JC, Lazar N, Houston PC, et al. Prospective randomized trial of 10% povidone-iodine versus 0.5% tincture of chlorhexidine as cutaneous antisepsis for prevention of central venous catheter infection. Clin Infect Dis. 2000;31:1001–7. doi: 10.1086/318145. [DOI] [PubMed] [Google Scholar]
- 18.Sherertz RJ, Raad II, Belani A, Koo LC, Rand KH, Pickett DL, et al. Three-year experience with sonicated vascular catheter cultures in a clinical microbiology laboratory. J Clin Microbiol. 1990;28:76–82. doi: 10.1128/jcm.28.1.76-82.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liñares J, Sitges-Serra A, Garau J, Pérez JL, Martín R. Pathogenesis of catheter sepsis: A prospective study with quantitative and semiquantitative cultures of catheter hub and segments. J Clin Microbiol. 1985;21:357–60. doi: 10.1128/jcm.21.3.357-360.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


