Abstract
The purpose of this study was to determine the contribution of hamstrings and quadriceps fatigue to quadriceps inhibition following lumbar extension exercise. Regression models were calculated consisting of the outcome variable: quadriceps inhibition and predictor variables: change in EMG median frequency in the quadriceps and hamstrings during lumbar fatiguing exercise. Twenty-five subjects with a history of low back pain were matched by gender, height and mass to 25 healthy controls. Subjects performed two sets of fatiguing isometric lumbar extension exercise until mild (set 1) and moderate (set 2) fatigue of the lumbar paraspinals. Quadriceps and hamstring EMG median frequency were measured while subjects performed fatiguing exercise. A burst of electrical stimuli was superimposed while subjects performed an isometric maximal quadriceps contraction to estimate quadriceps inhibition after each exercise set. Results indicate the change in hamstring median frequency explained variance in quadriceps inhibition following the exercise sets in the history of low back pain group only. Change in quadriceps median frequency explained variance in quadriceps inhibition following the first exercise set in the control group only. In conclusion, persons with a history of low back pain whose quadriceps become inhibited following lumbar paraspinal exercise may be adapting to the fatigue by using their hamstring muscles more than controls.
Key Points.
A neuromuscular relationship between the lumbar paraspinals and quadriceps while performing lumbar extension exercise may be influenced by hamstring muscle fatigue.
QI following lumbar extension exercise in persons with a history of LBP group may involve significant contribution from the hamstring muscle group.
More hamstring muscle contribution may be a necessary adaptation in the history of LBP group due to weaker and more fatigable lumbar extensors.
Key words: Superimposed burst technique, electromyography, spectral median frequency, correlation and regression, low back pain
Introduction
Muscle inhibition is defined as the reduced ability to completely activate a muscle and can occur following muscle fatigue (Walton et al., 2002) or joint injury (Hurley et al., 1994; Lewek et al., 2004; Mizner et al., 2003; Snyder-Mackler et al., 1994). Quadriceps weakness and inhibition may be present in persons with knee injuries such as patellofemoral joint pain (Suter et al., 1998) or osteoarthritis (Mizner et al., 2003) and is associated with a reduction in force production capability of a muscle (Mizner et al., 2003). Quadriceps inhibition (QI) may affect the ability to absorb impact forces during activity and may, therefore expose lower extremity joints to injury. Identifying contributing factors to quadriceps inhibition may provide better understanding of lower extremity overuse or degenerative joint injuries that may result from muscle inhibition.
Low back pain (LBP) may occur in persons with weak (Bayramoglu et al., 2001) or unbalanced (Lee et al., 1999) trunk muscles with quicker rates of fatigue during exercise (Kankaanpää et al., 1998). There is evidence of altered hip and low back muscle recruitment patterns during gait in persons with LBP (Vogt et al., 2003). These neuromuscular differences observed in persons with LBP may be unfavorable during prolonged and intense activity or sport.
Isometric lumbar extension endurance exercise is commonly used to strengthen the low back extensor muscles (Verna et al., 2002) and can be used to predict persons likely to experience an episode of LBP (Biering-Sorensen, 1984) due to poor lumbar paraspinal endurance. However, hip extensor and hamstring fatigue (Kankaanpää et al., 1998; Plamondon et al., 2004) is likely to occur while performing prone, isometric extension exercise and may therefore contribute to task failure, especially in the presence of weak lumbar extensors. We (Hart, 2005; Hart et al., 2005) previously observed QI following isometric lumbar extension exercise in persons who have a history of LBP and controls. Since weak musculature is common in persons with LBP, is important to determine the extent to which hamstring fatigue is contributing to this relationship between persons with LBP and controls.
The purpose of this study is to determine the contribution of the change in median frequency, measured with surface electromyography (EMG) for the hamstring and quadriceps muscles to quadriceps inhibition following fatiguing isometric lumbar extension exercise between persons with a history of LBP and controls.
Methods
This study consisted of repeated measures, time-series design. We measured QI following two sets of isometric lumbar extension exercise. We also measured muscle fatigue as the change in EMG median frequency of quadriceps and hamstring muscles while performing prone, isometric lumbar extension exercise. Multiple regression models were developed with 1 outcome variable: quadriceps inhibition; and 2 predictor variables: fatigue in the quadriceps and hamstring muscles while performing isometric lumbar extension exercise.
Subjects
Twenty- five subjects with a history of LBP including 13 females (age = 21.7 ± 1.9yrs., height = 1.69 ± 0. 07m, mass = 64.6 ± 6.8kg) and 12 males (age = 22.8 ± 3.5yrs., height = 1.80 ± 0.07m, mass = 80.5 ± 8.3kg) were matched by gender, height and mass with 13 female (age = 20.9 ± 1.5yrs., height = 1.71 ± 0.06m, mass = 64.2 ± 7.5kg) and 12 male (age = 23.8 ± 3.5yrs., height = 1.82 ± 0.07m, mass = 79.9 ± 11.7kg) control subjects, N = 50. All subjects voluntarily participated after they read and signed and informed consent form. This study was approved by our University’s Institutional Review Board.
Subjects were all recreationally active (exercised at least three days per week for at least 30 minutes per session) and did not report current knee pain or a history of knee injury or surgery. All subjects reported no history of vertebral disc injury, cancer, neurological injury or radicular symptoms in the lower extremity, vertebral fracture, spine surgery, lower extremity joint surgery, ligament deficiencies or any lower extremity joint injury within the past 6-months. Subjects were included in the history of LBP group if they reported at least 3 episodes of low back pain in the past 3 years or 5 episodes in their lifetime that was sufficient enough to cause them to modify or limit their daily activities.
Instruments
The force of knee extension was measured with a dynamometer (Biodex System 3 #900-550, Biodex Medical Systems, Inc., Shirley, NY). Signal from the dynamometer were exported from a remote access port through to a universal interface module (UIM100c) and digitized at 200Hz with a 16-bit data acquisition system (Biopac MP150, Biopac Systems, Inc., Goleta, CA).
The S88 dual output square pulse stimulator with the SIU8T transformer stimulus isolation unit (Grass-Telefactor, West Warwick, RI) was used to deliver a percutaneous 100ms train of 10 square-wave pulses at an intensity of 125V to the quadriceps muscles through two 8X14cm rubber electrodes placed at the proximal and distal thigh. Individual pulse duration was 600 µs delivered at a carrier frequency of 100pps.
A lumbar hyperextension chair (Wynmor, Inc.) was used to allow subjects to comfortably perform isometric lumbar paraspinal muscle contractions. The chair’s footpads provided leverage so the torso, proximal to the anterior-superior iliac spines, would be unsupported by any part of the chair (Figure 1).
Figure 1.

A subject performing fatiguing lumbar extension exercise.
Electrical activity in the lumbar paraspinal, hamstring and quadriceps muscles was collected with surface electromyography (EMG). Signals were amplified with a high gain, differential input, biopotential amplifier with a gain of 1000 and digitized with a 16-bit data acquisition system (Biopac Systems, Inc., Goleta, CA) at 2000Hz with a common-mode rejection ratio of 110dB, an input impedance of 1.0 MΩ and a noise voltage of 0.2µV.
Procedures
Prior to data collection, subjects were screened for group assignment. After screening, a physical exam was performed for all subjects. During the exam, an experienced, licensed and certified athletic trainer (JMH) performed a lower extremity dermatome/ myotome and deep tendon reflex (patellar and achilles) evaluation and a bilateral straight leg raise test(Magee, 1997). Subjects were also asked to perform active standing lumbar extension. Subjects were excluded if they displayed any lower quarter neurological bilateral asymmetry, intolerable pain (>3/10) with standing lumbar extension, the inability to extend the spine at least 15 degrees comfortably or a positive straight leg test.
Subject preparation
Subject preparation began by placing low back, quadriceps and hamstring EMG electrodes and quadriceps stimulating electrodes. To minimize skin resistance during signal acquisition, skin was shaved, lightly debrided with fine sandpaper and cleaned thoroughly with isopropyl alcohol prior to electrode placement. Self adhesive, round, small diameter (35mm), pre-gelled Ag-AgCl surface electrodes collected signal from muscle groups of interest. EMG electrodes were placed over active muscle by palpating the muscle during an active contraction. The quadriceps electrodes were placed over the vastus lateralis and biceps femoris muscle belly. The lumbar paraspinal muscle electrodes were placed over active muscle tissue, verified during an isometric contraction at approximately the L4-L5 level. All electrodes were placed parallel to muscle fiber orientation with an inter-electrode distance of approximately 2cm. A ground electrode was placed on the anterior mid-tibia. Then, two, 8x14cm, rubber electrodes coated with aqueous conductive gel were secured to the proximal-lateral and distal-medial thigh with a compression wrap. The subject was then secured to chair and dynamometer arm for baseline assessment of quadriceps inhibition (QI) (Figure 2).
Figure 2.
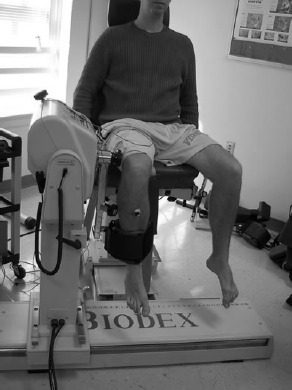
Subject setup for measuring knee extension force.
Baseline QI measurement
QI was measured using the superimposed burst technique (Mizner et al., 2003; Stevens et al., 2003). During QI measurements, a researcher manually triggered an electrical stimulus to the quadriceps while subjects performed a maximal, voluntary isometric contraction (MVIC). The superimposed stimulus was intended to recruit all the motor units in the quadriceps motor neuron pool thus causing a transient increase of force called a superimposed burst (SB). We calculated quadriceps inhibition with formula (1).
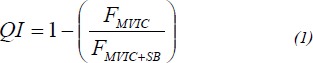 |
This ratio is 1-[the central activation ratio] has been used previously (Behm et al., 2001; Kent-Braun et al., 1996; Mizner et al., 2003; Stevens et al., 2003) to calculate quadriceps activation. In the present study, we calculated QI as 1-quadriceps activation. QI values are presented as percentages and indicate the percent of subjects’ quadriceps motor neuron pool that cannot be voluntarily activated (Figure 3).
Figure 3.
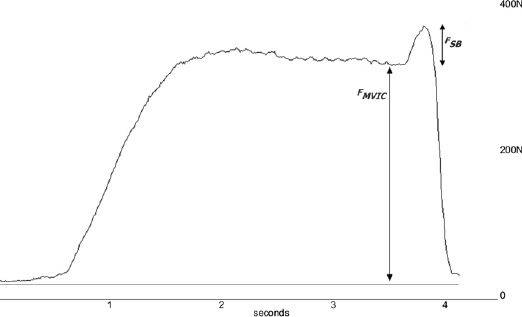
Sample tracing of force and EMG measured during the SIB technique with labels showing MVC and ST force components of the curve.
Following a baseline measure of quadriceps activation, subjects moved to a lumbar extension machine where they performed a set of lumbar extension exercise. Following both exercise sets, subjects were moved back to the dynamometer for post-exercise measures of QI.
Isometric lumbar extension exersise
The fatiguing isometric lumbar extension exercise consisted of repeated 10-second periods of isometric contractions followed by 10-second rest. Subjects were verbally encouraged to maintain a position of the trunk parallel to the floor. Muscle activity of the right side lumbar paraspinals, lateral hamstrings and lateral quadriceps was recorded during each active repetition for one second. The first repetition of the first set, representing baseline muscle activity, and the last repetition of each set were saved and used for analysis. For analysis, we calculated the median frequency (MedF) for each saved repetition and calculated the percent change in MedF from the baseline measure to the last repetition of each set.
EMG analysis
Quadriceps and hamstring EMG were analyzed post hoc to calculate the change in MedF during the exercise sets. Lumbar paraspinal EMG was analyzed during the exercise sets and was used as a physiologic marker to determine when the desired level of fatigue was achieved. We recorded the steps necessary to calculate the EMG MedF using macro software (Macro-Magic, Iolo Technologies, Los Angeles, CA) which executed the necessary commands at 500-times speed. This provided us the ability to record EMG from the paraspinals and calculate the MedF from all repetitions. The first exercise set ended once we observed a downward shift in the MedF from a repetition to approximately 15% that of the baseline MedF. The second exercise set ended once the MedF from a repetition shifted to approximately 25% compared to the baseline MedF. For example if the baseline MedF was 100Hz, then the subject was instructed to stop the exercise set once the MedF from a repetition fell to approximately 85Hz for the first exercise set and approximately 75Hz for the second set. MedF changes are calculated as a percent shift from baseline with negative values indicating a leftward (decreasing MedF) shift in the skewness of the frequency spectrum and positive values indicating a rightward (increasing MedF) shift in the skewness of the EMG frequency spectrum. We decided to use 15% and 25% shift in lumbar paraspinal EMG MedF since it generally represented mild and moderate lumbar paraspinal fatigue during pilot data collection.
All EMG signals were digitally filtered with a bandwidth of 10-500Hz and decomposed to the frequency domain using a fast Fourier transformation algorithm using a Hamming window. A 2048-point fast Fourier transform was performed (ie: 211 points) consisting of 2000 data points plus 48 zero pads. Then, the median of the frequency spectrum was calculated as recommended by the software manufacturer (Biopac systems, Inc., Goleta, CA).
Statistical analysis
Multiple regression equations were created using QI following both sets of fatiguing exercise as the outcome variable and the change in lateral hamstring ( ∆HMedF) and lateral quadriceps MedF ( ∆QMedF) during both fatiguing exercise set as the predictor variables, respectively. Separate equations were created for each group. Group comparisons of the resultant regression coefficients were performed to test the null hypothesis that the predictor variables explained an equal amount of variance in QI following both exercise sets. We used stepwise multiple regression procedures for all analyses. All statistical analyses were performed with SPSS statistical package, version 12.0 (SPSS, Inc., Chicago, IL). The a priori alpha-level was p ≤ 0.05.
Results
Means and standard deviations for QI measured at baseline, and following the first and second exercise sets along with hamstring, quadriceps and lumbar paraspinal MedF measurements are presented in Table 1. Relevant multiple regression results and correlations are presented in Table 2 and Table 3, respectively.
Table 1.
Means (±SD) of quadriceps inhibition (QI) over time and the change in lumbar paraspinal (LP) median frequency during the first and second exercise sets.
| Control (n=25) | History of LBP (n=25) | |
|---|---|---|
| BSL QI (%) | 9.92 (9.37) | 9.27 (94.0) |
| Post Set 1 QI(%) | 13.38 (9.80) | 11.91 (.28) |
| Post Set 2 QI(%) | 16.26 (9.85) | 14.20 (9.73) |
| Set 1 DLPMedF(%) | -17.10 (5.69) | -16.61 (5.63) |
| Set 2 DLPMedF(%) | -24.82 (8.37) | -23.72 (5.84) |
BSL: Baseline. ∆LPMedF: % change in lumbar paraspinal median frequency during exercise set.
Table 2.
Relevant multiple regression results including R2, standardized regression coefficient and associated t, P values for control and low back pain (LBP) groups after the first and second exercise sets.
| Group | R2 | Standardized Regression coefficient (b) | t | P-value | |
|---|---|---|---|---|---|
| First Set | Control | 0.18 | 0.43 * | 2.22 | 0.04 |
| LBP history | 0.22 | 0.46 † | 2.45 | 0.02 | |
| Second Set | Control | 0.07 | ‡ | ‡ | ‡ |
| LBP history | 0.21 | 0.46 § | 2.37 | 0.03 |
*Standardized regression coefficient for ∆QMedF
† Standardized regression coefficient for ∆MedF
‡ No predictor significantly explained variance in QI
§ Standardized regression coefficient for ∆HMedF
Table 3.
Correlations between variables used for multiple regression analysis.
| QI following first set | QI following second set | ||
|---|---|---|---|
| Control | DQMedF | 0.43* | -0.27 |
| DHMedF | -0.17 | -0.11 | |
| LBP history | DQMedF | -0.06 | 0.09 |
| DHMedF | 0.46* | 0.42* |
* p < 0.05. QI = quadriceps inhibition, LBP = low back pain.
QI following the first exercise set
The duration of the first exercise set was 187.2 ± 43.5 seconds (range: 120-240s) for the control group and 180.0 ± 77.5 seconds (range: 60-360s) for the history of LBP group.
∆QMedF during the first exercise set was moderately correlated with the amount of QI following the first exercise set for the control group only. ∆QMedF explained approximately 18% of the variance in QI, yielding the standardized regression model, equation (2):
The regression coefficient for ∆QMedF is a meaningful predictor in this model (t = 2.2, p = 0.04) but, the regression coefficient for ∆HMedF may have occurred by chance (t = -0.02, p = 0.99).
∆HMedF during the first exercise set was correlated with the amount of QI following the first exercise set in the history of LBP group only. ∆HMedF explained approximately 22% of the variance in QI, yielding the standardized regression model, equation (3):
The regression coefficient for ∆HMedF is a meaningful predictor in this model (t = 2.5, p = 0.02) but, the regression coefficient for ∆QMedF may have occurred by chance (t = -0.32, p = 0.75). QI following the second exercise set The duration of the first exercise set was 312.0 ± 94.9 seconds (range: 180-480s) for the control group and 321.6 ± 109.4 seconds (range: 120-540s) for the history of LBP group.
Neither ∆QMedF nor ∆HMedF were correlated with QI following the second fatiguing lumbar extension exercise set and neither variable contributed significantly in explaining variance in QI for the control subjects.
∆HMedF during the second exercise set was moderately correlated with the amount of QI following the second exercise set for the history of LBP subjects only. ∆HMedF explained approximately 21% of the variance in QI, yielding the standardized regression model, equation (4):
The regression coefficient for ∆HMedF is a meaningful predictor in this model (t = 2.4, p = 0.03) but, the regression coefficient for ∆QMedF may have occurred by chance (t = -0.97, p = 0.34).
Discussion
In our previous work, (Hart, 2005; Hart et al., 2005) we observed a statistically significant increase in QI after the first and second sets of lumbar paraspinal fatiguing exercise in persons with a history of LBP and controls. Therefore, in the current paper, our aim was to determine whether hamstring and quadriceps fatigue contributed to this increase in QI following prone, isometric lumbar extension exercise ∆HMedF contributes to increased QI in the history of LBP group following the first and second exercise sets. After the first exercise set, ∆QMedF was a meaningful predictor for QI in the control group only. The standardized regression suggests that for each standard deviation (SD) change in ∆QMedF we expect a 0.5 SD change in QI. This relationship was different for the history of LBP group. In the history of LBP group, ∆HMedF , not ∆QMedF , was a meaningful predictor of QI following the first and second exercise sets. The standardized regression equation for predicting QI in the history of LBP group following the first and second exercise sets suggest that for each SD change in ∆HMedF we expect a 0.5 SD change in QI. ∆QMedF was not a meaningful predictor of QI following the second exercise set for either group.
Both groups experienced a similar amount of lumbar paraspinal fatigue since all participants experienced similar shifts in LPMedF during the fatiguing exercise sets. Since persons with low back pain tend to have poor low back extension endurance, (Nourbakhsh et al., 2002; Latimer et al., 1999) the subjects in the current study probably needed to use the hip extensors in order to maintain the test position while reaching the desired downward shift in LPMedF. The hamstring muscles of subjects in the history of LBP group may be contributing more to completing the repetitive extension exercise than that of the control group. Based on the moderate correlation between QI and ∆HMedF, persons in the history of LBP group who experienced more hamstring MedF shift (more negative shift in ∆HMedF values) tended to experience less QI following the fatiguing exercise sets. This relationship makes sense functionally due to the reciprocal relationship between the knee flexors and extensors. If the lumbar paraspinals were weaker in persons with a history of low back pain, subjects in the history of LBP group would have had to rely more on the hip extensors to maintain the exercise position during the fatiguing sets. Greater contribution from the hamstring muscle group may change the way in which persons with history of LBP adapt to fatiguing exercise in a functional setting.
∆HMedF explained only 21% and 22% of the variance in QI following the first and second exercise sets, respectively for the history of LBP group. Therefore, it is important to note that more than 75% of the variance in QI is explained by something other than ∆HMedF. Low R2 values for the control group regression models also suggest that other factors, not included in these models contribute to QI. It is likely that lumbar paraspinal fatigue contributes to this relationship since we observed a significant reduction in QI following the fatiguing exercise sets previously (Hart et al., 2005). However, additional factors contributing to QI during exercise in persons with a history of LBP requires further research.
Contributing factors to QI in persons with history of LBP may help us to learn more about risk for lower extremity injuries during activity in this population. Hamstring tightness (Hultman et al., 1992; Jones et al., 2005; McClure et al., 1997), poor spinal flexibility (Hultman et al., 1992; Jones et al., 2005) and reduced lumbar lordosis (Hultman et al., 1992) in addition to weakness and imbalance(Nadler et al., 2000; 2001; 2000a) of the hip musculature commonly exist in persons with a history of low back pain. During prolonged, fatiguing exercise such as running or sport-specific maneuvers, this process may be more pronounced due to adaptations to muscle fatigue, weakness and/or inhibition. More research is needed to learn more about neuromuscular adaptations to fatiguing exercise in persons who are at risk for LBP during exercise (ie: persons with a history of LBP).
In the current study, we chose a group of subjects with a history of LBP due to the high likelihood of weaker trunk and hip muscles that may result in a different adaptive response to fatiguing exercise compared to controls. Previously, subjects were included in a “LBP ”group if they reported duration of LBP greater than 6-months (Suter et al., 2001) or had an episode of LBP that was treated within one year previous to research participation (Nadler et al., 2002b). This method does not exclude subjects who may have experienced a one-time episode of LBP due to an unusual activity or trauma Subjects may or may not have a chronic mechanical or muscular deficit. Although subjects in the history of LBP group in the current study were asymptomatic, we did not take into consideration the level of disability of individual subjects nor did we measure trunk muscle strength or balance to determine the severity of each subjects’ injury history. However, persons who have a history of multiple episodes of LBP are likely to experience subsequent episodes (Greene et al., 2001), possibly due to the fact that persons who have weak (Bayramoglu et al., 2001) or unbalanced (Lee et al., 1999) trunk muscles are likely to experience LBP. Therefore, since LBP pain commonly follows some sort of activity-related trauma it is important to learn more about neuromuscular adaptations to fatiguing exercise in asymptomatic persons with a history of LBP that may increase the risk for injury to the low back and other lower extremity joints.
Our finding that ∆QMedF was related to and explained variance in QI following the first exercise set in the control group should be interpreted with caution. It makes sense that changes in EMG muscle activity in the quadriceps would be related to the motorneuron pool excitability of the quadriceps. However, the moderate, positive relationship observed in the current data suggests that more negative ∆QMedF values correspond to smaller QI values. This does not make sense functionally since more fatigue in the quadriceps, represented here by a more negative ∆QMedF, would probably result in higher QI, measured by the superimposed burst technique. Quadriceps fatigue would probably cause less knee extension force production and a larger QI ratio. If the quadriceps were considerably fatigued during the prolonged exercise sets, it is also possible that the quadriceps were becoming less inhibited as a protective mechanism to preserve normal function. In addition, while performing prone lumbar extension, the quadriceps are not considerably challenged are most likely not considerably active. It is likely that the relationship between ∆QMedF and QI observed in the current study is spurious.
There are inherent limitations to the methods used in this study. First, the electrical stimulation used to measure QI with the superimposed burst technique likely does not recruit every single motor unit in the quadriceps muscle group. We addressed this methodological concern by using a within-subject design and using extreme caution to avoid electrode migration on subjects throughout the entire experiment. Second, it is difficult to generalize the results of our EMG recordings to an entire muscle or muscle group. However, EMG median frequency have been used previously to index muscular fatigue (Bilodeau et al., 2003; Gayda et al., 2005; Maisetti et al., 2002; Mathur et al., 2005) and have been shown to be reliable with repeated measures (Bilodeau et al., 1994; Daanen et al., 1990; Ng et al., 1996). Although EMG MedF is not commonly used as a marker of fatigue in “real time ”while subjects perform fatiguing exercise, it provides a reliable and easily controlled method to induce muscular fatigue over time. We chose to use lumbar paraspinal EMG MedF to determine when to stop an exercise in order to avoid other supraspinal contributors to task failure such as discomfort and effort which are difficult to control when inducing similar levels of muscular fatigue across subjects.
Conclusions
In conclusion, QI following lumbar extension exercise in the history of LBP group seems to involve significant contribution from the hamstring muscle group. More hamstring muscle contribution may be a necessary adaptation in the history of LBP group due to weaker and more fatigable lumbar extensors. QI following the first exercise set is related to the change in quadriceps MedF for the control group only however, this relationship may be spurious.
Biographies

Joseph M. Hart
Employment
University of Virginia, Department of Orthopaedic Surgery, Sports Medicine Division
Degree
PhD, ATC
Research interests
Lower extremity neuromuscular adaptations during fatiguing exercise in the presence of injury or muscle inhibition.
E-mail: joehart@virginia.edu

D. Casey Kerrigan
Employment
University of Virginia, Department of Physical Medicine and Rehabilitation
Degree
MD, MS
Research interests
Mechanics and physiology of walking and running

Julie M. Fritz
Employment
University of Utah Health Sciences Center, Division of Physical Therapy
Degree
PT, PhD, ATC
Research interests
Treatment for individuals with low back pain, matching the most effective treatments to various sub-groups of patients with low back pain

Ethan N. Saliba
Employment
University of Virginia, Athletics
Degree
PT, PhD, ATC, SCS
Research interests
Orthopaedic injuries in active/ athletic patient populations

Bruce Gansneder
Employment
University of Virginia, Department of Leadership, Foundations & Policy
Degree
PhD
Research interests
Research design and methodology including special interest in survey methodology

Christopher D. Ingersoll
Employment
University of Virginia, Department of Sports Medicine and Athletic Training
Degree
PhD, ATC
Research interests
Neuromechanical consequences of injury as it relates to muscle inhibition and neural control systems.
References
- Bayramoglu M., Akman M.N., Kilinc S., Cetin N., Yavuz N., Ozker R. (2001) Isokinetic measurement of trunk muscle strength in women with chronic low-back pain. American Journal of Physical Medicine and Rehabilitation 80, 650-655 [DOI] [PubMed] [Google Scholar]
- Behm D., Power K., Drinkwater E. (2001) Comparison of interpolation and central activation ratios as measures of muscle inactivation. Muscle and Nerve 24, 925-934 [DOI] [PubMed] [Google Scholar]
- Biering-Sorensen F. (1984) Physical measurements as risk indicators for low-back trouble over a one-year period. Spine 9, 106-119 [DOI] [PubMed] [Google Scholar]
- Bilodeau M., Arsenault A.B., Gravel D., Bourbonnais D. (1994) EMG power spectrum of elbow extensors: A reliability study. Electromyography and Clinical Neurophysiology 34, 149-158 [PubMed] [Google Scholar]
- Bilodeau M., Schindler-Ivens S., Williams D.M., Chandran R., Sharma S.S. (2003) EMG frequency content changes with increasing force and during fatigue in the quadriceps femoris muscle of men and women. Journal of Electromyography and Kinesiology 13, 83-92 [DOI] [PubMed] [Google Scholar]
- Biopac Systems. EMG Signal Analysis. Available from URL:http://biopac.com/AppNotes/app118EMG/emg.html [Google Scholar]
- Daanen H.A., Mazure M., Holewijn M., Van der Velde E.A. (1990) Reproducibility of the mean power frequency of the surface electromyogram. European Journal of Applied Physiology and Occupational Physiology 61, 274-277 [DOI] [PubMed] [Google Scholar]
- Gayda M., Merzouk A., Choquet D., Ahmaidi S. (2005) Assessment of skeletal muscle fatigue in men with coronary artery disease using surface electromyography during isometric contraction of quadriceps muscles. Archives of Physical Medicine and Rehabilitation 86, 210-215 [DOI] [PubMed] [Google Scholar]
- Greene H.S., Cholewicki J., Galloway M.T., Nguyen C.V., Radebold A. (2001) A history of low back injury is a risk factor for recurrent back injuries in varsity athletes. American Journal of Sports Medicine 29, 795-800 [DOI] [PubMed] [Google Scholar]
- Hart J.M. (2005) Quadriceps inhibition and gait kinetics following fatiguing isometric lumbar paraspinal exercise. PhD Dissertation, University of Virginia, Charlottesville, VA [Google Scholar]
- Hart J.M., Kerrigan D.C., Saliba E.N., Fritz J.M., Gansneder B.M., Ingersoll C.D. (2005) Reduced quadriceps activation following lumbar paraspinal fatiguing exercise. Journal of Athletic Training, in press [PMC free article] [PubMed] [Google Scholar]
- Hultman G., Saraste H., Ohlsen H. (1992) Anthropometry, spinal canal width, and flexibility of the spine and hamstring muscles in 45-55-year-old men with and without low back pain. Journal of Spinal Disorders and Techniques 5, 245-253 [DOI] [PubMed] [Google Scholar]
- Hurley M.V., Jones D.W., Newham D.J. (1994) Arthrogenic quadriceps inhibition and rehabilitation of patients with extensive traumatic knee injuries. Clinical Science (Lond) 86, 305-310 [DOI] [PubMed] [Google Scholar]
- Jones M.A., Stratton G., Reilly T., Unnithan V.B. (2005) Biological risk indicators for recurrent non-specific low back pain in adolescents. British Journal of Sports Medicine 39, 137-140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kankaanpää M., Taimela S., Laaksonen D., Hanninen O., Airaksinen O. (1998) Back and hip extensor fatigability in chronic low back pain patients and controls. Archives of Physical Medicine and Rehabilitation 79, 412-417 [DOI] [PubMed] [Google Scholar]
- Kent-Braun J.A., Le Blanc R. (1996) Quantitation of central activation failure during maximal voluntary contractions in humans. Muscle and Nerve 19, 861-869 [DOI] [PubMed] [Google Scholar]
- Latimer J., Maher C.G., Refshauge K., Colaco I. (1999) The reliability and validity of the biering-sorensen test in asymptomatic subjects and subjects reporting current or previous nonspecific low back pain. Spine 24, 2085-2089; discussion 2090 [DOI] [PubMed] [Google Scholar]
- Lee J.H., Hoshino Y., Nakamura K., Kariya Y., Saita K., Ito K. (1999) Trunk muscle weakness as a risk factor for low back pain. A 5-year prospective study. Spine 24, 54-57 [DOI] [PubMed] [Google Scholar]
- Lewek M.D., Rudolph K.S., Snyder-Mackler L. (2004) Quadriceps femoris muscle weakness and activation failure in patients with symptomatic knee osteoarthritis. Journal of Orthopaedic Research 22, 110-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee D.J. (1997) Orthopedic physical assessment. Philadelphia, PA, W.B. Saunders Company [Google Scholar]
- Maisetti O., Guevel A., Legros P., Hogrel J.Y. (2002) Prediction of endurance capacity of quadriceps muscles in humans using surface electromyogram spectrum analysis during submaximal voluntary isometric contractions. European Journal of Applied Physiology 87, 509-519 [DOI] [PubMed] [Google Scholar]
- Mathur S., Eng J.J., MacIntyre D.L. (2005) Reliability of surface emg during sustained contractions of the quadriceps. Journal of Electromyography and Kinesiology 15, 102-110 [DOI] [PubMed] [Google Scholar]
- McClure P.W., Esola M., Schreier R., Siegler S. (1997) Kinematic analysis of lumbar and hip motion while rising from a forward, flexed position in patients with and without a history of low back pain. Spine 22, 552-558 [DOI] [PubMed] [Google Scholar]
- Mizner R.L., Stevens J.E., Snyder-Mackler L. (2003) Voluntary activation and decreased force production of the quadriceps femoris muscle after total knee arthroplasty. Physical Therapy 83, 359-365 [PubMed] [Google Scholar]
- Nadler S.F., Malanga G.A., Bartoli L.A., Feinberg J.H., Prybicien M., Deprince M. (2002a) Hip muscle imbalance and low back pain in athletes: Influence of core strengthening. Medicine and Science in Sports and Exercise 34, 9-16 [DOI] [PubMed] [Google Scholar]
- Nadler S.F., Malanga G.A., DePrince M., Stitik T.P., Feinberg J.H. (2000) The relationship between lower extremity injury, low back pain, and hip muscle strength in male and female collegiate athletes. Clinical Journal of Sports Medicine 10, 89-97 [DOI] [PubMed] [Google Scholar]
- Nadler S.F., Malanga G.A., Feinberg J.H., Prybicien M., Stitik T.P., DePrince M. (2001) Relationship between hip muscle imbalance and occurrence of low back pain in collegiate athletes: A prospective study. American Journal of Physical Medicine and Rehabilitation 80, 572-577 [DOI] [PubMed] [Google Scholar]
- Nadler S.F., Moley P., Malanga G.A., Rubbani M., Prybicien M., Feinberg J.H. (2002b) Functional deficits in athletes with a history of low back pain: A pilot study. Archives of Physical Medicine and Rehabilitation 83, 1753-1758 [DOI] [PubMed] [Google Scholar]
- Ng J.K., Richardson C.A. (1996) Reliability of electromyographic power spectral analysis of back muscle endurance in healthy subjects. Archives of Physical Medicine and Rehabilitation 77, 259-264 [DOI] [PubMed] [Google Scholar]
- Nourbakhsh M.R., Arab A.M. (2002) Relationship between mechanical factors and incidence of low back pain. Journal of Orthopaedic and Sport Physical Therapy 32, 447-460 [DOI] [PubMed] [Google Scholar]
- Plamondon A., Trimble K., Lariviere C., Desjardins P. (2004) Back muscle fatigue during intermittent prone back extension exercise. Scandinavian Journal of Medicine and Science in Sports 14, 221-230 [DOI] [PubMed] [Google Scholar]
- Snyder-Mackler L., De Luca P.F., Williams P.R., Eastlack M.E., Bartolozzi A.R. (1994) Reflex inhibition of the quadriceps femoris muscle after injury or reconstruction of the anterior cruciate ligament. Journal of Bone and Joint Surgery: American Volume 76, 555-560 [DOI] [PubMed] [Google Scholar]
- Stevens J.E., Mizner R.L., Snyder-Mackler L. (2003) Quadriceps strength and volitional activation before and after total knee arthroplasty for osteoarthritis. Journal of Orthopaedic Research 21, 775-779 [DOI] [PubMed] [Google Scholar]
- Suter E., Herzog W., Bray R.C. (1998) Quadriceps inhibition following arthroscopy in patients with anterior knee pain. Clinical Biomechanics (Bristol, Avon) 13, 314-319 [DOI] [PubMed] [Google Scholar]
- Suter E., Lindsay D. (2001) Back muscle fatigability is associated with knee extensor inhibition in subjects with low back pain. Spine 26, E361-366 [DOI] [PubMed] [Google Scholar]
- Verna J.L., Mayer J.M., Mooney V., Pierra E.A., Robertson V.L., Graves J.E. (2002) Back extension endurance and strength: The effect of variable-angle roman chair exercise training. Spine 27, 1772-1777 [DOI] [PubMed] [Google Scholar]
- Vogt L., Pfeifer K., Banzer W. (2003) Neuromuscular control of walking with chronic low-back pain. Manual Therapy 8, 21-28 [DOI] [PubMed] [Google Scholar]
- Walton D.M., Kuchinad R.A., Ivanova T.D., Garland S.J. (2002) Reflex inhibition during muscle fatigue in endurance-trained and sedentary individuals. European Journal of Applied Physiology 87, 462-468 [DOI] [PubMed] [Google Scholar]


