Abstract
Background/Purpose
Aging is one of the major risk factors for white matter injury in cerebrovascular disease. However, the effects of age on the mechanisms of injury/repair in white matter remain to be fully elucidated. Here, we ask if compared to young brains, white matter regions in older brains may be more vulnerable in part due to decreased rates of compensatory oligodendrogenesis after injury.
Methods
A mouse model of prolonged cerebral hypoperfusion was prepared by bilateral common carotid artery stenosis in 2-month and 8-month old mice. Matching in vitro studies were performed by subjecting oligodendrocyte precursor cells (OPCs) to sub-lethal 7-day CoCl2 treatment to induce chemical hypoxic stress.
Results
Baseline myelin density in the corpus callosum was similar in 2-month and 8-month old mice. But after induction of prolonged cerebral hypoperfusion, older mice showed more severe white matter injury together with worse deficits in working memory. The numbers of newborn oligodendrocytes and their precursors were increased by cerebral hypoperfusion in young mice, whereas these endogenous responses were significantly dampened in older mice. Defects in CREB signaling may be involved because activating CREB with the type-III phosphodiesterase inhibitor cilostazol in older mice restored the differentiation of OPCs, alleviated myelin loss and improved cognitive dysfunction during cerebral hypoperfusion. Cell culture systems confirmed that cilostazol promoted the differentiation of OPCs.
Conclusions
An age-related decline in CREB-mediated oligodendrogenesis may compromise endogenous white matter repair mechanisms, and therefore, drugs that activate CREB signaling provide a potential therapeutic approach for treating white matter injury in aging brains.
Keywords: aging, white matter injury, prolonged cerebral hypoperfusion, oligodendrogenesis, CREB signaling
Introduction
Aging is one of the most important risk factors for developing white matter injury in stroke and cerebrovascular disease 1. The risk of stroke doubles every decade after age 55 2, and aged patients show less functional recovery from stroke compared to younger patients 3. However, the mechanisms that underlie the increased vulnerability of aging white matter remains poorly understood.
Increasingly, it has been proposed that CNS pathophysiology is significantly influenced by the balance between deleterious versus beneficial responses to the initial insult 4. Stroke and brain injury triggers a wide spectrum of neurovascular perturbations, glial activation, neuroinflammation and neuronal cell death cascades. But at the same time, many endogenous neuroprotective responses may also be induced at the same time. These include compensatory neurogenesis, angiogenesis, neuroplasticity and remodeling 5. Herein may lie a clue as to the effects of aging on CNS disease. Although the adult brain retains plastic capabilities for regeneration and recovery, aging may significantly dampen these endogenous protective mechanisms. In particular, the capacity for neurogenesis appears to diminish with age. This is primarily due to a general reduction of neuronal precursor cell proliferation because of age-related alterations in the cellular microenvironment - decline of neurotrophic factor expression 6, 7, increase in cell death rate of neuronal precursors and mature neurons 8, and decrease in the activation of cAMP response element binding protein (CREB) signaling 9.
Similar to neurogenesis, oligodendrogenesis and white matter homeostasis might also be affected by white matter senescence. In healthy young adult brains, myelin-forming mature oligodendrocytes (OLs) in the white matter can be newly generated from their precursor cells (oligodendrocyte precursor cells: OPCs). After white matter injury, OPCs rapidly proliferate and migrate to fill the demyelinated area, differentiate into mature OLs and restore myelin sheaths 10–12. Notably, however, myelin density along with cognitive function spontaneously declines with increasing age both in human and rodents 13, 14, indicating that the capacity for oligodendrogenesis may be associated with white matter senescence. In this study, we ask whether analogous declines in endogenous recovery mechanisms may also occur after CNS injury, thus mediating the age-related increase in white matter vulnerability in stroke and cerebrovascular disease.
Methods
Cerebral prolonged hypoperfusion model
All experiments were performed following an institutionally approved protocol in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Cerebral prolonged hypoperfusion stress was induced by bilateral common carotid artery stenosis. Male C57Bl/6 mice (2 and 8 months old, Charles River Institute) were anesthetized with 4.0% isoflurane and then maintained on 1.5% isoflurane in 70% N2O and 30% O2 using a small-animal anesthesia system. Through a midline cervical incision, both common carotid arteries were exposed. A microcoil with a diameter of 0.18 mm (Sawane Spring Co.) was applied to bilateral common carotid arteries, maintaining the rectal temperature between 36.5°C and 37.5°C using heating pad. All experimental groups were randomized, and investigators responsible for surgical procedures or drug treatments were blinded. End-point assessments (please see Supplementary Information for detailed methods of end-point assessments) were performed by investigators blinded to the groups for which each animal was assigned.
Cell culture
OPC cultures were prepared from rat neonatal cortex. Cultured OPCs were plated and maintained in Neurobasal medium containing glutamine, 1% penicillin/streptomycin, 10 ng/ml PDGF, 10 ng/ml FGF, and 2% B27 supplement onto poly-DL-ornithine-coated plates. Four to five days after plating, the OPCs were used for the experiments. To differentiate OPCs to myelin basic protein-positive OLs, the culture medium was switched to DMEM containing 1% penicillin/streptomycin, 10 ng/ml CNTF, 50 ng/ml T3, and 2% B27 supplement. To mimic chronic mild-hypoxic condition, OPCs were incubated with non-lethal CoCl2 (Sigma). Please see Supplementary Information for detailed methods of in vitro cell culture experiments.
Statistical analysis
Based on published and pilot data, power estimates were calculated based on α=0.05 and β=0.8 to obtain group sizes appropriate for detecting effect sizes in the range of 30–50% for in vivo models and 40–50% for cell cultures models. A one-way ANOVA followed by post-hoc Fisher-protected least significant difference test was used to determine the significant differences in various indices among the groups. A p-value of <0.05 was considered statistically significant.
Results
8-month old mice suffer more white matter injury than 2-month old mice after prolonged cerebral hypoperfusion
There were no clear differences in myelination and white matter integrity of the corpus callosum in young 2-month mice compared to older 8-month old mice (Suppl Fig S1). We then asked whether in spite of similar baseline conditions, older white matter would still be more vulnerable to injury. Mice were subjected to a standard model of prolonged cerebral hypoperfusion by using micro-coils to bilaterally narrow the luminal diameters of their common carotid arteries. There were no initial differences in carotid diameters (2-month old mice: 341.4 +/− 24.6 μm, 8-month old mice: 342.4 +/− 24.7 μm) and the surgical procedures produced similar degrees of cerebral hypoperfusion (2-month: 75.9 +/− 8.7%, 8-month: 78.0 +/− 6.6%, cerebral blood flow levels at 14 days relative to sham-operative animals).
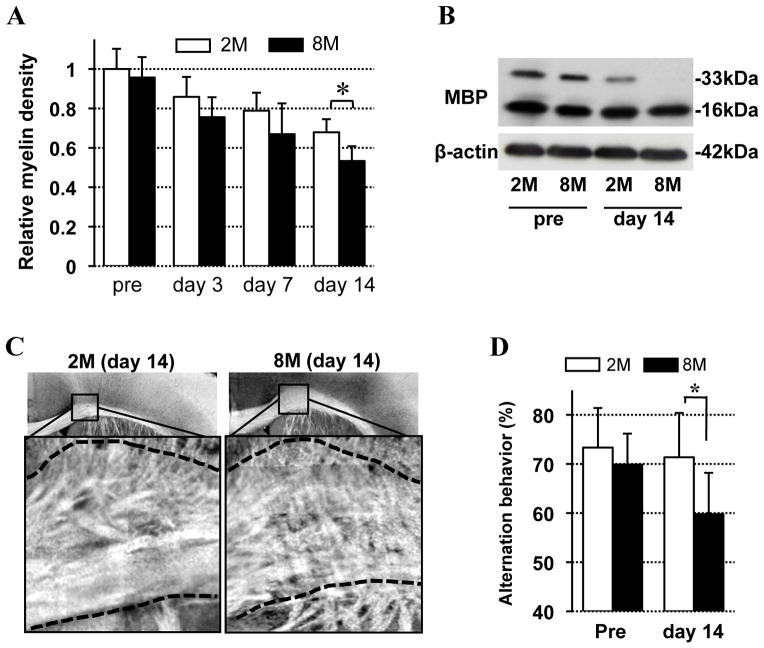
For 14 days after onset of cerebral hypoperfusion, white matter integrity was assessed with fluoromyelin staining, myelin-basic-protein (MBP) western blot, and spectral domain optical coherence microscopy (SD-OCM) imaging (Suppl Fig S2). As expected, cerebral hypoperfusion induced a progressive degradation of white matter integrity in the corpus callosum in all mice. But loss of myelin immunostaining (Fig 1A) and MBP western blot (Fig 1B) was more severe in older 8-month old brains compared with the younger 2-month old brains. SD-OCM imaging confirmed that the 8-month old brains showed increased myelin fiber derangement and white matter vacuoles (Fig 1C). Consistent with these morphological endpoints, 8-month old mice demonstrated more severe neurologic deficits compared to younger 2-month old mice, as measured with the standard Y-maze test. At pre-hypoperfusion baseline conditions, all mice showed normal Y-maze function. But after 2 weeks of cerebral hypoperfusion, Y-maze function was more severely affected in the older 8-month old mice (Fig 1D).
Figure 1. White matter lesion after prolonged cerebral hypoperfusion.
A. Relative density of fluoromyelin intensity in mouse corpus callosum. Values were calculated based on the density of pretretment in 2-month old mice. N=5. 2M: 2-month old mice, 8M: 8-month old mice. B. Western blot images for MBP expression in mouse corpus callosum before and 14-day after the stress onset. β-actin is an internal control. C. SD-OCM images of corpus callosum in 2-month old and 8-month old mice at day14 after the white matter injury. D. Alternation behavior (index of working/spatial memory) of 2-month and 8-month old mice at pretreatment and day 14 after the injury. N=10. Values are mean ± SD. *P<0.05.
8-month old mice show decreased oligodendrogenesis after prolonged cerebral hypoperfusion
To assess the hypothesis that older brains possess dampened endogenous repair capacities, we used 5-bromodeoxyuridine (BrdU) incorporation experiments to ask if oligodendrogenesis is suppressed in aging white matter (Suppl Fig S3). In young 2-month old white matter, the number of BrdU-incorporated cells was increased after cerebral hypoperfusion (Fig 2A). In older 8-month old white matter, the increase in BrdU-incorporated cells was detectable but significantly reduced compared to 2-month old white matter (Fig 2A). Double immunofluorescence labeling with cell specific markers revealed that the major cell type of BrdU-incorporated cells comprised NG2-positive oligodendrocyte precursor cells (OPCs) in both groups (Suppl Table S1).
Figure 2. OPC proliferation in vivo.
A. Number of BrdU–positive (BrdU+) cells in the corpus callosum. N=5. 2M: 2-month old mice, 8M: 8-month old mice. B. Representative images of NG2 staining in the lateral side of corpus callosum at day 14 after the white matter injury. Bar = 50 μm. C. Number of NG2–positive (NG2+) cells in mouse corpus callosum during the white matter damage. N=5. D. Ratio of ssDNA/NG2-double positive cells in total NG2 cells in the mouse corpus callosum at day 14. N=5.
To confirm these findings, immunostaining was used to quantify the number of NG-2 positive OPCs up to 14 days after cerebral hypoperfusion onset. In young 2-month old brains, the number of NG2-positive OPCs in the corpus callosum was gradually increased over the course of prolonged hypoperfusion (Fig 2B-C). In contrast, older 8-month old brains did not show a significant increase in OPC number over time (Fig 2B-C). The ratio of ssDNA/NG2 double positive cells (damaged OPCs) at day 14 was significantly larger in the 8-month old brains (Fig 2D), consistent with the observation that OPC numbers did not increase in this older group of mice.
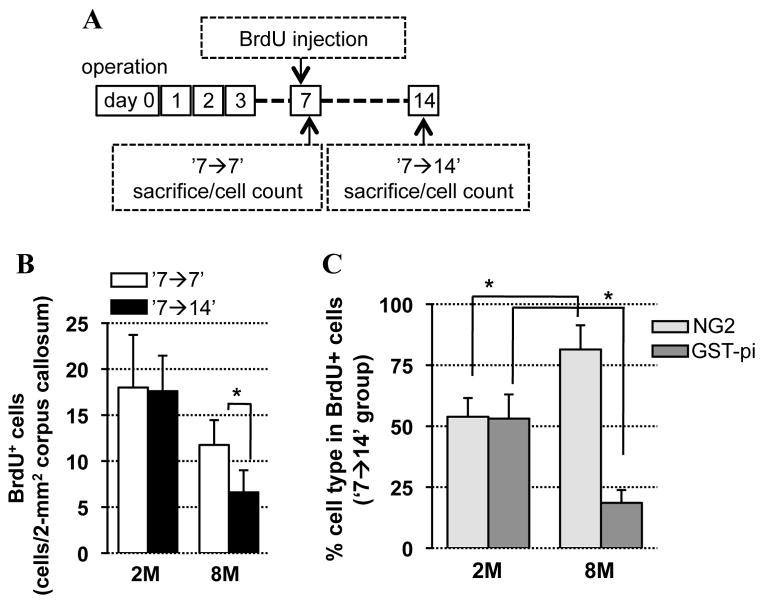
If there is a difference in OPC response in older brains, does this mean that the numbers of mature oliogodendrocytes would also be different? To answer this question, two BrdU treatment schedules were used – (i) BrdU was injected on day 7 after the hypoperfusion onset and brains were removed and examined on the same day (‘7→7’), and (ii) BrdU was injected on day 7 and brains were examined one week later (‘7→14’) (Fig 3A). In young 2-month old mice, the number of BrdU-positive cells at day 14 was similar to at day 7 (Fig 3B). In contrast, for 8-month old mice, the number of BrdU-positive cells at day 14 was less than that at day 7 (Fig 3B), indicating that newly generated OPCs post-hypoperfusion in the older brains would die in one week without maturing into OLs. Double immunofluorescence labeling with cell specific markers showed that some of newly generated OPCs at day 7 were successfully differentiated into mature OLs at day 14 in young mice. But the older 8-month old mice did not show equivalent levels of oligodendrogenesis (Fig 3C), indicating that OPCs in the older mice would die before differentiating into mature OLs.
Figure 3. OPC differentiation in vivo.
A. Experimental schedule to assess cell differentiation in the corpus callosum using BrdU. B. Number of BrdU–positive (BrdU+) cells in corpus callosum at days 7 and 14. N=5. 2M: 2-month old mice, 8M: 8-month old mice. C. Ratio of BrdU/NG2- and BrdU/GST-pi-double-labeled cells in total BrdU cells in corpus callosum at day 14 (BrdU injection: day 7). N=5. Values are mean ± SD. *P<0.05.
CREB signaling is involved in oligodendrogenesis after white matter injury
Older white matter regions may have a dampened recovery response to prolonged cerebral hypoperfusion compared to young brains. Thus far, our findings suggest that this may be due in part to a decline in the endogenous capacity for compensatory oligodendrogenesis. But what mechanisms are involved? Because CREB signaling is known to be generally important for neurogenesis and oligodendrogenesis, we asked whether differences in CREB responses may also be involved in our models. Immunostaining showed that, both in young and older white matter, phosphorylated CREB signaling was observed in mature OLs (CNPase-positive) and OPCs (PDGF-R-α-positive) under normal conditions, but phosphorylated CREB was primarily expressed within OPCs (Fig 4A, Suppl Fig S4). After prolonged cerebral hypoperfusion, phospho-CREB signals decreased in both groups, with a larger reduction present in the 8-month old brains (Fig 4B-C). Western blots confirmed that CREB phosphorylation was significantly lower in 8-month old brains compared to 2-month old brains (Fig 4D).
Figure 4. Phospho-CREB in white matter in vivo.
A. Double immunofluorescent staining of pCREB with CNPase (a marker for mature oligodendrocytes) or PDGFRα (a marker for OPCs) in 2-month old mice under normal conditions. Arrowheads are double positive cells. Bar = 25 μm. B. Representative images of pCREB staining in the lateral side of corpus callosum. Bar = 50 μm. 2M: 2-month old mice, 8M: 8-month old mice. C. Number of pCREB–positive (pCREB+) cells in the lateral side of corpus callosum during the hypoperfusion stress. N=5. D. Western blot image of pCREB expression in the corpus callosum. β-actin is an internal control. Values are mean ± SD. *P<0.05.
The PDE III inhibitor cilostazol promoted OPC differentiation in vitro and rescued OPC recovery in vivo
Differences in CREB-mediated OPC proliferation and differentiation may in part explain why older white matter was more vulnerable to prolonged cerebral hypoperfusion. So we next asked whether promoting CREB signaling with the phosphodiesterase (PDE) III inhibitor cilostazol could restore these compensatory OPC responses after prolonged stress and injury. To answer this question, we performed both cell culture and in vivo experiments.
First, primary OPCs were cultured from rat neonatal brain cortex. Under normal growth conditions, these OPCs matured over 7 days and decreased the expression of NG2, a marker for OPCs. In contrast, GST-pi and MBP expression was increased (Fig 5A), indicating that our cultured OPCs successfully differentiated into mature OLs in this model system. To mimic the hypoxic state of prolonged cerebral hypoperfusion, OPCs were treated with 0.01 to 1 μM of CoCl2 for 7 days. As expected, 7 days of CoCl2 treatment increased HIF1α expression in our cultured OPCs in a dose dependent manner (Fig 5A). CREB signaling may also be involved as the phosphorylation level of CREB was also decreased by CoCl2-inuced chemical hypoxia (Fig 5A). Correspondingly, OPC maturation was inhibited by the chronic CoCl2 treatment (Fig 5A). LDH assays showed that 0.01 to 1 μM of CoCl2 did not induce cell death in our OPCs (Fig 5B), indicating that the reduction of OPC maturation by CoCl2 was not merely due to cell death. Next, we asked whether activating CREB with cilostazol could rescue OPC maturation after prolonged chemical hypoxia. Cilostazol alone did not change OPC state (Suppl Fig S5), but significantly increased CREB phosphorylation and promoted OPC differentiation under CoCl2-induced hypoxic conditions (Fig 5C–D, Suppl Fig S5).
Figure 5. Cilostazol-induced OPC proliferation/differentiation in vitro.
A. Western blot images using samples from cultured rat OPCs with 7-day CoCl2 treatment. HIF1α is a marker for hypoxic conditions, NG2 is a marker for OPCs, GST-pi and MBP are markers for mature OLs, and β-actin is an internal control. B. LDH assays showed that 0.01 to 1 μM of CoCl2 (7-day treatment) did not induce cell death in our OPCs. N=6. Values are mean ± SD. C. Representative images of NG2, GST-pi and MBP staining in cultured rat OPCs at day 7 after the CoCl2 treatment with or without cilostazol. Bar = 25 μm. D. Representative images of pCREB, PDGFR-α and CNPase staining in cultured rat OPCs at day 7 after CoCl2 treatment with or without cilostazol. Bar = 25 μm.
Finally, we turned back to our in vivo model of prolonged cerebral hypoperfusion. Cilostazol treatment (10 mg/Kg/day for 14 days) was performed by intraperitoneal injection in 8-month old mice subjected to cerebral hypoperfusion. This treatment schedule was previously reported to effectively inhibit PDE III in brain in vivo 15. As expected, mice treated with cilostazol showed larger numbers of phopho-CREB positve cells than vehicle-treated group at day 14 (Suppl Fig S6). Correspondingly, the cilostazol-treated mice exhibited less white matter injury (Fig 6A, Suppl Fig S6), larger number of OPCs (Fig 6B, Suppl Fig S6), and better cognitive function (Fig 6C). The BrdU-incorporation/differentiation assay (Suppl Fig S7) revealed that cilostazol promoted the proliferation of white matter cells (Suppl Fig S8) as well as the differentiation of OPCs into mature OLs (Fig 6D).
Figure 6. Cilostazol-induced white matter repairing in middle-aged mice.

A. Representative images of fluoromyelin staining in 8-month old mice with or without cilostazol treatment at day 14. Bar = 100 μm. Quantitative data are shown in Supple Fig S6D. B. Representative images of NG2 staining in the lateral side of corpus callosum in 8-month old mice with or without cilostazol (10 mg/Kg/day for 14 days, i.p.) at day 14 after the white matter injury. Bar = 50 μm. Quantitative data are shown in Supple Fig S6E. C. Alternation behavior (index of working/spatial memory) of 8-month old mice with or without cilostazol treatment at day 14. N=8. D. Ratio of BrdU/NG2- and BrdU/GST-pi-double-labeled cells in total BrdU cells in 8-month old mice corpus callosum at day 14. N=5. Veh; vehicle group, Cilo; cilostazol-treatment group, Values are mean ± SD. *P<0.05. Experimental schedule for the OPC differentiation assay is shown in Supple Fig S7.
Discussion
Demyelination and cognitive decline are major pathological hallmarks of ischemic white matter diseases, which are mostly associated with increasing age. Although adult brains retain neuroplasticity and regenerative capacities to compensate for lost brain cells, aged brains tend to slowly lose these endogenous repair systems. Our current study demonstrated that white matter regions in aging 8-month-old mice are more vulnerable to prolonged cerebral hypoperfusion, and this is caused in part by a loss of endogenous CREB-mediated oligodendrogenesis. Therefore, drugs that can activate PKA-CREB signaling cascade may provide novel therapeutic approaches for white matter injury in vascular dementia and stroke.
Aging lowers the endogenous capacities for brain regeneration and remodeling. Past studies have demonstrated that aging reduces the differentiation of neuronal precursor cells into neuronal phenotypes 16, increases the number of dying/dead brain cells 17, and decreases CREB activation in neurons 18. Our current data may expand these findings - the capability of oligodendrogeneis in the white matter also decreases with aging through deactivation of CREB signaling. In this study, middle-aged mice showed lower phospho-CREB level in the white matter than young mice under pathologic conditions. Moreover, activating CREB by a PDE III inhibitor cilostazol alleviated the white matter dysfunction in the middle-aged mice by protecting newly generated OPCs and enhancing OPC proliferation/maturation. Cilostazol has been approved for the treatment of intermittent claudication and improves pain-free walking distance in patients with peripheral arterial disease in the world-wide 19 and the secondary prevention of ischemic stroke in Japan 20. Our findings provide initial proof-of-concept that cilostazol (or other PDE III inhibitors) might be a promising drug for age-related white matter diseases. But of course, much more extensive preclinical studies are required before testing this approach can be translated into clinical applications.
Although our current findings demonstrate that aged white matter possesses less capacity in oligodendrogenesis, many important caveats remain. First, we focused on only oligodendrocyte lineage cells in this study. But there are other cell types in white matter, including endothelial cells and astrocytes. The concept of the neurovascular unit suggests that interactions between different cell types maintain brain function under normal conditions, and facilitates brain remodeling after injury in the grey matter 5. In white matter, cell-cell trophic coupling might similarly participate in white matter homeostasis. Indeed, both cerebral endothelial cells and astrocytes show supportive effects for OLs/OPCs 21. Hence, future studies should examine if the trophic coupling between OLs and neighboring cells diminishes with aging in the white matter. Second, our current study demonstrates that loss of CREB activation in the aging 8-month old white matter leads to deficits in oligodendrogenesis. But what factors/mechanisms lower the CREB signaling in the middle-aged white matter? CREB activation is regulated by several growth factors such as BDNF, and growth factor expressions are decreasing in aging 22. Hence, reduction of growth factor expression may fail the CREB activation during the stress in aged white matter. These questions should be explored in future studies. Finally, it is important to acknowledge that trying to correlate aging mouse models to the aging human brain is not straightforward. Thus far, most aging studies with rodents have focused on differences between young (2~3 months) and very old (>12~15 months) brains 23. But it might be possible that the age-related decline in oligodendrogenesis would occur even before obvious declines in myelin density or cognitive function. In this study, we compared young 2-month old mice to older 8-month mice. It is possible that our models may mimic “middle-aged” humans. But further studies are warranted to carefully track the temporal profile of these CREB-mediated mechanisms in a wider range of aged mice.
In conclusion, our current study demonstrates novel mechanisms by which aging white matter in mouse brain is more vulnerable to prolonged cerebral hypoperfusion and hypoxic stress. Importantly, these phenomena occurred in mice that had normal myelin density and cognitive function at baseline. No deficits were present yet. But these aging regions had lost their ability to recruit CREB-mediated oligodendrogenesis for responding to injury and stress. Hence, drugs that can activate CREB signaling may be a promising approach for aging patients with white matter-related diseases such as vascular dementia or stroke.
Supplementary Material
Acknowledgments
Funding Sources: Supported in part by the Deane Foundation, MGH ECOR Fund for Medical Discovery, American Heart Association, National Institutes of Health, Research Abroad from the Uehara Memorial Foundation, National Research Foundation of Korea, the World Class University Program, and the Global Research Laboratory Program.
Footnotes
Disclosures: None
Note: Please see Supplementary Information for methods and additional data.
References
- 1.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marini C, Triggiani L, Cimini N, Ciancarelli I, De Santis F, Russo T, et al. Proportion of older people in the community as a predictor of increasing stroke incidence. Neuroepidemiology. 2001;20:91–95. doi: 10.1159/000054766. [DOI] [PubMed] [Google Scholar]
- 3.Bejot Y, Rouaud O, Jacquin A, Osseby GV, Durier J, Manckoundia P, et al. Stroke in the very old: Incidence, risk factors, clinical features, outcomes and access to resources--a 22-year population-based study. Cerebrovasc Dis. 2010;29:111–121. doi: 10.1159/000262306. [DOI] [PubMed] [Google Scholar]
- 4.Lo EH. A new penumbra: Transitioning from injury into repair after stroke. Nat Med. 2008;14:497–500. doi: 10.1038/nm1735. [DOI] [PubMed] [Google Scholar]
- 5.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: Mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaplan MS, Bell DH. Mitotic neuroblasts in the 9-day-old and 11-month-old rodent hippocampus. J Neurosci. 1984;4:1429–1441. doi: 10.1523/JNEUROSCI.04-06-01429.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walter J, Keiner S, Witte OW, Redecker C. Age-related effects on hippocampal precursor cell subpopulations and neurogenesis. Neurobiol Aging. 2011;32:1906–1914. doi: 10.1016/j.neurobiolaging.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 9.Asanuma M, Nishibayashi S, Iwata E, Kondo Y, Nakanishi T, Vargas MG, et al. Alterations of camp response element-binding activity in the aged rat brain in response to administration of rolipram, a camp-specific phosphodiesterase inhibitor. Brain Res Mol Brain Res. 1996;41:210–215. doi: 10.1016/0169-328x(96)00098-8. [DOI] [PubMed] [Google Scholar]
- 10.Miyamoto N, Tanaka R, Shimura H, Watanabe T, Mori H, Onodera M, et al. Phosphodiesterase iii inhibition promotes differentiation and survival of oligodendrocyte progenitors and enhances regeneration of ischemic white matter lesions in the adult mammalian brain. J Cereb Blood Flow Metab. 2010;30:299–310. doi: 10.1038/jcbfm.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skihar V, Silva C, Chojnacki A, Doring A, Stallcup WB, Weiss S, et al. Promoting oligodendrogenesis and myelin repair using the multiple sclerosis medication glatiramer acetate. Proc Natl Acad Sci U S A. 2009;106:17992–17997. doi: 10.1073/pnas.0909607106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gensert JM, Goldman JE. Endogenous progenitors remyelinate demyelinated axons in the adult cns. Neuron. 1997;19:197–203. doi: 10.1016/s0896-6273(00)80359-1. [DOI] [PubMed] [Google Scholar]
- 13.Doucette JR, Jiao R, Nazarali AJ. Age-related and cuprizone-induced changes in myelin and transcription factor gene expression and in oligodendrocyte cell densities in the rostral corpus callosum of mice. Cell Mol Neurobiol. 2010;30:607–629. doi: 10.1007/s10571-009-9486-z. [DOI] [PubMed] [Google Scholar]
- 14.Sim FJ, Zhao C, Penderis J, Franklin RJ. The age-related decrease in cns remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. J Neurosci. 2002;22:2451–2459. doi: 10.1523/JNEUROSCI.22-07-02451.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka Y, Tanaka R, Liu M, Hattori N, Urabe T. Cilostazol attenuates ischemic brain injury and enhances neurogenesis in the subventricular zone of adult mice after transient focal cerebral ischemia. Neuroscience. 2010;171:1367–1376. doi: 10.1016/j.neuroscience.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci. 1998;18:3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lemaire V, Koehl M, Le Moal M, Abrous DN. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci U S A. 2000;97:11032–11037. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charalampopoulos I, Alexaki VI, Tsatsanis C, Minas V, Dermitzaki E, Lasaridis I, et al. Neurosteroids as endogenous inhibitors of neuronal cell apoptosis in aging. Ann N Y Acad Sci. 2006;1088:139–152. doi: 10.1196/annals.1366.003. [DOI] [PubMed] [Google Scholar]
- 19.Barnett AH, Bradbury AW, Brittenden J, Crichton B, Donnelly R, Homer-Vanniasinkam S, et al. The role of cilostazol in the treatment of intermittent claudication. Curr Med Res Opin. 2004;20:1661–1670. doi: 10.1185/030079904X4464. [DOI] [PubMed] [Google Scholar]
- 20.Shinohara Y, Katayama Y, Uchiyama S, Yamaguchi T, Handa S, Matsuoka K, et al. Cilostazol for prevention of secondary stroke (csps 2): An aspirin-controlled, double-blind, randomised non-inferiority trial. Lancet Neurol. 2010;9:959–968. doi: 10.1016/S1474-4422(10)70198-8. [DOI] [PubMed] [Google Scholar]
- 21.Arai K, Lo EH. Oligovascular signaling in white matter stroke. Biol Pharm Bull. 2009;32:1639–1644. doi: 10.1248/bpb.32.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rage F, Silhol M, Biname F, Arancibia S, Tapia-Arancibia L. Effect of aging on the expression of bdnf and trkb isoforms in rat pituitary. Neurobiol Aging. 2007;28:1088–1098. doi: 10.1016/j.neurobiolaging.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 23.Popa-Wagner A, Buga AM, Kokaia Z. Perturbed cellular response to brain injury during aging. Ageing Res Rev. 2011;10:71–79. doi: 10.1016/j.arr.2009.10.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.