Abstract
Previous investigations indicate that diminished functional expression of voltage-dependent K+ (KV) channels impairs control of coronary blood flow in obesity/metabolic syndrome. The goal of this investigation was to test the hypothesis that KV channels are electromechanically coupled to CaV1.2 channels and that coronary microvascular dysfunction in obesity is related to subsequent increases in CaV1.2 channel activity. Initial studies revealed that inhibition of KV channels with 4-aminopyridine (4AP, 0.3 mM) increased intracellular [Ca2+], contracted isolated coronary arterioles and decreased coronary reactive hyperemia. These effects were reversed by blockade of CaV1.2 channels. Further studies in chronically instrumented Ossabaw swine showed that inhibition of CaV1.2 channels with nifedipine (10 μg/kg, iv) had no effect on coronary blood flow at rest or during exercise in lean swine. However, inhibition of CaV1.2 channels significantly increased coronary blood flow, conductance, and the balance between coronary flow and metabolism in obese swine (P < 0.05). These changes were associated with a ~50 % increase in inward CaV1.2 current and elevations in expression of the pore-forming subunit (α1c) of CaV1.2 channels in coronary smooth muscle cells from obese swine. Taken together, these findings indicate that electromechanical coupling between KV and CaV1.2 channels is involved in the regulation of coronary vasomotor tone and that increases in CaV1.2 channel activity contribute to coronary microvascular dysfunction in the setting of obesity.
Keywords: Coronary, Exercise, CaV1.2 channels, Obesity, Swine
Introduction
Ion channels play a critical role in the regulation of microvascular resistance and consequently coronary blood flow. Multiple channels function in dynamic equilibrium to effect changes in coronary smooth muscle membrane potential (EM) and vascular tone in response to metabolic needs of the surrounding myocardium. Thus, modulation of key coronary ion channels preserves the balance between myocardial oxygen delivery and consumption (MVO2) [17]. Although the mechanisms by which this balance is regulated are not fully understood, previous investigations implicate voltage-gated Ca2+ (CaV1.2) channels as a predominant mediator of extracellular Ca2+ influx and coronary vascular resistance [17, 26, 33]. Activation of CaV1.2 channels occurs in response to various stimuli, including changes in smooth muscle EM elicited by voltage-dependent K+ (KV) channels, which have been shown to contribute to the regulation of coronary blood flow at rest [16], during increases in MVO2 [5, 40], and following myocardial ischemia [8]. However, the extent to which this “electromechanical coupling” between KV and CaV1.2 channels modulates coronary microvascular tone and reactivity has not been specifically examined.
Recent studies indicate that obesity and the metabolic syndrome (MetS) markedly attenuate coronary vascular function and the ability of the coronary circulation to adequately balance blood flow with myocardial metabolism [6]. We recently documented that this impairment is related, at least in part, to diminished expression and activity of coronary KV channels [5]. These findings are intriguing in relation to earlier studies which demonstrated that inhibition of KV channels depolarizes smooth muscle within the activation threshold for CaV1.2 channels and elevates cytosolic [Ca2+] [35], an effect abolished by removal of extracellular Ca2+ [29]. Accordingly, we hypothesize that diminished functional expression of KV channels in obesity impairs coronary microvascular function, at least in part, via increases in CaV1.2 channel activity. This hypothesis is supported by recent data from our laboratory which found that obesity increases intra-cellular [Ca2+] in coronary smooth muscle, augments coronary vasoconstriction to the CaV1.2 channel agonist BayK 8644 and increases coronary vasodilation in response to the CaV1.2 channel antagonist nicardipine [10, 26]. Our findings provide novel insight into the physiologic regulation of the coronary circulation and mechanisms of obesity-induced microvascular dysfunction.
Materials and methods
Ossabaw swine model of obesity
All experimental procedures and protocols used in this investigation were approved by the Institutional Animal Care and Use Committee in accordance with the Guide for the Care and Use of Laboratory Animals. Lean control swine were fed ~2,200 kcal/day of standard chow (5L80, Purina Test Diet, Richmond, IN, USA) containing 18 % kcal from protein, 71 % kcal from complex carbohydrates, and 11 % kcal from fat. Obese swine were fed an excess ~8,000 kcal/day high fat/fructose, atherogenic diet containing 16 % kcal from protein, 41 % kcal from complex carbohydrates, 19 % kcal from fructose, and 43 % kcal from fat (mixture of lard, hydrogenated soybean oil, and hydrogenated coconut oil), and supplemented with 2.0 % cholesterol and 0.7 % sodium cholate by weight (KT324, Purina Test Diet, Richmond, IN, USA) [5, 11]. Both lean and obese castrated male swine were fed their respective diets for 16 weeks prior to surgical instrumentation. Additional lean animals were also utilized for acute open-chest and coronary functional/molecular experiments.
Isolated vessels
Epicardial coronary arteries were isolated, cleaned of adventitia, cut into 3 mm rings, and mounted in organ baths containing Krebs buffer (37 °C) for isometric tension studies as previously described [36]. Coronary segments were adjusted to optimal length (~3.5 g tension) as determined by <10 % change in active tension in response to 60 mM KCl before administration of either BayK 8644 (10 μM, n = 6 lean) or KCl (20 mM, n = 4 lean/obese) directly to the organ bath. Following a wash, administration of respective drugs was then repeated in the presence of the selective KV channel antagonist 4-aminopyridine (4AP, 0.3 mM). Active tension development was measured using Iox software (Emka, Falls Church, VA, USA). Remaining coronary arteries not used for tension studies were enzymatically digested to disperse smooth muscle cells or frozen for subsequent molecular experiments.
Subepicardial coronary arterioles (n = 3; 50–150 μM diameter) were also isolated from the left ventricular apex of lean swine, cannulated, and pressurized to 60 cmH2O, as described previously [4]. Intraluminal diameter was measured continuously with a video micrometer and recorded on a MacLab workstation. Arterioles that were free from leaks were allowed to equilibrate for ~1 h at 37 °C with the bath solution changed every 15 min. Arterioles that did not develop at least 20 % spontaneous tone were excluded. Following development of tone, arterioles were treated with 4AP (0.3 mM). Once a stable diameter was achieved with 4AP, the CaV1.2 channel antagonist nifedipine (10 μM) was added to the vessel bath. Diameter responses were normalized to maximal arteriolar diameter determined at the end of each experiment by changing the bath solution to Ca2+-free physiological salt solution.
Cellular and molecular studies
Coronary myocytes were isolated as described previously [10, 44] for microfluorimetry, patch-clamp, flow cytometry and fluorescent microscopy. Cells from lean animals were loaded with Fura-2 (2.5 μM, n = 59 cells from three animals), placed in a superfusion chamber and observed with a monochromator-based imaging system (TILL Photonics, Martinsried, Germany) equipped with a DU885 charge-coupled device camera (Andor Technology plc, South Windsor, CT, USA) used to monitor Fura-2 wavelengths. Fura-2 fluorescence was excited at 345 and 380 nM. Emitted light was collected using a 510-nm long-pass filter. Data were analyzed using TILLvisION software (TILL Photonics, Hillsboro, OR) and reported as the change in baseline F345/380 in response to KCl (120 mM) or KCl + 4AP (0.3 mM).
Coronary smooth muscle cells from lean and obese swine were isolated from proximal segments of the LAD and patch-clamp recordings were performed within 8 h of cell dispersion. Whole-cell CaV1.2 currents were measured at room temperature with the conventional dialyzed configuration of the patch-clamp technique. Bath solution contained (in mM) 138 NaCl, 5 KCl, 2 BaCl2, 1 MgCl2, 10 glucose, 10 HEPES, and 5 Tris (pH 7.4). Pipettes had tip resistances of 2–4 MΩ when filled with solution containing (in mM) 140 CsCl, 3 Mg-ATP, 0.1 Na-GTP, 0.1 EGTA, 10 HEPES, and 5 Tris (pH 7.1). After whole-cell access was established, series resistance and membrane capacitance were compensated. Current voltage relationships were assessed by 400-ms step pulses from −60 to +60 mV in 10-mV increments from a holding potential of −80 mV.
Viable samples (as determined by trypan blue) of dispersed coronary myocytes from lean (n = 2) and obese (n = 3) swine were also fixed and permeabilized using the cytofix/cytoperm kit (BD). Cells were blocked in perm-wash containing 10 mg/ml BSA and stained for smooth muscle actin (SMA, R&D, 10 μl/sample) and CaV1.2 α1c (Santa Cruz, 0.4 μg/sample) or concentration matched isotype controls (R&D, Calbiochem, respectively) followed by AlexaFluor 488 donkey anti-goat secondary antibody (Invitrogen) if required. Samples were run using CellQuest Pro software on a FACSCalibur (BD) and viable SMA+ cells were gated and analyzed for CaV1.2 α1c expression with approximately 10,000 total events collected per sample. Samples were uniformly gated (viability and SMA+) and analyzed with FlowJo (Tree Star Inc.) flow cytometry software. Remaining cells were cytospun onto slides, mounted with prolong (Invitrogen), permeabilized and stained for α1c using the same primary anti-body as above (1:50 dilution for 1 h). Cells were subsequently washed, conjugated with AlexaFluor 488 and imaged using epifluorescence.
CaV1.2 α1c expression was also obtained from homogenized lean (n = 5) and obese (n = 5) coronary arteries using a previously described Western blot technique [5]. Equivalent amounts of protein (40 μg) were loaded onto 7.5 % acrylamide gels and transferred to nitrocellulose membranes. Membranes were blocked for 1 h at ambient temperature prior to 24 h incubation at 4 °C with rabbit polyclonal antibodies (Santa Cruz Biotech) directed against the CaV1.2 α1c (1:150) subunit. The primary antibody was added to blocking buffer containing 0.1 % Tween 20 and mouse anti-actin antibody (MP Biomedicals, 1:20,000). Blots were washed and incubated for 1 h with IRDye 800 donkey anti-goat (1:10,000) and IRDye 700 donkey anti-mouse (1:20,000) secondary antibodies. Immunoreactivity for CaV1.2 channel subunits was determined with the Li-Cor Odyssey system (Li-Cor Biosciences) and expressed relative to actin (loading control).
Using a previously described technique [31], total RNA was isolated from lean (n = 5) and obese (n = 5) coronary arteries and mRNA expression changes assessed using qRT-PCR. Aliquots (5.0 μl of 1:50 dilutions) of reverse transcription reactions (0.5 μg total RNA) were combined with the appropriate primers (human) for α1c or the beta-actin endogenous control (TaqMan® Gene Expression Assays; Applied Biosystems, Foster City, CA) in the presence of PCR reagents (FastStart Universal Probe Master Mix; Roche, Indianapolis, IN, USA). Reactions were run in triplicate on an Applied Biosystems 7500 Real-Time PCR System using relative quantification (ddCt) with dual-labeled (FAM/MGB) probes as the detection method. Standard cycling conditions were used with amplification extended to 50 cycles. Differences in PCR product yields between lean and obese were determined by normalizing data to a lean control and comparing fold changes of beta-actin normalized samples.
In vivo coronary blood flow studies
Lean Ossabaw swine (n = 5) utilized for reactive hyperemia experiments were prepared as described previously [9]. Blood pressure was monitored via catheters in the right femoral artery. Arterial blood samples were analyzed to maintain blood gas parameters within physiologic limits via ventilatory adjustments and/or intravenous sodium bicarbonate administration. Following thoracotomy, the LAD was isolated to measure coronary blood flow using a perivascular flow transducer (Transonic Systems Inc.) and a snare was used for occlusions of the LAD. Once hemodynamics were stabilized (~15–30 min) and baseline measurements acquired, reactive hyperemia was assessed via 15-s coronary occlusions in the presence/absence of diltiazem (0.3 mg/kg, iv) to inhibit CaV1.2 channels and/or 4-aminopyridine (0.3 mg/kg iv) to block KV channels.
Lean (n = 7) and obese (n = 6) Ossabaw swine were instrumented as previously reported for exercise experiments [5]. Briefly, utilizing a sterile technique, a left lateral thoracotomy was performed in the fifth intercostal space. Pressure monitoring catheters (Edwards LifeSciences) were implanted in the descending thoracic aorta for blood pressure measurements and arterial blood sampling. Another catheter was placed in the coronary interventricular vein for coronary venous blood sampling and intravenous drug infusions. A perivascular flow transducer (Transonic Systems Inc.) was placed around the left anterior descending (LAD) coronary artery to measure coronary blood flow. Catheters/wires were externalized, the chest closed in layers, and appropriate post-operative analgesics/antibiotics administered. Following recovery from surgery, experiments were conducted in lean (n = 7) and obese (n = 6) Ossabaw swine under resting conditions and during graded treadmill exercise (~2 and ~5 mph) before and during inhibition of CaV1.2 channels with nifedipine (10 μg/kg, iv). Arterial and coronary venous blood samples were collected simultaneously in heparinized syringes when hemodynamic variables were stable and analyzed in duplicate with an automatic blood gas analyzer (Instrumentation Laboratories GEM Premier 3000) and associated CO-oximeter (682) system. Each exercise period was ~2 min in duration, and the animals were allowed to rest sufficiently between each level for hemodynamic variables to return to baseline.
Statistical analyses
Data are presented as mean ± SE. Statistical comparisons were made by unpaired t test (phenotype data) or by two-way analysis of variance (ANOVA) for within-group analysis (Factor A: drug treatment; Factor B: exercise level) and between-group analysis (Factor A: diet with drug treatment; Factor B: exercise level) as appropriate. When significance was found with ANOVA, a Student–Newman–Keuls multiple comparison test was performed to identify differences between groups and treatment levels. Multiple linear regression analysis was used to compare slopes of coronary blood flow plotted vs. MVO2. If the slopes of the regression lines were not significantly different, an analysis of covariance (ANCOVA) was used to adjust response variables for linear dependence on MVO2. Hyperemic volume was determined by calculating the area under the curve using Prism software (GraphPad). For all statistical comparisons, P < 0.05 was considered significant.
Results
Phenotype of Ossabaw swine
Phenotypic characteristics of lean and obese swine are given in Table 1. Consistent with our recent studies [5, 10, 14], we found that obese swine exhibited significant increases in body weight, glucose, total cholesterol, and LDL/HDL ratio, relative to their lean counterparts. In particular, relative to their lean counterparts, obese swine exhibited significant increases in body weight, glucose, total cholesterol, and LDL/HDL ratio. Blood samples obtained from swine at the time of exercise experiment (non-fasted) revealed modest increases in triglyceride levels and insulin concentrations. Homeostatic model assessment (HOMA) index values were also 1.6-fold higher in obese swine (P = 0.13).
Table 1.
Phenotypic characteristics of lean and obese Ossabaw swine
| Lean | Obese | |
|---|---|---|
| Body weight (kg) | 47 ± 2 | 72 ± 3* |
| Heart wt./body wt. (×100) | 0.37 ± 0.02 | 0.40 ± 0.03 |
| Glucose (mg/dl) | 75 ± 3 | 87 ± 5* |
| Insulin (μU/ml) | 21 ± 4 | 30 ± 8 |
| HOMA index | 3.9 ± 0.8 | 6.2 ± 1.2 |
| Total cholesterol (mg/dl) | 85 ± 6 | 439 ± 74* |
| LDL/HDL ratio | 1.6 ± 0.1 | 4.5 ± 0.4* |
| Triglycerides (mg/dl) | 41 ± 5 | 70 ± 15 |
Values are mean ± SE for lean (n = 7) and obese (n = 5) swine
P < 0.05 vs. lean
KV-dependent modulation of coronary CaV1.2 channels
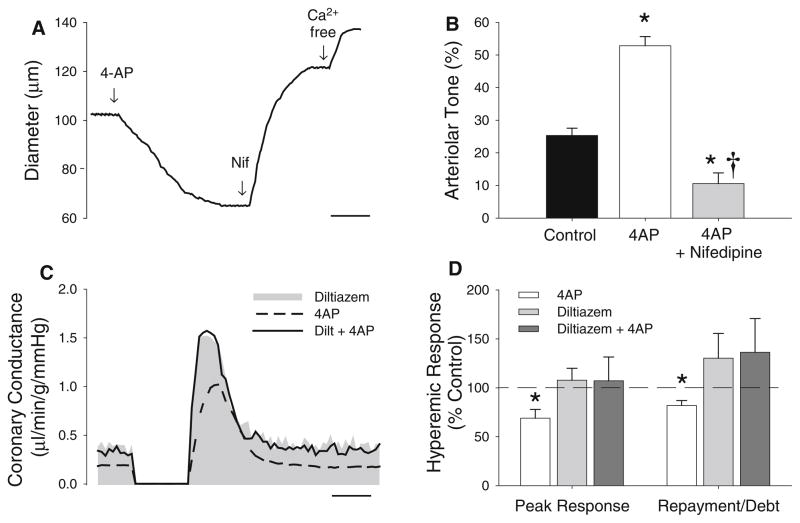
Activation of CaV1.2 channels by KCl (120 mM) increased baseline F345/380 ~20 % in coronary smooth muscle myocytes from lean animals (P < 0.05). This response was elevated ~twofold by subsequent addition of 4AP (Fig. 1b, P < 0.01). Isometric tension experiments in epi-cardial coronary artery segments support that inhibition of KV channels increases CaV1.2-mediated constriction as active tension development in response to the selective CaV1.2 channel agonist BayK 8644 (10 μM) was also increased ~twofold following the administration of 4AP (Fig. 1c, P < 0.01). The contribution of functional coupling between coronary KV and CaV1.2 channels to the control of coronary microvascular resistance is demonstrated in Fig. 2. Inhibition of KV channels in isolated coronary microvessels (pressurized diameter 102 ± 8 μm) resulted in a ~twofold increase in arteriolar tone (Fig. 2b, P < 0.01), as evidenced by a significant reduction in arteriolar diameter (Fig. 2a). Subsequent administration of the CaV1.2 channel antagonist nifedipine (10 μM) reversed arteriolar constriction to 4AP (Fig. 2b, P < 0.01).
Fig. 1.
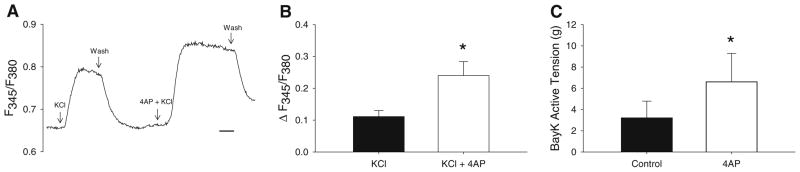
KV channel inhibition increases intracellular Ca2+ and coronary active tension. a Time course tracing of Fura-2 experiments in isolated coronary myocytes showing effect of 4AP on F345/F380 in response to 120 mM KCl (scale bar 30 s). b 4AP (0.3 mM) significantly increased KCl-mediated elevations in intracellular Ca2+ (P < 0.05). c Active tension development in isolated coronary artery segments in response to the CaV1.2 channel agonist BayK 8644 (10 μM) was significantly increased by 4AP (*P < 0.01)
Fig. 2.
Functional coupling between coronary KV and CaV1.2 channels. a Protocol tracing of isolated subepicardial coronary microvessels during 4AP and nifedipine treatments (scale bar 2 min). b Elevations in microvascular tone by 4AP were abolished by inhibition of CaV1.2 channels with nifedipine. c Representative tracing from reactive hyperemia experiments in open-chest anesthetized swine showing effects of additive CaV1.2 and KV channel blockade with diltiazem and 4AP on ischemic vasodilation (scale bar 20 s). d Addition of diltiazem to 4AP abolished the decrease in the hyperemia peak and repayment to debt responses relative to treatment with 4AP alone (data from [9]). Diltiazem administration did not significantly influence the reactive hyperemic response relative to untreated controls. *P < 0.05 vs. control, †P < 0.05 vs. 4AP and control
To examine the potential for coupling between KV and CaV1.2 channels in vivo in lean swine, coronary reactive hyperemia experiments were performed before and after the inhibition of KV channels with 4AP and/or CaV1.2 channels with diltiazem. A representative tracing demonstrating the marked reduction in coronary reactive hyperemia following 4AP administration is shown in Fig. 2c [9]. We found that diltiazem alone did not significantly alter the reactive hyperemic response relative to untreated control hearts (tracing not shown; P value for repayment to debt ratio = 0.24). In agreement with data from isolated coronary microvessels (Fig. 2b), we determined that administration of diltiazem prior to the inhibition KV channels completely prevented 4AP-mediated reductions in coronary reactive hyperemia (Fig. 2c). Specifically, diltiazem abolished 4AP-induced reductions in both the peak hyperemic response and the overall repayment of coronary flow debt (Fig. 2d).
Alterations in KV and CaV1.2 channels in obesity
Additional isometric tension studies were performed on isolated coronary arteries from lean and obese swine. Active tension development in response to KCl (20 mM) was elevated ~twofold in arteries from obese relative to lean swine under untreated control conditions (Fig. 3a, P = 0.02). In lean swine, the response to 20 mM KCl was augmented ~40 % by the administration of 4AP (Fig. 3a, P < 0.05). Importantly, inhibition of KV channels had no effect on active tension development to KCl in arteries from obese swine (Fig. 3a, P = 0.83). In agreement with these results, we found that 4AP significantly increased baseline coronary vascular resistance in conscious, instrumented lean but not obese swine (Fig. 3b, P < 0.05). Furthermore, inhibition of CaV1.2 channels with nifedipine (10 μg/kg, iv) had no effect on baseline vascular resistance in lean animals, but significantly reduced resistance in obese swine (Table 2; Fig. 3b, P < 0.02).
Fig. 3.
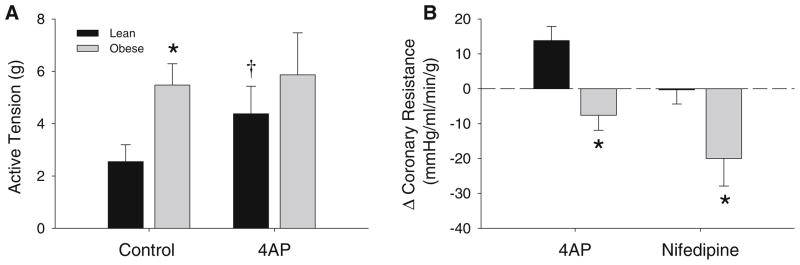
KV and CaV1.2 coupling in lean and obese swine. a Active tension development in response to KCl (20 mM) is elevated in arteries from obese relative to lean swine. 4AP increased this response in arteries from lean but not obese swine. b Baseline coronary resistance is increased by 4AP in lean swine while the change in response to nifedipine is markedly elevated in obese swine. *P < 0.05 vs. lean, †P < 0.05 vs. control
Table 2.
Hemodynamic and blood gas variables at rest and during graded treadmill exercise in lean and obese Ossabaw swine with and without Nifedipine
| Rest | Exercise
|
||
|---|---|---|---|
| Level 1 | Level 2 | ||
| Systolic blood pressure (mmHg) | |||
| Lean | 116 ± 7 | 114 ± 3 | 126 ± 4 |
| Lean + nifedipine | 109 ± 4 | 113 ± 4 | 119 ± 4 |
| Obese | 126 ± 6 | 136 ± 7† | 151 ± 1† |
| Obese + nifedipine | 116 ± 4 | 125 ± 4† | 133 ± 4*,† |
| Diastolic blood pressure (mmHg) | |||
| Lean | 76 ± 5 | 76 ± 2 | 84 ± 4 |
| Lean + nifedipine | 73 ± 4 | 74 ± 3 | 78 ± 3 |
| Obese | 88 ± 11 | 82 ± 4 | 92 ± 5 |
| Obese + nifedipine | 76 ± 3 | 76 ± 3 | 80 ± 6 |
| Mean aortic pressure (mmHg) | |||
| Lean | 98 ± 6 | 97 ± 3 | 106 ± 3 |
| Lean + nifedipine | 91 ± 3 | 95 ± 3 | 100 ± 3 |
| Obese | 102 ± 3 | 109 ± 5 | 125 ± 10† |
| Obese + nifedipine | 97 ± 4 | 101 ± 3 | 107 ± 5* |
| Heart rate (beats/min) | |||
| Lean | 148 ± 11 | 187 ± 8 | 201 ± 18 |
| Lean + nifedipine | 145 ± 11 | 183 ± 8 | 205 ± 7 |
| Obese | 149 ± 6 | 201 ± 19 | 194 ± 9 |
| Obese + nifedipine | 145 ± 5 | 186 ± 8 | 203 ± 8 |
| Coronary blood flow (ml/min/g) | |||
| Lean | 1.01 ± 0.11 | 1.35 ± 0.11 | 1.64 ± 0.13 |
| Lean + nifedipine | 1.01 ± 0.12 | 1.39 ± 0.14 | 1.60 ± 0.15 |
| Obese | 0.96 ± 0.07 | 1.25 ± 0.10 | 1.41 ± 0.10 |
| Obese + nifedipine | 1.11 ± 0.11 | 1.39 ± 0.11 | 1.62 ± 0.15* |
| Coronary conductance (μl/min/g/mmHg) | |||
| Lean | 10.3 ± 1.0 | 13.9 ± 0.9 | 15.7 ± 1.5 |
| Lean + nifedipine | 11.3 ± 1.4 | 14.7 ± 1.4 | 16.1 ± 1.5 |
| Obese | 9.5 ± 0.9 | 11.6 ± 0.9 | 11.5 ± 1.0† |
| Obese + nifedipine | 11.5 ± 1.1 | 13.8 ± 1.1* | 15.3 ± 1.4* |
| Myocardial O2 consumption (μl O2/min/g) | |||
| Lean | 116 ± 14 | 176 ± 24 | 231 ± 24 |
| Lean + nifedipine | 111 ± 13 | 163 ± 17 | 201 ± 26* |
| Obese | 126 ± 14 | 165 ± 14 | 182 ± 21 |
| Obese + nifedipine | 111 ± 13 | 178 ± 12 | 185 ± 11 |
| Arterial pH | |||
| Lean | 7.57 ± 0.01 | 7.55 ± 0.01 | 7.56 ± 0.02 |
| Lean + nifedipine | 7.59 ± 0.02* | 7.60 ± 0.01* | 7.57 ± 0.02 |
| Obese | 7.54 ± 0.02 | 7.52 ± 0.01 | 7.51 ± 0.01† |
| Obese + nifedipine | 7.52 ± 0.01† | 7.52 ± 0.02† | 7.50 ± 0.02† |
| Coronary venous pH | |||
| Lean | 7.47 ± 0.01 | 7.49 ± 0.01 | 7.48 ± 0.01 |
| Lean + nifedipine | 7.49 ± 0.01 | 7.51 ± 0.01 | 7.50 ± 0.01 |
| Obese | 7.44 ± 0.02 | 7.45 ± 0.01† | 7.45 ± 0.01 |
| Obese + nifedipine | 7.47 ± 0.01 | 7.48 ± 0.02 | 7.47 ± 0.01 |
| Arterial PCO2 (mmHg) | |||
| Lean | 31 ± 1 | 31 ± 2 | 29 ± 2 |
| Lean + nifedipine | 29 ± 3 | 28 ± 2 | 30 ± 2 |
| Obese | 31 ± 2 | 30 ± 2 | 29 ± 2 |
| Obese + nifedipine | 33 ± 2 | 31 ± 2 | 30 ± 2 |
| Coronary venous PCO2 (mmHg) | |||
| Lean | 51 ± 1 | 43 ± 3 | 45 ± 2 |
| Lean + nifedipine | 44 ± 2* | 42 ± 2 | 44 ± 2 |
| Obese | 46 ± 3 | 44 ± 2 | 41 ± 3 |
| Obese + nifedipine | 41 ± 2* | 41 ± 3 | 40 ± 2 |
| Arterial PO2 (mmHg) | |||
| Lean | 97 ± 3 | 100 ± 3 | 101 ± 5 |
| Lean + nifedipine | 102 ± 4 | 105 ± 4 | 99 ± 5 |
| Obese | 92 ± 4 | 91 ± 3 | 93 ± 3 |
| Obese + nifedipine | 88 ± 2† | 90 ± 3† | 94 ± 2 |
| Coronary venous PO2 (mmHg) | |||
| Lean | 17.8 ± 0.8 | 17.1 ± 1.1 | 16.2 ± 1.0 |
| Lean + nifedipine | 17.9 ± 1.4 | 16.4 ± 1.1 | 16.4 ± 1.1 |
| Obese | 14.2 ± 1.4† | 12.3 ± 1.3† | 12.4 ± 1.1† |
| Obese + nifedipine | 15.4 ± 1.9 | 13.6 ± 1.3* | 14.0 ± 1.2* |
| Arterial hematocrit (%) | |||
| Lean | 34 ± 1 | 38 ± 1 | 39 ± 1 |
| Lean + nifedipine | 33 ± 2 | 33 ± 2* | 37 ± 1 |
| Obese | 36 ± 2 | 38 ± 2 | 37 ± 1 |
| Obese + nifedipine | 32 ± 2 | 35 ± 2 | 33 ± 1 |
Values are mean ± SE for lean (n = 7) and obese (n = 6) swine
P < 0.05 vs. untreated control, same diet/condition
P < 0.05 vs. lean, same treatment
Coronary and cardiovascular response to exercise
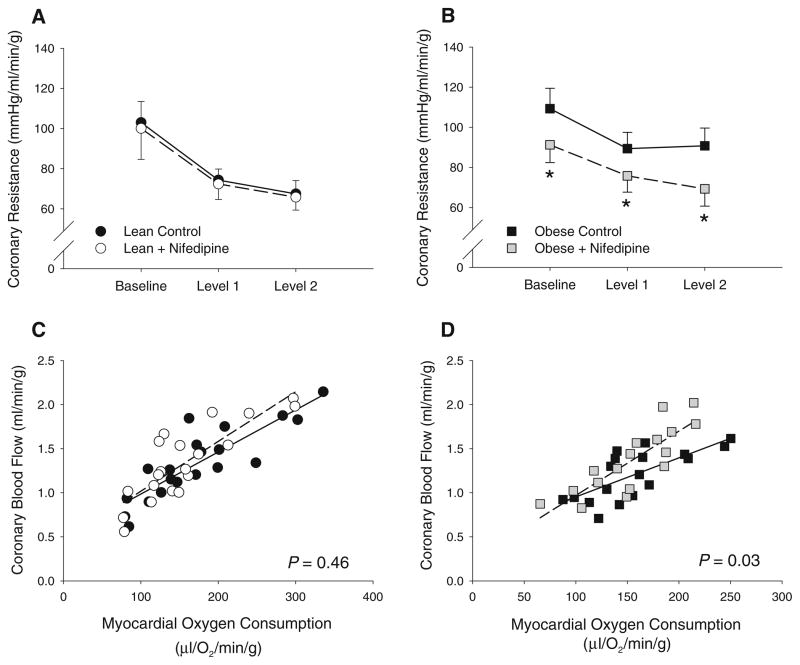
Hemodynamic and blood gas data for lean and obese Ossabaw swine at rest and during exercise are summarized in Table 2. Systolic, diastolic and mean aortic pressure were not significantly different in obese vs. lean swine under baseline/resting conditions in either treated or untreated groups. However, the systemic pressure response to exercise was elevated in obese swine as both systolic and mean aortic pressure were significantly increased during exercise relative to their lean counterparts, a finding that occurred in the absence of differences in heart rate. Despite this increase in arterial pressure, absolute coronary blood flow was similar in lean vs. obese swine. However, coronary venous PO2 (index of myocardial tissue PO2) was significantly decreased at rest and during exercise in obese swine (Table 2). Obesity also reduced coronary conductance (flow/pressure) ~25 % at the highest level of exercise. Evaluation of coronary vascular resistance revealed that obesity significantly increased vascular tone relative to lean swine with increasing exercise levels (Fig. 4a vs. b).
Fig. 4.
Effects of CaV1.2 channel inhibition on exercise-mediated coronary vasodilation. a Inhibition of CaV1.2 channels with nifedipine did not decrease coronary vascular resistance in lean swine. b Nifedipine significantly reduced coronary vascular resistance in obese swine at rest and during exercise. Administration of nifedipine produced a significant upward shift in the slope relationship between coronary blood flow and MVO2 in obese (d) but not lean swine (c). *P < 0.05 vs. control
Role of CaV1.2 channels in coronary and cardiovascular response to exercise
Effects of CaV1.2 channel inhibition with nifedipine (10 μg/kg, iv) on hemodynamic and blood gas variables at rest and during exercise are also summarized in Table 2. Administration of nifedipine significantly reduced systolic and mean aortic pressure during exercise in obese, but not lean swine. Similar to untreated conditions, heart rate was unaffected by nifedipine in either group. Coronary vascular resistance and coronary blood flow were not significantly altered by nifedipine administration in lean swine at rest or during exercise (Table 2; Fig. 4a, c). In contrast, nifedipine markedly increased coronary blood flow and reduced resistance during exercise in obese swine (Table 2; Fig. 4b, d). Regression analysis demonstrated that nifedipine significantly increased the slope of the relationship between coronary blood flow and MVO2 in obese (P = 0.03), but not lean swine (P = 0.46) (Fig. 4c, d). Importantly, no differences in slope were noted between obese swine treated with nifedipine and untreated control lean swine, i.e., inhibition of CaV1.2 channels in obese swine restored exercise-mediated increases in coronary blood flow to levels similar to that observed in lean swine. These findings are further supported by significant increases in the relationship between myocardial oxygen delivery vs. MVO2 and coronary venous PO2 vs. MVO2 in obese swine treated with nifedipine (Supplemental Figure).
Functional and molecular expression of coronary CaV1.2 channels in obesity
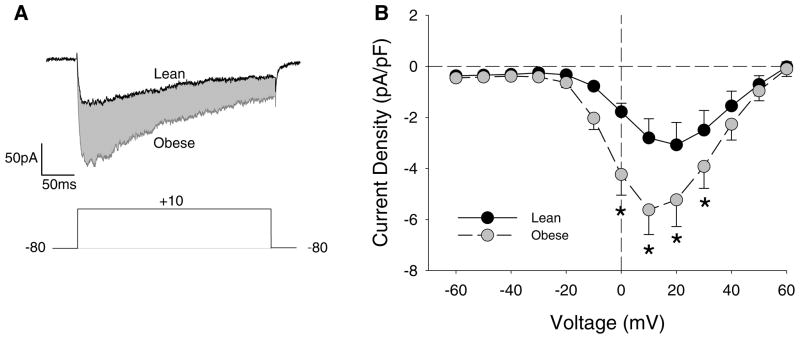
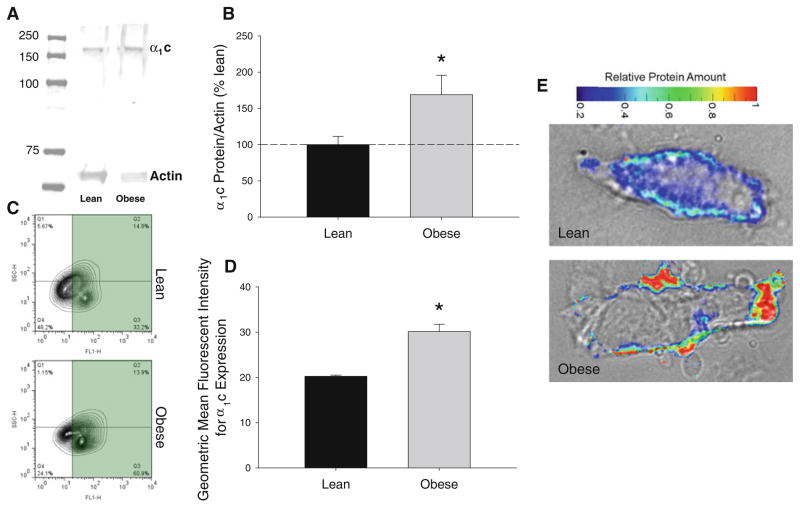
Whole-cell patch-clamp recordings (Fig. 5a) demonstrated a significant 35–60 % increase in native coronary CaV1.2 currents at 0–30 mV potentials (Fig. 5b, P < 0.05) over a voltage range consistent with CaV1.2 channel activity [12, 13]. Western blotting revealed a ~70 % increase in expression of the α1c pore-forming subunit of the CaV1.2 channel in obese coronary arteries (Fig. 6b, P < 0.05). Additional flow cytometry studies indicate that this increase in α1c expression was located in vascular smooth muscle cells as there were statistically significant increases in both the percentage of cells that stained positive for both α1c and α-smooth muscle actin as well as in the mean fluorescence intensity of expression levels in obese vs. lean coronary arteries (Fig. 6c, d, P < 0.05). Fluorescence imaging suggests that the increased expression of the α1c subunit was localized to the plasma membrane of obese smooth muscle cells (Fig. 6e). While marked elevations in protein expression were observed, no differences in coronary α1c mRNA expression were noted in obese arteries (88 ± 15 % vs. lean, P = 0.79).
Fig. 5.
Whole-cell voltage-dependent CaV1.2 current in coronary smooth muscle of lean and obese swine. a Representative current tracings from lean and obese coronary myocytes. Voltage template is 400 ms. b Group I–V data demonstrate a significant increase in inward CaV1.2 channel current at potentials greater than −10 mV. *P < 0.01 vs. lean, same voltage
Fig. 6.
Expression and membrane localization of the coronary CaV1.2 channel α1c subunit are increased by obesity. a Representative lean and obese Western blots for CaV1.2 α1c subunit and actin. b Results of Western blots for α1c expression showing ~75 % increase in the pore-forming subunit. c Representative flow cytometry contour plots for percent double positive smooth muscle actin and α1c stained cells. d Flow cytometry for CaV1.2 demonstrating significant increases in α1c subunit expression (mean fluorescence intensity) in coronary arteries from obese vs. lean swine. e Overlay of bright field and corresponding fluorescence images of α1c subunit from lean and obese smooth muscle cells. *P < 0.05 vs. lean
Discussion
The goal of this investigation was to examine the functional significance of electromechanical coupling between coronary KV and CaV1.2 channels and the contribution of CaV1.2 channels to the regulation of coronary blood flow at rest and during physiologically induced increases in myocardial metabolism. In addition, we assessed the influence of obesity on the interaction between coronary KV and CaV1.2 channels, the role of CaV1.2 channels in metabolic control of coronary blood flow as well as CaV1.2 channel current and expression in health and disease. Specifically, we hypothesized that diminished functional expression of KV channels contributes to increased CaV1.2 channel activity and coronary dysfunction in obesity. This hypothesis is supported by previous investigations in our laboratory demonstrating that the contribution of KV channels to local-metabolic control of coronary blood flow and ischemic coronary vasodilation is impaired by obesity [5, 8] and that these alterations are associated with increases in intracellular [Ca2+] in coronary artery smooth muscle cells of obese swine [10]. However, whether increases in CaV1.2 channel activity in obesity are attributable to reductions in KV channel function or result from alterations in CaV1.2 channel expression/localization is unclear. The major findings of this study are: (1) increases in coronary smooth muscle [Ca2+]i and vascular tone in response to KV channel inhibition are attributable to activation of CaV1.2 channels; (2) attenuated KV channel function in obesity is associated with elevated CaV1.2 channel activity; (3) enhanced CaV1.2 channel activity in obesity is accompanied by increased expression of the α1c pore-forming subunit of the CaV1.2 channel; (4) inhibition of CaV1.2 channels significantly augments exercise-mediated coronary vasodilation in obese but not lean swine. Taken together, these data demonstrate that electromechanical coupling between KV and CaV1.2 channels is involved in the regulation of coronary vasomotor tone and that increases in CaV1.2 channel activity contribute to coronary microvascular dysfunction in the setting of obesity.
Functional coupling of coronary KV and CaV1.2 channels
Evidence to date supports a prominent role for KV channels in the control of smooth muscle EM and coronary blood flow [7, 17]. In addition, CaV1.2 channels represent a significant source of extracellular Ca2+ influx and coronary smooth muscle contraction [33, 42]. Since CaV1.2 channels are activated within the range for smooth muscle EM, we hypothesized that alterations in EM elicited by KV channels modulate CaV1.2 channel activity and control coronary blood flow. The potential for functional interactions between these channels is important given the ability for modest alterations in smooth muscle EM to have large effects on CaV1.2-mediated Ca2+ conductance and vascular tone. Opening of only a few CaV1.2 channels during depolarization in the presence of large transmembrane Ca2+ gradients can cause significant (~10-fold) increases in [Ca2+]i [25]. Thus, even modest fluctuations in EM (~3 mV) are capable of producing ~twofold changes in [Ca2+]i [33, 34]. Accordingly, potential voltage-dependent interactions between KV and CaV1.2 channels may have profound consequences on the control of coronary vascular resistance and blood flow.
In agreement with the premise of coupling between KV and CaV1.2 channels, administration of 4AP has been shown to markedly increase [Ca2+]i in various cell types [15, 21, 39, 46]. Although we now demonstrate this in coronary smooth muscle (Fig. 1a), discrepancies exist as to whether increases in [Ca2+]i from 4AP are the result of voltage-dependent activation of CaV1.2 channels or IP3-mediated mobilization of SR stores and subsequent opening of store-operated calcium channels [2, 20, 29, 45]. Specifically, previous studies have shown that 4AP can increase [Ca2+]i in the absence of extracellular Ca2+ in isolated neurons, astrocytes, and skeletal muscle [21]. While a parallel role for 4AP-induced SR Ca2+ release in smooth muscle cannot be disregarded [19], it is unlikely that this mechanism contributes to coronary vasoconstriction given that SR Ca2+ release mainly functions as a negative feedback mechanism to contraction, i.e., spark-induced activation of calcium-sensitive K+ channels [24, 43]. Importantly, our data demonstrate that the increase in [Ca2+]i during KV channel inhibition potentiates CaV1.2- mediated coronary vasoconstriction in response to BayK 8644 (Fig. 1b). In addition, administration of 4AP alone is sufficient to induce marked CaV1.2-sensitive vasoconstriction in isolated microvessels (Fig. 2a), thus supporting that increases in [Ca2+]i and arteriolar tone are the direct result of KV-dependent modulation of extracellular Ca2+ influx via CaV1.2 channels.
These data are the first to demonstrate that the influence of KV and CaV1.2 channel interactions on the regulation of [Ca2+]i and vascular tone shown in vitro also play an important role in the control of coronary blood flow in vivo. Our laboratory findings have previously demonstrated that inhibition of KV channels significantly impairs coronary reactive hyperemia as the repayment to debt ratio is reduced ~30 % by 4AP (representative tracing, Fig. 3c) [16]. Experiments performed in the present study in lean swine show that these reductions in the hyperemic response during KV channel inhibition are abolished by prior inhibition of CaV1.2 channels with diltiazem (Fig. 3d). Taken together, these findings indicate that reductions in coronary blood flow induced by KV channel inhibition are attributable to the activation of CaV1.2 channels and thus support the functional importance of the coupling between KV and CaV1.2 in the regulation of coronary vasomotor tone in vivo.
Pathophysiologic implications of the coupling between KV and CaV1.2 channels are particularly important given the growing body of evidence indicating that K+ channel function is altered in various disease states [5, 9, 17]. Findings from our laboratory demonstrate that obesity/MetS reduce KV channel current, expression of coronary KV 1.5 channels, and the contribution of KV channels to local-metabolic coronary vasodilation [5]. Based on these previous studies and our current findings, we hypothesized that decreases in KV channel function in obesity may contribute to the impairment of coronary blood flow control via increases in CaV1.2 channel activity. This hypothesis is supported by significant increases in coronary active tension and diminished effects of 4AP in obese vs. lean coronary artery segments (Fig. 3a). Likewise, coronary vascular resistance was not increased by 4AP but was significantly reduced by nifedipine in obese swine, results which are opposite to lean counterparts and indicative of impaired KV and elevated CaV1.2 channel function (Fig. 3b). The specific KV channel subtypes responsible for this coupling requires further investigation.
Effects of obesity on function and expression of coronary CaV1.2 channels
Findings from the present investigation demonstrate that obesity impairs the regulation of coronary blood flow via a CaV1.2 channel-dependent mechanism. These results are consistent with previous studies on individual components of the MetS (i.e., obesity, hypertension, dyslipidemia) which documented that elevations in vascular tone are associated with increases in intracellular [Ca2+] and calcium channel current [3, 27, 28, 30, 37, 38]. However, the extent to which potential alterations in CaV1.2 channel function contribute to impaired coronary vasomotor responses to physiologic stimuli in obesity has not been previously studied. In agreement with findings from Bache et al. [1], we found that nifedipine did not alter coronary blood flow or resistance in lean animals at rest or during exercise, nor were these variables altered relative to changes in MVO2 (Fig. 4a, c). In contrast to the lack of coronary and cardiovascular responses to CaV1.2 channel inhibition during exercise in lean swine (at a dose that did not affect blood pressure or heart rate in lean swine), nifedipine significantly decreased microvascular resistance (Fig. 4b) and increased the slope of the relationship between coronary blood flow and MVO2 in obese swine (Fig. 4d). These findings indicate that the functional contribution of CaV1.2 channels to the regulation of coronary blood flow is augmented in the setting of obesity. Although the precise mechanisms responsible for this increased contribution remain to be elucidated, we propose that therapies targeted at diminishing coronary vasoconstrictor pathways such as alpha-adrenoceptors [22], which we have documented to be upregulated in obesity and diabetes [18, 41], decrease the incidence and severity of myocardial ischemia [23] in this at-risk patient population.
In order to determine whether increases in CaV1.2 channel function are the result of alterations in CaV1.2 regulatory pathways (i.e., KV channels) or intrinsic to changes within the channels themselves, we evaluated the effects of obesity on CaV1.2 channel current and expression. Obesity significantly increased inward CaV1.2 channel current 35–60 % without altering thresholds for activation/inactivation (Fig. 5). Accordingly, we speculated that increases in CaV1.2 channel current in obese swine are likely the result of augmented expression of channel subunits in vascular smooth muscle. This contention is supported by data from our Western blot analyses which noted that expression of the α1c pore-forming subunit was increased ~70 % in coronary arteries from obese swine (Fig. 6). Our flow cytometry and immunocytochemistry studies also suggest that this increase in α1c expression is localized in the membrane of coronary vascular smooth muscle cells. However, because no change at the transcriptional level was observed, alterations in subunit-dependent membrane trafficking or degradation may underlie the observed increases in α1c expression and warrants further investigation.
Limitations of the investigation
We acknowledge that the use of conduit arteries for patch-clamp, Western and mRNA analyses, as opposed to coronary arterioles which were functionally assessed for reactivity studies, is a limitation. However, this concern is moderated by our directionally consistent findings regarding augmented CaV1.2-mediated constriction in the coronary microcirculation and increased CaV1.2 channel current and α1c expression in large coronary arteries. In addition, it is also important to recognize that the specific subtypes of K+ and Ca2+ channels implicated in this investigation are limited to those effectively inhibited by the antagonists utilized in this investigation, i.e., channels sensitive to 4AP and nifedipine. Thus, further studies are needed to determine the precise voltage-dependent channels responsible for smooth muscle electromechanical coupling in the coronary circulation.
Implications and conclusions
Results obtained from this investigation demonstrate that the coupling between KV and CaV1.2 channels is an important mechanism by which coronary blood flow is regulated in health and disease. Given the prominent role of KV channels and the effect diminished coronary KV channel function can have on the regulation of coronary blood flow, establishing the functional consequences of coupling between these channels is vital to our understanding of coronary (patho-)physiology. The overall relevance of this coupling is further supported by the effects of obesity on CaV1.2 channel activity and expression which occurs independent of alterations in other K+ channels, i.e., KCa channels [10, 11]. We propose that future studies to elucidate the mechanisms underlying alterations in the functional expression of voltage-dependent K+ and Ca2+ channels could significantly improve cardiovascular outcomes in obese patients. The potential need for such studies is directly supported by a recent clinical trial by Murthy et al. [32] which demonstrated coronary vasodilator dysfunction to be a powerful, independent correlate of cardiac mortality among diabetics and non-diabetics.
Supplementary Material
Acknowledgments
This work was supported by AHA grants 10PRE4230035 (ZCB) and NIH grants HL092245 (JDT), HL115140 (JDT and AGO), T32DK007519; O’Leary (HB), T32HL079995; Goodwill (KM), and T32DK064466; Owen (PR).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00395-013-0370-0) contains supplementary material, which is available to authorized users.
Conflict of interest The authors have no conflicts to disclose.
Contributor Information
Zachary C. Berwick, Department of Cellular and Integrative Physiology, Indiana University School of Medicine, 635 Barnhill Drive, Indianapolis, IN 46202, USA
Gregory M. Dick, Department of Exercise Physiology, West Virginia University School of Medicine, Morgantown, USA. Center for Cardiovascular and Respiratory Sciences, West Virginia University School of Medicine, Morgantown, USA
Heather A. O’Leary, Department of Microbiology and Immunology, Indiana University School of Medicine, Indianapolis, USA
Shawn B. Bender, Department of Internal Medicine, University of Missouri School of Medicine, Columbia, MO, USA
Adam G. Goodwill, Department of Cellular and Integrative Physiology, Indiana University School of Medicine, 635 Barnhill Drive, Indianapolis, IN 46202, USA
Steven P. Moberly, Department of Cellular and Integrative Physiology, Indiana University School of Medicine, 635 Barnhill Drive, Indianapolis, IN 46202, USA
Meredith Kohr Owen, Department of Cellular and Integrative Physiology, Indiana University School of Medicine, 635 Barnhill Drive, Indianapolis, IN 46202, USA.
Steven J. Miller, Department of Cellular and Integrative Physiology, Indiana University School of Medicine, 635 Barnhill Drive, Indianapolis, IN 46202, USA. Department of Surgery, Indiana University School of Medicine, Indianapolis, USA
Alexander G. Obukhov, Department of Cellular and Integrative Physiology, Indiana University School of Medicine, 635 Barnhill Drive, Indianapolis, IN 46202, USA
Johnathan D. Tune, Email: jtune@iupui.edu, Department of Cellular and Integrative Physiology, Indiana University School of Medicine, 635 Barnhill Drive, Indianapolis, IN 46202, USA
References
- 1.Bache RJ, Quanbeck D, Homans DC, Dai XZ. Effects of nifedipine on coronary reactive and exercise induced hyperaemia. Cardiovasc Res. 1987;21:766–771. doi: 10.1093/cvr/21.10.766. [DOI] [PubMed] [Google Scholar]
- 2.Barbar E, Rola-Pleszczynski M, Payet MD, Dupuis G. Protein kinase C inhibits the transplasma membrane influx of Ca2+ triggered by 4-aminopyridine in Jurkat T lymphocytes. Biochim Biophys Acta. 2003;1622:89–98. doi: 10.1016/s0304-4165(03)00120-x. 10.1016/S0304-4165 (03)00120-X. [DOI] [PubMed] [Google Scholar]
- 3.Belin de Chantemele EJ, Ali MI, Mintz J, Stepp DW. Obesity induced-insulin resistance causes endothelial dysfunction without reducing the vascular response to hindlimb ischemia. Basic Res Cardiol. 2009;104:707–717. doi: 10.1007/s00395-009-0042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bender SB, Tune JD, Borbouse L, Long X, Sturek M, Laughlin MH. Altered mechanism of adenosine-induced coronary arteriolar dilation in early-stage metabolic syndrome. Exp Biol Med (Maywood) 2009;234:683–692. doi: 10.3181/0812-RM-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berwick ZC, Dick GM, Moberly SP, Kohr MC, Sturek M, Tune JD. Contribution of voltage-dependent K(+) channels to metabolic control of coronary blood flow. J Mol Cell Cardiol. 2011;52:912–919. doi: 10.1016/j.yjmcc.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berwick ZC, Dick GM, Tune JD. Heart of the matter: coronary dysfunction in metabolic syndrome. J Mol Cell Cardiol. 2011;52:848–856. doi: 10.1016/j.yjmcc.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berwick ZC, Moberly SP, Kohr MC, Morrical EB, Kurian MM, Dick GM, Tune JD. Contribution of voltage-dependent K+ and Ca2+ channels to coronary pressure-flow autoregulation. Basic Res Cardiol. 2012;107:264. doi: 10.1007/s00395-012-0264-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berwick ZC, Payne GA, Lynch B, Dick GM, Sturek M, Tune JD. Contribution of adenosine A(2A) and A(2B) receptors to ischemic coronary dilation: role of K(V) and K(ATP) channels. Microcirculation. 2010;17:600–607. doi: 10.1111/j.1549-8719.2010.00054.x. 10.1111/j.1549-8719.2010. 00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borbouse L, Dick GM, Payne GA, Berwick ZC, Neeb ZP, Alloosh M, Bratz IN, Sturek M, Tune JD. Metabolic syndrome reduces the contribution of K+ channels to ischemic coronary vasodilation. Am J Physiol Heart Circ Physiol. 2010;298:H1182–H1189. doi: 10.1152/ajpheart.00888.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borbouse L, Dick GM, Asano S, Bender SB, Dincer UD, Payne GA, Neeb ZP, Bratz IN, Sturek M, Tune JD. Impaired function of coronary BK(Ca) channels in metabolic syndrome. Am J Physiol Heart Circ Physiol. 2009;297:H1629–H1637. doi: 10.1152/ajpheart.00466.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borbouse L, Dick GM, Payne GA, Payne BD, Svendsen MC, Neeb ZP, Alloosh M, Bratz IN, Sturek M, Tune JD. Contribution of BKCa channels to local metabolic coronary vasodilation: effects of metabolic syndrome. Am J Physiol Heart Circ Physiol. 2009;298:H966–H973. doi: 10.1152/ajpheart.00876.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bowles DK, Heaps CL, Turk JR, Maddali KK, Price EM. Hypercholesterolemia inhibits L-type calcium current in coronary macro-, not microcirculation. J Appl Physiol. 2004;96:2240–2248. doi: 10.1152/japplphysiol.01229.2003. [DOI] [PubMed] [Google Scholar]
- 13.Bowles DK, Maddali KK, Ganjam VK, Rubin LJ, Tharp DL, Turk JR, Heaps CL. Endogenous testosterone increases L-type Ca2+ channel expression in porcine coronary smooth muscle. Am J Physiol Heart Circ Physiol. 2004;287:H2091–H2098. doi: 10.1152/ajpheart.00258.2004. [DOI] [PubMed] [Google Scholar]
- 14.Bratz IN, Dick GM, Tune JD, Edwards JM, Neeb ZP, Dincer UD, Sturek M. Impaired capsaicin-induced relaxation of coronary arteries in a porcine model of the metabolic syndrome. Am J Physiol Heart Circ Physiol. 2008;294:H2489–H2496. doi: 10.1152/ajpheart.01191.2007. [DOI] [PubMed] [Google Scholar]
- 15.del Carmen GM, Torres M, Sanchez-Prieto J. The modulation of Ca2+ and K+ channels but not changes in cAMP signaling contribute to the inhibition of glutamate release by cannabinoid receptors in cerebrocortical nerve terminals. Neuropharmacology. 2005;48:547–557. doi: 10.1016/j.neuropharm.2004.11.012. 10.1016/j.neuropharm.2004. 11.012. [DOI] [PubMed] [Google Scholar]
- 16.Dick GM, Bratz IN, Borbouse L, Payne GA, Dincer UD, Knudson JD, Rogers PA, Tune JD. Voltage-dependent K+ channels regulate the duration of reactive hyperemia in the canine coronary circulation. Am J Physiol Heart Circ Physiol. 2008;294:H2371–H2381. doi: 10.1152/ajpheart.01279.2007. [DOI] [PubMed] [Google Scholar]
- 17.Dick GM, Tune JD. Role of potassium channels in coronary vasodilation. Exp Biol Med (Maywood) 2010;235:10–22. doi: 10.1258/ebm.2009.009201. [DOI] [PubMed] [Google Scholar]
- 18.Dincer UD, Araiza AG, Knudson JD, Molina PE, Tune JD. Sensitization of coronary alpha-adrenoceptor vasoconstriction in the prediabetic metabolic syndrome. Microcirculation. 2006;13:587–595. doi: 10.1080/10739680600885228. [DOI] [PubMed] [Google Scholar]
- 19.Fan ZW, Zhang ZX, Xu YJ. Inhibition of voltage-gated K+ current in rat intrapulmonary arterial smooth muscle cells by endothelin-1. Yao Xue Xue Bao. 2004;39:9–12. [PubMed] [Google Scholar]
- 20.Fink RH, Stephenson DG. Ca2+-movements in muscle modulated by the state of K+-channels in the sarcoplasmic reticulum membranes. Pflugers Arch. 1987;409:374–380. doi: 10.1007/BF00583791. [DOI] [PubMed] [Google Scholar]
- 21.Grimaldi M, Atzori M, Ray P, Alkon DL. Mobilization of calcium from intracellular stores, potentiation of neurotransmitter-induced calcium transients, and capacitative calcium entry by 4-aminopyridine. J Neurosci. 2001;21:3135–3143. doi: 10.1523/JNEUROSCI.21-09-03135.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heusch G, Deussen A. Nifedipine prevents sympathetic vasoconstriction distal to severe coronary stenoses. J Cardiovasc Pharmacol. 1984;6:378–383. doi: 10.1097/00005344-198405000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Heusch G, Guth BD, Seitelberger R, Ross J., Jr Attenuation of exercise-induced myocardial ischemia in dogs with recruitment of coronary vasodilator reserve by nifedipine. Circulation. 1987;75:482–490. doi: 10.1161/01.cir.75.2.482. [DOI] [PubMed] [Google Scholar]
- 24.Jaggar JH, Porter VA, Lederer WJ, Nelson MT. Calcium sparks in smooth muscle. Am J Physiol Cell Physiol. 2000;278:C235–C256. doi: 10.1152/ajpcell.2000.278.2.C235. [DOI] [PubMed] [Google Scholar]
- 25.Jones SW. Calcium channels: unanswered questions. J Bioenerg Biomembr. 2003;35:461–475. doi: 10.1023/b:jobb.0000008020.86004.28. 10.1023/B:JOBB.000000 8020.86004.28. [DOI] [PubMed] [Google Scholar]
- 26.Knudson JD, Dincer UD, Bratz IN, Sturek M, Dick GM, Tune JD. Mechanisms of coronary dysfunction in obesity and insulin resistance. Microcirculation. 2007;14:317–338. doi: 10.1080/10739680701282887. [DOI] [PubMed] [Google Scholar]
- 27.Kubo T, Taguchi K, Ueda M. L-type calcium channels in vascular smooth muscle cells from spontaneously hypertensive rats: effects of calcium agonist and antagonist. Hypertens Res. 1998;21:33–37. doi: 10.1291/hypres.21.33. [DOI] [PubMed] [Google Scholar]
- 28.Lassaletta AD, Chu LM, Robich MP, Elmadhun NY, Feng J, Burgess TA, Laham RJ, Sturek M, Sellke FW. Overfed Ossabaw swine with early stage metabolic syndrome have normal coronary collateral development in response to chronic ischemia. Basic Res Cardiol. 2012;107:243. doi: 10.1007/s00395-012-0243-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mandegar M, Yuan JX. Role of K+ channels in pulmonary hypertension. Vascul Pharmacol. 2002;38:25–33. doi: 10.1016/s1537-1891(02)00123-4. [DOI] [PubMed] [Google Scholar]
- 30.McCarty MF. PKC-mediated modulation of L-type calcium channels may contribute to fat-induced insulin resistance. Med Hypotheses. 2006;66:824–831. doi: 10.1016/j.mehy.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 31.Miller SJ, Norton LE, Murphy MP, Dalsing MC, Unthank JL. The role of the renin-angiotensin system and oxidative stress in spontaneously hypertensive rat mesenteric collateral growth impairment. Am J Physiol Heart Circ Physiol. 2007;292:H2523–H2531. doi: 10.1152/ajpheart.01296.2006. [DOI] [PubMed] [Google Scholar]
- 32.Murthy VL, Naya M, Foster CR, Gaber M, Hainer J, Klein J, Dorbala S, Blankstein R, Di Carli MF. Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation. 2012;126:1858–1868. doi: 10.1161/CIRCULATIONAHA.112.120402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nelson MT, Patlak JB, Worley JF, Standen NB. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol. 1990;259:C3–18. doi: 10.1152/ajpcell.1990.259.1.C3. [DOI] [PubMed] [Google Scholar]
- 34.Nelson MT, Standen NB, Brayden JE, Worley JF., III Noradrenaline contracts arteries by activating voltage-dependent calcium channels. Nature. 1988;336:382–385. doi: 10.1038/336382a0. [DOI] [PubMed] [Google Scholar]
- 35.Park WS, Firth AL, Han J, Ko EA. Patho-, physiological roles of voltage-dependent K+ channels in pulmonary arterial smooth muscle cells. J Smooth Muscle Res. 2010;46:89–105. doi: 10.1540/jsmr.46.89. [DOI] [PubMed] [Google Scholar]
- 36.Payne GA, Borbouse L, Kumar S, Neeb Z, Alloosh M, Sturek M, Tune JD. Epicardial perivascular adipose-derived leptin exacerbates coronary endothelial dysfunction in metabolic syndrome via a protein kinase C-beta pathway. Arterioscler Thromb Vasc Biol. 2010;30:1711–1717. doi: 10.1161/ATVBAHA.110.210070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pesic A, Madden JA, Pesic M, Rusch NJ. High blood pressure upregulates arterial L-type Ca2+ channels: is membrane depolarization the signal? Circ Res. 2004;94:e97–104. doi: 10.1161/01.RES.0000131495.93500.3c. [DOI] [PubMed] [Google Scholar]
- 38.Pratt PF, Bonnet S, Ludwig LM, Bonnet P, Rusch NJ. Upregulation of L-type Ca2+ channels in mesenteric and skeletal arteries of SHR. Hypertension. 2002;40:214–219. doi: 10.1161/01.HYP.0000025877.23309.36. [DOI] [PubMed] [Google Scholar]
- 39.Ryan D, Drysdale AJ, Lafourcade C, Pertwee RG, Platt B. Cannabidiol targets mitochondria to regulate intracellular Ca2+ levels. J Neurosci. 2009;29:2053–2063. doi: 10.1523/JNEUROSCI.4212-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saitoh S, Zhang C, Tune JD, Potter B, Kiyooka T, Rogers PA, Knudson JD, Dick GM, Swafford A, Chilian WM. Hydrogen peroxide: a feed-forward dilator that couples myocardial metabolism to coronary blood flow. Arterioscler Thromb Vasc Biol. 2006;26:2614–2621. doi: 10.1161/01.ATV.0000249408.55796.da. [DOI] [PubMed] [Google Scholar]
- 41.Setty S, Sun W, Martinez R, Downey HF, Tune JD. Alpha-adrenoceptor-mediated coronary vasoconstriction is augmented during exercise in experimental diabetes mellitus. J Appl Physiol. 2004;97:431–438. doi: 10.1152/japplphysiol.01122.2003. [DOI] [PubMed] [Google Scholar]
- 42.Thorneloe KS, Nelson MT. Ion channels in smooth muscle: regulators of intracellular calcium and contractility. Can J Physiol Pharmacol. 2005;83:215–242. doi: 10.1139/Y05-016. [DOI] [PubMed] [Google Scholar]
- 43.Wellman GC, Nelson MT. Signaling between SR and plasmalemma in smooth muscle: sparks and the activation of Ca2+-sensitive ion channels. Cell Calcium. 2003;34:211–229. doi: 10.1016/S0143-4160(03)00124-6. [DOI] [PubMed] [Google Scholar]
- 44.Witczak CA, Wamhoff BR, Sturek M. Exercise training prevents Ca2+ dysregulation in coronary smooth muscle from diabetic dyslipidemic Yucatan swine. J Appl Physiol. 2006;101:752–762. doi: 10.1152/japplphysiol.00235.2006. [DOI] [PubMed] [Google Scholar]
- 45.Wood PG, Gillespie JI. In permeabilised endothelial cells IP3-induced Ca2+ release is dependent on the cytoplasmic concentration of monovalent cations. Cardiovasc Res. 1998;37:263–270. doi: 10.1016/S0008-6363(97)00207-1. [DOI] [PubMed] [Google Scholar]
- 46.Yuan JX, Aldinger AM, Juhaszova M, Wang J, Conte JV, Jr, Gaine SP, Orens JB, Rubin LJ. Dysfunctional voltage-gated K+ channels in pulmonary artery smooth muscle cells of patients with primary pulmonary hypertension. Circulation. 1998;98:1400–1406. doi: 10.1161/01.cir.98.14.1400. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.