Abstract
DNA ends exposed after introduction of double-strand breaks (DSBs) undergo 5′-3′ nucleolytic degradation to generate single-stranded DNA (ssDNA), the substrate for binding by the Rad51 protein to initiate homologous recombination. This process is poorly understood in eukaryotes, but several factors have been implicated, including the Mre11 complex (Mre11-Rad50-Xrs2/NBS1), Sae2/CtIP/Ctp1 and Exo1. Here we demonstrate that yeast Exo1 nuclease and Sgs1 helicase function in alternate pathways for DSB processing. Novel, partially resected intermediates accumulate in a double mutant lacking Exo1 and Sgs1 that are poor substrates for homologous recombination. The early processing step that generates partially resected intermediates is dependent on Sae2. When Sae2 is absent, in addition to Exo1 and Sgs1, unprocessed DSBs accumulate and homology-dependent repair fails. These results suggest a two-step mechanism for DSB processing during homologous recombination. First, the Mre11 complex and Sae2 remove a small oligonucleotide(s) from the DNA ends to form an early intermediate. Second, Exo1 and/or Sgs1 rapidly process this intermediate to generate extensive tracts of ssDNA that serve as substrate for Rad51.
DSBs are potentially lethal lesions that can occur spontaneously during normal cell metabolism or by treatment of cells with DNA-damaging agents. If unrepaired or repaired inappropriately, DSBs can lead to mutagenic events such as chromosome loss, deletions, duplications or translocations. The Mre11 complex (Mre11-Rad50-Xrs2/NBS1) is rapidly recruited to DSBs, signals checkpoint activation via the Tel1/ATM kinase and regulates 5′-3′ resection of the DNA ends1–3. The intrinsic Mre11 nuclease activity is essential for processing ends blocked by covalent adducts, such as Spo11 or hairpin-capped ends, but appears to be less important for processing HO endonuclease-induced DSBs4–7. Sae2/CtIP/Ctp1 interacts with the Mre11 complex and Sae2 deficiency is phenotypically similar to loss of the Mre11 nuclease activity6–10. The functions of the Mre11 complex and Sae2 in processing DSBs are partially redundant with the 5′-3′ double-strand exonuclease, Exo111. In the absence of Exo1 and Sae2, 5′-3′ resection of DSBs is reduced, but resection and homologous recombination still occur suggesting the existence of at least one other factor in DSB processing12. Here we demonstrate a function for the Saccharomyces cerevisiae RecQ helicase homolog, Sgs1, in DSB processing. As the Sgs1 homologue in human, BLM, maintains genome integrity and guards against cancer predisposition13, our findings suggest that some of the defects observed in individuals with Bloom’s syndrome could be due to altered processing of DSBs.
Sae2 and Exo1 function at different steps in resection
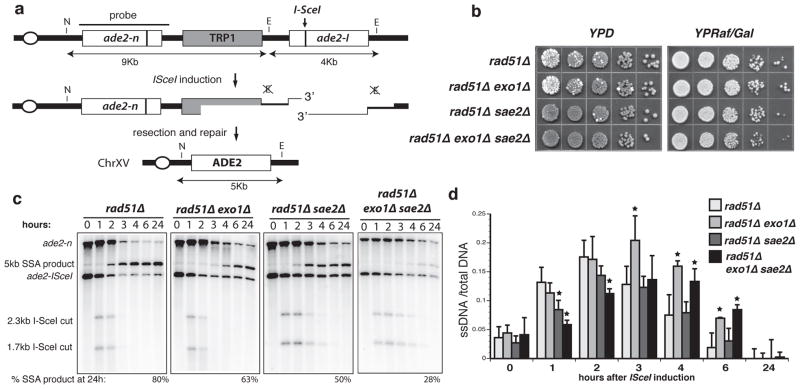
We developed a single-strand annealing assay (SSA), which is inherently sensitive to defects in DSB resection, to identify genes required for this process14. The assay employs tandem repeats of the ade2 gene, one of which contains a recognition site for the I-SceI endonuclease (Fig. 1a)15. The strain also harbors a GAL1p-I-SCEI fusion to provide regulated expression of the nuclease. Following induction of I-SceI, homology-dependent repair can occur by gene conversion directed by the uncut allele, or by SSA4. SSA requires extensive 5′-3′ degradation (~ 7-kb in this case) to expose complementary single-stranded DNA (ssDNA) followed by Rad52-dependent annealing of the repeats. After trimming residual single-stranded DNA tails and gap filling, recombinants form with loss of one of the repeats and the intervening sequence. In the rad51 mutant gene conversion is eliminated, leaving repair of the DSB by SSA as the alternate mode16. The efficiency of SSA can be assessed by the plating efficiency of strains on medium containing galactose (YPRaf/Gal) to induce constitutive expression of I-SceI. Changes in the kinetics of SSA can be evaluated by Southern blot analysis of NheI/EagI-digested genomic DNA.
Figure 1. Sae2 and Exo1 function at different steps in DSB resection.
a. Map of the chromosome XV region containing the ade2 direct repeat. The ade2-I allele is cleaved by I-SceI to yield the 2.3-kb and 1.7-kb cut fragments that disappear over time concomitant with repair to the 5-kb SSA product. b. SSA efficiency as indicated by sectoring on YPD plates and survival on YPRaf/Gal plates. Ade+ (white) colonies arise from ade2 (red) colonies by SSA during growth on YPD due to leaky expression of I-SceI. c. SSA physical assay: Monitoring of resection and repair of the I-SceI induced DSB by southern blot analysis of NheI/EagI digested genomic DNA. d. Quantitation of the ADE2 hybridization signal on native versus denatured DNA samples bound on nylon membrane. The means from multiple experiments from three independent inductions are presented, error bars indicate s.d.. * Denotes statistical significance of P<0.02 (unpaired t-test).
The plating efficiency of the rad51Δ strain on YPRaf/Gal was the same as on medium containing glucose (YPD) and Ade+ recombinants were evident by formation of white colonies (Fig. 1b). By physical analysis, DSB fragments appeared 1-h after inducing I-SceI expression and were then processed to form the SSA product visible at 2-h and accumulating to 80% of the total DNA after 24-h (Fig. 1c). 5′-3′ resection past the EagI and NheI sites results in loss of the cut fragments. Therefore, increased stability of the cut fragments indicates a delay or reduced rate of processing. Although the DSB fragments produced in the rad51Δ exo1Δ mutant were processed with similar kinetics as in the rad51Δ mutant, there was a delay in SSA product formation and reduced yield. In the rad51Δ sae2Δ strain, the DSB fragments persisted for longer, but the deletion products still appeared at 2-h and accumulated to 50% of the total. The phenotypes of sae2Δ and exo1Δ mutants, stability of cut fragments and delay in product formation, respectively, were additive in the rad51Δ exo1Δ sae2Δ mutant strain resulting in a substantial defect in SSA and a 2-fold reduction in plating efficiency on YPRaf/Gal plates (Fig. 1). To characterize the formation of ssDNA, DNA samples were applied to nylon membranes in the native or denatured state and tested for ability to hybridize to a probe adjacent to the DSB. This analysis revealed similar amounts of ssDNA adjacent to the break site in rad51Δ exo1Δ and rad51Δ strains at early time points, whereas a decrease was observed in the rad51Δ sae2Δ mutant (Fig. 1d). ssDNA persisted in the rad51Δ exo1Δ mutant, consistent with the observed delay in SSA product formation. These results are in line with a model in which Sae2 commences DSB processing to produce an early intermediate that is rapidly matured by the processive nucleolytic activity of Exo1. In the absence of Sae2, a smaller sub-set of DSBs is acted on directly by Exo1 yielding SSA products at the same time, but with lower yield than found in the presence of Sae2.
Sgs1 is required for DSB processing in the absence of Exo1
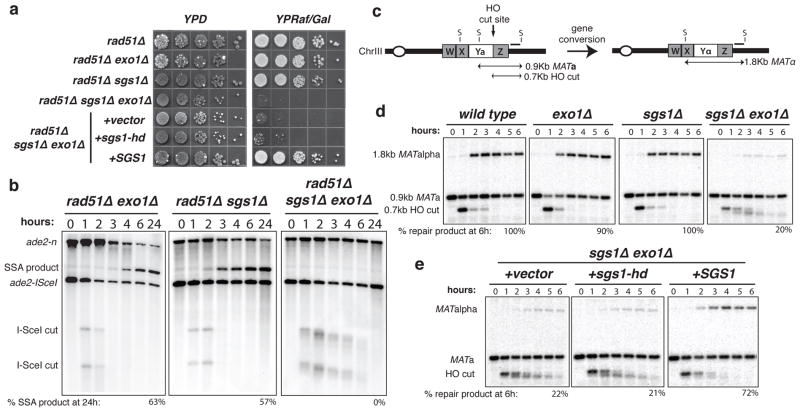
The presence of SSA products in the exo1Δ sae2Δ mutant suggests at least one other activity is operative in DSB processing. The missing factor is not Mre11 because SSA products still form in mre11Δ, mre11Δ exo1Δ, and mre11Δ exo1Δ sae2Δ mutants (Supplementary Fig. 1). The nuclease-defective mre11-H125N mutant was slightly less defective than sae2Δ, by itself or with exo1Δ (data not shown). One possible mechanism for DSB processing would involve a helicase in conjunction with a single-strand specific endo- or exonuclease. In Escherichia coli, the RecQ 3′-5′ helicase and RecJ 5′-3′ exonuclease function in DSB resection in the absence of the dominant RecBCD processing activity17. Therefore, we considered the possibility that the RecQ homologue of S. cerevisiae, Sgs118,19, might fulfill a similar redundant function. The rad51Δ sgs1Δ mutant showed a delay in SSA (Fig. 2 and Supplementary Fig. 2), however, the rad51Δ exo1Δ sgs1Δ strain was completely deficient as evidenced by the >1000-fold decrease in plating efficiency on YPRaf/Gal medium (Fig. 2a). Furthermore, by Southern blot analysis there was no detectable deletion product, even 24-h after I-SceI induction (Fig. 2b). Notably, in the rad51Δ exo1Δ sgs1Δ mutant novel intermediates were observed with slightly faster mobility than the DSB cut fragments and these became more smeared over time suggesting very slow processing. We were unable to analyze the sae2Δ sgs1Δ double mutant because of a synthetic growth defect 20.
Figure 2. Sgs1 and Exo1 function redundantly in DSB resection.
a. SSA plating efficiency as described in Fig. 1b. b. SSA physical assay as described in Fig. 1c. c. Schematic representation of MAT switching: the 0.9-kb MATa StyI fragment is cleaved by HO resulting in a 0.7-kb cut fragment that disappears over time concomitant with repair to an 1.8-kb MATα fragment. d. MAT switching assay: HO cut fragment processing and gene conversion efficiency as monitored by southern blot analysis of StyI digested genomic DNA. e. MAT switching assay on sgs1Δ exo1Δ cultures complemented with mutant or wild-type SGS1.
The SSA assay requires more extensive resection than is likely to be required for Rad51-dependent strand invasion. To determine whether the partially processed intermediates that accumulate in exo1Δ sgs1Δ strains are proficient for gene conversion, we analyzed DSB processing in RAD51 derivatives by a mating type (MAT) switching assay. In this assay, DNA samples are digested with StyI to detect HO endonuclease cleavage and gene conversion products by Southern blot analysis (Fig. 2c)21. The exo1Δ and sae2Δ mutants exhibited slight defects in the efficiency of repair, whereas the sgs1Δ single mutant appeared normal (Supplementary Fig. 3 and Fig. 2d). In the exo1Δ sgs1Δ double mutant the DSB fragment was processed to yield an intermediate with faster mobility than the cut fragment and this persisted for several hours, as we had observed in the SSA assay. Despite the defect in DSB processing, the 1.8-kb gene conversion product was still detected though with reduced efficiency (20%) compared with wild type and the single mutants. This suggests very short ssDNA tails are sufficient to initiate Rad51-dependent gene conversion. To determine whether the helicase activity of Sgs1 is important for DSB processing, plasmids expressing wild type or helicase- defective alleles of SGS1 were used to transform the exo1Δ sgs1Δ double mutant and the resulting strains tested for MAT switching or SSA proficiency22. As anticipated the SGS1 plasmid rescued the SSA and gene conversion defects of the sgs1Δ exo1Δ mutant, but the helicase-defective sgs1-K706A allele failed to complement sgs1Δ in these assays (Fig. 2). Previous studies have shown the steady state level of the Sgs1-K706A protein to be similar to wild type22. Therefore, the helicase activity is required for DSB processing by Sgs1. The lack of full complementation by SGS1 in the MAT-switching assay could be due to plasmid loss in some of the cells in the population.
Resection is severely attenuated in the exo1Δ sgs1Δ strain
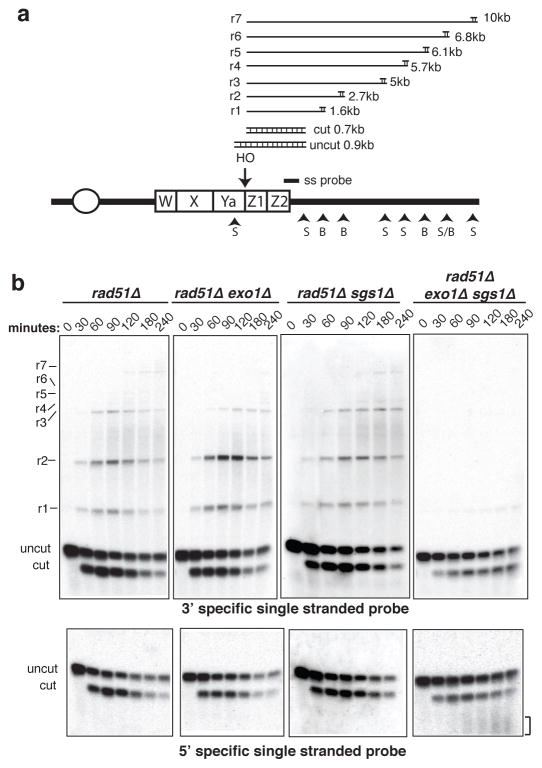
To measure resection of 5′ strands directly we analyzed HO-induced DSB fragments by restriction digestion and alkaline gel electrophoresis (Fig. 3a). Processing of the HO-induced break renders the DNA single-stranded and resistant to digestion with restriction enzymes resulting in a ladder of higher molecular weight bands21. By using RNA probes specific for the 3′ and 5′ strands to the right of the HO cut site, resection of the strands can be monitored. A rad51Δ background was used for this analysis to prevent engagement of the intermediates in repair.
Figure 3. Single stranded intermediates fail to form in the absence of Exo1 and Sgs1.
a. Schematic representation of the method used to detect single stranded intermediates after 5′-3′ resection of the MAT locus, showing the positions of the HO cut site, the probe and the StyI (S) and BstXI (B) sites. Processing of the HO-induced break renders the DNA single-stranded and resistant to digestion with restriction enzymes, giving rise to distinct sets of fragments21. b. Alkaline electrophoresis of StyI/BstXI digested genomic DNA: ssDNA intermediates formed after the resection of the HO-induced break were detected by using 3′ or 5′ specific riboprobes. The bracket indicates the smeared 5′-terminated strands observed in the rad51Δ exo1Δ sgs1Δ mutant.
The probe specific for 3′ strands detected extensive resection of the HO-induced break in the rad51Δ strain (Fig. 3b). In the rad51Δ exo1Δ mutant the first two ssDNA intermediates were formed with normal kinetics, but the products indicative of extensive resection were delayed resulting in accumulation of the r2 intemediate, suggesting Sgs1-dependent resection is less processive (Supplementary Fig. 4)5. The extent and timing of resection in the rad51Δ sgs1Δ mutant were similar to the rad51Δ strain. In contrast, only a faint band corresponding to resection beyond the first StyI site was detected in the rad51Δ exo1Δ sgs1Δ strain. Detection of a discrete band corresponding to degradation beyond the first StyI site and failure to detect products smaller than the cut fragment suggest the 3′ ends are intact in the rad51Δ exo1Δ sgs1Δ mutant. This was confirmed by using a probe specific for the 5′ strand. No intermediates were detected in the rad51Δ exo1Δ or rad51Δ sgs1Δ mutants, presumably due to the rapid degradation of 5′ ends. However, a smeared product was detected with faster migration than the 700-bp cut fragment in the rad51Δ exo1Δ sgs1Δ mutant, consistent with the severe resection defect. Interestingly, the smear does not occur immediately below the cut fragment suggesting there is an initial endonuclease cleavage step that removes about 50–100 nucleotides from the 5′ end.
Sae2 and Exo1 or Sgs1 function sequentially in DSB processing
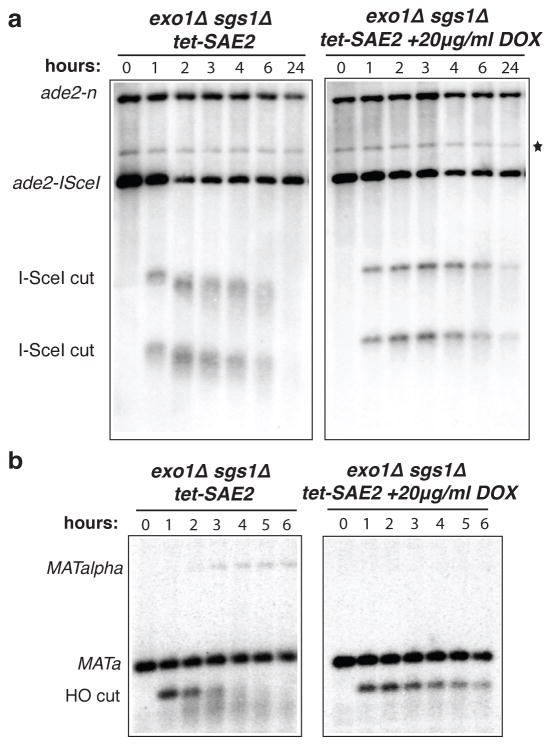
The results presented above suggest that after formation of a DSB, the ends are trimmed by an endonuclease to an intermediate form, which is subsequently processed by Exo1 or Sgs1. The Mre11 complex and Sae2 are likely to be involved in the initial processing step because DSB fragments are stabilized in mre11Δ and sae2Δ mutants, and recent studies have shown Mre11-dependent processing of DSBs to form short ssDNA oligonucleotides23. Furthermore, Sae2 has an endonuclease activity that is stimulated by the Mre11 complex24. We attempted to generate exo1Δ sgs1Δ mre11Δ or exo1Δ sgs1Δ sae2Δ triple mutants, but these were inviable (Supplementary Fig. 5a, b); the exo1Δ sgs1Δ mre11-H125N triple mutant was also inviable. A conditional sae2 allele was created in the exo1Δ sgs1Δ background by expressing endogenous SAE2 from the Ptet promoter in a strain expressing the tetracycline-controlled trans-activator (tTA) to repress transcription by growth in the presence of doxycycline25. The exo1Δ sgs1Δ Ptet-SAE2 strain was viable in the absence of doxycycline indicating normal function. By western blot analysis the Sae2 protein was depleted 4-h after addition of doxycycline to the growth medium (Supplementary Fig. 5c). In the absence of doxycycline, the DSB fragment generated by HO cleavage at the MATa locus was still processed to the faster mobility form, but in the presence of doxycycline the unprocessed 0.7-kb cut fragment persisted for more than 6-h and no MATα repaired products were detected (Fig. 4a). The cut fragments were also stabilized in the SSA assay in the absence of Sae2, even 24-h after I-SceI induction, confirming the block to resection (Fig. 4b). These results show the initial processing step is Sae2 dependent. Gene conversion occurs in the sgs1Δ Ptet-SAE2 strain in the presence of doxycycline confirming Exo1 is sufficient for DSB processing in the absence of Sgs1 (Supplementary Fig. 5d).
Figure 4. Sae2 is required for creating the minimally resected intermediates.
SSA assay (a) and mating type switching assay (b) in cells treated or untreated with doxycycline before I-SceI or HO expression. *Refers to a cross-hybridizing band, which is present at time 0 h and if it were due to recombinants in the population would allow survival of cells on YPRaf/Gal medium.
Conclusions
We have identified the key factors that are essential for generating 3′ single-stranded tails in DSB processing. In agreement with previous studies, the Mre11 complex and Sae2/CtIP play a critical role, but our findings suggest this function is limited to an early step in the reaction (Fig. 5). It is possible that the Mre11 complex and Sae2 fail to bind efficiently to the processed ends preventing repeated cycles of cleavage. This initial cleavage event could be important to prevent binding by the Ku complex and subsequent repair by end joining, and also to provide a substrate for Sgs1, which shows higher affinity for 3′ tailed substrates than blunt ends26. The intermediate thus formed is rapidly processed by either the 5′-3′ exonuclease activity of Exo1 or by Sgs1 helicase (Fig. 5). We assume Sgs1 functions with a single-strand specific nuclease to remove the 5′ strand. The essential nuclease, Dna2, is a candidate to function in resection because conditional mutations exhibit synthetic growth defects with exo1Δ and sgs1Δ27. That Sgs1 acts at this early step is unexpected because genetic studies have implied a role for it after Rad51 action, and biochemical studies have shown the human BLM protein to dissociate D-loops and double Holliday junction intermediates28–30. However, BLM localizes rapidly to sites of laser light-induced DSBs, suggesting an early role in repair in addition to dissolution of recombination intermediates31. As all of the factors involved in resection in yeast are evolutionarily conserved, these findings suggest a general mechanism for DSB processing in eukaryotes.
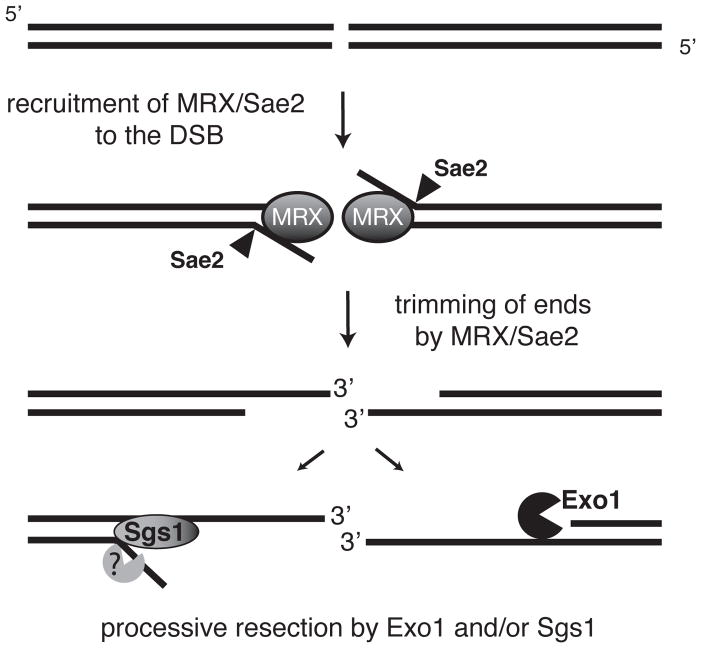
Figure 5. Two-step mechanism for DSB resection.
After a DSB is formed and recognized by the MRX complex, Sae2, in collaboration with MRX, trims the ends to create a minimally resected intermediate that is ‘compatible’ for processive resection by the 5′-3′ exonucleolytic activity of Exo1 or Sgs1 helicase and a single-strand specific nuclease. Cells lacking both the nuclease and the helicase activity accumulate the intermediates from MRX/Sae2 cleavage. Because mre11 and sae2 mutants still show DSB processing, Exo1 and Sgs1 must be able to access unprocessed DNA ends, but with reduced efficiency.
METHODS
Yeast strains and plasmids
The strains used were derived from W30332 and are listed in Supplementary Table 1. Strain LSY1092 was made by one-step gene replacement of W1588-4A with EcoRI/SphI-digested pMJ536 (gift of M. Lichten) to replace the SAE2 coding region with the kanMX6 cassette33. Strain LSY1975 was constructed by transformation of W1588-4C with a PCR fragment to generate a deletion allele of SGS1. PCR was performed on an hphMX4 cassette plasmid, pAG32 (gift of J. McCusker)34. Details of the primers used for gene disruption and confirmation are available on request. To create strain LSY2041, a PCR fragment amplifying the tet-SAE2 cassette from YAR1006 (gift of A. Rattray) was transformed into W1588-4C. A PCR fragment amplifying a 13-Myc tag from plasmid pFA6a-13Myc-TRP135 was transformed into LSY2041 to generate strain LSY2077. Strains containing the ade2 direct repeat were generated by crossing LSY143015 to strains within the lab collection to produce haploid progeny of the indicated genotypes.
Plasmids containing the wild type or helicase defective SGS1 were kindly provided by R. Rothstein. pEM-SGS1 and pEM-sgs1-K706A were created by moving the SGS1 containing KpnI/SacI fragment from plasmids pSM100 and pRS415-sgs1-K706A, respectively, into the multiple cloning site of pRS316.
Media, growth conditions, and genetic methods
Media, growth conditions and genetic methods are as described previously36. Hygromycin B (Sigma) to 300 μg/ml was used for selection of the hphMX4 cassette. Doxycycline (Sigma) was used to 20 μg/ml final concentration in the conditional Sae2 experiments. G418 (Sigma) to 200 μg/ml was used for selection of the kanMX cassettes.
Physical analysis of mating type switching
Strains to be tested were transformed to Trp+ with HO expressing plasmid, pFH80037. Trp+ transformants were grown in 5 ml of SC medium lacking tryptophan (SC-Trp) for 18 h. Cells were harvested, washed with water and used to inoculate 200 ml of SCRaf-Trp. Cultures were grown to an OD600 of 0.3 to 0.4, a 50ml sample was removed (t=0h) and galactose was added to the medium to a final concentration of 2%. One hour later, the cultures were harvested, washed and resuspended in 200 ml of SCRaff-Trp + 2% glucose. Samples (40 ml) were removed at 1-h intervals after induction for DNA analysis. Cells were harvested by centrifugation and the cell pellets were stored at −20°. DNA was extracted, digested with StyI, and DNA fragments were separated by electrophoresis through 1% agarose gels. DNA fragments were transferred to nylon membranes and hybridized with a PCR fragment generated by amplification of MAT sequences distal to the HO-cut site (coordinates 201176 to 201580 on the ChrIII sequence). For the conditional Sae2 experiments, the same protocol was followed with the exception that before addition of galactose, the cells were pre-treated with 20 μg/ml doxycycline for 6h to ensure full repression of Sae2 expression.
To quantitate the repair efficiency we compared the intensity of the gene conversion product to the total intensity of the lane, using ImageJ (NIH, USA). This ratio was then corrected for the HO cutting efficiency for each experiment. The cutting efficiency was quantitated using a set of primers that specifically anneal to Ya and Z2 sequences and amplify only the uncut substrate. PCR conditions for log-linear region amplification were used and PCR performed using as templates genomic DNA from t=0h and t=2h. In the same PCR reaction a second set of primers with the same annealing temperature but unrelated target locus were used as loading control. The PCR products were analyzed on agarose gels and the intensity of the bands was quantitated using ImageJ. For each time point we calculated the ratio of uncut MAT/control, designated as x. The ratio of x2h/x0h provides the percentage of uncut MATa that when subtracted from 1 gives the cutting efficiency.
Physical analysis of single strand annealing
Strains to be tested were grown in 5 ml of SC-Trp. Cells were harvested, washed with water and used to inoculate 200 ml of YPLactate (3%) medium. Cultures were grown to an OD600 of 0.3 to 0.4, a 50ml sample was removed (t=0h) and 16.8ml of 20% galactose were added to the medium. One hour later, the cultures were harvested, washed and resuspended in 200 ml of YPLactate + 2% glucose. Samples were removed at 1-h intervals after induction for DNA analysis as described above. DNA was digested with NheI/EagI, and DNA fragments were separated by electrophoresis through 0.8% agarose gels. DNA fragments were transferred to nylon membranes and hybridized with a 3.7kb BglII fragment of the ADE2 locus. The final SSA product was quantitated by the ratio of the intensity of the SSA product to the whole intensity of the lane. For the conditional Sae2 experiments, the same protocol was followed with the exception that before addition of galactose, the cells were pre-treated with 20 μg/ml doxycycline for 6h.
Dot blot analysis
DNA samples were applied to nylon membranes in the native or denatured state. 1 μg versus 0.1 μg of total genomic DNA was used for the native and denatured samples, respectively, after adjusting the concentration to 10xSSC. Samples were transferred to nylon membranes using a dot blot apparatus. The membrane was UV cross-linked and the blots were hybridized with the ADE2 probe. The signal of the dots was quantified by scanning the optic density of each dot using ImageJ.
Alkaline electrophoresis
ssDNA intermediates were analyzed by alkaline gel electrophoresis as described21 and the blots hybridized with single stranded probes complementary to the 5′ or 3′ strand. The probes were obtained by in vitro transcription using Epicentre Riboscribe T7 synthesis system and plasmids pEM-MAT or pEM-TAM as templates. The plasmids were obtained by cloning a MAT locus PCR fragment (coordinates 201176 to 201580 on the ChrIII sequence) into pGEM-T Easy (Promega) in either orientation. To quantitate the r2 band over time in different strain backgrounds we plotted the ratio of intensity of the r2 band to the intensity of an unrelated locus as a loading control.
Western blot analysis
Whole cells extracts were analyzed by SDS-PAGE electrophoresis and Sae-Myc was detected using anti-c-myc antibody (Sigma).
Supplementary Material
Acknowledgments
We thank M. Lichten, J. McCusker, A. Rattray and R. Rothstein for generous gifts of strains and plasmids, and W.K. Holloman and R. Rothstein for comments on the manuscript. This study was supported by a grant from the NIH.
Footnotes
Author Contributions. E.P.M. and L.S.S. designed experiments and wrote the paper; experiments were carried out by E.P.M.
References
- 1.Lee SE, et al. Saccharomyces Ku70, mre11/rad50 and RPA proteins regulate adaptation to G2/M arrest after DNA damage. Cell. 1998;94:399–409. doi: 10.1016/s0092-8674(00)81482-8. [DOI] [PubMed] [Google Scholar]
- 2.Lisby M, Barlow JH, Burgess RC, Rothstein R. Choreography of the DNA damage response: spatiotemporal relationships among checkpoint and repair proteins. Cell. 2004;118:699–713. doi: 10.1016/j.cell.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 3.Nelms BE, Maser RS, MacKay JF, Lagally MG, Petrini JH. In situ visualization of DNA double-strand break repair in human fibroblasts. Science. 1998;280:590–2. doi: 10.1126/science.280.5363.590. [DOI] [PubMed] [Google Scholar]
- 4.Krogh BO, Symington LS. Recombination proteins in yeast. Annu Rev Genet. 2004;38:233–71. doi: 10.1146/annurev.genet.38.072902.091500. [DOI] [PubMed] [Google Scholar]
- 5.Llorente B, Symington LS. The Mre11 nuclease is not required for 5′ to 3′ resection at multiple HO-induced double-strand breaks. Mol Cell Biol. 2004;24:9682–94. doi: 10.1128/MCB.24.21.9682-9694.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lobachev KS, Gordenin DA, Resnick MA. The Mre11 complex is required for repair of hairpin-capped double-strand breaks and prevention of chromosome rearrangements. Cell. 2002;108:183–93. doi: 10.1016/s0092-8674(02)00614-1. [DOI] [PubMed] [Google Scholar]
- 7.Rattray AJ, McGill CB, Shafer BK, Strathern JN. Fidelity of mitotic double-strand-break repair in Saccharomyces cerevisiae: a role for SAE2/COM1. Genetics. 2001;158:109–22. doi: 10.1093/genetics/158.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clerici M, Mantiero D, Lucchini G, Longhese MP. The Saccharomyces cerevisiae Sae2 protein promotes resection and bridging of double strand break ends. J Biol Chem. 2005;280:38631–8. doi: 10.1074/jbc.M508339200. [DOI] [PubMed] [Google Scholar]
- 9.Limbo O, et al. Ctp1 is a cell-cycle-regulated protein that functions with Mre11 complex to control double-strand break repair by homologous recombination. Mol Cell. 2007;28:134–46. doi: 10.1016/j.molcel.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sartori AA, et al. Human CtIP promotes DNA end resection. Nature. 2007;450:509–14. doi: 10.1038/nature06337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tran PT, Erdeniz N, Symington LS, Liskay RM. EXO1-A multi-tasking eukaryotic nuclease. DNA Repair (Amst) 2004;3:1549–59. doi: 10.1016/j.dnarep.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 12.Clerici M, Mantiero D, Lucchini G, Longhese MP. The Saccharomyces cerevisiae Sae2 protein negatively regulates DNA damage checkpoint signalling. EMBO Rep. 2006;7:212–8. doi: 10.1038/sj.embor.7400593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hickson ID. RecQ helicases: caretakers of the genome. Nat Rev Cancer. 2003;3:169–78. doi: 10.1038/nrc1012. [DOI] [PubMed] [Google Scholar]
- 14.Vaze MB, et al. Recovery from checkpoint-mediated arrest after repair of a double-strand break requires Srs2 helicase. Mol Cell. 2002;10:373–85. doi: 10.1016/s1097-2765(02)00593-2. [DOI] [PubMed] [Google Scholar]
- 15.Mozlin AM, Fung CW, Symington LS. Role of the Saccharomyces cerevisiae Rad51 paralogs in sister chromatid recombination. Genetics. 2008;178:113–26. doi: 10.1534/genetics.107.082677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ivanov EL, Sugawara N, Fishman-Lobell J, Haber JE. Genetic requirements for the single-strand annealing pathway of double-strand break repair in Saccharomyces cerevisiae. Genetics. 1996;142:693–704. doi: 10.1093/genetics/142.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amundsen SK, Smith GR. Interchangeable parts of the Escherichia coli recombination machinery. Cell. 2003;112:741–4. doi: 10.1016/s0092-8674(03)00197-1. [DOI] [PubMed] [Google Scholar]
- 18.Gangloff S, McDonald JP, Bendixen C, Arthur L, Rothstein R. The yeast type I topoisomerase Top3 interacts with Sgs1, a DNA helicase homolog: a potential eukaryotic reverse gyrase. Mol Cell Biol. 1994;14:8391–8. doi: 10.1128/mcb.14.12.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watt PM, Louis EJ, Borts RH, Hickson ID. Sgs1: a eukaryotic homolog of E. coli RecQ that interacts with topoisomerase II in vivo and is required for faithful chromosome segregation. Cell. 1995;81:253–60. doi: 10.1016/0092-8674(95)90335-6. [DOI] [PubMed] [Google Scholar]
- 20.Pan X, et al. A DNA integrity network in the yeast Saccharomyces cerevisiae. Cell. 2006;124:1069–81. doi: 10.1016/j.cell.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 21.White CI, Haber JE. Intermediates of recombination during mating type switching in Saccharomyces cerevisiae. EMBO J. 1990;9:663–73. doi: 10.1002/j.1460-2075.1990.tb08158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mullen JR, Kaliraman V, Brill SJ. Bipartite structure of the SGS1 DNA helicase in Saccharomyces cerevisiae. Genetics. 2000;154:1101–14. doi: 10.1093/genetics/154.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jazayeri A, Balestrini A, Garner E, Haber JE, Costanzo V. Mre11-Rad50-Nbs1-dependent processing of DNA breaks generates oligonucleotides that stimulate ATM activity. EMBO J. 2008 doi: 10.1038/emboj.2008.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lengsfeld BM, Rattray AJ, Bhaskara V, Ghirlando R, Paull TT. Sae2 is an endonuclease that processes hairpin DNA cooperatively with the Mre11/Rad50/Xrs2 complex. Mol Cell. 2007;28:638–51. doi: 10.1016/j.molcel.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baron U, Bujard H. Tet repressor-based system for regulated gene expression in eukaryotic cells: principles and advances. Methods Enzymol. 2000;327:401–21. doi: 10.1016/s0076-6879(00)27292-3. [DOI] [PubMed] [Google Scholar]
- 26.Bennett RJ, Keck JL, Wang JC. Binding specificity determines polarity of DNA unwinding by the Sgs1 protein of S. cerevisiae. J Mol Biol. 1999;289:235–48. doi: 10.1006/jmbi.1999.2739. [DOI] [PubMed] [Google Scholar]
- 27.Budd ME, et al. A network of multi-tasking proteins at the DNA replication fork preserves genome stability. PLoS Genet. 2005;1:e61. doi: 10.1371/journal.pgen.0010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gangloff S, Soustelle C, Fabre F. Homologous recombination is responsible for cell death in the absence of the Sgs1 and Srs2 helicases. Nat Genet. 2000;25:192–4. doi: 10.1038/76055. [DOI] [PubMed] [Google Scholar]
- 29.van Brabant AJ, et al. Binding and melting of D-loops by the Bloom syndrome helicase. Biochemistry. 2000;39:14617–25. doi: 10.1021/bi0018640. [DOI] [PubMed] [Google Scholar]
- 30.Wu L, Hickson ID. The Bloom’s syndrome helicase suppresses crossing over during homologous recombination. Nature. 2003;426:870–4. doi: 10.1038/nature02253. [DOI] [PubMed] [Google Scholar]
- 31.Karmakar P, et al. BLM is an early responder to DNA double-strand breaks. Biochem Biophys Res Commun. 2006;348:62–9. doi: 10.1016/j.bbrc.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 32.Zou H, Rothstein R. Holliday junctions accumulate in replication mutants via a RecA homolog-independent mechanism. Cell. 1997;90:87–96. doi: 10.1016/s0092-8674(00)80316-5. [DOI] [PubMed] [Google Scholar]
- 33.Borde V, Wu TC, Lichten M. Use of a recombination reporter insert to define meiotic recombination domains on chromosome III of Saccharomyces cerevisiae. Mol Cell Biol. 1999;19:4832–42. doi: 10.1128/mcb.19.7.4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldstein AL, McCusker JH. Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast. 1999;15:1541–53. doi: 10.1002/(SICI)1097-0061(199910)15:14<1541::AID-YEA476>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 35.Longtine MS, et al. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14:953–61. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 36.Sherman F, Fink G, Hicks J. Methods in Yeast Genetics. Cold Spring Harbor Laboratory; Cold Spring Harbor, N.Y: 1986. [Google Scholar]
- 37.Nickoloff JA, Singer JD, Hoekstra MF, Heffron F. Double-strand breaks stimulate alternative mechanisms of recombination repair. J Mol Biol. 1989;207:527–41. doi: 10.1016/0022-2836(89)90462-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.