Abstract
Preferential motor reinnervation (PMR) is the tendency for motor axons regenerating after repair of mixed nerve to reinnervate muscle nerve and/or muscle rather than cutaneous nerve or skin. PMR may occur in response to the peripheral nerve pathway alone in juvenile rats (Brushart, 1993; Redett et al., 2005), yet the ability to identify and respond to specific pathway markers is reportedly lost in adults (Uschold et al., 2007). The experiments reported here evaluate the relative roles of pathway and end organ in the genesis of PMR in adult rats. Fresh and 2-week predegenerated femoral nerve grafts were transferred in correct or reversed alignment to replace the femoral nerves of previously unoperated Lewis rats. After 8 weeks of regeneration the motoneurons projecting through the grafts to recipient femoral cutaneous and muscle branches and their adjacent end organs were identified by retrograde labeling. Motoneuron counts were subjected to Poisson regression analysis to determine the relative roles of pathway and end organ identity in generating PMR. Transfer of fresh grafts did not result in PMR, whereas substantial PMR was observed when predegenerated grafts were used. Similarly, the pathway through which motoneurons reached muscle had a significant impact on PMR when grafts were predegenerated, but not when they were fresh. Comparison of the relative roles of pathway and end organ in generating PMR revealed that neither could be shown to be more important than the other. These experiments demonstrate unequivocally that adult muscle nerve and cutaneous nerve differ in qualities that can be detected by regenerating adult motoneurons and that can modify their subsequent behavior. They also reveal that two weeks of Wallerian degeneration modify the environment in the graft from one that provides no modality-specific cues for motorneurons to one that actively promotes PMR.
Keywords: motoneuron, regeneration, peripheral nerve, retrograde labeling, rat, specificity, basal lamina, preferential motor reinnervation, Fluoro-Gold, WGA-ruby
INTRODUCTION
The intrinsic ability of transected peripheral axons to reinnervate functionally and topographically appropriate targets has been the subject of debate since the work of Ramon y Cajal (1928). Much of this controversy has resulted from the use of experimental models that vary substantially in design and often generate conflicting results. A prominent example is the debate over Ramon y Cajal's theory of neurotropism, which states that regenerating axons can be guided by diffusible, target-derived factors. Ramon y Cajal based his theory on a wide variety of experimental manipulations in which axon regeneration was not constrained. The theory was subsequently attacked by Weiss, who constrained regenerating axons within chambers fashioned from arterial allograft (Weiss and Taylor, 1944; Weiss and Edds, 1945). Later, models designed to minimize non-neural influences such as allograft rejection were used to confirm the action of neurotropism both in vitro and in vivo (Gunderson and Barrett, 1979; Lundborg et al., 1982).
Preferential motor reinnervation (PMR), the tendency for motor axons regenerating after repair of mixed nerve to reinnervate muscle nerve and/or muscle rather than cutaneous nerve and/or skin, was described on the basis of experiments performed in the rat femoral nerve model (Brushart, 1988). This model was chosen to explore sensory/motor specificity because of its anatomical characteristics. At the site of nerve repair, proximally within the femoral trunk, axons destined for skin and muscle intermingle. Regenerating motor axons will thus have access to Schwann cell tubes that lead to both skin and muscle as they reinnervate the distal nerve stump. Distally, the nerve bifurcates into distinct muscle and cutaneous branches that are well-matched as targets for regenerating axons. All motor axons normally project to the muscle branch; those that regenerate into the cutaneous branch can be identified by retrograde tracing as having made pathfinding errors.
The phenomenon of PMR has been confirmed in other laboratories by using retrograde tracing techniques in rodents (Madison et al., 1996; Al-Majed et al., 2000; Franz et al., 2005) and electrophysiologic techniques in the primate (Madison et al., 1999). Debate continues, however, as to the relative roles of pathway and end organ in generating PMR. A direct role for pathway involvement was demonstrated early on by the observation of PMR in young animals even when axons were prevented from contacting cutaneous or muscle end organs (Brushart, 1993). The ability of motor axons to recognize and respond to pathway identity was confirmed by the observation that motoneurons in juvenile rats maintain more collaterals in cutaneous than in muscle nerve, even when end organ contact is denied (Redett et al., 2005). Similarly, when motor and sensory axon regeneration were compared through grafts of ventral root and cutaneous nerve, ventral root preferentially supported motor axon regeneration while cutaneous nerve provided the best support for sensory axon regeneration (Hoke et al., 2006). Candidates for the molecular determinant of motor pathway identity include the HNK-1 carbohydrate (Martini et al., 1994) and unique combinations of growth factors (Hoke et al., 2006).
The relative roles of pathway and end organ have also been explored in adult rodents by transecting and blocking one or both femoral branches at varying distances from the repair site (summarized in Uschold et al., 2007). The changes in PMR elicited by these maneuvers led the authors to conclude that the accuracy of motoneuron regeneration depended upon the relative balance of generalized trophic support provided by pathways and end organs, and not on specific pathway identity.
The present experiments were designed to evaluate the relative roles of pathway and end organ in the genesis of PMR in adult rats. To minimize experimental variables such as unilateral blocking of regeneration, femoral nerve grafts were placed in correct or reversed alignment, so that only pathway identity was varied. Additionally, pathways were pre-degenerated in some experiments to more accurately model clinical reality. PMR was not generated within freshly-harvested grafts, and reversing their cutaneous and muscle pathways did not influence regeneration specificity. When grafts had been predegenerated, however, PMR was substantial and was eliminated by graft reversal. These experiments demonstrate unequivocally that, when predegenerated, adult rat muscle and cutaneous nerve differ in qualities that can be detected by adult motoneurons and that can influence regeneration specificity.
MATERIALS AND METHODS
Experiments were performed on adult (150–200 gm) female Lewis rats so that grafts could be exchanged between individuals without the need for immunosuppression. Rats were anaesthetized by intraperitoneal injection of a mixture of ketamine (100 mg/Kg) and xylazine (6 mg/Kg) in normal saline. Procedures were approved by the Animal Care and Use Committee of the Johns Hopkins Medical Institutions.
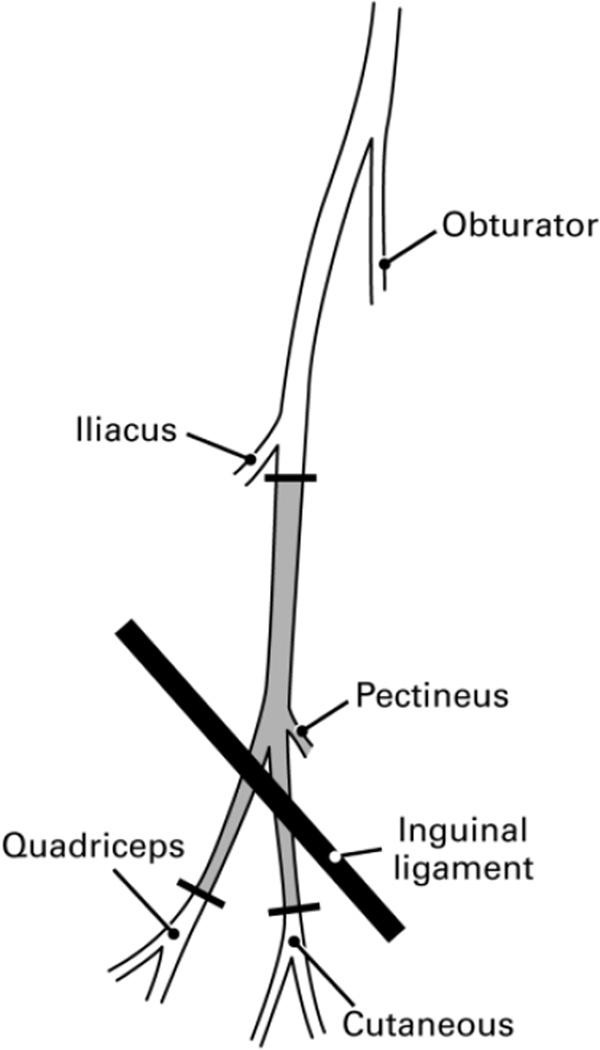
In all experimental animals both femoral nerves were replaced with femoral nerve graft harvested from separate donor animals. These grafts extended from the femoral trunk just distal to the origin of the iliacus nerve at their proximal end to the level of quadriceps innervation by the femoral muscle branch distally (Figure 1). Left femoral nerve grafts were always used to reconstruct the left femoral nerve, and vice versa, to allow anatomical matching of the graft and recipient proximal stump.
Figure 1.
The right femoral nerve of the rat as seen through the ilio-inguinal approach. Femoral nerve grafts used in these experiments extended from just distal to the iliacus innervation to the distal-most unbranched portions of the femoral motor and sensory tributaries, the portion of the nerve shaded grey. The small pectineus branch was tied off at its origin. Sensory and motor axons intermingle within the nerve at the site of proximal transection. Distally, motor axons segregate into the quadriceps muscle branch, and are not normally found in the cutaneous branch.
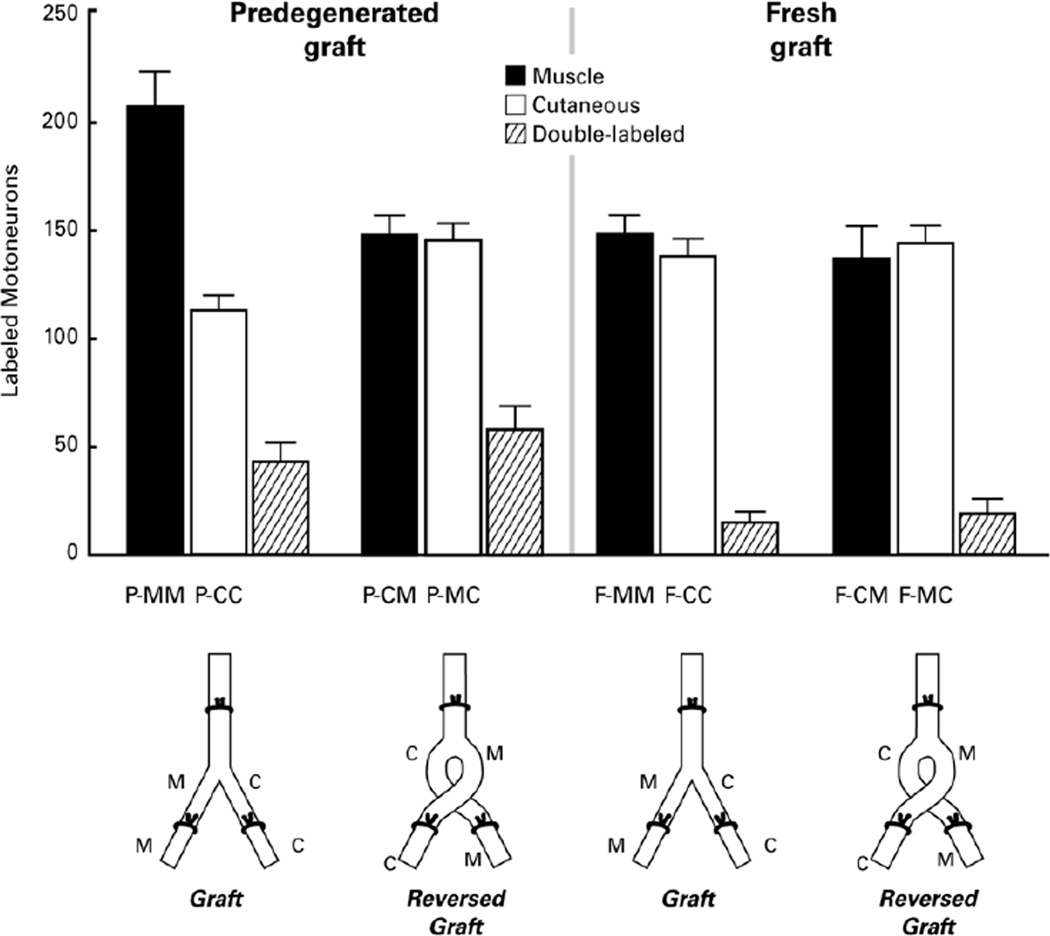
Four groups of 12 nerves each were created by varying the environment within the graft (fresh or predegenerated) and the alignment of the distal repairs (correct alignment vs crossed alignment), leading to 8 possible combinations of donor graft branch and recipient nerve (Figure 2). Each of these 8 combinations is signified by a three-letter identifier. The first letter indicates the graft environment (F=fresh, P=predegenerated), the second describes which branch of the graft is involved (M=muscle branch, C=cutaneous branch), and the third denotes the recipient nerve (M=muscle nerve, C=cutaneous nerve). Fresh grafts were harvested from previously unoperated donor animals and placed in recipient animals in one of two possible orientations. Correct matching of cutaneous and muscle branches of the distal graft with their corresponding structures in the recipient animals (n=12) joined previously motor Schwann cell tubes within the graft to recipient Schwann cell tubes that led to muscle (F-MM), and previously cutaneous Schwann cell tubes within the graft to those leading to skin in the recipient (F-CC). Reversal of the distal graft branches, in contrast (n=12), aligned previously motor Schwann cell tubes in the graft with distal Schwann cell tubes leading to skin (F-MC), and previously cutaneous Schwann cell tubes in the graft with those leading to recipient muscle (F-CM).
Figure 2.
The four types of experimental preparation and the regeneration specificity resulting from each. Grafts were either Fresh (F) or Predegenerated for 2 weeks (P), and were either aligned correctly (MM- graft Muscle branch joined to recipient Muscle nerve; CC- graft Cutaneous branch joined to recipient Cutaneous nerve) or reversed (CM- graft Cutaneous nerve to recipient Muscle nerve; MC- graft Muscle nerve to recipient Cutaneous nerve). Vertical bars represent the mean number of motoneurons in each group that correctly reinnervated the muscle branch (black bar), incorrectly reinnervated the cutaneous branch (open bar), or projected axon collaterals to both (striped bar). After 8 weeks of regeneration, PMR was pronounced in the correctly-aligned, predegenerated grafts, but not in other groups.
An additional 24 grafts were predegenerated in donor animals by ligating and transecting the proximal femoral nerve through a limited lateral retroperitoneal approach to minimize the perineural scarring that results from the more extensive exposure required for the ventral approach. Two weeks later these grafts were harvested and placed in the beds of freshly-axotomized femoral nerves in recipient animals. Grafts were aligned correctly (n=12; P-MM, P-CC) or were reversed (n=12; P-CM, P-MC). The consequences of correct and reversed alignment for the matching of graft and recipient Schwann cell tubes were the same as those described for fresh grafts, with the exception that the graft pathways had been predegenerated.
All nerve junctures were aligned within microrenathane tubes (Braintree Science, Braintree Mass.) using 10-0 nylon sutures to maintain the alignment and contact of nerve stumps. Two-millimeter-long tubes of 0.04 inch inner diameter were used to align the recipient femoral trunk and the proximal end of the graft, and 2-mm-long tubes of 0.35 inches inner diameter were used to align the distal limbs of the grafts with the distal stumps of the axotomized femoral branches. Eight weeks were then allowed for axons to regenerate through the grafts and reinnervate recipient nerve and end organs.
At the completion of the regeneration period the recipient femoral cutaneous and muscle branches were axotomized by crushing them with jewelers forceps, then injected with retrograde tracers to identify the motoneurons that had reinnervated them. In each nerve preparation one branch, determined randomly, was injected with a 3% solution of Fluoro-Gold (Fluorochrome, Denver CO) (Schmued and Fallon, 1986) and the other was injected with a 5% solution of a conjugate of Alexa Fluor 555 and wheat germ agglutinin (Invitrogen, Carlsbad California, catalog # W32464). A Picospritzer (Parker Hannefin, Fairfield NJ) set at a pressure of 20 mm Hg delivered the tracers through glass micropipettes into the crushed area of the nerve. In six control rats, one femoral muscle branch was injected with Fluoro-Gold and the contralateral muscle branch was injected with Alexa Fluor 555-WGA to compare their labeling efficacies.
Forty-eight hours after tracer injection rats were anaesthetized deeply and perfused through the left ventricle with 200 cc of warm saline followed by 500 cc of 4% paraformaldehyde in 0.1 M sodium acetate buffer over a period of one-half hour. The lumbar spinal cords were then removed, post-fixed for 12 hours in 4% paraformaldehyde, and stored in 20% sucrose in Sorensen's phosphate buffer. Spinal cords were sectioned transversely at 40µ with a freezing microtome. The resulting sections were mounted serially on gelatin-coated glass slides, dried, and overlaid with coverslips using DPX (Aldrich, Milwaukee WI) to minimize background fluorescence.
Spinal cord sections were viewed with fluorescent light (Fluoro-Gold: 323 nm excitation, 408 nm emission; Alexa-Fluor 555-WGA: 555 nm excitation, 580 nm emission) by an observer who was unaware of the experimental treatment. Counts were prepared for each nerve of Fluoro-Gold-labeled motoneurons, Alexa-Fluor labeled motoneurons, and motoneurons that contained both tracers (double-labeled). Once the counts had been completed, group identity and pathway-tracer matching were determined for each specimen. Each of the four groups was then characterized by the mean number of motoneurons that projected correctly to the recipient muscle branch and muscle, the mean number that projected incorrectly to the recipient cutaneous branch and skin, and the mean number that projected collateral axons to both branches simultaneously (Figure 2). Individual counts were then subjected to longitudinal Poisson regression analysis, using each animal as a cluster, to evaluate each group for PMR, and to determine the influence of graft predegeneration on PMR (Figure 3), the pathway contribution to PMR (Figure 4), the pathway effect on muscle reinnervation (Figure 5), and the relative contributions of pathway and end organ to PMR (figure 6). Of the categories of regression analysis, the Poisson approach is appropriate for count data, the analysis is longitudinal because it deals with the same system at two time periods (before and after predegeneration), and each animal is used as a cluster because there could be a relationship between the two results obtained within a single animal.
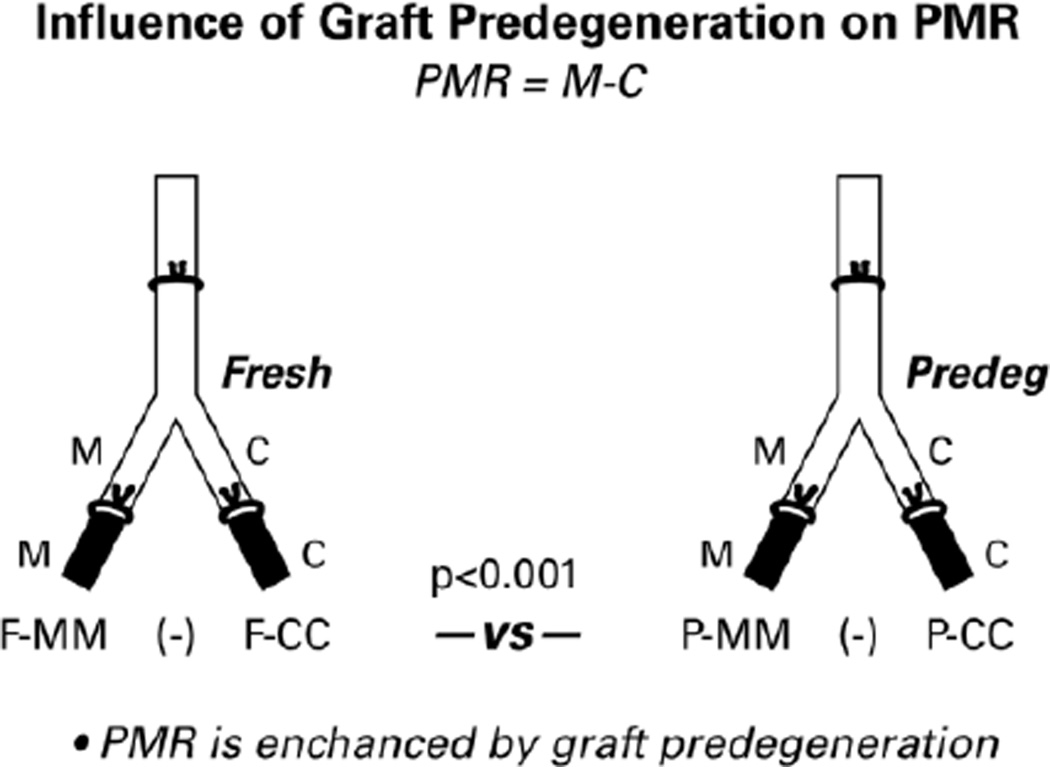
Figure 3.
Diagram of the preparations that were used to determine the influence of pathway predegeneration on specificity generation. Data were obtained by retrograde labeling the distal pathways shown in black. Resulting motoneuron counts were subjected to longitudinal Poisson regression analysis. PMR is the difference between the number of motoneurons correctly reinnervating distal muscle nerve and/or muscle and the number incorrectly reinnervating distal cutaneous nerve and/or skin. PMR was significantly greater (p<0.001) when the pathway had been predegenerated.
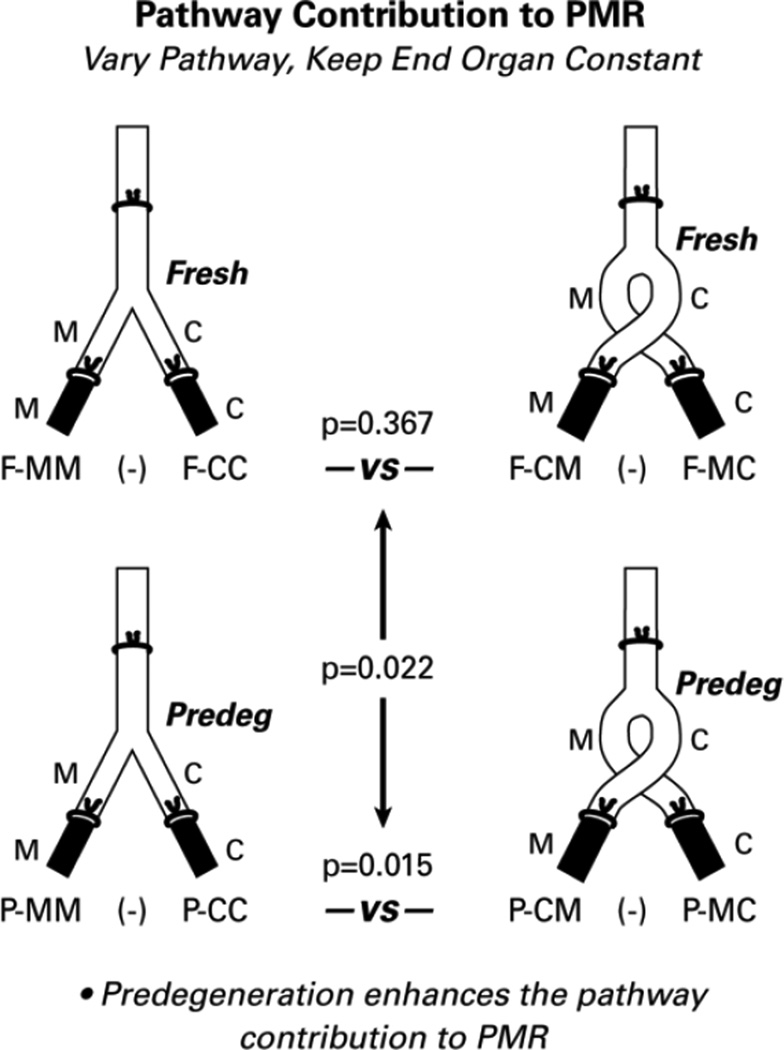
Figure 4.
To determine the contribution of the graft pathway to PMR, we analyzed the effects of reversing the alignment of the cutaneous and muscle branches of the graft while maintaining the constant alignment of the recipient nerve/end organ components. The pathway did not influence PMR when it was fresh (p=0.367), but influenced PMR significantly when it had been predegenerated for 2 weeks (p=0.15). When comparing these two effects, the pathway contribution to PMR was enhanced significantly by predegeneration (p=0.022).
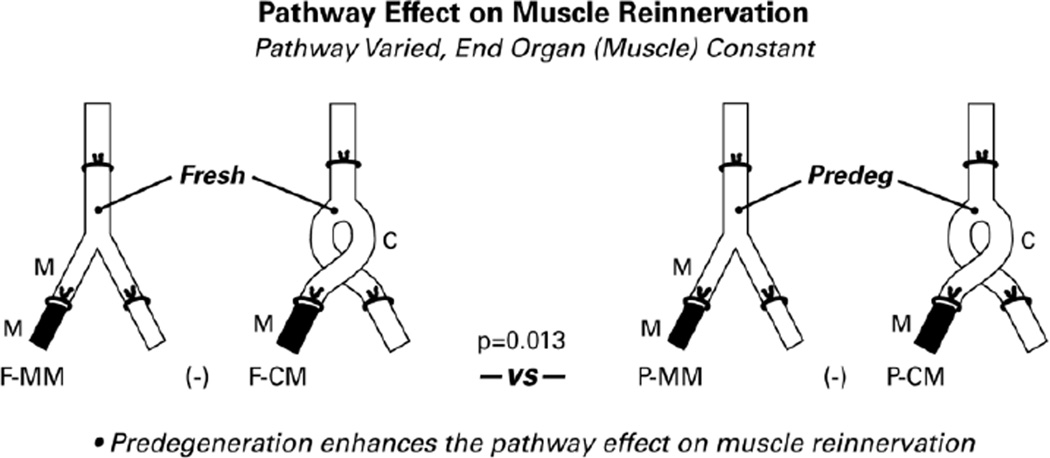
Figure 5.
Evaluation of the pathway effect on muscle reinnervation alone, without reference to motoneurons that project elsewhere. As in other diagrams, the pathways that provide data for the comparisons are solid black. Significantly more motoneurons reinnervated muscle through graft muscle nerve than through graft cutaneous nerve when the graft had been predegenerated (p=0.003), but not when it was fresh (p=0.442). Overall, predegeneration significantly enhanced the pathway effect on muscle reinnervation (p=0.013).
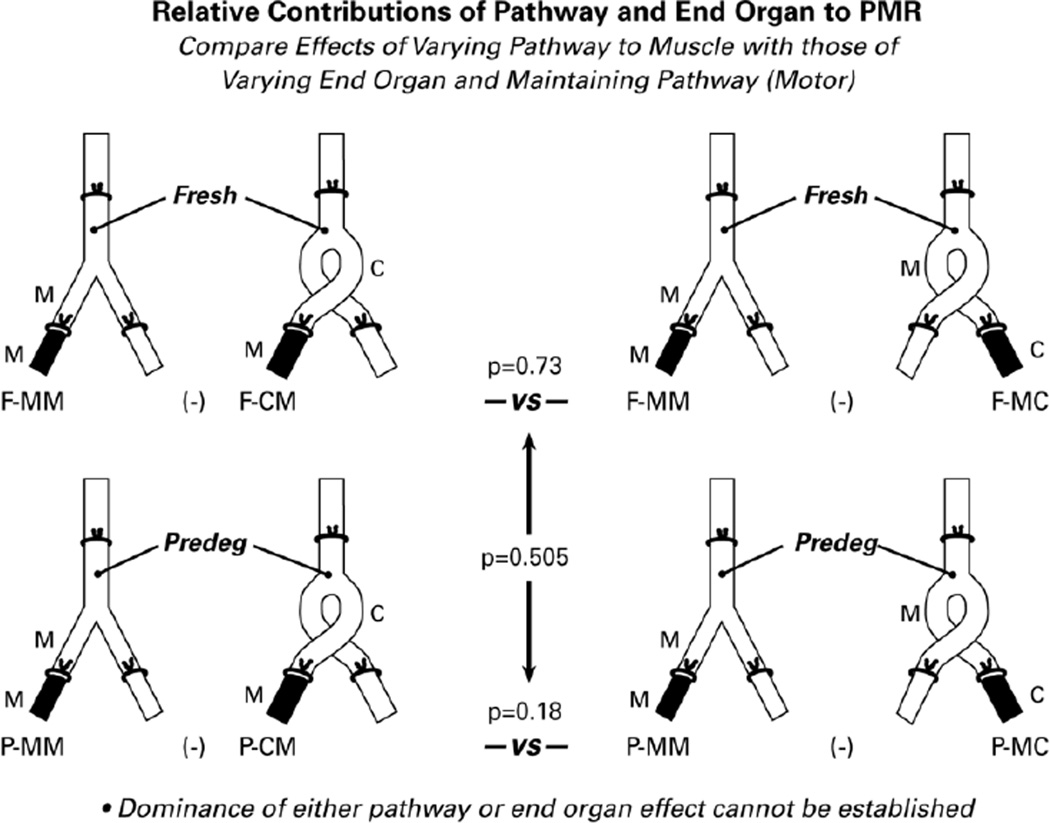
Figure 6.
The relative contributions of graft pathway and recipient muscle to PMR were evaluated by comparing the effects of varying the pathway to muscle (on the left) with those of maintaining the muscle nerve pathway and varying the end organ (on the right). A significant difference between pathway and end organ contributions to PMR could not be demonstrated with the current data set.
RESULTS
Preparations were of sufficient quality to obtain motoneuron counts from 18 nerves in the predegenerated groups (9 in the group with correct alignment and 9 in the group with reversed alignment) and from 22 in the fresh graft groups (11 in the group with correct alignment and 11 in the group with reversed alignment; Figure 2). Preferential motor reinnervation (PMR) in the rat femoral nerve is quantified as the number of motoneurons that project correctly to muscle minus the number that project incorrectly to skin. In the correctly-aligned fresh grafts, there was no significant difference between the number of motoneurons projecting correctly through the muscle branch to muscle (F-MM) and the number projecting incorrectly through the cutaneous branch to skin (F-CC) (p=0.26), indicating a lack of PMR. In the correctly-aligned predegenerated grafts, however, the difference between P-MM and P-CC was highly significant (p < 0.001). Comparison of the degree of PMR between the two groups (F-MM minus F-CC vs P-MM minus P-CC; Figure 3) revealed that PMR was significantly more prominent in the predegenerated grafts (p<0.001). Pathway modifications that are brought about by 2 weeks of graft predegeneration thus promote regeneration specificity.
Graft predegeneration could enhance PMR directly by modifying the interaction between regenerating axons and the pathway within the graft, or indirectly by influencing the delivery of regenerating axons to muscle. To isolate the contributions of the graft pathway to PMR, we analyzed the effects of varying the pathway while keeping the end organs constant (Figure 4). In fresh grafts, in which PMR was not observed, switching pathways had no effect (F-MM minus FCC vs F-CM minus F-MC; p=0.367). In the predegenerated grafts, however, the PMR observed when pathway and end organ were aligned correctly was lost when graft polarity was reversed (P-MM minus P-CC vs P-CM minus P-MC; p=0.015). In adult rats, regenerating motor axons are thus able to recognize and respond to pathway identity under appropriate conditions. Subsequent comparison of the pathway effect in fresh and predegenerated grafts revealed that it was significantly more prominent if the pathway had been predegenerated (p=0.022).
To further explore the role of the peripheral nerve pathway in specificity generation, we asked whether pathway identity within each experimental group influenced the number of motoneurons that correctly reinnervated muscle. In these comparisons, the pathway is again varied while the end organ, muscle, remains constant (Figure 5). In this comparison, however, we are looking only at reinnervation of the recipient muscle branch/muscle complex without reference to what is happening within the recipient cutaneous branch. In the fresh grafts, the difference between F-MM and F-CM was not significant (p=0.442); similar numbers of motoneurons reinnervated muscle regardless of whether they regenerated through previously muscle or previously cutaneous Schwann cell tubes. In the predegenerated grafts, in contrast, the difference between P-MM and P-CM was highly significant (p=0.003); significantly more motoneurons reinnervated muscle through previously muscle than through previously cutaneous pathways. Comparison of the pathway effect revealed that it was significantly greater in predegenerated than in fresh grafts (p=0.013).
To complete our analysis, we compared the role of graft muscle pathways to that of muscle itself in the generation of PMR in adult animals (Figure 6). In fresh grafts, comparing the effects of varying the pathway through which muscle is reinnervated (F-MM minus F-CM) with those of varying the effectiveness with which skin and muscle are reinnervated through muscle pathways (F-MM minus F-MC) failed to identify a difference between the two (p=0.73). Similarly, there was no significant difference if the pathways were predegenerated (P-MM minus P-CM vs P-MM minus P-MC; p=0.18). When the relative effects of pathway and end-organ were compared in fresh and predegenerated grafts, there were no significant differences (p=0.505). Pathway identity and contact with muscle thus both influence PMR, and it is not possible to establish the dominance of either with the current data set.
The analyses above were performed using counts of motoneurons that projected singly to either the recipient muscle branch/muscle or to the cutaneous branch/skin after 8 weeks of regeneration. In each experimental group, however, some motoneurons projected collaterals to both branches, and were thus “double-labeled.” The mean number of double-labeled motoneurons was significantly greater (p<0.01) in both predegenerated graft groups than it was in either fresh graft group. These findings are consistent with an increase in growth factor production by Schwann cells that have been denervated for 2 weeks (Hoke et al., 2006) and a resultant increase in collateral sprout formation in response to this environment (Redett et al., 2005).
The labeling efficacies of Fluoro-Gold and Alexa Fluor 555-WGA were compared in the right and left femoral muscle branches of six rats. Fluoro-Gold labeled a mean of 345 motoneurons, and Alexa Fluor 555-WGA labeled a mean of 341 motoneurons (p=0.89). The labeling efficacies of these tracers are thus equivalent in the rat femoral nerve model.
DISCUSSION
These experiments explore the relative contributions of pathway and end organ to PMR. They demonstrate that, in adult rats, PMR is not observed when femoral motor axons regenerate through freshly-harvested femoral nerve grafts. If the grafts have been predegenerated for 2 weeks, however, PMR is dramatic. Furthermore, reversal of the graft insertions, so that the femoral muscle branch leads to skin and the cutaneous branch leads to muscle, demonstrates a clear role for pathway identity in generating PMR within predegenerated grafts, but not within fresh grafts.
The idea that regenerating motor axons might receive more support from previously motor Schwann cell tubes than from previously sensory tubes has been challenged by recent experimental evidence, all obtained with freshly transected or harvested nerve. Madison and co-workers varied the relative lengths of the rat femoral cutaneous and muscle branches by transecting and blocking off one or both pathways at varying distances from the femoral bifurcation (Robinson and Madison 2004, 2006; Uschold et al., 2007). They found that motoneurons preferentially reinnervated the longest branch, regardless of whether it was muscle nerve or cutaneous nerve. On the basis of these findings, they rejected the concept of specific pathway identity, advancing in its place the hypothesis that the "relative level of trophic support" would determine motoneuron destination, and could be manipulated by shortening or lengthening the pathway. A role for pathway identity was also challenged by experiments in which freshly-harvested grafts of the femoral cutaneous and muscle branches were used to reconstruct defects in the rat tibial nerve and femoral motor branch, and performed equally as assessed by axon counts (Neubauer et al., 2010; Kawamura et al., 2010).
The current experiments demonstrate that, in adult animals, predegeneration of the distal nerve stump facilitates PMR. Since the work of Ramon y Cajal (1928), predegeneration of distal pathways has been known to enhance the overall process of regeneration. In the rat, predegeneration of sciatic nerve grafts was found to reduce the delay in distal stump reinnervation (Kerns et al., 1993), to increase the amplitude of the nerve action potential and the number of large axons 4 weeks after repair (Sorenson and Windebank, 1993), and to promote the reinnervation of grafts placed in the dorsal columns (Oudega et al., 1994). In other rat models, predegeneration of the rat facial nerve enhanced its reinnervation by freshly-axotomized hypoglossal motoneurons (Guntinsa-Lichius et al., 2000) and predegeneration of the ulnar nerve sped the return of finger flexion when it was used to graft a defect in the median nerve (Bertelli et al., 2006).
Based on these observations, predegeneration of the distal nerve stump can be seen to have a positive effect on regeneration. Exploration of the factors that contribute to this positive effect, however, reveals a rapidly evolving menu of pathway components that influence regeneration in both positive and negative ways. On the positive side, denervated Schwann cells upregulate laminin and growth factors, both of which may enhance regeneration (Figure 7). Laminin is a glycoprotein that is deposited on the adaxonal basal lamina by adjacent Schwann cells (Cornbrooks et al., 1983) and that signals through integrin receptors to promote regeneration. In the motor system, interfering with integrin signaling impairs facial nerve regeneration (Werner et al., 2000). DRG neurons upregulate integrins after peripheral but not central axotomy, require integrin signaling for the conditioning effect both in vitro and in vivo, and respond to transgenic overexpression of integrin with enhanced regeneration (Condic, 2001; Ekstrom et al., 2003; Wallquist et al., 2004). Upregulation of growth factors is biphasic, with rapid expression of some factors soon after injury, followed by more gradual upregulation of a more diverse group over ensuing weeks (Figure 7). The expression patterns of several factors and their potential links to regeneration have been examined recently (Hoke et al., 2006; Brushart et al., 2013).
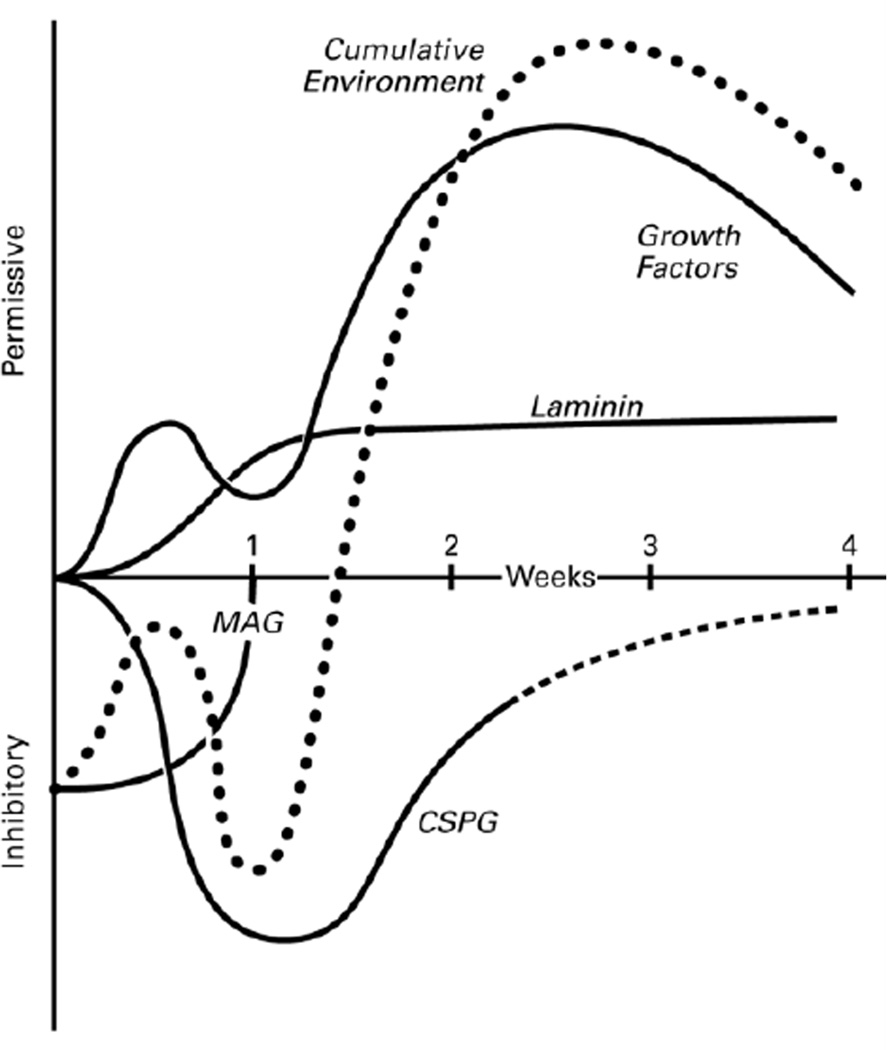
Figure 7.
The evolution of the distal nerve stump. As Wallerian degeneration proceeds, factors that promote regeneration, laminin and growth factors, are upregulated. MAG, an inhibitor of regeneration, is cleared within the first week, and the inhibitory CSPGs are upregulated transiently. The net effect (dotted line) is an initially inhibitory environment that becomes progressively more supportive of regeneration. The relative changes within each factor are derived from experimental data (see DISCUSSION); the relative impact of each factor in relation to the others is hypothetical .
The two inhibitory influences that have received the most attention are the myelin-associated glycoprotein (MAG) and the chondroitin sulfate proteoglycans (CSPGs). MAG, normally found on the inner, adaxonal membrane of myelinating Schwann cells, is cleared from the distal stump by Wallerian degeneration and can no longer be detected 1 week after injury (Schafer et al., 1996). Soluble MAG inhibits early reinnervation of the distal nerve stump (Torigoe and Lundborg, 1998), while systemic treatment with MAG antibodies dramatically enhances PMR (Mears et al., 2003). Unlike MAG, the CSPGs are primarily localized to the endoneurium outside of the Schwann cell tube and are upregulated by nerve injury (Tona et al., 1993; Zuo et al., 1998). Breakdown of CSPGs with the enzyme chondroitinase ABC increases both the speed and volume of distal stump reinnervation (Zuo et al., 2002; English, 2005).
PMR may be influenced by the condition of the distal pathway, as one would expect based on the above discussion, but also by the regeneration state of the neuron, neuronal phenotype, and the immediate repair environment (reviewed in Brushart, 2011). The net effect of these factors is evident even after routine end-to-end rat femoral nerve repair. Reinnervation of the distal stump occurs gradually, with many neurons crossing the repair site in the first week after repair, but others not crossing until 3–4 weeks have passed (Brushart et al., 2002). The early crossers project randomly to cutaneous and muscle nerve, but later projections all accumulate within the muscle branch (Brushart, 1993). PMR thus varies over time within an individual nerve repair, influencing the destination of delayed projections, but unable to redirect motor axons that project only to the cutaneous branch early on.
The individual contributions of motoneuron, repair site, and distal pathway to PMR have been explored in further experiments in the femoral nerve model. Neuronal regeneration state has been shown to decrease with aging (Cai et al, 2001). In experiments in which femoral nerve grafts were exchanged between young and old rats, neuronal age was the dominant factor in predicting PMR, as only young motoneurons could generate PMR through a graft from an old animal (Le et al., 2001). Similarly, increasing neuronal regeneration state by brief electrical stimulation at the time of nerve repair increased the regeneration specificity of both motoneurons and DRG neurons (Brushart et al., 2002, 2005). The phenotype of regenerating motoneurons has also been shown to influence PMR. Motoneurons in transgenic mice that do not express polysialic acid (PSA) generate fewer regenerative collaterals, inhibiting their ability to sample multiple distal pathways, and do not generate PMR (Franz et al., 2005). Similarly, motoneurons of non-femoral origin that do not normally express PSA fail to develop PNR when they regenerate through he femoral nerve (Franz et al., 2008). At the repair site, blocking the inhibitory effects of MAG significantly enhanced PMR (Mears et al., 2003), as did replacing suture repair with fibrin glue repair (Robinson and Madison, 2003). Modification of the femoral graft environment by proximal nerve crush 4 and 2 weeks before graft transfer in young rats was also found to enhance PMR dramatically (Brushart et al., 1998). In the current experiments, predegeneration of adult femoral nerve grafts for 2 weeks had similar effects.
CONCLUSIONS
PMR is a multifactoral phenomenon that can be promoted or defeated by subtle changes in the regeneration state of the motoneuron, the permissiveness of the repair site, or the environment within the distal nerve stump. In adult animals, this balance has shifted towards inhibition when regenerating axons are confronted with a distal stump in the early stages of Wallerian degeneration. After 2 weeks of predegeneration, however, once MAG has been removed and growth factors upregulated, the balance has shifted back to one that is conducive to PMR. Although non-physiologic modifications of the system such as changes in pathway length may influence PMR, it is normally modulated by more subtle physiologic cues. Identification of these cues and their manipulation to enhance regeneration specificity should ultimately result in improved clinical outcomes.
Highlights.
Fresh nerve graft does not promote Preferential Motor Reinnervation (PMR)
Predegenerated grafts promote PMR to a highly significant degree
Degenerating nerve evolves from an inhibitory to a permissive substrate
The state of the neuron, repair site, and pathway all influence PMR
The clinical standard of immediate nerve repair defeats PMR
ACKNOWLEDGMENTS
The authors thank Ms Catherine Kiefe for preparation of the illustrations. This work was supported by NIH RO-1 NS034484.
Abbreviations
- PMR
preferential motor reinnervation
- MAG
myelin-associated glycoprotein
- CSPG
chondroitin sulfate proteoglycans
- F
fresh
- P
predegenerated
- MM
graft muscle nerve branch joined to recipient muscle nerve
- CC
graft cutaneous branch joined to recipient cutaneous nerve
- MC
graft muscle branch joined to recipient cutaneous nerve
- CM
graft cutaneous branch joined to recipient muscle branch
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Al-Majed AA, Neumann CM, Brushart TM, Gordon T. Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J.Neurosci. 2000;20:2602–2608. doi: 10.1523/JNEUROSCI.20-07-02602.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertelli JA, Taleb M, Mira JC, Ghizoni MF. Functional recovery improvement is related to aberrant reinnervation trimming. A comparative study using fresh or predegenerated nerve grafts. Acta Neuropathol.(Berl.) 2006;111:601–609. doi: 10.1007/s00401-005-0005-0. [DOI] [PubMed] [Google Scholar]
- Brushart TM. Preferential reinnervation of motor nerves by regenerating motor axons. J.Neurosci. 1988;8:1026–1031. doi: 10.1523/JNEUROSCI.08-03-01026.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brushart TM. Motor axons preferentially reinnervate motor pathways. J.Neurosci. 1993;13:2730–2738. doi: 10.1523/JNEUROSCI.13-06-02730.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brushart TM, Gerber J, Kessens P, Chen Y-G, Royall R. Contributions of pathway and neuron to preferential motor reinnervation. J. Neurosci. 1998;18:8674–8681. doi: 10.1523/JNEUROSCI.18-21-08674.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brushart TM, Hoffman PN, Royall RM, Murinson BB, Witzel C, Gordon T. Electrical stimulation promotes motoneuron regeneration without increasing its speed or conditioning the neuron. J. Neurosci. 2002;22:6631–6638. doi: 10.1523/JNEUROSCI.22-15-06631.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brushart TM, Jari R, Verge V, Rohde C, Gordon T. Electrical stimulation restotes the specificity of sensory axon regeneration. Exp. Neurol. 2005;194:221–229. doi: 10.1016/j.expneurol.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Brushart TM. Nerve Repair. New York: Oxford University Press; 2011. [Google Scholar]
- Brushart TM, Aspalter M, Griffin JW, Redett R, Hameed H, Zhou C, Wright M, Vyas A, Hoke A. Schwann cell phenotype is regulated by axon modality and central-peripheral location, and persists in vitro. Exp. Neurol. 2013;247:272–281. doi: 10.1016/j.expneurol.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai CM, Qiu J, Cao ZX, McAtee M, Bregman BS, Filbin MT. Neuronal cyclic AMP controls the developmental loss in ability of axons to regenerate. J. Neurosci. 2001;21:4731–4739. doi: 10.1523/JNEUROSCI.21-13-04731.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condic ML. Adult neuronal regeneration induced by transgenic integrin expression. J Neurosci. 2001;21:4782–4788. doi: 10.1523/JNEUROSCI.21-13-04782.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornbrooks CJ, Carey DJ, McDonald JA, Timpl R, Bunge RP. In vivo and in vitro observations on laminin production by Schwann cells. Proc Natl Acad Sci U S A. 1983;80:3850–3854. doi: 10.1073/pnas.80.12.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom PA, Mayer U, Panjwani A, Pountney D, Pizzey J, Tonge DA. Involvement of alpha7beta1 integrin in the conditioning-lesion effect on sensory axon regeneration. Mol. Cell Neurosci. 2003;22:383–395. doi: 10.1016/s1044-7431(02)00034-9. [DOI] [PubMed] [Google Scholar]
- English AW. Enhancing axon regeneration in peripheral nerves also increases functionally inappropriate reinnervation of targets. J.Comp.Neurol. 2005;490:427–441. doi: 10.1002/cne.20678. [DOI] [PubMed] [Google Scholar]
- Franz CK, Rutishauser U, Rafuse VF. Polysialylated neural cell adhesion molecule is necessary for selective targeting of regenerating motor neurons. J.Neurosci. 2005;25:2081–2091. doi: 10.1523/JNEUROSCI.4880-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz CK, Rutishauser U, Rafuse VF. Intrinsic neuronal properties control selective targeting of regenerating motoneurons. Brain. 2008;131:1492–1505. doi: 10.1093/brain/awn039. [DOI] [PubMed] [Google Scholar]
- Gunderson RW, Barrett JN. Neuronal chemotaxis: Chick dorsal root axons turn toward high concentrations of nerve growth factor. Science. 1979;206:1079–1080. doi: 10.1126/science.493992. [DOI] [PubMed] [Google Scholar]
- Guntinas-Lichius O, Effenberger K, Angelov DN, Klein J, Streppel M, Stennert E, Neiss WF. Delayed rat facial nerve repair leads to accelerated and enhanced muscle reinnervation with reduced collateral axonal sprouting during a definite denervation period using a cross-anastomosis paradigm. Exp.Neurol. 2000;162:98–111. doi: 10.1006/exnr.2000.7309. [DOI] [PubMed] [Google Scholar]
- Hoke A, Redett R, Hameed H, Jari R, Li JB, Griffin JW, Brushart TM. Schwann cells express motor and sensory phenotypes that regulate axon regeneration. J.Neurosci. 2006;26:9646–9655. doi: 10.1523/JNEUROSCI.1620-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura DH, Johnson PJ, Moore AM, Magill CK, Hunter DA, Ray WZ, Tung TH, Mackinnon SE. Matching of motor-sensory modality in the rodent femoral nerve model shows no enhanced effect on peripheral nerve regeneration. Exp Neurol. 2010;223:496–504. doi: 10.1016/j.expneurol.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns JM, Danielsen N, Holmquist B, Kanje M, Lundborg G. The influence of predegeneration on regeneration through peripheral nerve grafts in the rat. Exp.Neurol. 1993;122:28–36. doi: 10.1006/exnr.1993.1104. [DOI] [PubMed] [Google Scholar]
- Le TB, Aszmann O, Chen Y-G, Royall RM, Brushart TM. Effects of pathway and neuronal aging on the specificity of motor axon regeneration. Exp. Neurol. 2001;167:126–132. doi: 10.1006/exnr.2000.7538. [DOI] [PubMed] [Google Scholar]
- Lundborg G, Dahlin LB, Danielsen N, Johannesson A, Hansson HA. Clinical Applications of Biomaterials. Chichester U.K.: John Wiley & Sons, Ltd.; 1982. Regenerating nerve fibers in preformed mesothelial chambers: influence of the distal segment of a transected nerve on growth an direction; pp. 323–329. [Google Scholar]
- Madison RD, Archibald SJ, Lacin R, Krarup C. Factors contributing to preferential motor reinnervation in the primate peripheral nervous system. J.Neurosci. 1999;19:11007–11016. doi: 10.1523/JNEUROSCI.19-24-11007.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini R, Schachner M, Brushart TM. The L2/HNK-1 carbohydrate is preferentially expressed by previously motor axon-associated Schwann cells in reinnervated peripheral nerves. J. Neurosci. 1994;14:7180–7191. doi: 10.1523/JNEUROSCI.14-11-07180.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mears S, Schachner M, Brushart TM. Antibodies to myelin-associated glycoprotein accelerate preferential motor reinnervation. JPNS. 2003;8:91–99. doi: 10.1046/j.1529-8027.2003.03012.x. [DOI] [PubMed] [Google Scholar]
- Neubauer D, Graham JB, Muir D. Nerve grafts with various sensory and motor fiber compositions are equally effective for the repair of a mixed nerve defect. Exp Neurol. 2010;223:203–206. doi: 10.1016/j.expneurol.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Oudega M, Varon S, Hagg T. Regeneration of adult rat sensory axons into intraspinal nerve grafts: Promoting effects of conditioning lesion and graft predegeneration. Exp.Neurol. 1994;129:194–206. doi: 10.1006/exnr.1994.1161. [DOI] [PubMed] [Google Scholar]
- Ramon y Cajal S. Degeneration and Regeneration of the Nervous System. London: Oxford University Press; 1928. [Google Scholar]
- Redett R, Jari R, Crawford T, Chen YG, Rohde C, Brushart TM. Peripheral pathways regulate motoneuron collateral dynamics. J.Neurosci. 2005;25:9406–9412. doi: 10.1523/JNEUROSCI.3105-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson GA, Madison RD. Preferential motor reinnervation in the mouse: comparison of femoral nerve repair using a fibrin sealant or suture. Muacle Nerve. 2003;28:227–231. doi: 10.1002/mus.10422. [DOI] [PubMed] [Google Scholar]
- Robinson GA, Madison RD. Motor neurons can preferentially reinnervate cutaneous pathways. Exp. Neurol. 2004;190:407–413. doi: 10.1016/j.expneurol.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Robinson GA, Madison RD. Developmentally regulated changes in femoral nerve regeneration in the mouse and rat. Exp Neurol. 2006;197:341–346. doi: 10.1016/j.expneurol.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Schafer M, Fruttiger M, Montag D, Schachner M, Martini R. Disruption of the gene for the myelin-associated glycoprotein improves axonal regrowth along myelin in C57BL/Wld mice. Neuron. 1996;16:1107–1113. doi: 10.1016/s0896-6273(00)80137-3. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Fallon J. Fluoro-gold, a new fluorescent retrograde axonal tracer with numerous unique properties. Brain Res. 1986;377:147–154. doi: 10.1016/0006-8993(86)91199-6. [DOI] [PubMed] [Google Scholar]
- Sorenson E, Windebank A. Relative importance of basement membrane and soluble growth factors in delayed and immediate regeneration of rat sciatic nerve. J.Neuropathology. 1993;52:216–222. doi: 10.1097/00005072-199305000-00005. [DOI] [PubMed] [Google Scholar]
- Tona A, Perides G, Rahemtulla F, Dahl D. Extracellular matrix in regenerating rat sciatic nerve: A comparative study on the localization of laminin, hyaluronic acid, and chondroitin sulfate proteoglycans, including versican. J. Histochem. Cytochem. 1993;41:593–599. doi: 10.1177/41.4.8450198. [DOI] [PubMed] [Google Scholar]
- Torigoe K, Lundborg G. Selective inhibition of early axonal regeneration by myelin-associated glycoprotein. Exp.Neurol. 1998;150:254–262. doi: 10.1006/exnr.1997.6775. [DOI] [PubMed] [Google Scholar]
- Uschold T, Robinson GA, Madison RD. Motor neuron regeneration accuracy: balancing trophic influences between pathways and end-organs. Exp. Neurol. 2007;205:250–256. doi: 10.1016/j.expneurol.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Wallquist W, Zelano J, Plantman S, Kaufman SJ, Cullheim S, Hammarberg H. Dorsal root ganglion neurons up-regulate the expression of laminin-associated integrins after peripheral but not central axotomy. J. Comp. Neurol. 2004;480:162–169. doi: 10.1002/cne.20345. [DOI] [PubMed] [Google Scholar]
- Weiss P, Edds MV. Sensory-motor nerve crosses in the rat. J.Neurophysiol. 1945;30:173–193. [Google Scholar]
- Weiss P, Taylor AC. Further experimental evidence against neurotropism in nerve regeneration. J.Exp.Zool. 1944;95:233–257. [Google Scholar]
- Werner A, Willem M, Jones LL, Kreutzberg GW, Mayer U, Raivich G. Impaired axonal regeneration in α7 integrin-deficient mice. J.Neurosci. 2000;20:1822–1830. doi: 10.1523/JNEUROSCI.20-05-01822.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo J, Hernandez YJ, Muir D. Chondroitin sulfate proteoglycan with neurite-inhibiting activity is up-regulated following peripheral nerve injury. J.Neurobiol. 1998;34:41–54. [PubMed] [Google Scholar]
- Zuo J, Neubauer D, Graham J, Krekoski CA, Ferguson TA, Muir D. Regeneration of axons after nerve transection repair is enhanced by degradation of chondroitin sulfate proteoglycan. Exp.Neurol. 2002;176:221–228. doi: 10.1006/exnr.2002.7922. [DOI] [PubMed] [Google Scholar]