Abstract
This study investigated the effect of mechanical strain on solute diffusion in human TMJ discs (mean cadaver age 77.8) using the electrical conductivity method. The electrical conductivity, as well as small ion diffusivity, of male and female TMJ discs was determined under three compressive strains. In the male group, the average disc electrical conductivity (mean ± sd) at 0% strain was 5.14±0.97 mS/cm, decreased to 4.50±0.91 mS/cm (−12.3%) at 10% strain, and 3.93±0.81 mS/cm (−23.5%) at 20% compressive strain. Correspondingly, the average disc relative ion diffusivity at 0% strain was 0.44±0.08, decreased to 0.40±0.08 (−8.9%) at 10% strain, and 0.36±0.08 (−16.7%) at 20% compressive strain. In the female group, the average disc electrical conductivity at 0% strain was 5.84±0.59 mS/cm, decreased to 5.01±0.50 mS/cm (−14.2%) at 10% strain, and 4.33±0.46 mS/cm (−25.8%) at 20% compressive strain. Correspondingly, the average disc relative ion diffusivity at 0% strain was 0.49±0.05, decreased to 0.43±0.04 (−11.3%) at 10% strain, and 0.39±0.04 (−19.9%) at 20% compressive strain. The results indicated that mechanical strain significantly impeded solute diffusion through the disc. This mechanical strain effect was larger in the female than in the male human TMJ disc. This study may provide new insights into TMJ pathophysiology.
Keywords: Temporomandibular joint (TMJ) disorders, TMJ disc, Mechanical strain, Electrical conductivity, Ion diffusivity, Tissue porosity
INTRODUCTION
Temporomandibular joint (TMJ) disorders exert significant impact with estimated minimum annual health care and societal costs of $4 billion 29. Women are about 3 times more likely to be afflicted than men based on the recent OPPERA study 27. The TMJ is a load bearing joint with a unique articular structure and function 31. The TMJ disc, a dense fibrocartilaginous tissue, distributes stress and aids in lubrication of the joint 20. In approximately 30% of TMJ disorder patients, mechanical dysfunction of the TMJ disc, especially displacement due to tissue degeneration, is a common event 1, 25. Degenerative changes generally occur more than a decade earlier in the TMJ than other human joints (e.g., knee and hip) 21, 28. It is generally believed that pathological mechanical loading (e.g. sustained jaw clenching or traumatic impact) triggers a cascade of molecular events leading to TMJ disc degeneration 18. However, the link between pathologic mechanical loading at the tissue level and the resulting biological response at the cellular/molecular level remains poorly understood.
The normal adult human TMJ disc is a large avascular structure and the nutrients required by the disc cells are supplied through diffusion by blood vessels and synovial fluid at the margins of the disc 16, 24. A nutrient concentration gradient is established across the TMJ disc based on diffusion from the nutrient rich periphery to the interior of the disc and is balanced by the rate of nutrient consumption from the disc cells. The concentration gradient has been shown to impact disc cell viability, matrix synthesis and composition, along with response to inflammatory factors 30, 32. This suggests deviations from physiological levels may initiate tissue remodeling and matrix degeneration. The rate of solute diffusion in tissue is governed by solute diffusivities which are affected by the composition and structure of the matrix, as well as mechanical strains on the tissue. Recently, we examined the effect of mechanical loading on small ion diffusivity in porcine TMJ discs using the electrical conductivity method. The results indicated that solute diffusivities in the TMJ disc are much lower than the values in other cartilaginous tissues, and compressive strain significantly impeded solute transport in the porcine TMJ disc 13. Moreover, our cell metabolic studies have shown that porcine TMJ discs have higher cell densities and nutrient consumption rates than articular cartilage and the intervertebral disc (IVD) 12. Therefore, it is likely that a steeper nutrient gradient may exist in TMJ discs and is vulnerable to pathological events which impede nutrient supply, including sustained joint loading due to jaw clenching. Although pigs have been considered the best TMJ biomechanical experimental model 8, 19, the mechanical and transport properties of TMJ discs have not been fully compared due to the lack of human material property data. Especially, to our knowledge, the transport properties (e.g., solute diffusivity) and the effect of mechanical loading on these properties, in the human TMJ disc have not been determined. Such knowledge is important for translating the research findings from an animal model to human.
The electrical conductivity method has been employed to determine the effect of mechanical strains on ion diffusivities in hydrogels, IVD, and porcine TMJ discs 6, 9, 13. In these studies, a relationship between ion diffusivities and the porosity of the material were also determined. In this study, we adopted this method to study the impact of mechanical loading on the solute transport in human TMJ discs. We hypothesized that the electrical conductivity of the human TMJ disc was mechanical strain-dependent due to changes in tissue porosity caused by tissue compression. In addition, we sought to determine the electrical conductivity in both male and female subjects to investigate if there were sex differences. Therefore, the objective of this study was to measure the electrical conductivity of male and female human TMJ discs under three compressive strains in five disc regions.
MATERIALS AND METHODS
Specimen preparation
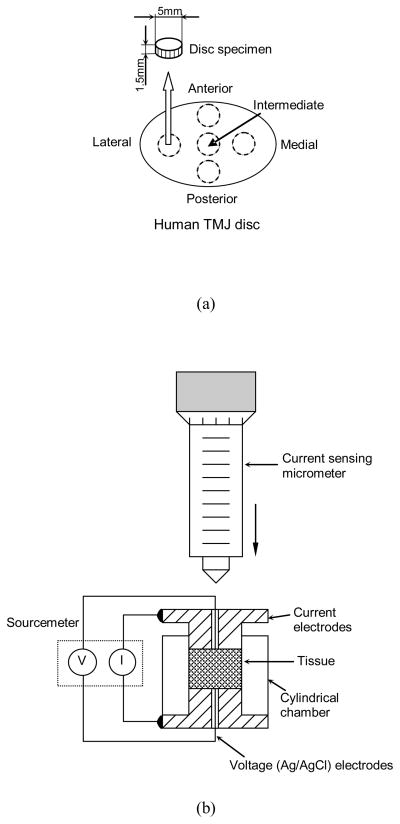
Human TMJ discs were harvested from the left TMJ of fresh cadavers in the MUSC gross anatomy laboratory with institutional approval. Discs with visible signs of degeneration or trauma were removed, leaving 12 male and 12 female discs for the study. The mean cadaver age was 76.0±7.0 for males and 79.6±10.5 for females. The extracted TMJ disc was wrapped in cellophane and placed into plastic bags. Saline moistened gauze was added to the bag to maintain humidity and prevent specimen dehydration. The disc sample was immediately used for porosity and conductivity measurement. Care was taken to prevent specimen dehydration during specimen preparation. Cylindrical plugs were punched from five disc regions (anterior, lateral, intermediate, medial, and posterior) using a 5mm corneal trephine (Figure 1a). The plugs were microtomed on a freezing stage to remove the natural concave tissue shape and to allow for a flat surface during electrical conductivity measurements. Each time, one plug was punched and microtomed in a moisture-controlled dissection hood. The rest of the disc was wrapped and placed in the same sealed plastic bag. Prior to the water content measurement, the specimen was thawed at room temperature for 20 minutes in a sealed plastic vial. The initial height of the specimen was measured by a custom-designed electrical current sensing micrometer to avoid sample compression. The prepared specimens (n=60 for male, n=60 for female) had an average height of 1.49±0.46 mm. Three conductivity measurements were made on each specimen corresponding to the three levels of compressive strain (0%, 10%, and 20%) based on the initial height. Both finite element analysis and MRI imaging data have shown that the change of human TMJ disc thickness during normal joint motion is about 15–20% 11. The sustained mechanical loading, such as bruxism, may induce 20–30% change of the disc thickness.
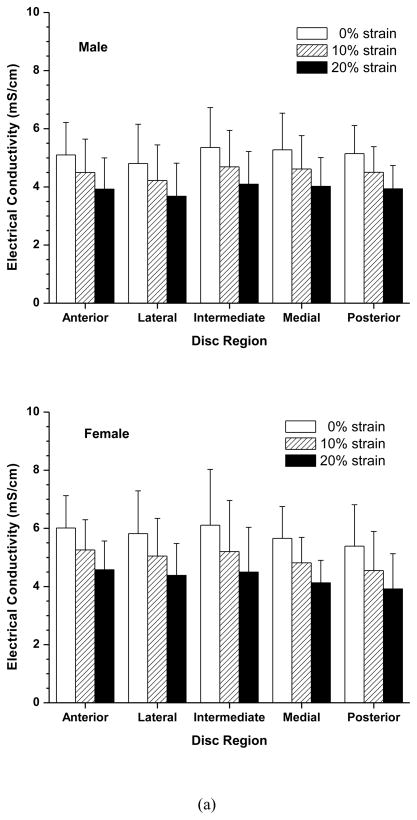
Figure 1.
(a) Schematic of specimen preparation. The region and size of test specimens are shown. (b) Schematic of apparatus for measuring electrical conductivity, consisting of two stainless steel current electrodes, two Ag/AgCl voltage-sensing electrodes, a nonconductive Plexiglass chamber, a current sensing micrometer, and a sourcemeter.
Porosity (water volume fraction) measurement
The porosity or water volume fraction of specimens at the undeformed state ( ) was determined using a buoyancy method 33. Briefly, the weight of the specimens in air (Wwet) and the weight in phosphate buffered saline (PBS) solution (WPBS) were measured using the density-determination kit of a Sartorius analytical balance (Sartorius YDK01, Germany). After measuring electrical conductivity, specimens were lyophilized and the dry weights (Wdry) were recorded. According to Archimedes’ principle and the biphasic assumption, the water volume fraction was determined by:
| (1) |
where ρPBS is the mass density of PBS (1.005 g/ml), and ρw is the mass density of water. The TMJ disc tissue was considered as a biphasic mixture, consisting of solid phase and free water phase. Both phases are assumed to be incompressible. Thus, the water volume fraction of the specimen at different compression levels (φw) can be calculated by 15:
| (2) |
where e is the tissue dilatation. Considering one-dimensional confined compression in this study, the tissue dilatation is equal to the compressive strain.
Electrical conductivity measurement
In this study, the impact of mechanical strain on electrical conductivity of human TMJ disc was examined in the superior-inferior direction only. This was achieved by using a confined chamber to ensure that the applied mechanical strain and measured electrical conductivity were aligned in the same direction. The superior-inferior direction is the main transport route in the TMJ disc due to its short diffusion distance compared to the anterior-posterior and medial-lateral directions. The electrical conductivity was measured based on the principle of a four wire resistance test using a Keithley Sourcemeter (Model 2400, Keithley Instruments, Inc., Cleveland, OH) and a custom designed conductivity chamber reported previously 4, 13. Briefly, the conductivity apparatus consists of two stainless steel current electrodes coaxial to two Teflon-coated Ag/AgCl voltage electrodes placed on the top and bottom of a cylindrical nonconductive Plexiglass chamber (5mm diameter). The specimen was placed inside the chamber for measurement and compression (Figure 1b). The resistance (R) values across the specimens were measured at a low, constant current density of 0.015 mA/cm2. The height of the specimen was measured with an electrical current sensing micrometer. The electrical conductivity ( ) values of the specimens were calculated by:
| (3) |
where h and A are the height and cross-sectional area of the specimens, respectively. The precision for the resistance measurements was 0.5 Ω while the height measured with an accuracy of ±1.0 μm. The electrical conductivity of the specimen was measured at 0%, 10%, and 20% compression levels. The confined compression of the tissue specimen was achieved by lowering the micrometer to the desired height (Figure 1b) and fluid was allowed to exude from the sample through a small clearance between the chamber and the current electrodes. A resistance measurement was taken at a 15 minute interval after the specimen was compressed to ensure the sample had reached equilibrium with no fluid exudation. The influence of the order of the strain level on the electrical conductivity was not examined in this study, since the order of the strain level is not expected to affect the equilibrium response. All conductivity tests were performed in a bathing solution of PBS at a physiological concentration of 0.15M (pH 7.4) at room temperature (22°C).
Ion diffusivity calculation
Under a zero fluid flow condition, the measured electrical conductivity ( ) of a tissue in NaCl solution is related to intrinsic cation and anion diffusivities (Di, i = +, − ) by 3, 17:
| (4) |
where Fc is the Faraday constant, R is the gas constant, T is the temperature, c+ is the cation ion concentration, and c− is the anion concentration. The TMJ disc was considered as uncharged in this study, since biochemical studies have shown that the GAG content of human and porcine TMJ discs is very low (< 4% dry weight) compared to hyaline cartilage and the IVD 2, 14. More importantly, the high ionic concentration of bathing solution (0.15 M) assured a small osmotic effect on the diffusion process. For uncharged tissues in NaCl solution, the relative diffusivity (D/D0) of NaCl is simply related to the relative conductivity by 6:
| (5) |
where D is the mean ion diffusivity of Na+ and Cl− in tissue, D0 is the mean ion diffusivity in the bathing solution, χ is the electrical conductivity of the tissue, and χ0 is the conductivity of the bathing solution. In our analysis, Na+ and Cl− were assumed to carry the electrical current because these ions are the primary ionic components of PBS.
Statistical analysis
The electrical conductivity, porosity, and relative ion diffusivity were determined for each specimen (5 regions per disc) under three compressive strains. Mixed effects analysis of variance (ANOVA) was used to investigate the main and interaction effects on each outcome of disc region, strain, sex, and age. The models accommodated correlations among measurements from the same disc both within and between the three strains, allowing that measurements on a disc from the same region may be more highly correlated than measurements from different regions. SAS/STAT® software version 9.3 (SAS Institute Inc., Cary, NC) was used for statistical analyses and statistical significance was determined at p-values < 0.05. Pilot data analyses showed that none of the main and interaction effects involving region or age emerged as statistically significant. Hence subsequent analyses were performed using the average response among the 5 regions for each disc at each strain. Two-way mixed effects ANOVA incorporating a random effect for disc was used to assess the effects of strain and sex on this average outcome.
RESULTS
Electrical conductivity
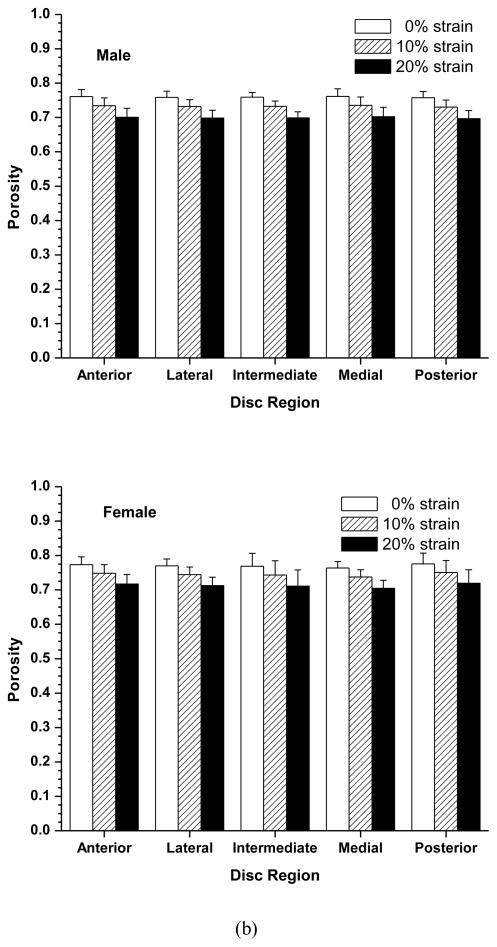
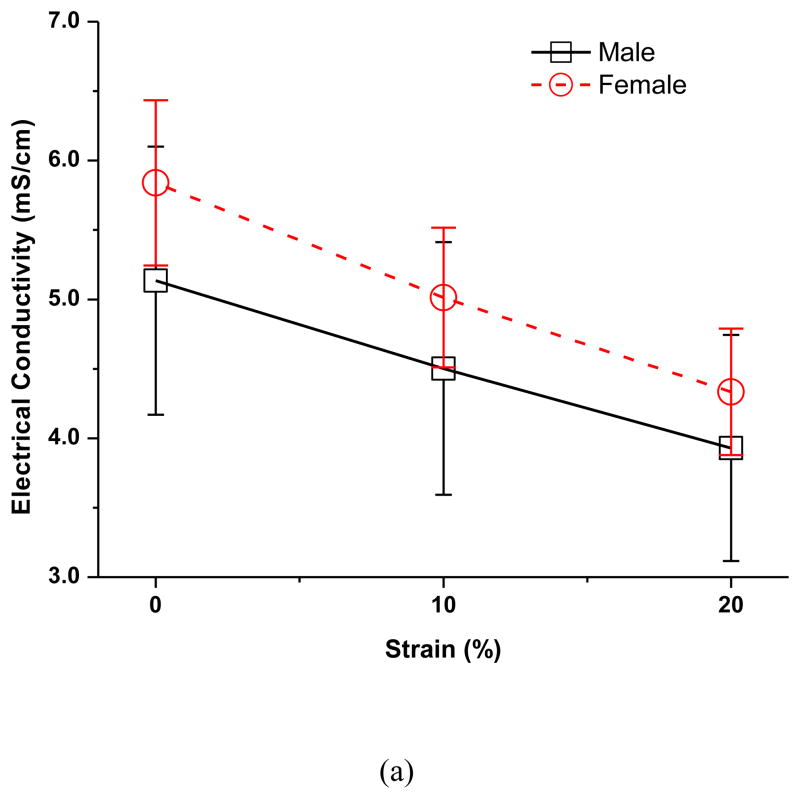
The effects of compressive strain on electrical conductivity in the five disc regions are shown in Figure 2a. No significant regional variation was detected at all strain levels in both the male and female groups. However, there was a significant decrease in electrical conductivity with increases of compressive strain in all five disc regions in both the male and female groups (p<0.0001). In the male group, the average disc electrical conductivity (mean ± sd) at 0% strain was 5.14±0.97 mS/cm, decreased to 4.50±0.91 mS/cm (−12.3%) at 10% strain, and 3.93±0.81 mS/cm (−23.5%) at 20% strain (Figure 3a). In the female group, the average disc electrical conductivity at 0% strain was 5.84±0.59 mS/cm, decreased to 5.01±0.50 mS/cm (−14.2%) at 10% strain, and 4.33±0.46 mS/cm (−25.8%) at 20% strain (Figure 3a). The electrical conductivity was higher in the female than in the male TMJ disc, although the differences were not statistically significant (p=0.08). Interestingly, the interaction between strain and sex was found to be statistically significant (p=0.0004). The impact of the mechanical strain on the electrical conductivity was higher in the female TMJ disc compared to the male.
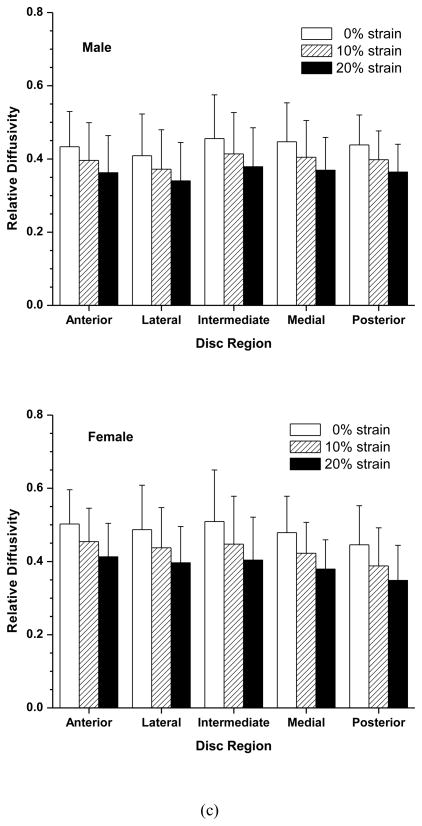
Figure 2.
Effect of compressive strains on regional distribution of electrical conductivity (a), porosity (water volume fraction) (b), and relative ion diffusivity of human TMJ disc (c). Mixed effects ANOVA as described in the Statistical Analysis section confirmed the lack of significant difference in outcomes among the disc regions and among two-factor interactions between disc region and strain or sex or age of disc donor.
Figure 3.
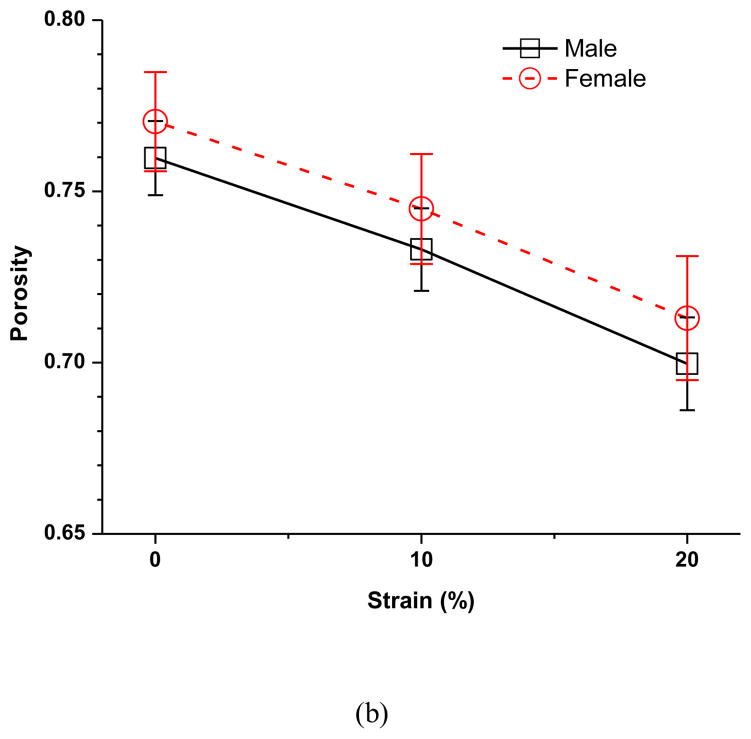
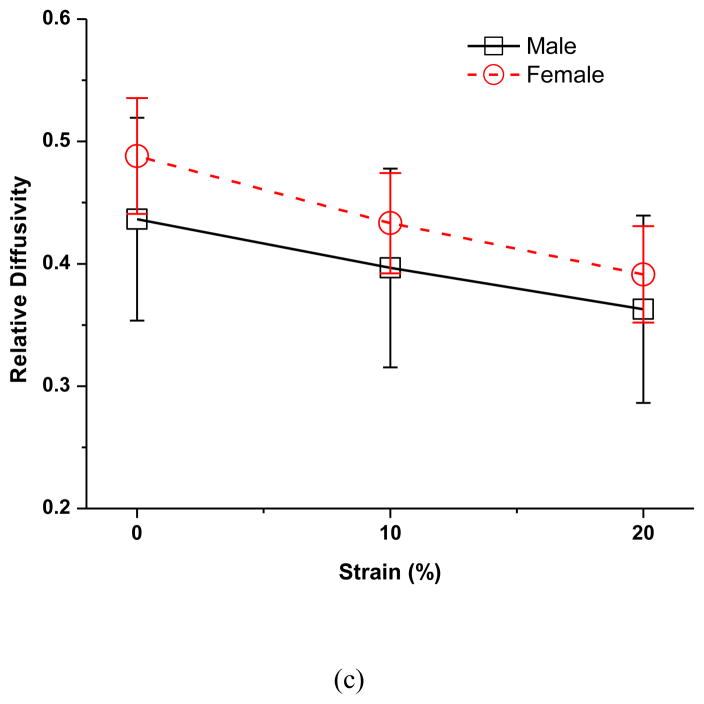
The effect of sex on the strain-dependent electrical conductivity (a), porosity (water volume fraction) (b), and relative ion diffusivity of human TMJ disc (c). Note that the average value of the five regions from the same disc (male n=12, female n=12) were used to represent the outcomes of that disc since the regional variations were found not statistically significant in this study.
Porosity (water volume fraction)
The effect of compressive strain on tissue porosity (water volume fraction) in the five disc regions is shown in Figure 2b. No significant regional variation was detected at all strain levels in both the male and female groups. However, there was a significant decrease in tissue porosity with increases of compressive strain in all five disc regions in both the male and female groups (p<0.0001). In the male group, the average disc porosity at 0% strain was 0.760±0.011, decreased to 0.733±0.012 (−3.6%) at 10% strain, and 0.700±0.014 (−7.9%) at 20% strain (Figure 3b). In the female group, the average disc porosity at 0% strain was 0.770±0.014, decreased to 0.745±0.016 (−3.2%) at 10% strain, and 0.713±0.018 (−7.4%) at 20% strain (Figure 3b). The porosity was higher in the female than in the male TMJ disc, although the differences were not statistically significant (p=0.0533). The interaction between strain and sex was found statistically significant (p=0.0218), even though the magnitude of this effect appears very small.
Ion diffusivity
Electrical conductivities of PBS at 22°C were measured using an Orion conductivity meter (Model 150Aplus, Beverly, MA). The value of 15.5 mS/cm for solution conductivity was used to normalize the measured conductivity data of tissues to calculate the relative ion diffusivity. The effect of compressive strain on relative ion diffusivity in the five disc regions is shown in Figure 2c. No significant regional variation was detected at all strain levels in both the male and female groups. However, there was a significant decrease in ion diffusivity with increases of compressive strain in all five disc regions (p<0.0001). In the male group, the average disc relative ion diffusivity at 0% strain was 0.44±0.08, decreased to 0.40±0.08 (−8.9%) at 10% strain, and 0.36±0.08 (−16.7%) at 20% strain (Figure 3c). In the female group, the average disc relative ion diffusivity at 0% strain was 0.49±0.05, decreased to 0.43±0.04 (−11.3%) at 10% strain, and 0.39±0.04 (−19.9%) at 20% strain (Figure 3c). The relative ion diffusivity was higher in the female than in the male TMJ disc, although the differences were not statistically significant (p=0.1502). The interaction between strain and sex was found statistically significant (p=0.0004). The impact of the mechanical strain on the relative ion diffusivity was higher in the female TMJ disc compared to the male.
DISCUSSION
The objective of this study was to investigate the effect of mechanical strain on small ion diffusivity in human TMJ discs using the electrical conductivity method. The measured electrical conductivity and calculated ion diffusivity from this study indicated that compressive mechanical strain significantly impeded solute diffusion through the disc. Our results further indicated that this mechanical strain effect was larger in the female than in the male human TMJ disc.
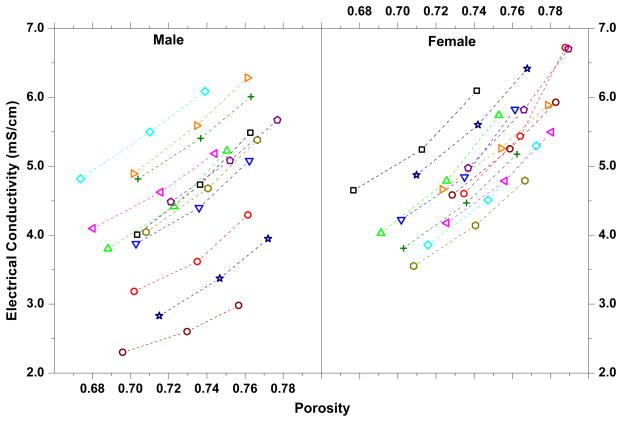
The decrease in electrical conductivity with increasing mechanical strain in the human TMJ disc was mainly due to the porosity (water volume fraction) reduction caused by increasing compression (Figure 4). Tissue compression caused fluid exudation and a corresponding decrease in tissue porosity. The reduction in porosity resulted in decreased ion diffusivity in the tissue and subsequently decreased electrical conductivity 6, 9. This is in agreement with previous studies reporting decreasing diffusivity of solutes with increasing static compressive strain in articular cartilage 22, 23, IVD tissues 10, 34, and porcine TMJ discs 13. The strain-dependent electrical conductivity and ion diffusivity indicated that mechanical strain impeded solute transport in human TMJ discs. Moreover, the impact of mechanical strain on ion diffusivity was larger in the female than in the male TMJ disc. Figure 4 showed the association between the electrical conductivity and the porosity for the male and female human TMJ discs. It seems that the electrical conductivity of the female disc is more sensitive to the change of the tissue porosity due to the mechanical strain. This suggested that a subtle tissue structure difference might exist between the male and female TMJ discs. However, to our knowledge, there is no study to quantitatively compare the biochemical composition (e.g., water, GAG, and collagen contents) and tissue structure (e.g., fiber density and orientation) in human male and female TMJ discs. This will be a very interesting thing to look at in our next study. The pathological implication of this finding needs to be further explored. Although the recent OPPERA study confirmed a long standing report of 3 to1 incidence of females to males for TMJ disorders 27, the causes of such a sex disparity are still poorly understood.
Figure 4.
Association between the electrical conductivity and the porosity (water volume fraction). Each dashed line depicts the data for one disc.
In our previous study of porcine TMJ discs, transport properties were region-dependent with the electrical conductivity and ion diffusivity in the anterior region significantly higher than in the posterior region. This regional difference is likely due to the significant differences of tissue porosity between these two regions in the porcine TMJ disc. In contrast, no significant regional variation of tissue porosity was found in the human TMJ disc. The diffusive transport in both porcine and human TMJ discs was dependent upon tissue composition. Consequently, the electrical conductivity and ion diffusivity were not region-dependent in the human TMJ disc.
The electrical conductivity and tissue porosity of cartilaginous tissues were compared and are listed in Table 1. The electrical conductivity in human TMJ discs was the lowest compared with human articular cartilage and human annulus fibrosis. The lower solute diffusion in the human TMJ disc is likely due to a lower tissue porosity compared to other human cartilaginous tissues. The normal adult TMJ disc is a large avascular structure 16, 24, so the nutrients required by disc cells are supplied through diffusion. This study showed that solute diffusivities in the human TMJ disc are lower than the values in other human cartilaginous tissues, and compressive mechanical strain can further impede solute diffusion in the human TMJ disc. Moreover, the TMJ disc has a higher cell density and nutrient consumption rates than in articular cartilage and the IVD 12. Therefore, it is likely that a steeper nutrient gradient may exist in human TMJ discs and might be vulnerable to pathological events which impede nutrient supply, including sustained joint loading due to jaw clenching. The strain distribution in the TMJ disc in vivo is complex and can be determined through finite element analysis 11. The strain-dependent diffusivity established in this study needs to be incorporated into finite element models to assess the overall impact of physiological/pathological loading on solute transport in human TMJ discs.
Table 1.
Comparison of electrical conductivity and tissue porosity in an unstrained state between the TMJ disc and other cartilaginous tissues (mean±sd).
| Electrical conductivity (mS/cm) | Water volume faction (porosity) | Reference | |
|---|---|---|---|
| Human TMJ disc (male) | 5.14±0.97 | 0.760±0.011 | Present study |
| Human TMJ disc (female) | 5.84±0.59 | 0.770±0.014 | Present study |
| Porcine TMJ disc | 2.97±0.90 | 0.726±0.038 | Reference [13] |
| Human articular cartilage | 6–10 | ~0.8 | Reference [7] |
| Porcine annulus fibrosis | 5.60±0.89 | 0.74±0.03 | Reference [5] |
| Human annulus fibrosis | 7.5±0.8 | 0.80±0.02 | Reference [9] |
The electrical conductivity and tissue porosity of the human TMJ disc in this study were much higher than the values of porcine TMJ discs (Table 1). It is unclear whether this difference is an intrinsic difference between the species, or due to sample ages. The human TMJ discs were harvested from old donors (>70 yr), while the porcine TMJ discs were collected from young pigs (6–8 month) 13. The donor age may affect the tissue composition and structure of human TMJ disc, resulting in the change of the material properties, such as electrical conductivity. Thus, it is necessary to further examine the age effect in future studies. In this study, the electrical conductivity was measured in the superior-inferior direction. However, considering the fiber structure of the TMJ disc, the diffusion in the TMJ disc is significantly anisotropic 26. Although the superior-inferior direction is the main transport route in TMJ disc, the impact of mechanical strain on solute diffusion in the anterior-posterior and medial-lateral directions still needs to be examined. It is also necessary to determine the impact of mechanical strain on the diffusivities of other essential solutes, such as oxygen and glucose. In summary, the effect of mechanical strain on small ion diffusivity in human TMJ discs was investigated using electrical conductivity methods. The results indicated that mechanical strain significantly impeded solute diffusion through the disc. This mechanical strain effect was larger in the female than in the male human TMJ disc. Although pigs have been considered as a good animal model to study human TMJ disc mechanics, the similarity in terms of tissue material properties still needs further validation.
Acknowledgments
This project was supported by NIH grants DE018741, DE021134, and AR055775, a NIH F31 training grant DE023486 to GJW, a NIH F31 training grant DE020230 to JK, and a NSF RII grant predoctoral fellowship (EPS-00903795) to CS.
Footnotes
CONFLICT OF INTEREST
None of the authors of this paper have a conflict of interest that might be construed as affecting the conduct or reporting of the work presented.
References
- 1.Ahmad M, Hollender L, Anderson Q, Kartha K, Ohrbach R, Truelove EL, John MT, Schiffman EL. Research diagnostic criteria for temporomandibular disorders (RDC/TMD): development of image analysis criteria and examiner reliability for image analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:844–860. doi: 10.1016/j.tripleo.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almarza AJ, Bean AC, Baggett LS, Athanasiou KA. Biochemical analysis of the porcine temporomandibular joint disc. Br J Oral Maxillofac Surg. 2006;44:124–128. doi: 10.1016/j.bjoms.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Frank EH, Grodzinsky AJ, Phillips SL, Grimshaw PE. Physiochemical and bioelectrical determinants of cartilage material properties. In: Mow VC, Wood DO, Woo SL, editors. Biomechanics of Diarthrodial Joints. Spring-Verlag; New York: 1990. pp. 261–282. [Google Scholar]
- 4.Gu WY, Justiz MA. Apparatus for measuring the swelling dependent electrical conductivity of charged hydrated soft tissues. J Biomech Eng. 2002;124:790–793. doi: 10.1115/1.1516571. [DOI] [PubMed] [Google Scholar]
- 5.Gu WY, Justiz MA, Yao H. Electrical conductivity of lumbar annulus fibrosis: Effects of porosity and fixed charge density. Spine. 2002;27:2390–2395. doi: 10.1097/00007632-200211010-00014. [DOI] [PubMed] [Google Scholar]
- 6.Gu WY, Yao H, Vega AL, Flagler D. Diffusivity of ions in agarose gels and intervertebral disc: Effect of porosity. Ann Biomed Eng. 2004;32:1710–1717. doi: 10.1007/s10439-004-7823-4. [DOI] [PubMed] [Google Scholar]
- 7.Hasegawa I, Kuriki S, Matsuno S, Matsumoto G. Dependence of electrical conductivity on fixed charge density in articular cartilage. Clin Orthop Relat Res. 1983:283–288. [PubMed] [Google Scholar]
- 8.Herring SW, Decker JD, Liu ZJ, Ma T. Temporomandibular joint in miniature pigs: anatomy, cell replication, and relation to loading. Anat Rec. 2002;266:152–166. doi: 10.1002/ar.10049. [DOI] [PubMed] [Google Scholar]
- 9.Jackson AR, Travascio F, Gu WY. Effect of mechanical loading on electrical conductivity in human intervertebral disk. J Biomech Eng. 2009;131:054505. doi: 10.1115/1.3116152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson AR, Yuan TY, Huang CY, Travascio F, Yong GW. Effect of compression and anisotropy on the diffusion of glucose in annulus fibrosus. Spine. 2008;33:1–7. doi: 10.1097/BRS.0b013e31815e4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koolstra JH, van Eijden TM. Combined finite-element and rigid-body analysis of human jaw joint dynamics. J Biomech. 2005;38:2431–2439. doi: 10.1016/j.jbiomech.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 12.Kuo J, Shi C, Cisewski S, Zhang L, Kern MJ, Yao H. Regional Cell Density Distribution and Oxygen Consumption Rates in Porcine TMJ Discs: An Explant Study. Osteoarthritis Cartilage. 2011;19:911–918. doi: 10.1016/j.joca.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuo J, Wright GJ, Bach DE, Slate EH, Yao H. Effect of mechanical loading on electrical conductivity in porcine TMJ discs. J Dent Res. 2011;90:1216–1220. doi: 10.1177/0022034511415275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuo J, Zhang L, Bacro T, Yao H. The region-dependent biphasic viscoelastic properties of human temporomandibular joint discs under confined compression. J Biomech. 2010;43:1316–1321. doi: 10.1016/j.jbiomech.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai WM, Hou JS, Mow VC. A triphasic theory for the swelling and deformation behaviors of articular cartilage. J Biomech Eng. 1991;113:245–258. doi: 10.1115/1.2894880. [DOI] [PubMed] [Google Scholar]
- 16.Leonardi R, Lo ML, Bernasconi G, Caltabiano C, Piacentini C, Caltabiano M. Expression of vascular endothelial growth factor in human dysfunctional temporomandibular joint discs. Arch Oral Biol. 2003;48:185–192. doi: 10.1016/s0003-9969(02)00207-8. [DOI] [PubMed] [Google Scholar]
- 17.Maroudas A. Physicochemical properties of cartilage in the light of ion exchange theory. Biophys J. 1968;8:575–595. doi: 10.1016/S0006-3495(68)86509-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milam SB. Pathogenesis of degenerative temporomandibular joint arthritides. Odontology. 2005;93:7–15. doi: 10.1007/s10266-005-0056-7. [DOI] [PubMed] [Google Scholar]
- 19.Mills DK, Daniel JC, Herzog S, Scapino RP. An animal model for studying mechanisms in human temporomandibular joint disc derangement. J Oral Maxillo fac Surg. 1994;52:1279–1292. doi: 10.1016/0278-2391(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 20.Nickel JC, McLachlan KR. In vitro measurement of the frictional properties of the temporomandibular joint disc. Arch Oral Biol. 1994;39:323–331. doi: 10.1016/0003-9969(94)90124-4. [DOI] [PubMed] [Google Scholar]
- 21.Pullinger AG, Seligman DA, Solberg WK. Temporomandibular disorders. Part I: Functional status, dentomorphologic features, and sex differences in a nonpatient population. J Prosthet Dent. 1988;59:228–235. doi: 10.1016/0022-3913(88)90019-4. [DOI] [PubMed] [Google Scholar]
- 22.Quinn TM, Kocian P, Meister JJ. Static compression is associated with decreased diffusivity of dextrans in cartilage explants. Arch Biochem Biophys. 2000;384:327–334. doi: 10.1006/abbi.2000.2077. [DOI] [PubMed] [Google Scholar]
- 23.Quinn TM, Morel V, Meister JJ. Static compression of articular cartilage can reduce solute diffusivity and partitioning: implications for the chondrocyte biological response. J Biomech. 2001;34:1463–1469. doi: 10.1016/s0021-9290(01)00112-9. [DOI] [PubMed] [Google Scholar]
- 24.Rees LA. The structure and function of the mandibular joint. Br Dent J. 1954;96:125–133. [Google Scholar]
- 25.Schiffman EL, Truelove EL, Ohrbach R, Anderson GC, John MT, List T, Look JO. The Research Diagnostic Criteria for Temporomandibular Disorders. I: overview and methodology for assessment of validity. J Orofac Pain. 2010;24:7–24. [PMC free article] [PubMed] [Google Scholar]
- 26.Shi C, Kuo J, Bell PD, Yao H. Anisotropic solute diffusion tensor in porcine TMJ discs measured by FRAP with spatial Fourier analysis. Ann Biomed Eng. 2010;38:3398–3408. doi: 10.1007/s10439-010-0099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slade GD, Bair E, By K, Mulkey F, Baraian C, Rothwell R, Reynolds M, Miller V, Gonzalez Y, Gordon S, Ribeiro-Dasilva M, Lim PF, Greenspan JD, Dubner R, Fillingim RB, Diatchenko L, Maixner W, Dampier D, Knott C, Ohrbach R. Study methods, recruitment, sociodemographic findings, and demographic representativeness in the OPPERA study. J Orofac Pain. 2011;12:T12–26. doi: 10.1016/j.jpain.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solberg WK, Hansson TL, Nordstrom B. The temporomandibular joint in young adults at autopsy: a morphologic classification and evaluation. J Oral Rehabil. 1985;12:303–321. doi: 10.1111/j.1365-2842.1985.tb01285.x. [DOI] [PubMed] [Google Scholar]
- 29.Stowell AW, Gatchel RJ, Wildenstein L. Cost-effectiveness of treatments for temporomandibular disorders: biopsychosocial intervention versus treatment as usual. J Am Dent Assoc. 2007;138:202–208. doi: 10.14219/jada.archive.2007.0137. [DOI] [PubMed] [Google Scholar]
- 30.Tojyo I, Yamaguchi A, Nitta T, Yoshida H, Fujita S, Yoshida T. Effect of hypoxia and interleukin-1beta on expression of tenascin-C in temporomandibular joint. Oral Dis. 2008;14:45–50. doi: 10.1111/j.1601-0825.2006.01344.x. [DOI] [PubMed] [Google Scholar]
- 31.Werner JA, Tillmann B, Schleicher A. Functional anatomy of the temporomandibular joint. A morphologic study on human autopsy material. Anat Embryol (Berl) 1991;183:89–95. doi: 10.1007/BF00185839. [DOI] [PubMed] [Google Scholar]
- 32.Yamaguchi A, Tojyo I, Yoshida H, Fujita S. Role of hypoxia and interleukin-1beta in gene expressions of matrix metalloproteinases in temporomandibular joint disc cells. Arch Oral Biol. 2005;50:81–87. doi: 10.1016/j.archoralbio.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Yao H, Justiz MA, Flagler D, Gu WY. Effects of Swelling Pressure and Hydraulic Permeability on Dynamic Compressive Behavior of Lumbar Annulus Fibrosus. Ann Biomed Eng. 2002;30:1234–1241. doi: 10.1114/1.1523920. [DOI] [PubMed] [Google Scholar]
- 34.Yuan TY, Jackson AR, Huang CY, Gu WY. Strain-dependent oxygen diffusivity in bovine annulus fibrosus. J Biomech Eng. 2009;131:074503. doi: 10.1115/1.3127254. [DOI] [PMC free article] [PubMed] [Google Scholar]