Abstract
Background
The prevalence of atrial fibrillation substantially increases after 70 years of age. However, the effect of rate-control versus rhythm-control strategies on outcomes in these patients remains unclear.
Methods
In the randomized Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) trial, 4060 patients (mean age, 70, range, 49–80 years) with paroxysmal and persistent atrial fibrillation were randomized to rate-control versus rhythm-control strategies. Of these, 2248 were 70–80 years, of whom 1118 were in the rate-control group. Propensity scores for rate-control strategy were estimated for each of the 2248 patients and were used to assemble a cohort of 937 pairs of patients receiving rate-control versus rhythm-control strategies, balanced on 45 baseline characteristics.
Results
Matched patients had a mean age of 75 years, 45% were women, 7% were non-white, and 47% had prior hospitalizations due to arrhythmias. During 3.4 years of mean follow-up, all-cause mortality occurred in 18% and 23% of matched patients in the rate-control and rhythm-control groups, respectively (hazard ratio {HR} associated with rate-control, 0.77; 95% confidence interval {CI}, 0.63–0.94; p=0.010). HRs (95% CIs) for cardiovascular and non-cardiovascular mortality associated with rate-control were 0.88 (0.65–1.18) and 0.62 (0.46–0.84), respectively. All-cause hospitalization occurred in 61% and 68% of rate-control and rhythm-control patients, respectively (HR, 0.76; 95% CI, 0.68–0.86). HRs (95% CIs) for cardiovascular and non-cardiovascular hospitalization were 0.66 (0.56–0.77) and 1.07 (0.91–1.27).
Conclusion
In septuagenarian patients with atrial fibrillation, compared with rhythm-control, a rate-control strategy was associated with significantly lower mortality and hospitalization.
Keywords: atrial fibrillation, rate control, rhythm control, hospitalization, mortality, propensity score, older adults
In the randomized Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) trial, although there was no significant reduction in all-cause mortality among patients in the rate-control group, compared to those in the rhythm-control group (P=0.08), a subgroup analysis suggested that among those 65 to 80 years of age there was a significant reduction in mortality in the rate-control strategy group.1 However, baseline characteristics of this older subgroup were not presented and it remained unknown whether the beneficial effect of a rate-control strategy among older AFFRIM patients may have been confounded by between-group imbalances in potential baseline confounders. The prevalence and incidence of atrial fibrillation increase after the eighth decade of life,2,3 and yet, the optimal management strategy for atrial fibrillation in these patients has not been fully defined.4 Therefore, in the current analysis, we compared the effect of rate versus rhythm-control strategies on outcomes in a propensity-matched cohort of AFFIRM participants 70 to 80 years of age.5
MATERIALS AND METHODS
Study Design and Participants
The current analysis is based on a public-use copy of the AFFIRM data obtained from the National, Heart, Lung and Blood Institute. The design and results of the AFFIRM trial have been previously reported.1,6-9 Briefly, 4060 patients 65–80 years of age with paroxysmal and persistent atrial fibrillation were randomized to receive rate-control (n=2027) versus rhythm-control (n=2033) strategies. To be eligible, patients <65 years of age had to have one of the following risk factors for stroke or death: hypertension, diabetes, heart failure, previous stroke, previous transient ischemic attack, systemic embolism, left atrial enlargement by echocardiography, or reduced left ventricular ejection fraction. Patients were followed-up for 6 years (mean, 3.4 years, through October 31, 2001). The current study is restricted to 2248 AFFIRM patients 70–80 years of age, of whom 1118 were in the rate-control group.
Rate-Control versus Rhythm-Control Strategies
Patients in the rate-control group received beta-blockers, digoxin, verapamil, diltiazem, or a combination of these drugs. In the rate-control group, the therapeutic goal was to control heart rate to 80 beats per minute or less at rest and to 110 beats per minute or less during the six-minute walk test. Patients in the rhythm-control group received cardioversion and/or medication as necessary to maintain normal sinus rhythm. Medications used in the rhythm-control group included amiodarone, disopyramide, flecainide, moricizine, procainamide, propafenone, quinidine, sotalol, or a combination of these drugs, following specific guidelines for the use of anti-arrhythmic drugs.
Outcomes
The primary endpoint in the AFFIRM trial was all-cause mortality, which was also the primary outcome for the current analysis. Secondary outcomes included cause-specific mortality, all-cause and cause-specific hospitalization, stroke and major bleeding. Cardiovascular mortality referred to the death due to cardiac or vascular causes. Cardiac death included mortality resulting from cardiac ischemia, arrhythmia, and non-arrhythmic causes including heart failure. Vascular death included non-cerebral hemorrhage, vascular catastrophe, pulmonary embolism, and cerebrovascular events. Non-cardiovascular death referred to mortality occurring due to cancer, sepsis, trauma, pulmonary disease, non-cardiac surgery, suicide, or other specific non-cardiovascular cause.
Assembly of a Balanced Study Cohort
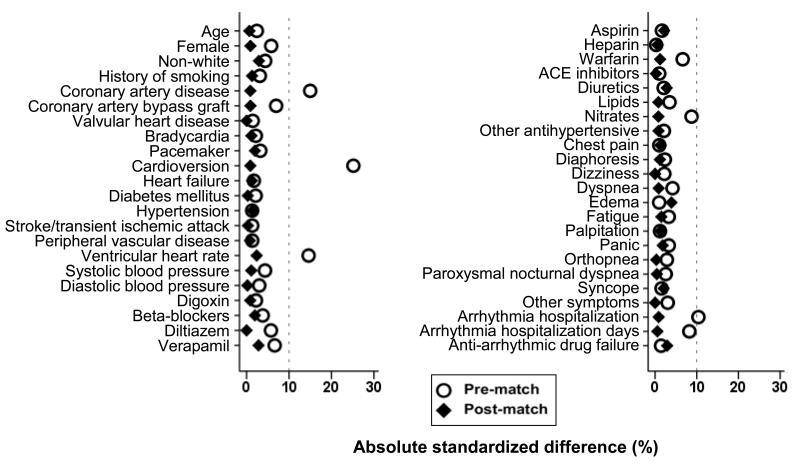
Considering that the between-group balance achieved by randomization may be lost, at least in part, in the subgroup of patients 70–80 years of age, we used propensity score approach to assemble a cohort in which the rate-control and the rhythm-control groups would be balanced on key measured baseline confounders.10,11 We began by estimating propensity scores for the receipt of rate-control strategy for each of the 2248 participants using a non-parsimonious multivariable logistic regression model.12,13 In the model, rate-control strategy was used as the dependent variable, and 45 clinically relevant baseline characteristics displayed in Figure 1 were included as covariates. Absolute standardized differences were estimated to evaluate the pre-match imbalance and post-match balance and presented as a Love plot.14,15 An absolute standardized difference of 0% indicates no residual bias and differences <10% are considered inconsequential.
Figure 1.
Absolute standardized differences comparing baseline characteristics of the subset of AFFRIM patients 70 to 80 years of age with atrial fibrillation randomized to rate-control versus rhythm-control strategies, before and after propensity score matching
Using a greedy matching protocol, we matched patients in the rate-control group with those in the rhythm-control group who had similar propensity scores to 5, 4, 3, 2 and 1 decimal places in five repeated steps.16 We began by multiplying the raw propensity scores by 100,000. For example, propensity scores of 0.56519791 and 0.56519653 for a pair of patients were converted to 56519.79 and 56519.65. Because propensity score for each patient is a unique number, we rounded them to nearest values divisible by 0.25 (e.g. 56519.75) and matched. We then removed all patients matched by 5 decimal points from the file and repeated the process to match the remaining patients by 4 decimal points by multiplying the raw propensity scores by 10,000. This process was then repeated three more times, each time, multiplying by 1000, 100, and 10 to match by 3, 2 and 1 decimal points. In all, we were able to match 937 patients in the rate-control group with 937 patients in the rhythm-control group who had similar propensity scores.
Statistical Analysis
For descriptive analyses, Pearson’s Chi-square test, Wilcoxon rank-sum test, McNemar’s test and paired sample t-test were used as appropriate for pre- and post-match between-group comparisons. To estimate the association between rate-control strategy and outcomes, we used Kaplan-Meier and Cox proportional hazard analyses. Proportional hazards assumptions were checked using log-minus-log scale survival plots. We conducted a formal sensitivity analysis to quantify the degree of a hidden bias that would need to be present to invalidate our main conclusions.17 Subgroup analyses were conducted to determine the homogeneity of the effect of rate-control versus rhythm-control strategy on outcomes. Finally, the association of rate-control strategy with all-cause mortality was also examined in the full pre-match cohort of 2248 participants using three different approaches: (1) unadjusted, (2) multivariable-adjusted (entering all covariates displayed in Figure 1) and (3) propensity score-adjusted. All statistical tests were two-tailed and a p-value <0.05 was considered significant. All data analyses were performed using SPSS 18 for Windows (SPSS, Inc., Chicago, Illinois).
RESULTS
Baseline Characteristics
Matched patients had a mean age of 75 years, 45% were women, 7% were non-white, and 47% had prior hospitalizations due to arrhythmias. Baseline characteristics of patients in the rate-control and rhythm-control strategies are displayed in Table 1. After matching, standardized differences for most measured covariates were <5% and the difference was <10% for all the covariates, suggesting substantial balance across the groups (Figure 1).
Table 1.
Baseline characteristics of the subset of AFFRIM patients 70 to 80 years with atrial fibrillation randomized to rate-control versus rhythm-control strategies, before and after propensity score matching
| Before propensity-matching (n=2248) | After propensity-matching(n=1874) | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Variables mean ± SD or n (%) |
Rate- control strategy (n=1118) |
Rhythm- control strategy (n=1130) |
P value |
Rate- control strategy (n=937) |
Rhythm- control strategy (n=937) |
P value |
| Age (years) | 75 ±3 | 75 ±3 | 0.568 | 75 ±3 | 75 ±3 | 0.891 |
| Female | 524 (47) | 497 (44) | 0.169 | 421 (45) | 425 (45) | 0.892 |
| Non-whites | 89 (8) | 77 (7) | 0.299 | 71 (8) | 64 (7) | 0.589 |
| History of smoking | 80 (7) | 72 (6) | 0.459 | 64 (7) | 61 (7) | 0.852 |
| Past medical history | ||||||
| Hypertension | 764 (68) | 779 (69) | 0.758 | 645 (69) | 639 (68) | 0.804 |
| Coronary artery disease | 404 (36) | 491 (44) | <0.001 | 378 (40) | 374 (40) | 0.879 |
| Heart failure | 259 (23) | 270 (24) | 0.684 | 219 (23) | 214 (23) | 0.825 |
| Diabetes mellitus | 197 (18) | 190 (17) | 0.613 | 162 (17) | 163 (17) | 1.000 |
| Cerebrovascular events | 160 (14) | 167 (15) | 0.753 | 134 (14) | 135 (14) | 1.000 |
| Valvular heart disease | 170 (15) | 166 (15) | 0.732 | 140 (15) | 140 (15) | 1.000 |
| Symptomatic bradycardia | 96 (9) | 104 (9) | 0.608 | 82 (9) | 85 (9) | 0.869 |
| Peripheral vascular disease | 87 (8) | 92 (8) | 0.753 | 77 (8) | 75 (8) | 0.933 |
| Cardioversion | 392 (35) | 535 (47) | <0.001 | 385 (41) | 381 (41) | 0.848 |
| Coronary artery bypass graft | 145 (13) | 174 (15) | 0.099 | 138 (15) | 141 (15) | 0.897 |
| Pacemaker implantation | 87 (8) | 98 (9) | 0.442 | 79 (8) | 74 (8) | 0.736 |
| Hospitalization for arrhythmia | 502 (45) | 566 (50) | 0.014 | 440 (47) | 444 (47) | 0.885 |
| Duration of hospitalization for arrhythmia |
2.4 ±3.7 | 2.7 ±3.7 | 0.052 | 2.5 ±3.8 | 2.5 ±3.6 | 0.901 |
| Symptoms during atrial fibrillation in the last 6 months | ||||||
| Fatigue | 622 (56) | 647 (57) | 0.438 | 542 (58) | 535 (57) | 0.778 |
| Dyspnea | 610 (55) | 593 (53) | 0.322 | 502 (54) | 506 (54) | 0.893 |
| Palpitation | 524 (47) | 523 (46) | 0.781 | 433 (46) | 427 (46) | 0.816 |
| Dizziness | 381 (34) | 397 (35) | 0.599 | 325 (35) | 325 (35) | 1.000 |
| Chest pain | 255 (23) | 253 (22) | 0.812 | 204 (22) | 209 (22) | 0.823 |
| Diaphoresis | 188 (17) | 200 (18) | 0.579 | 162 (17) | 158 (17) | 0.851 |
| Leg swelling | 234 (21) | 241 (21) | 0.818 | 206 (22) | 191 (20) | 0.427 |
| Orthopnea | 157 (14) | 170 (15) | 0.501 | 139 (15) | 140 (15) | 1.000 |
| Paroxysmal nocturnal dyspnea | 72 (6) | 80 (7) | 0.546 | 63 (7) | 62 (7) | 1.000 |
| Panic | 102 (9) | 114 (10) | 0.438 | 90 (10) | 85 (9) | 0.751 |
| Syncope | 46 (4) | 50 (4) | 0.716 | 40 (4) | 44 (5) | 0.728 |
| Other symptoms | 117 (11) | 108 (10) | 0.473 | 95 (10) | 95 (10) | 1.000 |
| Medications used within 6 months prior to randomization | ||||||
| Warfarin | 932 (83) | 969 (86) | 0.117 | 796 (85) | 800 (85) | 0.842 |
| Digoxin | 604 (54) | 598 (53) | 0.600 | 495 (53) | 499 (53) | 0.891 |
| Beta-blocker | 452 (40) | 478 (42) | 0.368 | 380 (41) | 389 (42) | 0.709 |
| Diuretics | 502 (45) | 519 (46) | 0.625 | 430 (46) | 417 (45) | 0.584 |
| Angiotensin-converting enzyme inhibitor |
415 (37) | 414 (37) | 0.813 | 346 (37) | 345 (37) | 1.000 |
| Diltiazem | 354 (32) | 328 (29) | 0.174 | 279 (30) | 279 (30) | 1.000 |
| Verapamil | 122 (11) | 101 (9) | 0.117 | 100 (11) | 92 (10) | 0.582 |
| Aspirin | 299 (27) | 294 (26) | 0.696 | 255 (27) | 246 (26) | 0.675 |
| Lipid lowering agents | 216 (19) | 234 (21) | 0.411 | 190 (20) | 193 (21) | 0.910 |
| Nitrate | 208 (19) | 250 (22) | 0.038 | 189 (20) | 192 (21) | 0.907 |
| Heparin | 195 (17) | 196 (17) | 0.952 | 164 (18) | 162 (17) | 0.950 |
| Anti-arrhythmic drug failure | 181 (16) | 177 (16) | 0.733 | 147 (16) | 157 (17) | 0.587 |
| Ventricular heart rate (beats per minute) | 74 ±14 | 72 ±14 | 0.001 | 73 ±14 | 73 ±14 | 0.585 |
| Systolic BP (mmHg) | 136 ±19 | 135 ±20 | 0.304 | 136 ±19 | 136 ±19 | 0.817 |
| Diastolic BP (mmHg) | 75 ±10 | 75 ±10 | 0.479 | 75 ±10 | 75 ±10 | 0.968 |
| International normalization ratio* | 2.3 ±0.7 | 2.3 ±0.7 | 0.484 | 2.3 ±0.7 | 2.3 ±0.7 | 0.461 |
based on available data from 1877 and 1578 pre- and post-match patients, respectively
Rate-Control Strategy and Mortality
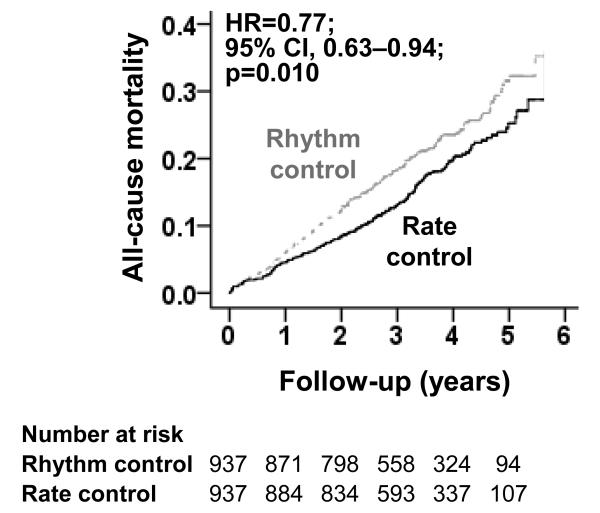
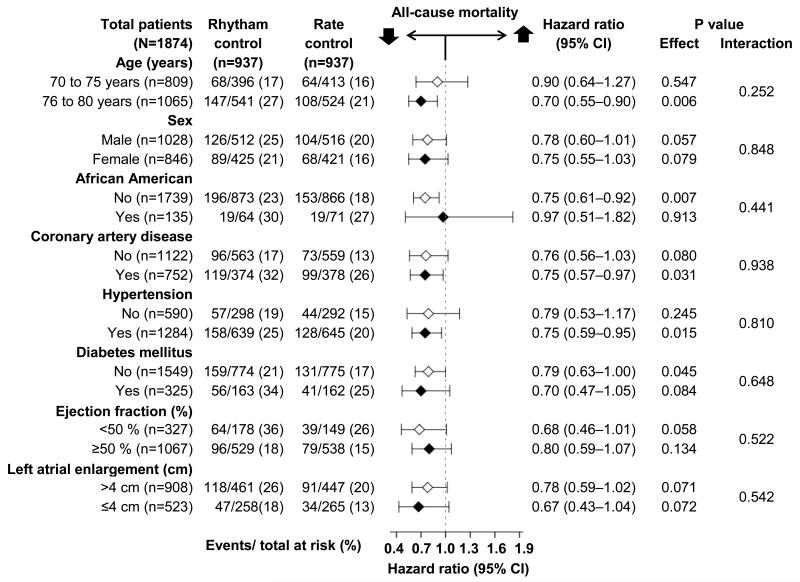
All-cause mortality occurred in 18% and 23% of matched patients randomized to receive rate-control versus rhythm-control strategies, respectively (hazard ratio {HR}when the rate-control strategy was compared with the rhythm-control strategy, 0.77; 95% confidence interval{CI}, 0.63–0.94; P=0.010; Table 2 and Figure 2). This association was homogeneous across various subgroups of patients (Figure 3). There was no association with cardiovascular mortality but there was a significant reduction in non-cardiovascular mortality (HR, 0.62; 95% CI, 0.46–0.84; P=0.002; Table 3). Unadjusted, multivariable-adjusted and propensity score-adjusted associations with all-cause mortality in the 2248 patients in pre-match cohort are presented in Table 2. Electrocardiographic data at 12 month were available from 1591 of the 1874 matched patients. Of these, 796 (50%) were in the rhythm-control group and 75.9% (604/796) were in sinus rhythm.
Table 2.
All-cause mortality in the subset of AFFRIM patients 70 to 80 years with atrial fibrillation
| Events (%) |
Absolute risk difference* |
Hazard ratio (95% CI) |
P value |
||
|---|---|---|---|---|---|
| Rate-control strategy |
Rhythm-control strategy |
||||
| Pre-match (N=2248) | n=1118 | n=1130 | |||
| Randomized, subgroup of patients age 70-80 years |
210 (19%) | 252 (22%) | −3% | 0.82 (0.69–0.99) | 0.039 |
| Additional multivariable- adjustmenta |
--- | --- | --- | 0.80 (0.66–0.96) | 0.018 |
| Additional adjustment for propensity scores |
--- | --- | --- | 0.83 (0.69–1.00) | 0.048 |
| Post-match (N=1874) | n=937 | n=937 | |||
| Propensity-matched | 172 (18%) | 215 (23%) | −5% | 0.77 (0.63–0.94) | 0.010 |
Absolute risk difference was calculated by subtracting the percentage of events in the rhythm control group from that in the rate control group.
Adjusted for all 45 baseline characteristics displayed in Figure 1
Figure 2.
Kaplan-Meier plot for all-cause mortality among a propensity-matched subset of AFFRIM patients 70 to 80 years with atrial fibrillation, by rate-control versus rhythm-control strategies (HR=hazard ratio; CI=confidence interval)
Figure 3.
Effect of a rate-control (vs. rhythm-control) strategy on all-cause mortality in subgroups of propensity-matched subset of AFFRIM patients 70 to 80 years with atrial fibrillation (CI=confidence interval)
Table 3.
Other outcomes among the subset of AFFRIM patients 70 to 80 years with atrial fibrillation
| Events (%) |
Absolute risk difference* |
Hazard ratio (95% CI) |
P value |
||
|---|---|---|---|---|---|
| Outcomes | Rate-control strategy (n=937) |
Rhythm-control strategy (n=937) |
|||
| Cardiovascular mortality | 84 (9%) | 92 (10%) | 1% | 0.88 (0.65–1.18) | 0.39 |
| Due to cardiac causes | 65 (7%) | 74 (8%) | 1% | 0.85 (0.61–1.18) | 0.33 |
| Arrhythmic | 35 (4%) | 45 (5%) | 1% | 0.75 (0.48–1.16) | 0.20 |
| Non-arrhythmic | 30 (3%) | 29 (3%) | 0% | 1.00 (0.60–1.66) | 1.00 |
| Due to vascular causes | 19 (2%) | 18 (2%) | 0% | 1.01 (0.53–1.93) | 0.97 |
| Non-cardiovascular mortality | 70 (8%) | 108 (12%) | 4% | 0.62 (0.46–0.84) | 0.002 |
| All-cause hospitalization | 571 (61%) | 641 (68%) | 7% | 0.76 (0.68–0.86) | <0.001 |
| Cardiovascular | 288 (31%) | 387 (41%) | 10% | 0.66 (0.56–0.77) | <0.001 |
| Non-cardiovascular | 283 (30%) | 254 (27%) | 3% | 1.07 (0.91–1.27) | 0.42 |
| Stroke | 41 (4%) | 44 (5%) | 1% | 0.90 (0.59–1.37) | 0.61 |
| Major bleeding** | 78 (8%) | 72 (8%) | 0% | 1.05 (0.77–1.45) | 0.75 |
Absolute risk difference was calculated by subtracting the percentage of events in the rate control group from that in the rhythm control group.
Major bleeding was defined as bleeding requiring transfusion and/or surgery.
Rate-Control Strategy and Hospitalization
All-cause hospitalization occurred in 61% and 68% of matched patients randomized to receive rate-control versus rhythm-control strategies, respectively (HR associated with the rate-control strategy, 0.76; 95% CI, 0.68–0.86; P<0.001; Table 3). There was a significant reduction in cardiovascular hospitalization (HR, 0.66; 95% CI, 0.56–0.77; P<0.001), but had no association with non-cardiovascular hospitalization (Table 3). There was no difference in incident stroke and major bleeding events between the two groups (Table 3).
DISCUSSION
Findings from the current study demonstrate that in septuagenarian patients with paroxysmal and persistent atrial fibrillation, compared to a rhythm-control strategy, the use of a rate-control strategy was associated with a significant reduction in all-cause mortality, which was mostly via a reduction in the non-cardiovascular deaths. A rate-control strategy was also associated with a significant reduction in the risk of all-cause hospitalization, which was mostly mediated by a reduction in cardiovascular hospitalization. There was no difference in incident stroke or major bleeding between the two treatment strategies. These findings suggest that the harmful effects of a rhythm-control strategy for atrial fibrillation management may be more pronounced among septuagenarians, a large and growing population at high risk for incident atrial fibrillation and its complications.
Our findings are consistent with the subgroup analysis that was presented by AFFIRM investigators in patients 65–80 years of age. Because none of the drugs used in the rate-control strategy has been shown to reduce mortality in patients with atrial fibrillation, the mortality difference observed in our study is likely an effect of the drugs used for rhythm control. Deaths due to anti-arrhythmic drugs are often attributed to their pro-arrhythmic properties and thus cardiovascular in nature. Thus, the higher risk of non-cardiovascular mortality in patients in the rhythm-control strategy group is rather intriguing. Although, amiodarone was the most commonly used rhythm-control drug, its use in AFFIRM patients with preexisting pulmonary disease was not associated with higher death due to pulmonary causes.18 It has been suggested that a careful selection of antiarrhythmic drugs, adjustment of their dosages based on hepatic and renal function, and close electrocardiographic monitoring may have resulted in lower arrhythmia-related adverse events.19 This observation is consistent with findings from other randomized trials in which a rhythm-control strategy was associated with significantly increased cardiovascular hospitalizations without increased mortality.20-22 Misclassification of deaths due to cardiovascular causes as non-cardiovascular is another potential explanation. In AFFIRM the prevalence of sinus rhythm in the rhythm-control group declined from 82% at 1 year to 63% at 5 years.1 The higher cardiovascular hospitalization in the rhythm-control group may in part be due to relapse from sinus rhythm to atrial fibrillation.23 However, the higher all-cause and cardiovascular hospitalization in the rhythm-control group may also be in part due to specific procedures used for rhythm-control strategy.
These findings have important implications for both the care of older adults with atrial fibrillation and for reduction of the health care costs. Controlling the cost of the Medicare through reduction of 30-day all-cause hospital readmission for older Medicare beneficiaries is a goal of the new U.S. healthcare reform law.24 Older patients with atrial fibrillation have high hospital admission and readmissions rates.25,26 Findings of the current analyses suggest that until further evidence emerges, older adults with atrial fibrillation may be best served by traditional rate-control rather than rhythm-control strategies. Despite the older age of the patients in our analysis, these trial-eligible patients may be healthier than their real-world counterparts.27-29 Whether the adverse effect of a rhythm-control strategy might be more pronounced in a broader population of older adults with atrial fibrillation and multiple comorbid conditions is unknown. Therefore, these findings would need to be confirmed in large prospective randomized clinical trials of real-world patients with atrial fibrillation.
Several limitations of our study need to be acknowledged. Although treatment strategy was randomized and the older subset used in the current analysis were further balanced via propensity matching, bias due to unmeasured confounders is possible. Findings from our sensitivity analysis suggest that a hidden covariate could potentially explain away this association if it would also increase the odds of rate-control by 6.8%. However, such an unmeasured covariate would need to be a near-perfect predictor of death and also could not be associated with any of the 45 measured balanced covariates, which is highly unlikely. We had no data on heart rate during follow-up. However, strict rate control has not been shown to be associated with better outcomes.30 The prevalence of use of beta-blockers was low and only 14 patients had ablation-based rhythm-control therapy, which may limit generalizability to contemporary younger patients with severe symptoms associated with atrial fibrillation. Although ablation can be useful in these patients, the long-term effect of this invasive procedure on mortality and morbidity has not been examined in a randomized controlled trial.31 Finally, AFFIRM did not enroll patients over the 80 years of age who have the highest prevalence of atrial fibrillation.
In conclusion, in septuagenarian patients with atrial fibrillation, the use of rate-control strategy was associated with a significantly lower risk of mortality and hospitalization than those treated with a rhythm-control strategy.
Clinical Significance Statement.
The role of rate-control versus rhythm control in patients 70 to 80 years of age with atrial fibrillation is not well known.
In these patients, compared with a rhythm-control strategy, a rate-control strategy using beta-blockers, digoxin, diltiazem and verapamil was superior in reducing the risk of mortality and hospitalization
Until further evidence emerges, patients with atrial fibrillation in the eighth decade of life may be better managed with a traditional rate-control strategy.
Acknowledgments
Funding: Dr. Ahmed was in part supported by the National Institutes of Health through grants (R01-HL085561, R01-HL085561-S and R01-HL097047) from the National Heart, Lung, and Blood Institute and a generous gift from Ms. Jean B. Morris of Birmingham, Alabama.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None
References
- 1.Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 2.Feinberg WM, Blackshear JL, Laupacis A, Kronmal R, Hart RG. Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch Intern Med. 1995;155:469–473. [PubMed] [Google Scholar]
- 3.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation: a major contributor to stroke in the elderly. The Framingham Study. Arch Intern Med. 1987;147:1561–1564. [PubMed] [Google Scholar]
- 4.Chinitz JS, Halperin JL, Reddy VY, Fuster V. Rate or rhythm control for atrial fibrillation: update and controversies. Am J Med. 2012;125:1049–1056. doi: 10.1016/j.amjmed.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Roy B, Desai RV, Mujib M, et al. Effect of warfarin on outcomes in septuagenarian patients with atrial fibrillation. Am J Cardiol. 2012;109:370–377. doi: 10.1016/j.amjcard.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corley SD, Epstein AE, DiMarco JP, et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2004;109:1509–1513. doi: 10.1161/01.CIR.0000121736.16643.11. [DOI] [PubMed] [Google Scholar]
- 7.Epstein AE, Vidaillet H, Greene HL, et al. Frequency of symptomatic atrial fibrillation in patients enrolled in the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study. J Cardiovasc Electrophysiol. 2002;13:667–671. doi: 10.1046/j.1540-8167.2002.00667.x. [DOI] [PubMed] [Google Scholar]
- 8.Pawar PP, Jones LG, Feller M, et al. Association between smoking and outcomes in older adults with atrial fibrillation. Arch Gerontol Geriatr. 2012;55:85–90. doi: 10.1016/j.archger.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baseline characteristics of patients with atrial fibrillation: the AFFIRM Study. Am Heart J. 2002;143:991–1001. doi: 10.1067/mhj.2002.122875. [DOI] [PubMed] [Google Scholar]
- 10.Rosenbaum PR, Rubin DB. The central role of propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 11.Rubin DB. Using propensity score to help design observational studies: Application to the tobacco litigation. Health Services and Outcomes Research Methodology. 2001;2:169–188. [Google Scholar]
- 12.Ahmed A, Husain A, Love TE, et al. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J. 2006;27:1431–1439. doi: 10.1093/eurheartj/ehi890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmed A, Fonarow GC, Zhang Y, et al. Renin-angiotensin inhibition in systolic heart failure and chronic kidney disease. Am J Med. 2012;125:399–410. doi: 10.1016/j.amjmed.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmed A, Rich MW, Zile M, et al. Renin-angiotensin inhibition in diastolic heart failure and chronic kidney disease. Am J Med. 2013;126:150–161. doi: 10.1016/j.amjmed.2012.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mujib M, Patel K, Fonarow GC, et al. Angiotensin-converting enzyme inhibitors and outcomes in heart failure and preserved ejection fraction. Am J Med. 2013;126:401–410. doi: 10.1016/j.amjmed.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmed MI, White M, Ekundayo OJ, et al. A history of atrial fibrillation and outcomes in chronic advanced systolic heart failure: a propensity-matched study. Eur Heart J. 2009;30:2029–2037. doi: 10.1093/eurheartj/ehp222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenbaum PR. Sensitivity to hidden bias. In: Rosenbaum PR, editor. Observational Studies. Vol. 1. Springer-Verlag; New York: 2002. pp. 105–170. [Google Scholar]
- 18.Olshansky B, Sami M, Rubin A, et al. Use of amiodarone for atrial fibrillation in patients with preexisting pulmonary disease in the AFFIRM study. Am J Cardiol. 2005;95:404–405. doi: 10.1016/j.amjcard.2004.09.044. [DOI] [PubMed] [Google Scholar]
- 19.Kaufman ES, Zimmermann PA, Wang T, et al. Risk of proarrhythmic events in the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study: a multivariate analysis. J Am Coll Cardiol. 2004;44:1276–1282. doi: 10.1016/j.jacc.2004.06.052. [DOI] [PubMed] [Google Scholar]
- 20.Carlsson J, Miketic S, Windeler J, et al. Randomized trial of rate-control versus rhythm-control in persistent atrial fibrillation: the Strategies of Treatment of Atrial Fibrillation (STAF) study. J Am Coll Cardiol. 2003;41:1690–1696. doi: 10.1016/s0735-1097(03)00332-2. [DOI] [PubMed] [Google Scholar]
- 21.Hohnloser SH, Kuck KH, Lilienthal J. Rhythm or rate control in atrial fibrillation--Pharmacological Intervention in Atrial Fibrillation (PIAF): a randomised trial. Lancet. 2000;356:1789–1794. doi: 10.1016/s0140-6736(00)03230-x. [DOI] [PubMed] [Google Scholar]
- 22.Van Gelder IC, Hagens VE, Bosker HA, et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834–1840. doi: 10.1056/NEJMoa021375. [DOI] [PubMed] [Google Scholar]
- 23.Saksena S, Slee A, Waldo AL, et al. Cardiovascular outcomes in the AFFIRM Trial (Atrial Fibrillation Follow-Up Investigation of Rhythm Management). An assessment of individual antiarrhythmic drug therapies compared with rate control with propensity score-matched analyses. J Am Coll Cardiol. 2011;58:1975–1985. doi: 10.1016/j.jacc.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tilson S, Hoffman GJ. In: Addressing Medicare Hospital Readmissions. CRS Report for Congress: Prepared for Members and Committees of Congress. Service CR, editor. Washington, D.C.: 2012. [Google Scholar]
- 25.Amin AN, Jhaveri M, Lin J. Temporal pattern and costs of rehospitalization in atrial fibrillation/atrial flutter patients with one or more additional risk factors. J Med Econ. 2012;15:548–555. doi: 10.3111/13696998.2012.664224. [DOI] [PubMed] [Google Scholar]
- 26.Amin AN, Jhaveri M, Lin J. Hospital readmissions in US atrial fibrillation patients: occurrence and costs. Am J Ther. 2013;20:143–150. doi: 10.1097/MJT.0b013e3182512c7e. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Kilgore ML, Arora T, et al. Design and rationale of studies of neurohormonal blockade and outcomes in diastolic heart failure using OPTIMIZE-HF registry linked to Medicare data. Int J Cardiol. 2011 doi: 10.1016/j.ijcard.2011.10.089. [Epub ahead of print]. doi:10.1016/j.ijcard.2011.10.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masoudi FA, Havranek EP, Wolfe P, et al. Most hospitalized older persons do not meet the enrollment criteria for clinical trials in heart failure. Am Heart J. 2003;146:250–257. doi: 10.1016/S0002-8703(03)00189-3. [DOI] [PubMed] [Google Scholar]
- 29.Fiocca L, Guagliumi G, Rossini R, et al. Characteristics and Outcomes of Patients With ST-Segment Elevation Myocardial Infarction Excluded from the Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) Trial. Am J Cardiol. 2013;111:196–201. doi: 10.1016/j.amjcard.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 30.Van Gelder IC, Groenveld HF, Crijns HJ, et al. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362:1363–1373. doi: 10.1056/NEJMoa1001337. [DOI] [PubMed] [Google Scholar]
- 31.Redberg RF. Clinical benefit of catheter ablation for atrial fibrillation: comment on “Discerning the incidence of symptomatic and asymptomatic episodes of atrial fibrillation before and after catheter ablation (DISCERN AF)”. JAMA Intern Med. 2013;173:157. doi: 10.1001/jamainternmed.2013.2308. [DOI] [PubMed] [Google Scholar]