Summary
The mTOR signaling pathway regulates many fundamental metabolic and physiological processes, including lipid metabolism. We explore recent findings on the role of mTOR in lipid homeostasis, with an emphasis on recent findings from in vivo models regarding the role of mTORC2 in lipolysis, lipogenesis and adipogenesis.
An introduction to mTOR
The mechanistic target of rapamycin (mTOR) is a PI3K-like serine/threonine protein kinase that is evolutionary conserved in all eukaryotes. mTOR is found in two complexes, each with distinct protein components as well as substrates. mTOR complex 1 (mTORC1), which is acutely sensitive to rapamycin, regulates processes such as ribosomal biogenesis, cap-dependent translation, lysosomal biogenesis, and autophagy via substrates that include S6K, 4E-BP1, TFEB1, and Ulk1. mTORC1 activity is regulated in part by the Rag family of GTPases, which promote the localization of mTORC1 to the lysosome in response to amino acids and glucose. At the lysosome, mTORC1 is activated by GTP-bound Rheb, which is itself regulated by the tuberous sclerosis complex (TSC 1/2) in response to AMPK, oxygen, and growth factor signaling. mTOR complex 2 (mTORC2), which is resistant to acute rapamycin treatment but can be disrupted by chronic rapamycin treatment in tissue culture as well as in vivo, is sensitive to growth factor signaling and regulates targets downstream of the insulin/IGF-1 receptor via substrates that include Akt, SGK, and PKCα. For a more complete treatment of the regulation of mTOR signaling in response to nutrients, we refer the reader to a recent comprehensive review (Laplante and Sabatini, 2012).
The past several years have seen an explosion of interest in the mTOR signaling pathway, spurred in large part by the finding that inhibition of mTORC1 signaling can significantly increase lifespan and protect from age-related diseases in mouse models (reviewed in (Lamming et al., 2013)). Genetically engineered mouse models have significantly added to our understanding of the role of mTOR in mammalian physiology. It is clear that mTOR signaling regulates lipid homeostasis, as treatment of rodents or humans with rapamycin leads to hyperlipidemia and hypercholesterolemia. This minireview will focus on the role of mTORC1 and mTORC2 in lipid physiology (Figure 1A).
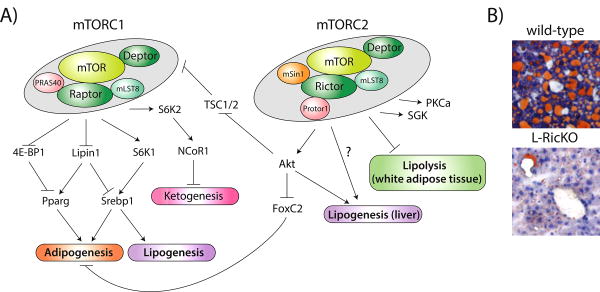
Figure 1.
mTOR in lipid homeostasis. A) A model of the actions of mTORC1 and mTORC2 in lipid metabolism, including adipogenesis, lipogenesis and lipolysis. mTORC1 also controls ketogenesis, which may have a reciprocal relationship with lipogenesis, via NCoR1, but the exact mechanism by which it regulates NCoR1 is unknown. The mechanism by which mTORC2 regulates lipogenesis and lipolysis are likewise not currently known, although Akt may be involved in the regulation of lipogenesis. B) Representative Oil-Red-O stained liver sections from wild-type or Rictor Liver knockout (L-RicKO) mice on a high-fat diet for 25 weeks.
Both mTOR complexes play a role in adipogenesis
It has been appreciated for some time that mTORC1 plays a role in adipogenesis. Tissue culture experiments starting in 2001 have shown that inhibition of mTORC1 signaling genetically or with rapamycin impairs adipogenesis, while increasing mTORC1 signaling by siRNA against TSC1/2 promotes adipogenesis. Deletion of TSC2 likewise promotes adipocyte differentiation (Zhang et al., 2009). This effect is mediated in part by 4E-BP1 via the regulation of the translation of PPARγ, but in vivo experiments have also pointed to an important role for S6K. S6K1 knockout mice show resistance to weight gain on a high fat diet due to impaired generation of adipocytes (Carnevalli et al., 2010). Mice with an adipose-specific deletion of Raptor, which is required for mTORC1 activity, phenocopy the S6K1 knockout mice (Polak et al., 2008). They are lighter, lean, and resistant to weight gain on a high-fat diet. Furthermore, their adipocytes are both smaller and less numerous. This was true even though raptor expression was lost only in mature adipocytes. Finally, as discussed in the next section, mTORC1 may also regulate adipogenesis in part via the regulation of the sterol regulatory element-binding proteins (SREBPs).
While it was initially assumed that mTORC2 also played a role in adipogenesis, as it is upstream of Akt, initial studies of adipose-specific Rictor knockout mice observed no defects in adipogenesis (Cybulski et al., 2009; Kumar et al., 2010). However, Yao and colleagues have recently uncovered a role for mTORC2 in adipogenesis (Yao et al., 2013). They discovered that the phosphorylation of Akt S473 by mTORC2 was dependent upon the interaction of Akt with phosphorylated BTSA (a BSD domain-containing protein). Moreover, they found that BSTA is a direct substrate of mTORC2, and phosphorylation of BSTA promotes the interaction with Akt, leading to its subsequent phosphorylation at S473. Embryonic stem cells lacking BSTA have defective Akt S473 phosphorylation, and are not capable of differentiating into adipocytes. Yao et al. discovered that this was due to induction of the transcription factor FoxC2, which inhibits white adipogenesis while potentiating brown adipogenesis. The combination of these studies suggest that mTORC2 may be essential for early adipogenesis, but does not affect adipogenesis when excised in mature adipocytes.
The DEP domain-containing mTOR-interacting protein (DEPTOR) interacts with both mTORC1 and mTORC2 (Peterson et al., 2009). DEPTOR is part of a quantitative trait locus linked to obesity in mice, and recently it was observed that overexpression of DEPTOR leads to the accumulation of white adipose tissue (Laplante et al., 2012). Increased expression of DEPTOR stimulates adipogenesis in vitro, in part by activating PPARγ (Laplante et al., 2012). DEPTOR expression also relieves the negative effect of mTORC1 on insulin signaling, activating the adipogenic functions of Akt. DEPTOR expression is significantly elevated in the adipose tissue of obese humans, suggesting that this mechanism may be conserved from mouse to man (Laplante et al., 2012).
Hepatic mTORC1 regulates ketogenesis and lipogenesis
It has long been known that during fasting, ketone bodies are produced in the liver as an energy source for peripheral tissues including the brain. While studying mice with a liver-specific deletion of TSC1, resulting in the constitutive activation of mTORC1, Sengupta et al. observed defective ketogenesis when fasted. Following a 24 hour fast, these mice had less than half the level of total serum ketones of control mice (Sengupta et al., 2010). The livers of aged mice similarly have increased mTORC1 activity and a defect in ketogenesis, which can be corrected by deletion of hepatic Raptor (Sengupta et al., 2010). While the mechanism was elusive, they found that mTORC1 regulates PPARα activity and gene expression by promoting the nuclear localization of NCoR1, a corepressor which interacts with PPARα. Kim et al. recently determined that S6K2, an effector of mTORC1 that is highly homologous to S6K1, regulates the nuclear localization of NCoR1 (Kim et al., 2012).
In 2008, Portsmann and colleagues were the first to identify a role for mTORC1 in lipogenesis, with the finding that rapamycin blocks the expression of genes involved in lipogenesis and impairs the nuclear accumulation of the sterol regulatory element-binding proteins (SREBPs) (Porstmann et al., 2008). Subsequent work by Düvel and colleagues identified the sterol regulatory element as the most highly enriched DNA motif in a gene expression study of rapamycin sensitive genes (Duvel et al., 2010). Although the exact mechanism by which SREBP1 and SREBP2 are regulated by mTORC1 is unclear, it is believed to be mediated by S6K1 (Duvel et al., 2010). Using a small molecule inhibitor of S6K, Owen et. al find that the transcriptional regulation of SREBP1c by insulin is not dependent on S6K, whereas post-transcriptional processing of SREBP1c is S6K dependent (Owen et al., 2012).
mTORC1 may also regulate the SREBP transcriptional network via the negative regulation of lipin1 (Peterson et al., 2011). Lipin1 is an mTORC1 substrate with multiple phosphorylation sites, including both rapamycin-sensitive sites and sites phosphorylated by mTORC1 that are relatively insensitive to rapamycin. Phosphorylation of lipin1 by mTORC1 regulates its subcellular localization, with phosphorylated lipin1 residing in the cytoplasm and dephosphorylated lipin1 accumulating in the nucleus. Nuclear lipin1 represses SREBP dependent gene transcription by reducing the nuclear SREBP protein levels (Peterson et al., 2011). Despite these recent advances in understanding how mTORC1 regulates lipogenesis, the molecular mechanisms by which S6K or lipin1 regulate SREBPs remains unknown. It should also be emphasized that mTORC1 signaling is essential but not sufficient to activate SREBP1c and hepatic lipid synthesis. Mice with a liver-specific deletion of TSC1 show increased mTORC1 activity, but have defective SREBP1c activation and lipogenesis due to an attenuation of Akt signaling (Yecies et al., 2011). As discussed below, signaling through mTORC2 is also essential for lipogenesis.
mTORC2, a newly identified regulator of lipid homeostasis
In comparison to mTORC1, much less is known about the substrates and functions of mTORC2 as it was discovered more recently. The best-characterized substrates of mTORC2 are members of the AGC family of kinases, including Akt, SGK1, and PKCα. mTORC2 mediates Akt activity by directly phosphorylating Akt S473 in response to growth factor signaling, and also phosphorylates T450 co-translationally. As Akt regulates mTORC1 via phosphorylation of TSC2 and PRAS40, mTORC2 indirectly regulates mTORC1 in response to growth factor signaling. mTORC2 is also required for phosphorylation of sites on PKCα, as well as SGK1. Additional substrates of mTORC2 may also include PKCδ, and are still being identified using phosphoproteomic techniques.
The regulation of Akt and SGK1 by mTORC2 suggests that mTORC2 may play a crucial role in the regulation of metabolism. Data from C. elegans has supported this concept, as Rictor-null worms have high levels of body fat on either a normal or high-fat diet (Jones et al., 2009; Soukas et al., 2009). While the exact mechanism underlying these effects is not clear, reports from both the Ashrafi and Ruvkun labs agree that these effects are at least partially dependent on SGK1 (Jones et al., 2009; Soukas et al., 2009).
Mammalian evidence for a role of mTORC2 in metabolism has been longer in coming due to the requirement for mTORC2 during development. Moreover, some of the first mouse models with tissue-specific inactivation of mTORC2, achieved through use of mice expressing a conditional allele of Rictor, showed minimal phenotypes. Bentzinger and colleagues found that mice lacking skeletal muscle Rictor (M-RicKO) had normal activation of Akt and normal phosphorylation of Akt T308, despite decreased phosphorylation of Akt S473 (Bentzinger et al., 2008). Subsequent studies of the role of mTORC2 in other tissues suggest that while mTORC2 may be dispensable for Akt activity in muscle, it is required for Akt activity in liver and adipose tissue (Kumar et al., 2008; Lamming et al., 2012; Yuan et al., 2012). While an in vivo role for mTORC2 in skeletal muscle has not been identified, in vitro data suggests that mTORC2 may play a role in the rapamycin-mediated skeletal muscle insulin resistance (Ye et al., 2012).
Adipose mTORC2 regulates lipolysis
Adipose-specific inactivation of mTORC2 has been researched independently by two groups, with largely similar conclusions. Adipose-specific deletion of Rictor (A-RicKO) mice trend towards being slightly heavier, with significantly increased lean mass (Cybulski et al., 2009). On a high-fat diet, these trends are exaggerated, with the livers of mTORC2-adipose knockout mice weighing 75% more than the livers of control mice after 10 weeks on a high-fat diet (Cybulski et al., 2009). The increased weight of these livers was partially due to a significant elevation in hepatic triglycerides compared with control mice, with a concomitant increase in hepatic steatosis.
While Cybulski and colleagues found A-RicKO mice to be glucose tolerant, Kumar and colleagues found that mTORC2-adipose-knockout mice to be quite severely glucose intolerant (Kumar et al., 2010). This apparent discrepancy in findings is easily explained by the different ages of the mice, as Kumar examined relatively old mice (greater than 9 months of age). At this age, A-RicKO mice displayed both adipose and skeletal muscle insulin resistance, likely due to significant lipid deposition in skeletal muscle (Kumar et al., 2010). Kumar and colleagues also found increased hepatic lipid deposition and steatosis in young and old A-RicKO mouse livers. These results suggest that many of the metabolic consequences of disrupting mTORC2 in adipose tissue are driven by lipid deposition in other tissues.
Interestingly, Kumar and colleagues found that mTORC2 regulates the insulin-mediated suppression of lipolysis in adipose tissue. Lipolysis, the breakdown of lipids into free fatty acids, should normally be suppressed with nutrients are high, but this does not occur in A-RicKO mice (Kumar et al., 2010). Moreover, A-RicKO mice have high levels of FFA, indicating that the absence of mTORC2 activity results in higher basal levels of lipolysis. While the mechanism has not been fully worked out, mTORC2 may normally regulate lipolysis by inhibiting the activation of PKA. In the absence of mTORC2, PKA is activated, and phosphorylates hormone-sensitive lipase, activating its lipolytic activity (Kumar et al., 2010). Finally, mTORC2 may also promote glucose uptake into adipose tissue, as mTORC2-adipose-knockout mice have decreased glucose uptake (Kumar et al., 2010). However, this will require further study, as adult mice in which Rictor has been depleted in the whole body show increased glucose uptake into Rictor-depleted adipose tissue (Lamming et al., 2012).
Hepatic mTORC2 regulates lipogenesis
In the past year, some exciting lessons have been learned about the role of mTORC2 in hepatic tissue, through the use of mice with a liver-specific deletion of Rictor (L-RicKO). One of the most prominent phenotypes of these mice is hepatic insulin resistance that leads to increased gluconeogenesis (Lamming et al., 2012). However, while hepatic insulin resistance is often associated with fatty liver, L-RicKO mice are actually protected against high-fat diet induced fatty liver (Hagiwara et al., 2012; Yuan et al., 2012). Hagiwara and colleagues, observed that in addition to increased gluconeogenesis, hepatocytes from L-RicKO mice have decreased glucose uptake, likely as a result of decreased expression of glucokinase. Moreover, L-RiKO mice have decreased levels of triglycerides, and Hagiwara and colleagues, as well as Yuan and colleagues, theorized that mTORC2 is required for hepatic lipogenesis.
Indeed, both groups found that insulin-stimulated hepatic lipogenesis was significantly impaired in L-RicKO mice. In the absence of mTORC2, hepatic expression of many key lipid synthesis genes, including acetyl CoA-carboxylase and fatty acid synthase, was reduced by 50% or more (Hagiwara et al., 2012; Yuan et al., 2012). L-RicKO mice also have decreased hepatic expression of SREBP1c and PPARγ. Interestingly, L-RicKO mice also have increased expression of genes related to fatty acid oxidation, including PPARα, and decreased expression of fatty acid uptake genes. The lack of hepatic lipids in mTORC2-deficient livers is therefore likely due to a combination of decreased lipid synthesis, decreased lipid uptake, and increased lipid consumption.
Both Hagiwara et al. and Yuan et al. performed in vivo genetic manipulations to try and determine the mechanisms behind the effects of Rictor deletion on gluconeogenesis and lipogenesis. Both groups found that expression of constitutively active Akt was sufficient to inhibit gluconeogenesis and restore normal glucose tolerance. However, while Hagiwara et al. reported that constitutively active Akt was sufficient to restore lipogenesis, Yuan et al. found that constitutively active Akt did not rescue the lipogenesis defect. The difference between these results may be explained by the fact that Hagiwara et al. observed gene expression and lipid synthesis in hepatocyte culture, while Yuan and colleagues looked in vivo at the effect of constitutively active Akt expression on serum triglycerides and hepatic triglyceride accumulation. In vivo, Yuan et al. demonstrate that activation of Akt alone is insufficient to correct the lipogenesis defect of L-RicKO mice, and they suggest that this may be due to decreased mTORC1 activity towards lipin1 in vivo in L-RicKO mice (Yuan et al., 2012). The observations of both groups are internally consistent with the observed effects on mTORC1 signaling in each system, as Hagiwara et al. did not observe decreased mTORC1 signaling in hepatocyte culture.
Conclusions
mTOR is a central regulator of lipid metabolism, regulating not only lipogenesis and lipolysis, but also adipogenesis. Most recently, mTORC2 in particular has emerged as a key controller of lipid metabolism, regulating lipogenesis in the liver, lipolysis in white adipose tissue, and controlling adipogenesis. These findings are summarized in Figure 1A. From a clinical perspective, the development of mTORC1-specific inhibitors may significantly decrease the side effects now associated with rapamycin. Specific inhibition of mTORC2 has the potential for real gains in the treatment of obesity and non-alcoholic fatty liver disease (NAFLD). As shown in Figure 1B, deletion of hepatic Rictor, resulting in the inhibition of mTORC2 signaling, provides dramatic protection against high fat diet induced fatty liver.
Clinically, inhibition of hepatic mTORC2 may promote the metabolism of excess hepatic lipids, while inhibition of mTORC2 during adipocyte differentiation could promote the gain of brown adipose tissue. Both of these properties could potentially be advantageous from the standpoint of treating obesity. However the side effects of mTORC2 inhibition, including hepatic insulin resistance, which may account for an increased incidence of new onset diabetes in patients treated with rapamycin, suggests that direct inhibition of mTORC2 will be undesirable. Identification of the ultimate mechanisms downstream of mTORC2 that regulate lipid metabolism, and the generation of new compounds that directly affect these mechanisms without disturbing glucose homeostasis, will be vital in safely translating these findings into the clinic.
Acknowledgments
We would like to thank Liron Bar-Peled, Joseph Baur and Mathieu Laplante for critical reading of the manuscript, Tom DiCesare for graphics design help, and all the members of the Sabatini lab for their support. DWL is supported by a K99/R00 award from the NIH/NIA (AG041765). DMS is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bentzinger CF, Romanino K, Cloetta D, Lin S, Mascarenhas JB, Oliveri F, Xia J, Casanova E, Costa CF, Brink M, et al. Skeletal muscle-specific ablation of raptor, but not of rictor, causes metabolic changes and results in muscle dystrophy. Cell Metab. 2008;8:411–424. doi: 10.1016/j.cmet.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Carnevalli LS, Masuda K, Frigerio F, Le Bacquer O, Um SH, Gandin V, Topisirovic I, Sonenberg N, Thomas G, Kozma SC. S6K1 plays a critical role in early adipocyte differentiation. Dev Cell. 2010;18:763–774. doi: 10.1016/j.devcel.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cybulski N, Polak P, Auwerx J, Ruegg MA, Hall MN. mTOR complex 2 in adipose tissue negatively controls whole-body growth. Proc Natl Acad Sci U S A. 2009;106:9902–9907. doi: 10.1073/pnas.0811321106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvel K, Yecies JL, Menon S, Raman P, Lipovsky AI, Souza AL, Triantafellow E, Ma Q, Gorski R, Cleaver S, et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell. 2010;39:171–183. doi: 10.1016/j.molcel.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara A, Cornu M, Cybulski N, Polak P, Betz C, Trapani F, Terracciano L, Heim MH, Ruegg MA, Hall MN. Hepatic mTORC2 activates glycolysis and lipogenesis through Akt, glucokinase, and SREBP1c. Cell Metab. 2012;15:725–738. doi: 10.1016/j.cmet.2012.03.015. [DOI] [PubMed] [Google Scholar]
- Jones KT, Greer ER, Pearce D, Ashrafi K. Rictor/TORC2 regulates Caenorhabditis elegans fat storage, body size, and development through sgk-1. PLoS Biol. 2009;7:e60. doi: 10.1371/journal.pbio.1000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Pyo S, Um SH. S6 kinase 2 deficiency enhances ketone body production and increases peroxisome proliferator-activated receptor alpha activity in the liver. Hepatology. 2012;55:1727–1737. doi: 10.1002/hep.25537. [DOI] [PubMed] [Google Scholar]
- Kumar A, Harris TE, Keller SR, Choi KM, Magnuson MA, Lawrence JC., Jr Muscle-specific deletion of rictor impairs insulin-stimulated glucose transport and enhances Basal glycogen synthase activity. Mol Cell Biol. 2008;28:61–70. doi: 10.1128/MCB.01405-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Lawrence JC, Jr, Jung DY, Ko HJ, Keller SR, Kim JK, Magnuson MA, Harris TE. Fat cell-specific ablation of rictor in mice impairs insulin-regulated fat cell and whole-body glucose and lipid metabolism. Diabetes. 2010;59:1397–1406. doi: 10.2337/db09-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamming DW, Ye L, Katajisto P, Goncalves MD, Saitoh M, Stevens DM, Davis JG, Salmon AB, Richardson A, Ahima RS, et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012;335:1638–1643. doi: 10.1126/science.1215135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamming DW, Ye L, Sabatini DM, Baur JA. Rapalogs and mTOR inhibitors as anti-aging therapeutics. J Clin Invest. 2013;123:980–989. doi: 10.1172/JCI64099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M, Horvat S, Festuccia WT, Birsoy K, Prevorsek Z, Efeyan A, Sabatini DM. DEPTOR cell-autonomously promotes adipogenesis, and its expression is associated with obesity. Cell Metab. 2012;16:202–212. doi: 10.1016/j.cmet.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen JL, Zhang Y, Bae SH, Farooqi MS, Liang G, Hammer RE, Goldstein JL, Brown MS. Insulin stimulation of SREBP-1c processing in transgenic rat hepatocytes requires p70 S6-kinase. Proc Natl Acad Sci U S A. 2012;109:16184–16189. doi: 10.1073/pnas.1213343109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson TR, Laplante M, Thoreen CC, Sancak Y, Kang SA, Kuehl WM, Gray NS, Sabatini DM. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 2009;137:873–886. doi: 10.1016/j.cell.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson TR, Sengupta SS, Harris TE, Carmack AE, Kang SA, Balderas E, Guertin DA, Madden KL, Carpenter AE, Finck BN, et al. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell. 2011;146:408–420. doi: 10.1016/j.cell.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak P, Cybulski N, Feige JN, Auwerx J, Ruegg MA, Hall MN. Adipose-specific knockout of raptor results in lean mice with enhanced mitochondrial respiration. Cell Metab. 2008;8:399–410. doi: 10.1016/j.cmet.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Porstmann T, Santos CR, Griffiths B, Cully M, Wu M, Leevers S, Griffiths JR, Chung YL, Schulze A. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 2008;8:224–236. doi: 10.1016/j.cmet.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta S, Peterson TR, Laplante M, Oh S, Sabatini DM. mTORC1 controls fasting-induced ketogenesis and its modulation by ageing. Nature. 2010;468:1100–1104. doi: 10.1038/nature09584. [DOI] [PubMed] [Google Scholar]
- Soukas AA, Kane EA, Carr CE, Melo JA, Ruvkun G. Rictor/TORC2 regulates fat metabolism, feeding, growth, and life span in Caenorhabditis elegans. Genes Dev. 2009;23:496–511. doi: 10.1101/gad.1775409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Suraokar M, Darnay BG, Hollier BG, Shaiken TE, Asano T, Chen CH, Chang BH, Lu Y, Mills GB, et al. BSTA promotes mTORC2-mediated phosphorylation of Akt1 to suppress expression of FoxC2 and stimulate adipocyte differentiation. Sci Signal. 2013;6:ra2. doi: 10.1126/scisignal.2003295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Varamini B, Lamming DW, Sabatini DM, Baur JA. Rapamycin has a biphasic effect on insulin sensitivity in C2C12 myotubes due to sequential disruption of mTORC1 and mTORC2. Frontiers in Genetics. 2012;3 doi: 10.3389/fgene.2012.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yecies JL, Zhang HH, Menon S, Liu S, Yecies D, Lipovsky AI, Gorgun C, Kwiatkowski DJ, Hotamisligil GS, Lee CH, et al. Akt stimulates hepatic SREBP1c and lipogenesis through parallel mTORC1-dependent and independent pathways. Cell Metab. 2011;14:21–32. doi: 10.1016/j.cmet.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M, Pino E, Wu L, Kacergis M, Soukas AA. Identification of Akt-independent regulation of hepatic lipogenesis by mammalian target of rapamycin (mTOR) complex 2. J Biol Chem. 2012;287:29579–29588. doi: 10.1074/jbc.M112.386854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HH, Huang J, Duvel K, Boback B, Wu S, Squillace RM, Wu CL, Manning BD. Insulin stimulates adipogenesis through the Akt-TSC2-mTORC1 pathway. PLoS One. 2009;4:e6189. doi: 10.1371/journal.pone.0006189. [DOI] [PMC free article] [PubMed] [Google Scholar]