Abstract
Diabetic retinopathy is a leading cause of blindness in the United States. Access to new animal models that exhibit retinal vasculopathies with short duration of diabetes, are vital for understanding the underlying mechanisms and examining the efficacy of new treatment modalities. Our previous studies demonstrated decreased expression of Thrombospondin-1 (TSP1), an endogenous inhibitor of angiogenesis, in eyes from both patients and rodents with diabetes. Here we examined whether TSP1 deficiency could exacerbate diabetic retinal vasculopathies. Akita/+ male mice reproducibly develop diabetes by 4 weeks of age. These mice demonstrated the early non-proliferative stages of diabetic retinopathy, including decreased numbers of pericytes and increased glial cell activation. However, Akita/+ male mice deficient in TSP1 (Akita/+; TSP1−/−) demonstrated more advanced stages of diabetic retinopathy with a 4-fold increase in acellular capillaries and increased fibronectin and GFAP expression. These vascular changes were not attributable to aberrant retinal vascular development in the absence of TSP1, since down-regulation of TSP1 postnatally in the endothelium also resulted in more severe retinopathy. In addition, lack of another endogenous inhibitor of angiogenesis, pigment epithelium derived factor (PEDF), also enhanced diabetic retinopathy in Akita/+ mice. Akita/+; PEDF−/− male mice demonstrated increased numbers of acellular capillaries compared to controls but at a level lower than that observed in Akita/+; TSP1−/− mice. Thus, the exacerbation of diabetic retinopathy in Akita/+; TSP1−/− mice will allow the study of retinal vasculopathies with a shorter duration of diabetes and facilitate future testing of treatment modalities that protect the retinal vasculature and preserve sight.
Keywords: Angiogenesis inhibitors, Diabetic retinopathy, Thrombospondin-1, PEDF
Introduction
Diabetic retinopathy, a secondary microvascular complication of diabetes mellitus, is a leading cause of blindness in the United States [1–3]. The earliest histological features of diabetic retinopathy include pericyte and endothelial cell loss and thickening of the vascular basement membrane, which go undetected by regular fundus examination. At advanced stages, neovascularization or proliferative diabetic retinopathy is observed, which if left untreated can lead to blindness as a result of bleeding and retinal detachment. There is now mounting evidence indicating that alterations in production of pro- and anti-angiogenic factors play a crucial role in the pathogenesis and progression of diabetic retinopathy [4–9]. However, the detailed mechanisms, and the target cells involved need further elucidation.
Thrombospondin-1 (TSP1) is a potent endogenous inhibitor of angiogenesis, and its expression in the endothelium promotes a quiescent, differentiated phenotype [10–12]. The role TSP1 plays in the development and progression of diabetic retinopathy remains elusive. We have previously shown that TSP1 is expressed at high levels in vitreous samples from various species including humans [5,13]. Loss of TSP1 in vitreous samples from diabetic rats was associated with the onset of early retinal vasculopathies [13]. In vitro, microvascular Endothelial Cells (EC) under high glucose conditions demonstrate decreased TSP1 expression [13]. Thus, down regulation of TSP1 produced by retinal EC during diabetes may lead to retinal vascular rarefaction.
Pigment Epithelium Derived Factor (PEDF) is a 50-kDa neurotrophic glycoprotein that acts as an endogenous inhibitor of angiogenesis [14,15]. Similar to TSP1, PEDF inhibits angiogenesis as well as EC proliferation and migration [16–18]. Our previous studies demonstrated that aberrant expression of a higher molecular weight PEDF isoform was associated with decreased levels of TSP1 [5]. Together these studies suggest that the aberrant expression of anti-angiogenic factors such as TSP1 and PEDF may contribute to the dysregulation of retinal vascular homeostasis and vasculopathies associated with diabetes.
Here we extended our previous studies to determine whether lack of TSP1 expression exacerbated the progression of diabetic retinopathy in male Akita/+ mice. The Akita spontaneous mutation (commonly referred to as MODY; Maturity-Onset Diabetes of the Young) is an autosomal dominant mutation in the insulin II gene (Ins2) [19] which causes male mice to reproducibly develop diabetes by 4 weeks of age. We observed the early stages of non-proliferative diabetic retinopathy in 6–10 month old male Akita/+ mice including decreased numbers of pericytes and increased activation of glial cells as previously reported [20]. In contrast, Akita/+ male mice lacking TSP1 (Akita/+; TSP1−/−) demonstrated more advanced stages of diabetic retinopathy with a 4-fold increase in acellular capillaries and a dramatic increase in fibronectin and Glial Fibrillary Acidic Protein (GFAP) expression. These changes were observed with a shorter duration of diabetes as compared to Akita/+ male mice. To ensure the vascular changes we observed were not attributable to aberrant vascular development in the absence of TSP1, we generated diabetic mice in which TSP1 expression could be down-regulated postnatally at will. In addition, we also made TSP1−/− adult mice diabetic with a Streptozotocin (STZ) injection. In both cases, diabetic mice lacking TSP1 demonstrated enhanced non-proliferative changes and increased numbers of acellular capillaries.
To determine whether loss of an endogenous inhibitor of angiogenesis other than TSP1 could enhance diabetic retinopathy in Akita/+ mice, we generated Akita/+ male mice deficient in PEDF (Akita/+; PEDF−/−). Akita/+; PEDF−/− male mice demonstrated increased numbers of acellular capillaries compared to controls, but at a level lower than that observed in Akita/+; TSP1−/− mice. Thus, loss of endogenous inhibitors of angiogenesis can make a significant contribution to the pathogenesis of diabetic retinopathy.
Materials and Methods
Animals
Ins2Akita heterozygous (Akita/+) male mice were obtained from Jackson Laboratories. The colony is maintained by breeding C57BL/6J inbred females with Ins2Akita heterozygous males. Control animals were C57BL/6J male littermates. Only male mice were used in the experiments described below. In all diabetic mice, diabetes was left untreated. All procedures were approved by the Animal Care and Use Committee of the University of Wisconsin School of Medicine and Public Health. Genomic DNA was prepared from tail biopsies and the transgenic Akita/+ mice were identified by PCR screening utilizing the following primers: 5′-TGCTGATGCC CTGGCCTGCT-3′ and 5′-TGGTCCCACATATGC ACATG-3′. The amplified fragments were digested with FNU 4 HI as recommended by Jackson Laboratories.
To generate Akita/+; TSP1−/− and Akita/+; PEDF−/− mice, Akita/+ male mice were bred to TSP1−/− or PEDF−/− female mice, respectively. The resulting Akita/+; TSP1+/− and Akita/+; PEDF+/− male mice were then bred to either TSP1−/− or PEDF−/− female mice, respectively. Akita/+; TSP1−/− and Akita/+; PEDF−/− male mice were determined by screening and the colony was maintained by breeding the respective male mice to TSP1−/− or PEDF−/− female mice. The litters were screened for Akita/+, PEDF−/− [21] and/or TSP1−/− [22,23], as previously described. TSP1−/− mice on a C57BL/6J background were generously provided by Dr. J. Lawler (Harvard University, Boston, MA) and screened as previously described [11]. The PEDF−/− mice on a C57BL/6J background were provided by Dr. S.J. Wiegand (Regenneron Pharmaceuticals, Tarrytown, NY) and screened as previously described [21].
Next, we generated a binary antisense TSP1 (ASTSP1) transgenic mouse model (TRE/ASTSP1 and TIE2/rtTA/Enh) driven by the tetracycline-responsive element (TRE) and the rtTA cDNA under the control of the TIE2 endothelial promoter. This facilitated conditional suppression of TSP1 expression in endothelial cells after doxycycline (Dox) treatment. We inserted an antisense TSP1 cDNA (a 1 Kbp 5′-fragment of mouse TSP1 cDNA) into the pTRE vector (BD-Clontech, Palo Alto, CA). The construct was purified and the transgenic mice (C57BL/6j background) were generated in our Biotechnology Center at the University of Wisconsin. The founders from these injections were screened by PCR analysis for the presence of the transgene, utilizing the following primers: 5′-TTAGTGAACCGTCAGATCGCC-3′ and 5′-AGAGCTGGTCAGTGAGCTG AAG-3′. The genotypes were verified by Southern blot analysis. TRE/ASTSP1 mice were mated to the Akita/+ male mice and screened to generate Akita/+; TRE/ASTSP1 mice.
Akita/+; TIE2/rtTA/Enh; TRE/ASTSP1 (Akita/+; TieTSP1) mice were then generated by mating the Akita/+; TRE/ASTSP1 mice to TIE2/rtTA/Enh mice [24]. These mice were screened as described above and also for TIE2/rtTA/Enh using the following primers: 5′-CAGACGACGA GGCTTGCAG-3′ and 5′-GGACACACGCGCA GACTGT-3′. Six week old male mice were fed regular mouse chow or mouse chow containing Doxycycline (600 mg/kg; Bio-Serv, Frenchtown, NJ) for the duration of the experiment.
STZ-induced diabetic mice
Wild-type and TSP1−/− male mice were made diabetic with a single intraperitoneal (i.p.) injection of streptozotocin (STZ; Sigma, St. Louis, MO; 180 mg/kg). STZ-injected animals were considered diabetic when blood glucose levels reached stable levels over 250 mg/dl. Diabetic and nondiabetic mice were sacrificed after 3 months of diabetes and the eyes were collected. Blood glucose levels were then measured with a portable glucometer (Glucometer Elite, Bayer Inc., Toronto, Ont.) using a drop of blood collected from a small incision at the tip of the tail.
Trypsin-digested retinal vessel preparation
Eyes were enucleated from mice, fixed in 4% paraformaldehyde for at least 24 h. The eyes were bisected equatorially and the entire retina was removed. Retinas were washed overnight in distilled water, and incubated in 3% trypsin (Trypsin 1:250, Difco) prepared in 0.1 M Tris, 0.1 M maleic acid, pH 7.8 containing 0.2 M NaF for approximately 1–1.5 h at 37°C. The digestion buffer contained 0.2 M sodium fluoride, to inhibit DNases contaminating the crude trypsin preparation. Following completion of digestion, retinal vessels were flattened by four radial cut and mounted on glass slides for Periodic Acid-Schiff (PAS) and hematoxylin staining. Nuclear morphology was used to distinguish pericytes from endothelial cells. The nuclei of endothelial cells are oval or elongated and lie within the vessel wall along the axis of the capillary, while pericytes nuclei are small, spherical and stain densely and generally have a protuberant position on the capillary wall. The stained and intact retinal wholemounts were coded and subsequent counting was performed in a masked fashion.
The number of EC and PC were determined by counting respective nuclei under the microscope at a x400 magnification. A mounting reticle (10 μm × 10 μm) was placed in one of the viewing oculars to facilitate counting. Only retinal capillaries were included in the cell count, which was performed in mid-zone of the retina. The number of EC and PC in four reticles from the four quadrants of each retina was determined. The total number of EC and PC for each retina was determined by adding the numbers from the four reticles. The ratio of EC to PC was then calculated.
Assessments of acellular capillaries
Retinal blood vessels were isolated by the trypsin digestion method, placed on a glass microscope slide, and stained with hematoxylin and PAS. Acellular capillaries were quantified in five field areas in the mid-retina (x200 magnification) in a masked fashion. Acellular capillaries were identified as capillary-sized vessel tubes having no nuclei anywhere along their length and reported per whole retinal area. Tubes with a diameter <20% of the diameter of adjacent capillaries were identified as strands and were not counted.
Immunohistochemical staining of frozen sections
Mice eyes were enucleated and embedded in Optimal Cutting Temperature (OCT) compound at −80°C. Sections (9 μm) were cut on a cryostat, placed on glass slides, and allowed to dry for 2 h. For fluorescence microscopy, sections were fixed in cold acetone (4°C) on ice for 10 min, followed by three washes with PBS, 5 minutes each. Sections were incubated in blocker (1% BSA, 0.2% skim milk, and 0.3% Triton X-100 in PBS) for 15 minutes at room temperature. Sections were then incubated with rabbit anti-mouse fibronectin (1:500; Millipore), anti-GFAP (1:500; DAKO, Carpinteria, CA), anti-occludin (1:200; Zymed, South San Francisco CA) or mouse-anti-human TSP1 (1:500; Clone A6.1, Neo Marker, Fremont, CA) overnight at 4°C in humid environment. After three washes in PBS, 5 min each, sections were incubated with appropriate secondary antibodies (Molecular probes; 1:500). As a control some sections were incubated only with secondary antibody. Sections were washed three times in PBS, covered with PBS: glycerol (2 vol/1 vol), and mounted with a coverslip. Retina sections were viewed by fluorescence microscopy and images were captured in digital format using a Zeiss microscope (Carl Zeiss, Chester, VA).
β-galactosidase staining
The entire PEDF gene was replaced with a targeting vector encoding a lacZ reporter cassette inserted downstream of the PEDF ATG site allowing determination of β-galactosidase. The β-galactosidase staining of frozen eye sections was performed as previously described [25]. Sections were fixed in 1.25% glutaraldehyde prepared in PBS for 10 min, and washed with PBS three times, 5 min each. Sections were incubated overnight at 37°C with 0.5 mg/ml 5-bromo-4-chloro-3-indolyl-b-D-pyranoside (X-Gal; Invitrogen) prepared in 44 mM HEPES, 3 mM potassium ferricyanide, 3 mM potassium ferrocyanide, 15 mM NaCl, and 1.3 mM MgCl2. After two washed in PBS, 5 min each, sections were incubated with Nuclear Fast Red (Invitrogen). Sections were washed three times in PBS, covered with PBS/glycerol (2 vol/1 vol), and mounted with a coverslip. Retina sections were viewed by microscopy and images were captured in digital format.
Statistical analysis
Statistical differences between control and treated samples were evaluated with student’s unpaired t-test (2-tailed) or two-way ANOVA with Bonferroni correction for multiple comparisons when appropriate. Mean ± SEM are shown. p values ≤ 0.05 were considered significant.
Results
Body weight and glucose levels of control, Akita/+ and Akita/+; TSP1 −/− mice
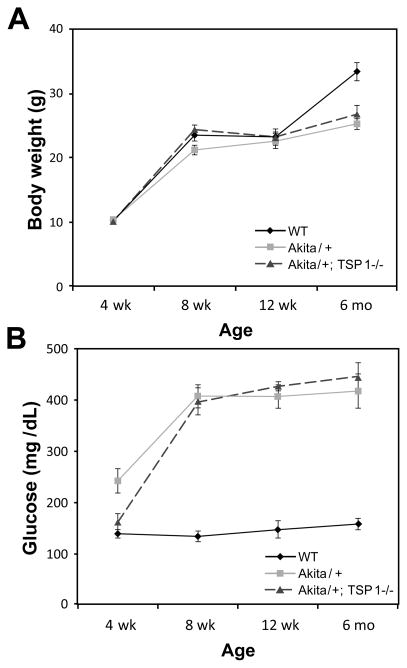
Male Akita/+ mice exhibit hyperglycemia, hypoinsulinemia, polydipsia, and polyuria beginning around 3–4 weeks of age [26]. Body weights remained similar in wild-type, Akita/+ and Akita/+; TSP1−/− male mice through 3 months of age (Figure 1A). At 6 months of age wild-type mice demonstrated a modest increase in weight compared to the diabetic mice (Figure 1A). As expected, blood glucose levels were significantly increased in Akita/+ and Akita/+; TSP1−/− male mice compared to their respective control mice (Figure 1B).
Figure 1.
Blood glucose and body weight in wild-type and Akita/+ male mice. Akita/+ male mice have spontaneous high glucose starting from 1 month. Body weight (A) and blood glucose levels (B) were monitored for up to 6 months in wild-type (WT), Akita/+ and Akita/+; TSP1−/− male mice (n=10). Note Akita/+ and Akita/+; TSP1−/− mice developed sustained hyperglycemia.
Increased endothelial/pericyte (EC/PC) ratio in Akita/+ mice
Retinal PC loss is one of the earliest morphological changes observed during diabetes [3,27]. Figure 2 is a representative photomicrograph of retinal trypsin digests from 7 month old wild-type and Akita/+ male mice. Although the vascular density appears similar in the retinal trypsin digests, 7 month old Akita/+ mice had decreased numbers of PC (Table 1) and moderately increased numbers of EC (Table 1) resulting in an increased EC/PC ratio compared to wild-type littermates (Table 1). Retinas from Akita/+ male mice demonstrated increased GFAP staining (Figure 2) consistent with glial cell activation that accompanies the progression of diabetic retinopathy. We also observed a modest change in occludin staining in Akita/+ male mice (Figure 2), consistent with increased vascular permeability observed during diabetic retinopathy. Together these data demonstrate that Akita/+ mice exhibit the early changes associated with diabetic retinopathy, as previously reported [20].
Figure 2.
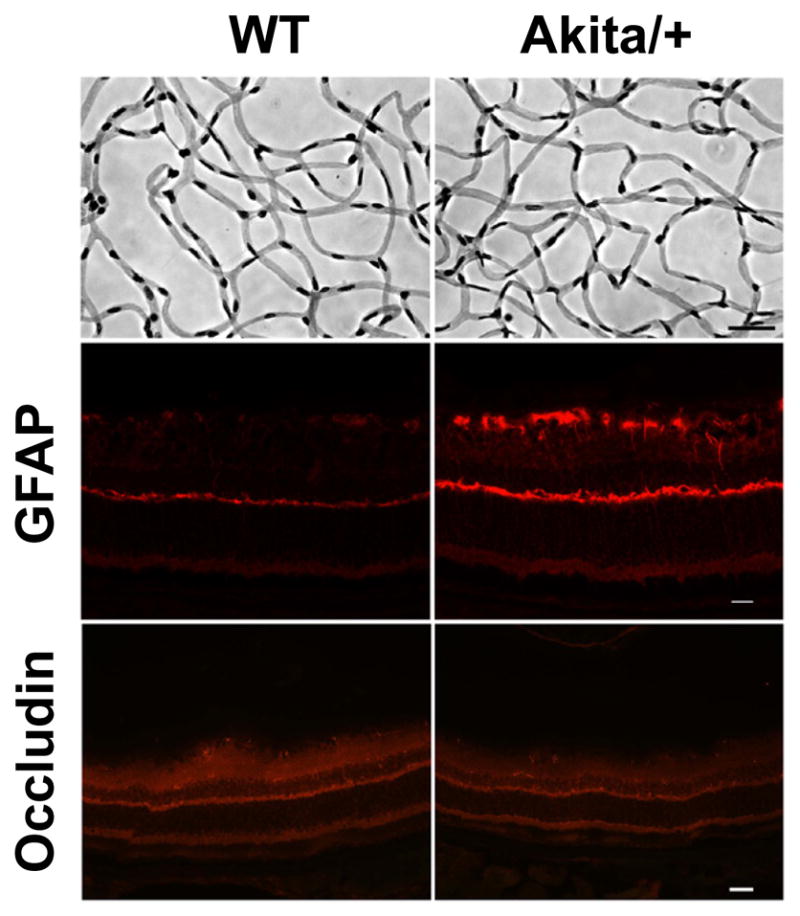
Decreased numbers of pericytes in Akita/+ male mice. Retinas from 7 month old wild-type and Akita/+ mice were prepared by trypsin digest as described in Materials and Methods (scale bar= 20 μM) [11,41]. GFAP and occludin staining of retinal sections from 9 month wild-type and Akita/+ male mice are also shown (scale bar= 50 μM). Note that GFAP expression increased in sections from Akita/+ male mice. Experiments were repeated with eyes from 5 mice with similar results.
Table 1.
Number of pericytes and endothelial cells per-reticle square (100μm2) in 7-month-old wild type (control) and Akita/+male mice (Density; Mean ± SD).
| Control | Akita/+ | |
|---|---|---|
| Pericytes (PC) | 46.7 ± 2.6 (6) | 34.7 ± 1.7 (6)* |
| Endothelial Cell (EC) | 110.8 ± 2.0 (6) | 122.1 ± 8.7 (6)* |
| EC/PC Ratio | 2.54 0.22 (6) | 3.13 ± 0.14 (6)* |
p<0.01. The number of retinas (mice) counted is given in parentheses
Akita/+; TSP1−/− mice exhibit acellular capillaries
As diabetic retinopathy progresses in humans, an increasing number of acellular capillaries are noted in the retina [2]. Unfortunately, known mouse models of diabetic retinopathy do not demonstrate significant levels of acellular capillaries with short duration of diabetes [28]. Our previous studies demonstrated decreased TSP1 expression in the vitreous and aqueous humor of diabetic rats [13]. To further delineate the role TSP1 plays during the initiation and early progression of diabetic retinopathy, we generated Akita/+; TSP1−/− mice. Akita/+; TSP1−/− male mice demonstrated a 4-fold increase in acellular capillaries compared to Akita/+ or wild-type male mice (Figure 3). Increased numbers of acellular capillaries were detected in Akita/+; TSP1−/− male mice by 7 months of age. Thus, loss of TSP1 expression in Akita/+ male mice hastens the progression of diabetic retinopathy.
Figure 3.
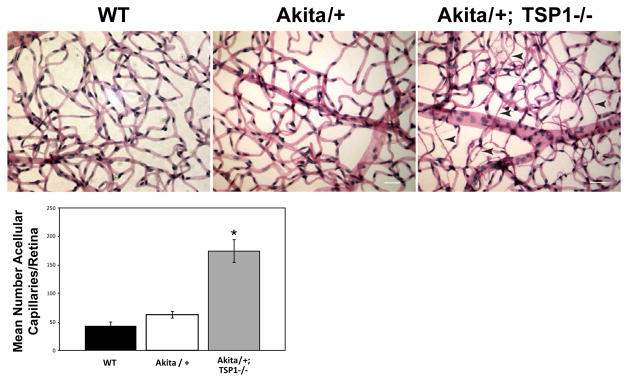
Increased acellular capillaries in Akita/+; TSP1−/− male mice. Trypsin digest of retinas from 7 month old wild-type, Akita/+ and Akita/+; TSP1−/− male mice were prepared and the number of acellular capillaries per retina were quantified (scale bar=50 μM). Arrow heads point to acellular capillaries. Please note more acellular capillaries in retinas from Akita/+; TSP1−/− male mice compared to either wild-type or Akita/+ male mice (*p<0.05: n ≥ 5 mice).
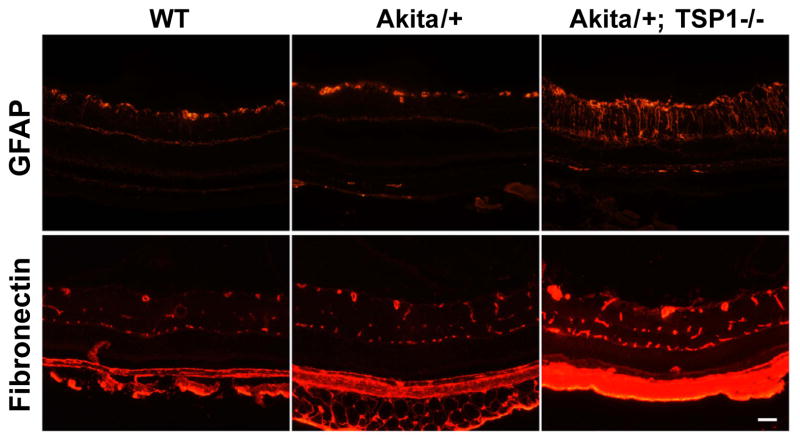
Increased fibronectin expression and glial cell activation in retinas from Akita/+; TSP1−/− mice
Diffuse thickening of retinal capillary basement membranes is a pathogenic morphological characteristic of diabetic retinopathy. Since increased fibronectin expression may contribute to vascular basement membrane thickening, we examined fibronectin staining of retinal sections from wild-type, Akita/+ and Akita/+; TSP1−/− male mice (Figure 4). We observed increased fibronectin staining in retinal sections from Akita/+; TSP1−/− compared to Akita/+ and wild-type male mice. We also observed a dramatic increase in glial cell activation in retinal sections from Akita/+; TSP1−/− male mice as indicated by increased GFAP staining (Figure 4). Thus, Akita/+; TSP1−/− mice exhibit many of the non-proliferative changes observed in Akita/+ mice but at significantly higher levels with relatively short duration of diabetes.
Figure 4.
Increased GFAP and fibronectin expression in retinas from Akita/+; TSP1−/− mice. GFAP and fibronectin staining of the retinal sections from 7 month wild-type, Akita/+ and Akita/+; TSP1−/− male mice are shown (scale bar= 50 μM). Please note the increase in GFAP and fibronectin expression in Akita/+; TSP1−/− male mice compared to wild-type or Akita/+ male mice. Experiments were repeated with eyes from 5 mice with similar results.
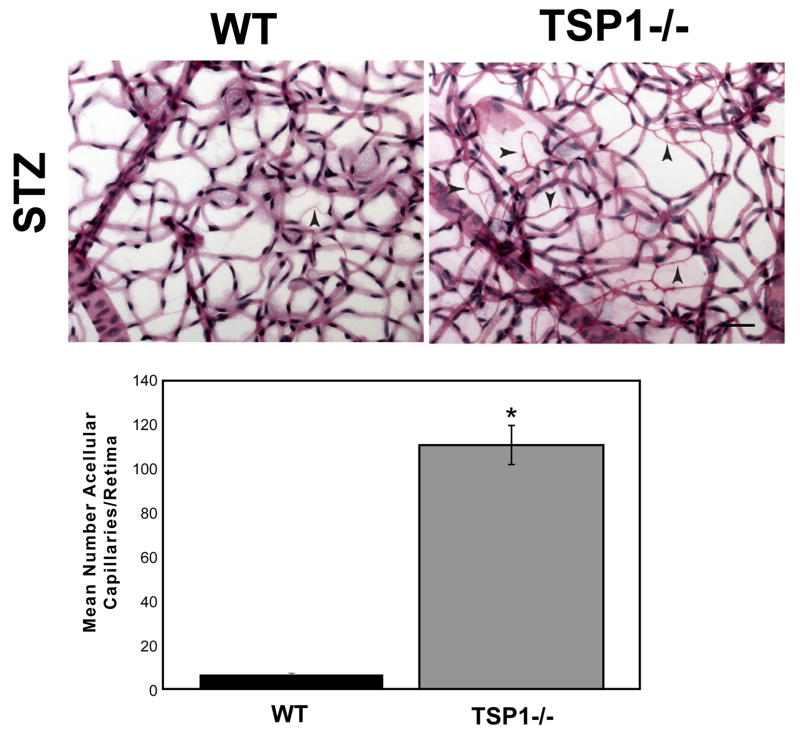
STZ-induced diabetic TSP1−/− mice exhibit increased acellular capillaries
To ensure that the acellular capillaries observed in the Akita/+; TSP1−/− male mice were not unique to this diabetic mouse model; we injected 8-week old wild-type and TSP1−/− male mice with STZ. Glucose values of STZ-injected diabetic wild-type and TSP1−/− male mice were similar. Trypsin digests of retinas from wild-type and TSP1−/− male mice were performed 3 months following STZ injection to determine the number of acellular capillaries. We observed a 20-fold increase in the number of acellular capillaries in STZ-injected TSP1−/− mice compared to the STZ-injected wild-type mice (Figure 5). Minimal numbers of acellular capillaries were noted in non-diabetic mice (not shown). Thus, TSP1−/− male mice are more susceptible to pathogenesis of diabetic retinopathy with significant non-proliferative changes after 3 months of diabetes.
Figure 5.
Increased acellular capillaries in STZ-injected TSP1−/− male mice. Wild-type and TSP1−/− 8 week old male mice were injected with STZ and trypsin digestion of retinal wholemounts were prepared 3 months later (5 months of age). Trypsin digest preparation of retinas from STZ-induced diabetic wild-type and TSP1−/− male mice were quantified for the number of acellular capillaries (scale bar= 50 μM). Arrow heads point to acellular capillaries. Please note increased numbers of acellular capillaries were observed in STZ-injected TSP1−/− male mice compared to their wild-type STZ-injected counterparts (*P < 0.05; n ≥ 5 mice).
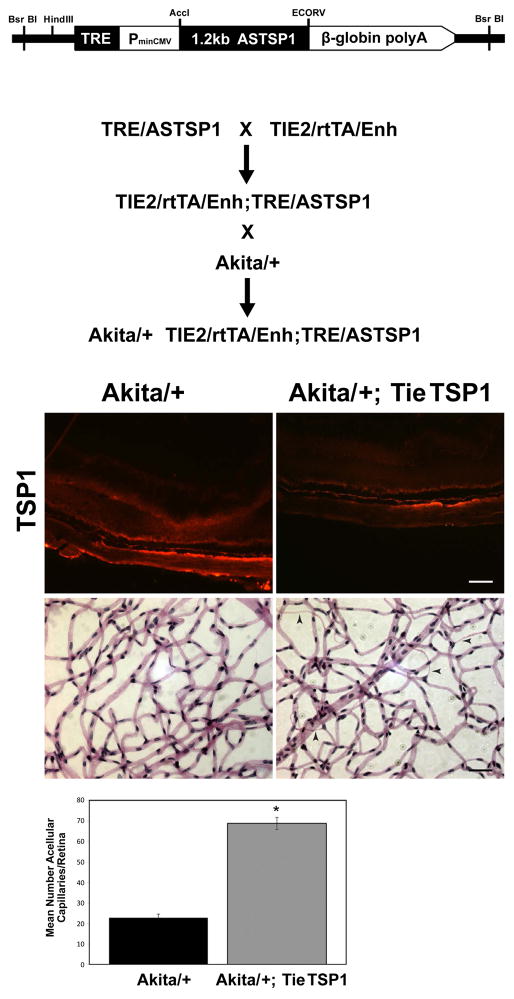
Endothelial-specific down-regulation of TSP1 following retinal development enhance the number of acellular capillaries during diabetes
To exclude the possibility that loss of TSP1 expression during retinal vascular development exacerbated diabetic retinopathy observed here, we generated male Akita/+ mice in which TSP1 expression was down-regulated by feeding doxycycline chow starting at 6 weeks of age. The construct and generation of Akita/+; TieTSP1 mice is detailed in figure 6A. TSP1 is produced by many cell types including retinal PC, astrocytes, and retinal pigment epithelial (RPE) cells, and is a secreted protein. Therefore, the examination of TSP1 level in Akita/+; TieTSP1 by western blotting of retinal extracts was not informative (not shown). As anticipated, TSP1 expression was decreased in retinal sections from Akita/+; TieTSP1 male mice which were fed doxycycline chow compared to Akita/+ mice (Figure 6B; upper panels). The significance of this is again somewhat masked by high levels of TSP1 produced by other ocular cell type including PC and RPE cells, and deposition in the Bruck’s membrane and vascular basement membranes.
Figure 6.
Akita/+ male mice with TSP1 inducibly down-regulated demonstrated increased numbers of acellular capillaries. The generation of Akita/+; TieTSP1 mice is detailed in Panel A. In Panel B TSP1 staining of the frozen sections from eyes of 8.5 month Akita/+ and Akita/+; TieTSP1 male mice are shown (scale bar= 50μM). Mice were feed doxocycline chow from age 6 weeks through the duration of the experiment (7 months). Note decreased TSP1 expression in retinas from Akita/+; TieTSP1 male mice compared to Akita/+ male mice. Arrow heads point to acellular capillaries. Retinal trypsin digests and the quantitative assessment of the number of acellular capillaries are shown in the lower panels. Please note that more acellular capillaries formed in Akita/+; TieTSP1 male mice compared to Akita/+ male mice (*P < 0.05; n ≥ 5 mice).
To determine the impact of endothelium specific down-regulation of TSP1 on formation of acellular capillaries, we prepared retinal trypsin digests from Akita/+ and Akita/+; TieTSP1 mice. We observed a 3-fold increase in acellular capillaries in Akita/+; TieTSP1 male mice at 3 months of age compared with Akita/+ (Figure 6B; lower panels) or Akita/+; TieTSP1 male mice with doxycycline free chow (not shown). Thus, increased numbers of acellular capillaries during diabetes in TSP1 deficient mice was not attributable to defects in retinal vascular development in the absence of TSP1. Furthermore, down regulation of TSP1 in retinal vascular endothelium was sufficient to exacerbate the pathogenesis of diabetic vasculopathies.
Akita/+; PEDF−/− mice exhibit increased numbers of acellular capillaries
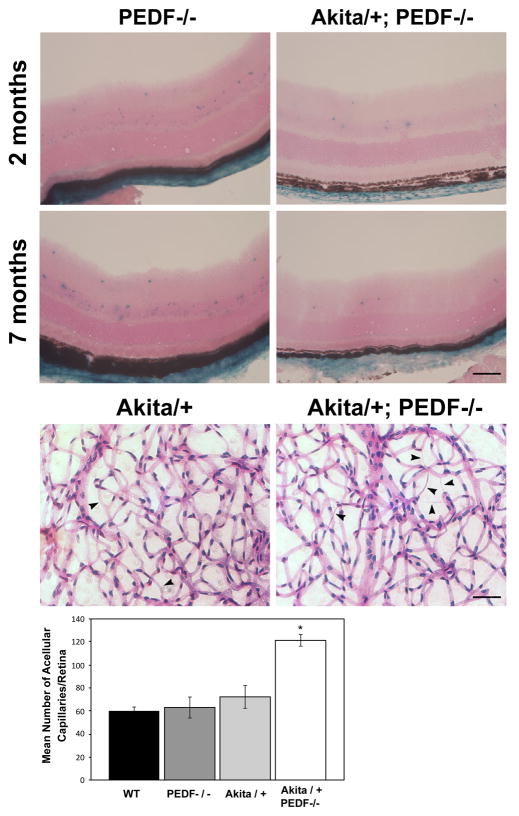
PEDF is another potent endogenous inhibitor of angiogenesis whose decreased expression is thought to contribute to the pathogenesis of diabetic retinopathy. To explore the role PEDF plays during the early progression of diabetic retinopathy, we generated Akita/+; PEDF−/− mice. Endogenous PEDF expression was determined by evaluating β-galactosidase activity, which is under the control of the endogenous PEDF promoter. With short (4 weeks of diabetes; 2 months of age) or long (6 months of diabetes; 7 months of age) duration of diabetes we observed decreased β-galactosidase expression in Akita/+; PEDF−/− male mice (Figure 7A). Figure 7B demonstrates that by 7 months of age Akita/+; PEDF−/− male mice demonstrated approximately a 2-fold increase in acellular capillaries compared to Akita/+ male mice. Therefore, lack of an endogenous inhibitor of angiogenesis such as PEDF enhances the progression of diabetic retinopathy.
Figure 7.
Increased acellular capillaries in retinas from Akita/+; PEDF−/− male mice. Retinal sections prepared from 2 and 7 month old PEDF−/− and Akita/+; PEDF−/− male mice were stained for β-galactosidase activity (Panel A). Note β-falactosidase activity in the inner and outer plexiform layer of retinas from Akita/+; PEDF−/− male mice was decreased compared with PEDF−/− male mice. In Panel B, trypsin digestion of the retinal vasculature were prepared from 9 month Akita/+ and Akita/+; PEDF−/− male mice and acellular capillaries were quantified. Arrow heads point to acellular capillaries. Please note that more acellular capillaries formed in Akita/+; PEDF−/− male mice compared to Akita/+ male mice (*P < 0.05; scale bar= 50 μM; n ≥ 5 mice).
Discussion
Diabetic retinopathy is a leading cause of blindness in the United States. Since treatments for this disease are limited, identification of rodent models that develop more characteristics of diabetic retinopathy will be useful tools. Akita/+ male mice reproducibly develop diabetes at 4 weeks of age. Obesity or insulitis does not accompany diabetes in these mice and they respond to exogenously administered insulin. Although Akita/+ male mice can serve as a substitute for mice made insulin-dependent by treatment with STZ, both models only exhibit the early stages of non-proliferative retinopathy. Even at 10 months of age, these models only develop modest numbers of acellular capillaries. Thus, animal models which exhibit many of the retinal vasculopathies associated with the development and progression of diabetes are highly desirable.
Aberrant modulation of anti- and pro-angiogenic factors can be a contributing factor in many disease states. Both TSP1 and PEDF are anti-angiogenic factors that have been reported to decrease during diabetes [4,5,8,29–32]. Unfortunately, it is not known whether aberrant regulation of anti-angiogenic factors is a contributing factor to diabetic retinopathy or merely a side effect of the disease. Previous work from this laboratory demonstrated down-regulation of TSP1 in samples from diabetic patients and rats [5,13]. Utilizing multiple approaches, we sought to determine whether lack of TSP1 expression was sufficient to exacerbate the pathogenesis of diabetic retinopathy. Akita/+; TSP1−/−, STZ-injected TSP1−/− and Akita/+; TieTSP1 male mice all exhibited increased numbers of retinal acellular capillaries with short duration of diabetes. Therefore, diabetic male mice lacking TSP1 may be a good model to test therapies aimed at maintaining retinal vascular integrity during diabetes.
One of the early changes observed in the retinal vasculature during diabetes is enhanced fibronectin expression in vascular cells within the vascular basement membrane [33,34]. This is thought to contribute to basement membrane thickening, impacting the proliferative and differentiated state of the endothelium. Here we demonstrated that the retinal vasculature of Akita/+; TSP1−/− male mice expressed higher levels of fibronectin compared to wild-type and Akita/+ male mice [12,35], consistent with the severe vasculopathies observed in these mice. We observed these vasculopathies, irrespective of the age of onset of diabetes or loss of TSP1 expression. However, we did observe differences in the severity of these vasculopathies. The greatest number of acellular capillaries were observed when TSP1 expression was absent during retinal vascularization (Akita/+; TSP1−/− and STZ treated TSP1−/−). When loss of TSP1 expression occurred following maturation of the retinal vasculature (Akita/+; TieTSP1) 2-fold less acellular capillaries were observed compared to Akita/+; TSP1−/− mice. Since the onset of diabetes was similar in these models, TSP1 expression during retinal vascular development may be protective. Alternatively, TSP1 expression in other retinal vascular cell types could moderate the effect since loss of TSP1 expression would only occur in the EC. Thus, endothelium TSP1 deficiency contributes to the development and progression of retinal vasculopathies.
PEDF is an endogenous inhibitor of angiogenesis that also plays an important role in retinal vascular homeostasis [21]. To further examine the impact dysregulation of this anti-angiogenic factor had on the progression of diabetic retinopathy Akita/+; PEDF−/− were generated. Akita/+; PEDF−/− mice demonstrated increased acellular capillaries although at a lesser degree than that observed in the absence of TSP1. Thus, aberrant modulation of anti-angiogenic factors during diabetes may enhance vascular dysfunction, exacerbating the pathogenesis of diabetic retinopathy.
TSP1 anti-angiogenic activity is mediated by directly acting on EC, independent of its role in modulation of TGF-β activity [10]. TSP1 induces apoptosis of EC through both intrinsic and extrinsic pathways [36]. We have shown that retinal EC prepared from TSP1−/− mice maintain a proangiogenic phenotype [12]. In addition, restoration of TSP1 expression in a highly angiogenic line of EC with reduced levels of TSP1 promotes a quiescent, differentiated state [10]. Thus, our results suggest an important role for aberrant modulation of TSP1 expression in the development and progression of diabetic retinopathy.
PEDF is also an inhibitor of angiogenesis and modulates proangiogenic properties of EC, most likely through its ability to interfere with Wnt signaling pathways [37]. PEDF is also shown to have anti-inflammatory and antioxidant activity [8,38]. We have recently shown that the level of PEDF is minimally affected in vitreous samples from diabetic patients [5]. However, we identified two isoforms of PEDF in vitreous samples from normal and diabetic patients. In addition, vitreous samples from diabetic patients which contained the high molecular weight isoform expressed significantly lower levels of TSP1. How PEDF isoforms and/or TSP1 modulate each other’s expression and/or activity requires further delineation. However, our data suggest a more significant role for changes in TSP1 during pathogenesis of diabetic retinopathy.
Endothelial dysfunction plays a key role in the inability of other organs to properly function during diabetes. In particular, diabetic nephropathy represents about half of the new cases of end-stage renal disease reported in the United States [39–41]. Our recent studies in kidney endothelial cells from Akita/+ male mice with short duration of diabetes (4 weeks) demonstrated attenuation of capillary morphogenesis, cell migration and VEGF expression, as well as aberrant expression of extracellular matrix proteins [34]. Thus, the angiogenic capacity of these endothelial cells was greatly diminished by diabetes. We also observed uncoupled eNOS/NO production that lead to increased oxidative stress. However, Akita/+ male mice needed to be diabetic for at least 8 weeks before we observed an inability of aortas to generate sprouting outgrowths [34]. Relief of oxidative stress by incubation with N-acetylcysteine facilitated sprouting outgrowths from these aortas. Thus, vascular damage incurred with increasing duration of diabetes may be difficult to repair due to limited capacity to undergo angiogenesis, thus, setting the stage for vascular rarefaction as the disease progresses.
In summary, we show that decreased expression of anti-angiogenic factors exacerbate the progression of diabetic retinopathy in the Akita/+ mouse model. Declining levels of these proteins in patients could be warning sign that uncontrolled diabetes is taking its toll on the integrity of the retinal vascular. Gaining a better understand of how anti-angiogenic factors are modulated during chronic hyperglycemia may open new therapeutic avenues of treatment to maintain vascular homeostasis and prevent vascular rarefaction, tissue ischemia, and proliferative neovascularization during diabetes.
Acknowledgments
This work was supported by grants EY016995, EY021357, and P30-EY16665 from the National Institutes of Health and an unrestricted departmental award from Research to Prevent Blindness. NS is a recipient of a Research Award from American Diabetes Association, 1-10-BS-160, Retina Research Foundation, and Juvenile Diabetes Research Foundation Int, 5-2005-1319. CMS is a recipient of a grant from American Heart Association, 0950057G.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Aiello LP, Gardner TW, King GL, Blankenship G, Cavallerano JD, et al. Diabetic retinopathy. Diabetes Care. 1998;21:143–156. doi: 10.2337/diacare.21.1.143. [DOI] [PubMed] [Google Scholar]
- 2.Frank RN. Diabetic retinopathy. N Engl J Med. 2004;350:48–58. doi: 10.1056/NEJMra021678. [DOI] [PubMed] [Google Scholar]
- 3.Hammes HP, Feng Y, Pfister F, Brownlee M. Diabetic retinopathy: targeting vasoregression. Diabetes. 2011;60:9–16. doi: 10.2337/db10-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang JJ, Zhang SX, Lu K, Chen Y, Mott R, et al. Decreased expression of pigment epithelium-derived factor is involved in the pathogenesis of diabetic nephropathy. Diabetes. 2005;54:243–250. doi: 10.2337/diabetes.54.1.243. [DOI] [PubMed] [Google Scholar]
- 5.Wang S, Gottlieb JL, Sorenson CM, Sheibani N. Modulation of thrombospondin 1 and pigment epithelium-derived factor levels in vitreous fluid of patients with diabetes. Arch Ophthalmol. 2009;127:507–513. doi: 10.1001/archophthalmol.2009.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boehm BO, Lang G, Volpert O, Jehle PM, Kurkhaus A, et al. Low content of the natural ocular anti-angiogenic agent pigment epithelium-derived factor (PEDF) in aqueous humor predicts progression of diabetic retinopathy. Diabetologia. 2003;46:394–400. doi: 10.1007/s00125-003-1040-9. [DOI] [PubMed] [Google Scholar]
- 7.Zhang SX, Wang JJ, Gao G, Parke K, Ma JX. Pigment epithelium-derived factor downregulates vascular endothelial growth factor (VEGF) expression and inhibits VEGF-VEGF receptor 2 binding in diabetic retinopathy. J Mol Endocrinol. 2006;37:1–12. doi: 10.1677/jme.1.02008. [DOI] [PubMed] [Google Scholar]
- 8.Zhang SX, Wang JJ, Gao G, Shao C, Mott R, et al. Pigment epithelium-derived factor (PEDF) is an endogenous antiinflammatory factor. FASEB J. 2006;20:323–325. doi: 10.1096/fj.05-4313fje. [DOI] [PubMed] [Google Scholar]
- 9.Sheibani N, Newman PJ, Frazier WA. Thrombospondin-1, a natural inhibitor of angiogenesis, regulates platelet-endothelial cell adhesion molecule-1 expression and endothelial cell morphogenesis. Mol Biol Cell. 1997;8:1329–1341. doi: 10.1091/mbc.8.7.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheibani N, Frazier WA. Thrombospondin-1 expression in transformed endothelial cells restores a normal phenotype and suppresses their tumorigenesis. Proc Natl Acad Sci U S A. 1995;92:6788–6792. doi: 10.1073/pnas.92.15.6788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang S, Wu Z, Sorenson CM, Lawler J, Sheibani N. Thrombospondin-1-deficient mice exhibit increased vascular density during retinal vascular development and are less sensitive to hyperoxia-mediated vessel obliteration. Dev Dyn. 2003;228:630–642. doi: 10.1002/dvdy.10412. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Wang S, Sheibani N. Enhanced proangiogenic signaling in thrombospondin-1-deficient retinal endothelial cells. Microvasc Res. 2006;71:143–151. doi: 10.1016/j.mvr.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Sheibani N, Sorenson CM, Cornelius LA, Frazier WA. Thrombospondin-1, a natural inhibitor of angiogenesis, is present in vitreous and aqueous humor and is modulated by hyperglycemia. Biochem Biophys Res Commun. 2000;267:257–261. doi: 10.1006/bbrc.1999.1903. [DOI] [PubMed] [Google Scholar]
- 14.Dawson DW, Volpert OV, Gillis P, Crawford SE, Xu H, et al. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 1999;285:245–248. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- 15.Stellmach V, Crawford SE, Zhou W, Bouck N. Prevention of ischemia-induced retinopathy by the natural ocular antiangiogenic agent pigment epithelium-derived factor. Proc Natl Acad Sci U S A. 2001;98:2593–2597. doi: 10.1073/pnas.031252398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L, Zhang SS, Barnstable CJ, Tombran-Tink J. PEDF induces apoptosis in human endothelial cells by activating p38 MAP kinase dependent cleavage of multiple caspases. Biochem Biophys Res Commun. 2006;348:1288–1295. doi: 10.1016/j.bbrc.2006.07.188. [DOI] [PubMed] [Google Scholar]
- 17.Ho TC, Chen SL, Yang YC, Liao CL, Cheng HC, et al. PEDF induces p53-mediated apoptosis through PPAR gamma signaling in human umbilical vein endothelial cells. Cardiovasc Res. 2007;76:213–223. doi: 10.1016/j.cardiores.2007.06.032. [DOI] [PubMed] [Google Scholar]
- 18.Kanda S, Mochizuki Y, Nakamura T, Miyata Y, Matsuyama T, et al. Pigment epithelium-derived factor inhibits fibroblast-growth-factor-2-induced capillary morphogenesis of endothelial cells through Fyn. J Cell Sci. 2005;118:961–970. doi: 10.1242/jcs.01686. [DOI] [PubMed] [Google Scholar]
- 19.Yoshioka M, Kayo T, Ikeda T, Koizumi A. A novel locus, Mody4, distal to D7Mit189 on chromosome 7 determines early-onset NIDDM in nonobese C57BL/6 (Akita) mutant mice. Diabetes. 1997;46:887–894. doi: 10.2337/diab.46.5.887. [DOI] [PubMed] [Google Scholar]
- 20.Barber AJ, Antonetti DA, Kern TS, Reiter CE, Soans RS, et al. The Ins2Akita mouse as a model of early retinal complications in diabetes. Invest Ophthalmol Vis Sci. 2005;46:2210–2218. doi: 10.1167/iovs.04-1340. [DOI] [PubMed] [Google Scholar]
- 21.Huang Q, Wang S, Sorenson CM, Sheibani N. PEDF-deficient mice exhibit an enhanced rate of retinal vascular expansion and are more sensitive to hyperoxia-mediated vessel obliteration. Exp Eye Res. 2008;87:226–241. doi: 10.1016/j.exer.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scheef EA, Huang Q, Wang S, Sorenson CM, Sheibani N. Isolation and characterization of corneal endothelial cells from wild type and thrombospondin-1 deficient mice. Mol Vis. 2007;13:1483–1495. [PubMed] [Google Scholar]
- 23.Scheef EA, Sorenson CM, Sheibani N. Attenuation of proliferation and migration of retinal pericytes in the absence of thrombospondin-1. Am J Physiol Cell Physiol. 2009;296:C724–734. doi: 10.1152/ajpcell.00409.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wall DS, Gendron RL, Good WV, Miskiewicz E, Woodland M, et al. Conditional knockdown of tubedown-1 in endothelial cells leads to neovascular retinopathy. Invest Ophthalmol Vis Sci. 2004;45:3704–3712. doi: 10.1167/iovs.03-1410. [DOI] [PubMed] [Google Scholar]
- 25.Emert MP, Sorenson CM, Basile DP, Rogers JG, Hammerman MR, et al. The human plasminogen activator inhibitor type I gene promoter targets to kidney. Am J Physiol. 1998;274:F405–412. doi: 10.1152/ajprenal.1998.274.2.F405. [DOI] [PubMed] [Google Scholar]
- 26.Yoshioka M, Kayo T, Ikeda T, Koizumi A. A novel locus, Mody4, distal to D7Mit189 on chromosome 7 determines early-onset NIDDM in nonobese C57BL/6 (Akita) mutant mice. Diabetes. 1997;46:887–894. doi: 10.2337/diab.46.5.887. [DOI] [PubMed] [Google Scholar]
- 27.Hayden MR, Yang Y, Habibi J, Bagree SV, Sowers JR. Pericytopathy: oxidative stress and impaired cellular longevity in the pancreas and skeletal muscle in metabolic syndrome and type 2 diabetes. Oxid Med Cell Longev. 2010;3:290–303. doi: 10.4161/oxim.3.5.13653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kern TS, Engerman RL. A mouse model of diabetic retinopathy. Arch Ophthalmol. 1996;114:986–990. doi: 10.1001/archopht.1996.01100140194013. [DOI] [PubMed] [Google Scholar]
- 29.Sheibani N, Sorenson CM, Frazier WA. Differential modulation of cadherin-mediated cell-cell adhesion by platelet endothelial cell adhesion molecule-1 isoforms through activation of extracellular regulated kinases. Mol Biol Cell. 2000;11:2793–2802. doi: 10.1091/mbc.11.8.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boehm BO, Lang G, Feldmann B, Kurkhaus A, Rosinger S, et al. Proliferative diabetic retinopathy is associated with a low level of the natural ocular anti-angiogenic agent pigment epithelium-derived factor (PEDF) in aqueous humor. a pilot study. Horm Metab Res. 2003;35:382–386. doi: 10.1055/s-2003-41362. [DOI] [PubMed] [Google Scholar]
- 31.Duh EJ, Yang HS, Haller JA, De Juan E, Humayun MS, et al. Vitreous levels of pigment epithelium-derived factor and vascular endothelial growth factor: implications for ocular angiogenesis. Am J Ophthalmol. 2004;137:668–674. doi: 10.1016/j.ajo.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 32.Funatsu H, Yamashita H, Nakamura S, Mimura T, Eguchi S, et al. Vitreous levels of pigment epithelium-derived factor and vascular endothelial growth factor are related to diabetic macular edema. Ophthalmology. 2006;113:294–301. doi: 10.1016/j.ophtha.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 33.Chen S, Khan ZA, Cukiernik M, Chakrabarti S. Differential activation of NF-kappa B and AP-1 in increased fibronectin synthesis in target organs of diabetic complications. Am J Physiol Endocrinol Metab. 2003;284:E1089–1097. doi: 10.1152/ajpendo.00540.2002. [DOI] [PubMed] [Google Scholar]
- 34.Grutzmacher C, Park S, Zhao Y, Morrison ME, Sheibani N, et al. Aberrant production of extracellular matrix proteins and dysfunction in kidney endothelial cells with a short duration of diabetes. Am J Physiol Renal Physiol. 2013;304:F19–30. doi: 10.1152/ajprenal.00036.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su X, Sorenson CM, Sheibani N. Isolation and characterization of murine retinal endothelial cells. Mol Vis. 2003;9:171–178. [PubMed] [Google Scholar]
- 36.Henkin J, Volpert OV. Therapies using anti-angiogenic peptide mimetics of thrombospondin-1. Expert Opin Ther Targets. 2011;15:1369–1386. doi: 10.1517/14728222.2011.640319. [DOI] [PubMed] [Google Scholar]
- 37.Park K, Lee K, Zhang B, Zhou T, He X, et al. Identification of a novel inhibitor of the canonical Wnt pathway. Mol Cell Biol. 2011;31:3038–3051. doi: 10.1128/MCB.01211-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang SX, Wang JJ, Dashti A, Wilson K, Zou MH, et al. Pigment epithelium-derived factor mitigates inflammation and oxidative stress in retinal pericytes exposed to oxidized low-density lipoprotein. J Mol Endocrinol. 2008;41:135–143. doi: 10.1677/JME-08-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanwar YS, Sun L, Xie P, Liu FY, Chen S. A glimpse of various pathogenetic mechanisms of diabetic nephropathy. Annu Rev Pathol. 2011;6:395–423. doi: 10.1146/annurev.pathol.4.110807.092150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Dijk C, Berl T. Pathogenesis of diabetic nephropathy. Rev Endocr Metab Disord. 2004;5:237–248. doi: 10.1023/B:REMD.0000032412.91984.ec. [DOI] [PubMed] [Google Scholar]
- 41.Wang S, Sorenson CM, Sheibani N. Attenuation of retinal vascular development and neovascularization during oxygen-induced ischemic retinopathy in Bcl-2−/− mice. Dev Biol. 2005;279:205–219. doi: 10.1016/j.ydbio.2004.12.017. [DOI] [PubMed] [Google Scholar]