Abstract
Neuroblastoma (NB) is the most prevalent pediatric solid tumor and a leading cause of cancer-related death in children. In the present study, a novel cytotoxic role for the dietary compounds, curcumin, andrographolide, wedelolactone, dibenzoylmethane, and tanshinone IIA was identified in human S-type NB cells, SK-N-AS and SK-N-BE(2). Mechanistically, cell death appeared apoptotic by flow cytometry; however, these effects proceeded independently from both caspase-3 and p53 activation, as assessed by both genetic (shRNA) and pharmacological approaches. Notably, cell death induced by both curcumin and andrographolide was associated with decreased NFκB activity and a reduction in Bcl-2 and Bcl-xL expression. Finally, curcumin and andrographolide increased cytotoxicity following co-treatment with either cisplatin or doxorubicin, two chemotherapeutic agents widely used in the clinical management of NB. Coupled with the documented safety in humans, dietary compounds may represent a potential adjunct therapy for NB.
Keywords: Ayurveda, Akt, pediatric cancer, nutrition, chemotherapy
1. Introduction
Neuroblastoma (NB) is a malignant tumor of the neural crest that arises within the sympathetic nervous system. NB is the most prevalent pediatric solid tumor (41% of all cases) with 7–12 cases per million children under 4 years of age and 25–51 cases per million in infants (Eisenberg et al., 1990; Stiller and Parkin, 1992). Despite advances in radiotherapy, chemotherapy, and surgery, high grade NB patients face a poor prognosis with a cure rate below 20% (Schmidt et al., 2000). Children recovering from NB frequently exhibit learning and memory deficits, as well as impairment of fine motor skills, due in part to the adverse effects of radiation and chemotherapy on the developing nervous system (Humpl, 1995). The grim prognoses coupled with a dearth of effective treatments that minimize long-term disability emphasize the need for improved therapeutics.
The cellular and molecular changes underlying NB development and progression remain poorly understood; however, the nuclear factor-κB (NFκB) transcription factor paradoxically influences both NB survival (Bian et al., 2002; Karacay et al., 2004) and cell death (Bian et al., 2001). NB are comprised of three phenotypically different cells types, N-type, I-type, and S-type cells (Biedler et al., 1973; Ross et al., 1983), which may explain the differential effects of NFκB on cellular viability. Neuroblastic (N-type) cells, the predominant cell type in NB, utilize NFκB as a death-inducing signal following cytotoxic treatment (Bian et al., 2001) whereas constitutive NFκB promotes cellular survival in Schwannian stromal (S-type) cells (Bian et al., 2002). Both N-type and S-type cells are derived from genetically-identical precursor cells and can transdifferentiate into the other cell type (Biedler et al., 1988; Mora et al., 2001); however, unlike N-type cells which are eradicated by medical therapies, stromal elements may persist and develop into chemoresistant clones, contributing to the frequent relapses observed in NB patients following cytotoxic treatment (Lavoie et al., 2009). Thus, directed targeting of S-type cells may represent an important, yet relatively unexplored strategy to reduce the likelihood of cancer remission.
Children with NB would ideally be treated with tumor-specific chemotherapeutic agents to reduce cancer growth without adversely influencing long-term neurodevelopment. Unfortunately, radiation and current chemotherapeutics are particularly toxic to the developing nervous system (Humpl, 1995), stressing the need for novel compounds to combat NB. Diet is an important influence on carcinogenesis and cancer progression (Kelloff et al., 2000) and over half of modern chemotherapeutic drugs are derived from natural substances (Cragg et al., 1997). Thus, dietary compounds may represent a valuable source of anti-cancer compounds with the added benefit of low toxicity in non-transformed cells. Ayurveda, a traditional Indian system of medicine incorporating the use of phytochemicals, has been used for centuries to treat a variety of ailments, including cancer (Balachandran and Govindarajan, 2005). Epidemiological studies in Asian countries, such as India and China, report significantly fewer cases of NB as compared to Western countries (Stiller and Parkin, 1992), suggesting genetic and/or environmental factors influence the development and progression of NB.
In the present study, dietary phytochemicals, including curcumin, andrographolide, wedelolactone, and dibenzoylmethane induced cell death in human S-type NB cells via a reduction in Akt-NFκB signaling. Inhibition of this pathway also increased cell death in S-type cells after treatment with cisplatin and doxorubicin, suggesting a novel role for dietary compounds as a potential adjunct to traditional chemotherapeutics in the treatment of NB.
2. MATERIALS AND METHODS
2.1.Reagents
Curcumin (>98% purity), salicylic acid, cisplatin, doxorubicin, dibenzoylmethane, and MTT were purchased from Sigma (St. Louis, MO). LY294002, pyrrolidine dithiocarbamate (PDTC), zVAD-fmk, pifithrin-α, tanshinone IIA (98% purity), and helenalin were from Biomol (Plymouth Meeting, PA). Wedelolactone was from EMD Biosciences (San Diego, CA). 4-hydroxy-3-methoxy-benzoic acid (vanillic acid) was purchased from Alfa Aesar (Pelham, NH) and 4-hydroxy-3-methoxycinnamic acid (ferulic acid) and andrographolide (97% purity) were purchased from Indofine (Hillsborough, NJ).
2.2. Cell Culture
All cell culture reagents, sera, and media were purchased from Hyclone (Carlsbad, CA). SK-N-AS and SK-N-BE(2) human neuroblastoma cells were obtained from ATCC (Manassas, VA) and were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum and antibiotics in a 37°C in a humidified cell culture incubator.
2.3. Cell viability assays
Cells (1 × 104 cells/well) were plated in 96-well plates overnight, and treated as detailed in Figure Legends. Cellular viability was assessed using the MTT reduction assay or lactate dehydrogenase (LDH) assays (Roche, Indianapolis, IN), as described by our laboratory (Dhandapani et al., 2007). Cell morphology was assessed with an Axiovert light microscope (Carl Zeiss, Thornwood, NY) equipped with a digital imaging system. To detect apoptotic or necrotic cell death, adherent and non-adherent cells were collected and washed following treatments. Cell suspensions were stained with annexin V-PE (BD Pharmigen, San Diego, CA), an early apoptotic marker, and with 7-aminoactinomycin D (7-AAD), a fluorescent, dead cell marker. The percentage of apoptotic cells was quantified using a FACScan flow cytometry.
2.4. Transfections
Cells were plated at ∼50% confluence in complete culture medium without antibiotics. 1 µg IκB superrepressor, dominant negative Akt (dnAkt), constitutively active Akt (myrAkt), or empty vector plasmids (Upstate Biotechnology; Lake Placid, NY) were complexed with Lipofectamine 2000 (Invitrogen, Carlsbad, CA), and added to cells overnight, as detailed by our laboratory (Dhandapani et al., 2007). Cells were then returned to culture media until experimental treatment.
2.5. shRNA-mediated gene knockdown
Stable gene silencing was performed using shRNA lentiviral particles (Santa Cruz Biotechnology, Santa Cruz, CA), per manufacturer’s recommended protocol. Briefly, SK-N-AS cells were transduced with p53, caspase-3, or control shRNA particles at a multiplicity of infection (MOI) of 1 in the presence of 5 µg/mL polybrene. Growth medium was replaced 18 hours after transduction. At three days post-transduction, stably transduced cells were selected using 2 µg/mL puromycin for one week. Following selection, Western blotting was performed to ensure complete protein knockdown.
2.6. Quantitative RT-PCR
RNA was isolated using a SV RNA Isolation Kit (Promega, Madison, WI) and qRT-PCR was performed on a SmartCycler II (Cepheid, Sunnyvale, CA) using the Superscript III Platinum SYBR One-Step qRT-PCR kit (Invitrogen, Carlsbad, CA). Primers were: Bcl-2: FP 5’-GAGGATTGTGGCCTTCTTTG-3’, RP 5’-ACAGTTCCACAAAGGCATCC-3’; Bcl-xL: 5’-GTAAACTGGGGTCGCATTGT-3’, RP 5’-TGCTGCATTGTTCCCATAGA-3’; Bax: FP 5’-TTTGCTTCAGGGTTTCATCC-3’; RP 5’-CAGTTGAAGTTGCCGTCAGA-3’. Expression levels were quantified using a cDNA standard curve (Rajeevan et al., 2001) and data was normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH), a housekeeping gene which was unaffected by experimental treatments, to correct for equal RNA loading.
2.7. Nuclear extracts and transcription factor activation
Cells were grown to ∼90–100% confluence in 10 cm plates, and then treated with dietary inhibitors for 3–6h. Nuclear extracts were harvested from cells using a Nuclear Extract Kit (Active Motif, Carlsbad, CA) (Dhandapani et al., 2007). 7.5 µg of nuclear extract were used to detect activated NFκB binding (p50 and p65) or Sp1 binding using a TransAm immunoassay (Active Motif, Carlsbad, CA), which possesses a detection limit <0.5 µg nuclear extract, and is up to five times more sensitive than a traditional electromobility shift assay (EMSA). Nuclear extracts from TPA-stimulated Jurkat cells (2.5 µg/well) were used as a positive control. To demonstrate binding specificity, a 20-fold excess of NFκB wild type consensus oligonucleotide (20 pmol/well) was used as a competitor to block specific NFκB binding to the well. Conversely, a mutated consensus NFκB oligonucleotide had no effect on NFκB binding, further demonstrating the specificity of the reaction (data not shown).
2.8. Western blotting
Whole cell lysates were collected in radioimmunoprecipitation buffer containing protease inhibitor cocktail, phosphatase-inhibitor cocktail and phenyl methane sulfonyl fluoride. Cell lysates were sonicated, centrifuged for 5 minutes at 14,000 rpm at 4°C, and protein concentrations were quantified by BCA protein assay kit (Pierce, Rockford, IL). 50 µg of protein were resolved on a 4–20% sodium dodecyl sulfate-polyacrylamide gel and transferred onto a polyvinylidene difluoride membrane. Blots were incubated overnight at 4°C in Bcl-2, Bcl-xL, or Bax (Cell Signaling Technology, Beverly, MA), β-actin (Abcam, Cambridge, MA), pro-caspase-3, (Santa Cruz Biotechnology, Santa Cruz, CA), or PARP (Trevigen, Gaithersburg, MD) primary antibody followed incubation with a corresponding Alexa Fluor 750 secondary antibody. Blots were visualized using the Li-Cor Odyssey near-infrared imaging system and quantified using Quantity One software (Bio-Rad, Foster City, CA).
2.9. Determination of Akt activation
Following treatments, cells were washed and collected in radioimmunoprecipitation buffer (1X phosphate-buffered saline, 1% IGEPAL, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate supplemented with phenyl-methylsulfonyl fluoride, aprotinin, and sodium orthovanadate). Lysates were cleared through a 21 gauge needle to shear DNA and then incubated on ice. Protein concentrations were determined using a BCA assay (Pierce, Rockford, IL). Levels of total Akt or phosphorylated AktSer473 were measured using enzyme-linked assays (Invitrogen, Carlsbad, CA), per our group (Dhandapani et al., 2007). Data was expressed as the ratio of AktSer473 (U/mL)/Total Akt (pg/mL).
2.10. Statistical analysis
The effects of treatments were analyzed using a one-way analysis of variance followed by Student Newman Keul’s post-hoc test. Unless otherwise noted, n≥5 and experiments were performed in triplicate to verify results. Results are expressed as mean ± SEM. A p value <0.05 was considered to be significant.
3. RESULTS
3.1. Dietary compounds inhibit human SK-N-AS neuroblastoma cell viability
Curcumin (IC50=24.9 µM), andrographolide (IC50=26.2 µM), wedelolactone (IC50=16.2 µM), dibenzoylmethane (IC50=15.9 µM), or tanshinone IIA (IC50=53.5 µM) treatment significantly decreased SK-N-AS cellular viability after a 48h treatment (FIGURE 1A). Curcumin elicited 52.3% cell death at a concentration of 25 µM and 100% cytotoxicity at concentrations above 50 µM, making this the most potent compound tested. Similarly, dibenzoylmethane, which structurally resembles curcumin, decreased viability by 52% at 25 µM. In contrast, the curcumin metabolites, ferulic acid and vanillic acid, did not significantly affect SK-N-AS viability (data not shown). Andrographolide had a minor, but statistically significant effect below 10 µM; however, cellular viability was reduced to 44%, 34%, 17% and 10% following treatment with 25, 50, 75 and 100 µM (p<0.01 vs. vehicle), respectively. Wedelolactone (25 µM) also attenuated cellular viability by 37%, although higher concentrations did not significantly increase cytotoxicity beyond this point. Tanshinone IIA (25 µM) was associated with a 22% reduction in cellular viability. Dietary compounds similarly reduced the viability of SK-N-BE(2) cells (FIGURE 1A), to a similar magnitude as was observed in SK-N-AS cells. None of the dietary compounds increased LDH release from primary, non-transformed mouse neuronal or glial cultures, documenting the specificity for these effects in cancer cells (data not shown)
FIGURE 1. Dietary phytochemicals reduce NB cell viability.
(A) SK-N-AS cells (wild type p53; left panel) or SK-N-BE(2) (mutated p53; right panel) cells were treated with various concentrations (5–100 µM) of curcumin (CUR), andrographolide (AND), tanshinone IIA (TAN), wedelolactone (WED), or dibenzoylmethane (DBM) for 48h prior to the determination of cellular viability by MTT assay. Drug treated groups were compared to vehicle-treated cultures, which were normalized to 100% viability. For all studies, *p<0.05, ** p<0.01, ***p<0.001 vs. vehicle. Data are representative of at least three independent trials (n=8/trial). (B) Cellular morphology was assessed in SK-N-AS cells following a 48h exposure to indicated compounds (50 µM). Digital images captured at 100x. Scale bar = 50 µm. (C) Quantification of apoptotic cell death in SK-N-AS cells using flow cytometry, as assessed by Annexin V staining. Cell viability was assessed following a 48h treatment with vehicle, andrographolide (ANDRO; 25 or 50 µM) or curcumin (CUR; 25 or 50 µM). Numbers within each panel represent both early and late apoptotic cells following treatment. Data are representative of three independent trials.
The MTT reduction assay does not discriminate between cell death and proliferation, thus cell death was determined using LDH release assays and morphological assessments. With the exception of tanshinone IIA, all compounds significantly promoted cell death, as assessed by LDH release and by an increase in the number of floating, dead cells (FIGURE 1B and data not shown). Furthermore, quantification of Annexin V labeling using flow cytometry revealed that curcumin (25, 50 µM) increased apoptotic cell death by 57% and 99%, respectively, after a 48h treatment (FIGURE 1C). Andrographolide (25, 50 µM) similarly elevated apoptotic cell death by 21% and 35%, respectively (FIGURE 1C). Dibenzoylmethane and wedelolactone increased cell death, albeit not as potently as either curcumin or andrographolide. In contrast, tanshinone IIA did not dramatically influence cytotoxicity, although fewer cells were observed, indicative of an anti-proliferative effect.
3.2. Dietary compounds induce caspase- and p53 independent cell death in NB cells
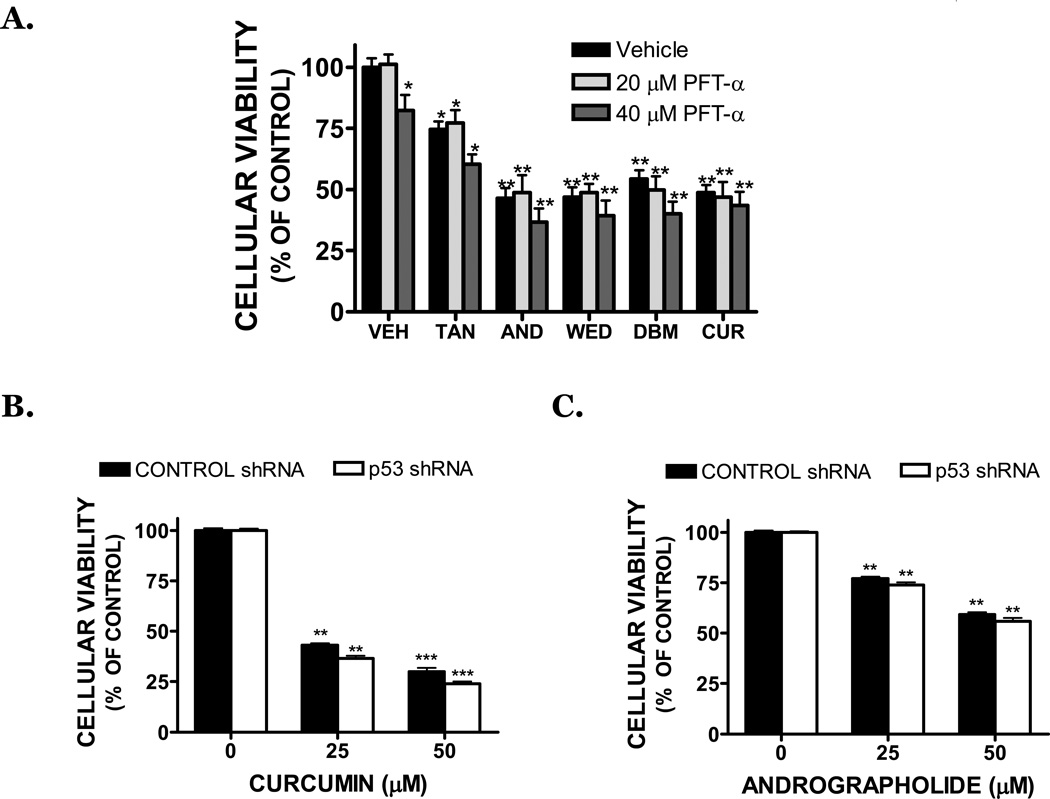
Dietary compounds induced cell death to an equal extent in both SK-N-AS, which contain a wild-type p53, and SK-N-BE(2) cells, which contain a mutated p53 gene (FIGURE 1A). To determine whether p53 functionally influences cell death after treatment, SK-N-AS cells were treated with pifithrin-α (PFT-α), an inhibitor of p53 transcriptional activity, in combination with dietary compounds. 20 µM PFT-α had no effect on basal cell viability, although treatment with 40 µM PFT-α induced a slight decrease in viability (FIGURE 2A). Notably, cell death induced by dietary compounds (25 µM) was not significantly reduced by co-treatment with PFT-α, consistent with a p53 independent mechanism of action. In further support of this possibility, p53 was stably repressed to undetectable levels in SK-N-AS using lentiviral-mediated shRNA gene knockdown. As was observed with PFT-α co-treatment, both curcumin (FIGURE 2B) and andrographolide (FIGURE 2C) (25, 50 µM) induced an equivalent degree of cell death in SK-N-AS following transduction with either control shRNA or p53 shRNA.
FIGURE 2. Dietary inhibitors induce p53-independent cell death in NB cells.
(A) SK-N-AS cells were pretreated for 30 minutes with vehicle (DMSO) or pifithrin-α (PFT-α), an inhibitor of p53-dependent transcriptional activation. Cells were then exposed to vehicle (VEH), tanshinone IIA (TAN), andrographolide (AND), wedelolactone (WED), dibenzoylmethane (DBM), or curcumin (CUR) in the continued presence of inhibitor. Cellular viability was assessed by MTT assay 48 hours after treatment. Drug-treated groups were compared to vehicle-treated cultures, which were normalized to 100% viability (*p<0.05, ** p<0.01 vs. vehicle). Data are representative of at least three independent trials (n=8/trial). SK-N-AS cells were stably transduced with a control shRNA (black bars) or with a p53 shRNA (white bars), which produced a complete knockdown of p53 expression. Cells were then treated for 48h with (B) curcumin or (C) andrographolide, followed by the assessment of cellular viability by MTT assay (**p<0.01, *** p<0.001 vs. vehicle treated cultures).
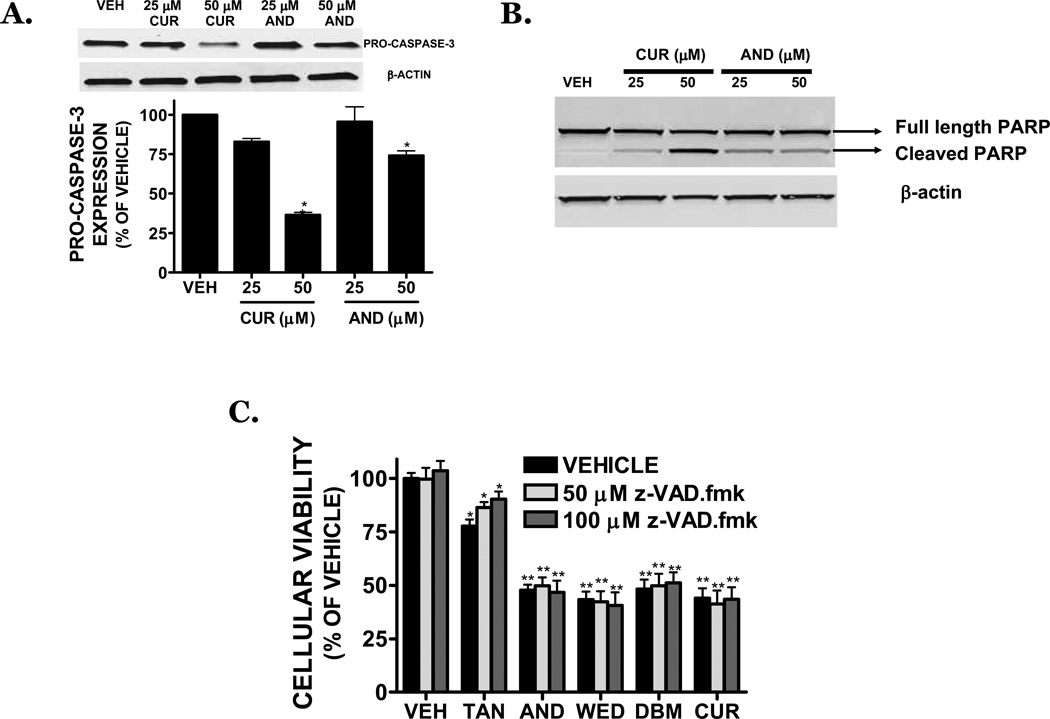
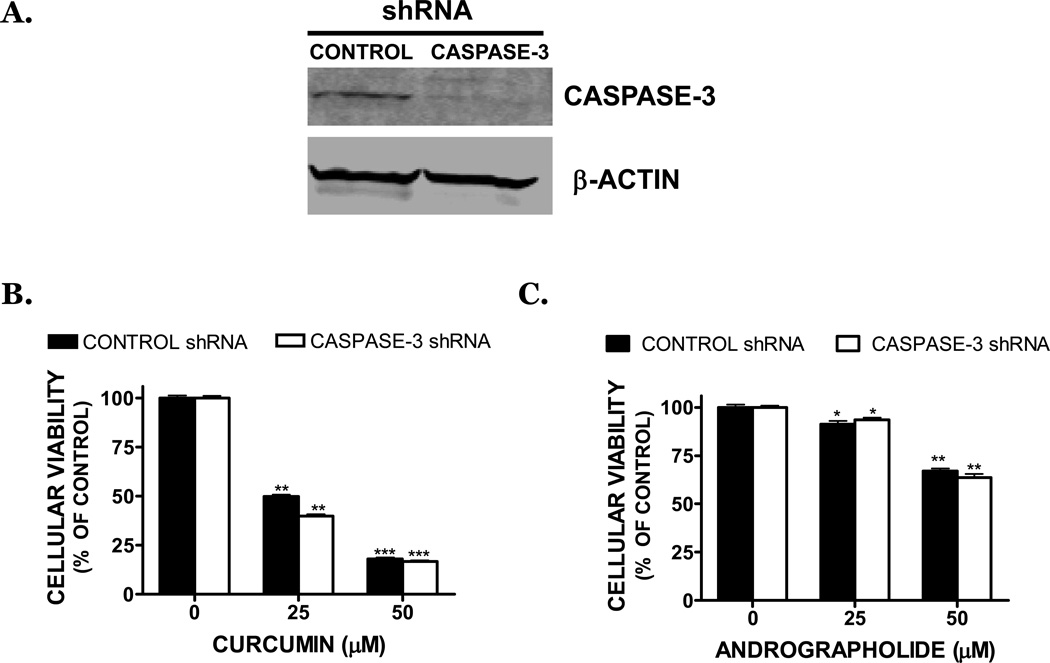
Caspase activation frequently mediates the apoptotic response. Consistent with this possibility, andrographolide and curcumin (50 µM) reduced pro-caspase-3 expression by 26% and 63%, respectively (p<0.05 vs. vehicle) (FIGURE 3A) and concomitantly increased the cleavage of caspase-3, indicative of enzyme activation (data not shown). In line with this possibility, the 89-kDa PARP cleavage product (a downstream target of caspase-3) was strongly increased after treatment with curcumin or andrographolide (25, 50 µM) (FIGURE 3B), suggesting a role for caspase activation in the cytotoxic effect of dietary compounds. However, addition of the irreversible, cell-permeable, pan-caspase inhibitor, z-VAD.fmk at concentrations (50–100 µM) that prevent caspase activation, did not significantly reverse cell death after exposure to dietary compounds (25 µM) (FIGURE 3C) and low molecular DNA fragmentation was not appreciable following treatment (data not shown). To confirm these findings using an alternative approach, SK-N-AS cells were stably transduced with caspase-3 shRNA to fully suppressed protein expression (FIGURE 4A). In contrast, cells transduced with a control shRNA exhibited similar caspase-3 expression, as compared to the non-transduced SK-N-AS cells. Notably, curcumin or andrographolide treatment of cells transduced with either control shRNA or caspase-3 shRNA produced an identical magnitude of cell death (FIGURE 4B,C). Together, these data suggest that cell death after exposure to dietary compounds may proceed independently from caspase-3 activation.
FIGURE 3. Dietary inhibitors induce caspase -independent cell death in SK-N-AS cells.
(A) (Top) Representative Western blot of pro-caspase-3, a hallmark of apoptotic cell death, following a 24h treatment with CUR (25, 50 µM) or AND (25, 50 µM). Blots were normalized to β-actin. (Bottom) Densitometry of pro-caspase-3 following CUR and AND treatment. (B) Representative Western blot showing PARP cleavage following a 24h treatment with CUR (25, 50 µM) or ANDRO (25, 50 µM). An increase in the lower, cleaved PARP band (89 KDa) is indicative of caspase activation. Bands were normalized to β-actin to demonstrate equal protein loading. Data are representative of three independent trials. (C) SK-N-AS cells were pretreated for 1h with vehicle or the irreversible pan-caspase inhibitor, z-VAD.fmk (z-VAD; 50 or 100 µM), then exposed to vehicle (VEH), tanshinone IIA (TAN), andrographolide (AND), wedelolactone (WED), dibenzoylmethane (DBM), or curcumin (CUR) in the continued presence of inhibitor.
FIGURE 4. Curcumin and andrographolide induced cell death is unaffected by genetic inhibition of caspase-3.
(A) Stable transduction of SK-N-AS cells with a caspase-3 shRNA completely reduced caspase-3 protein expression by Western blotting, as compared to cells transduced with a control shRNA. Data were normalized to β-actin to control for equal protein loading. SK-N-AS cells stably transduced with a control shRNA (black bars) or with a caspase-3 shRNA (white bars) were treated for 48h with (B) curcumin or (C) andrographolide. Cellular viability was assessed 48h later by MTT assay (*p<0.05, **p<0.01, *** p<0.001 vs. vehicle treated cultures).
3.3. Dietary compounds target the NFκB transcription factor
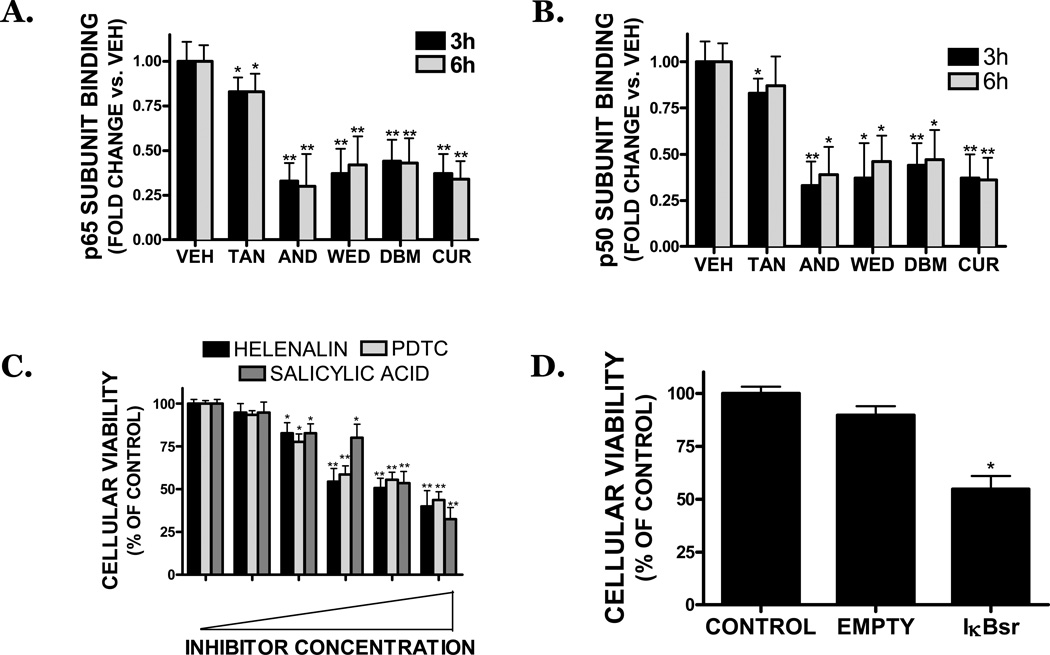
DNA binding activity of the p50 and p65 subunits of NFκB was significantly attenuated after a 3–6h treatment with dietary compounds, at concentrations that induced cell death (FIGURE 5A,B). As was observed in the cytotoxicity studies, tanshinone IIA was the least potent inhibitor of NFκB activity, reducing p65 and p50 binding by 17% and 13%, respectively. Andrographolide, wedelolactone, dibenzoylmethane, and curcumin significantly reduced NFκB activity by ∼65% at all time points. The reduction in NFκB binding was not a result of decreased expression of either p50 or p65 subunit, as both the gene and protein expression of each subunit were unaffected by experimental treatments (data not shown) and nuclear translocation of phosphorylated p65 was affected by treatment, as compared to vehicle treated cultures (data not shown). In contrast, constitutive, basal Sp1-DNA binding was unaffected by treatment with all dietary compounds, demonstrating the specificity of these compounds for NFκB (data not shown).
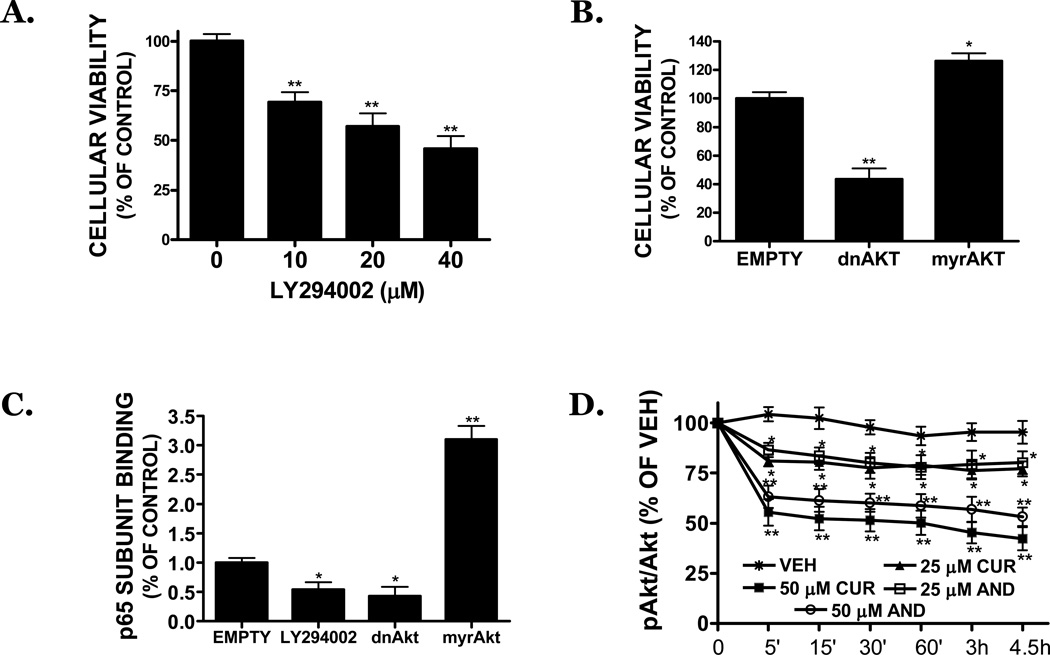
FIGURE 5. Dietary inhibitors reduce the viability of SK-N-AS cells via a reduction in NFκB activity.
SK-N-AS cells were treated with vehicle (VEH), tanshinone IIA (TAN), andrographolide (AND), wedelolactone (WED), dibenzoylmethane (DBM), or curcumin (CUR) for 3h (black bars) or 6h (grey bars). All compounds were used at a concentration of 25 µM. Following treatments, nuclear extracts were prepared and DNA binding of the NFκB subunits, (A) p65 or (B) p50 binding assessed. Treatment groups were compared with vehicle-treated cells. Data are representative of three independent trials, n=4 wells/trial. (C) Treatment of cells with pharmacological NFκB inhibitors reduced cell viability, as assessed by MTT assay, after a 48h treatment. Concentrations of inhibitors: Helenalin (0, 1, 2.5, 5, 10, 20 µM), pyrrolidine dithiocarbamate (PDTC; 0, 25, 50, 75, 100, 200 µM), salicylic acid (0, 1, 2.5, 5, 10, 20 mM). Data are representative of three independent trials, n=8/trial. (D) Overexpression of an IκB superrepressor (IκBsr) reduced cell viability, as assessed by MTT assay, as compared to control (mock transfected) and empty vector transfected cultures. Data are the mean ± SEM from three independent experiments, n=5/experiment. For all studies, * p<0.05, **p<0.01 vs. control.
Treatment with a p65 translocation inhibitor (helenalin), an IKK inhibitor (salicylic acid), or a NFκB inhibitor (PDTC) concentration-dependently reduced cellular viability after a 48h treatment (FIGURE 5C). Helenalin (10 µM) reduced viability by ∼50%, suggesting p50/p65 heterodimers contribute to the survival of SK-N-AS cells. (FIGURE 5C). Similarly, salicylic acid reduced cellular viability, implicating the IKK-IκB signaling pathway in NB cellular survival (FIGURE 5C). This possibility was further supported by a ∼54% reduction in viability following overexpression of an IκB superrrepressor, which retains NFκB in the cytosol and thereby prevents transcriptional activation. In contrast, mock transfection or overexpression of an empty vector did not affect cellular viability (FIGURE 5D).
3.4. Akt mediates the constitutive activity of NFκB in SK-N-AS
The PI3 kinase inhibitor, LY294002, significantly decreased cellular viability by 31%, 43%, and 54% after a 48h treatment with 10, 20, or 40 µM, respectively (p<0.05 vs. vehicle) (FIGURE 6A). Overexpression of a dominant negative Akt similarly reduced cell viability by 56%, as compared to empty vector transfected controls (FIGURE 6B). In contrast, overexpression of a constitutively activated Akt construct resulted in a modest (26%), but statistically significant increase in cellular viability. Basal activation of Akt was associated with NFκB activation, as overexpression of a dominant negative Akt construct reduced p65 subunit binding by 57%, an effect that was also achieved by treatment with LY294002 (20 µM) (46% inhibition). Conversely, overexpression of a constitutively active Akt construct increased p65 binding by 210%, as compared to empty vector transfected cultures (FIGURE 6C). Paralleling the reduction in NFκB activation and cellular viability, curcumin and andrographolide (25, 50 µM) reduced the phosphorylation of AktS473, a residue essential for kinase activation, by 45–58% and 37–47%, respectively, over the course of the study (FIGURE 6D).
FIGURE 6. Curcumin and andrographolide inhibit NFκB via a reduction in Akt signaling.
(A) LY294002, an inhibitor of Akt signaling, reduces the viability of SK-N-AS cells following a 48h treatment, as assessed by MTT assay. (B) Transfection of cells with a dominant negative Akt (dnAkt) attenuates cellular viability, as assessed by MTT assay, whereas overexpression of a constitutively active Akt (myrAkt) construction increased cell proliferation. (C) LY294002 (20 µM) or overexpression of dnAkt significantly reduced NFκB (p65) DNA binding. In contrast, myrAkt significantly increased p65 binding. Data are representative of at least three independent trials, with n=6/trial. (D) Curcumin (CUR; 25, 50 µM) or andrographolide (AND; 25,50 µM) reduced the basal activation of Akt in SK-N-AS within the first 4.5h of treatment. For all studies, * p<0.05, ** p<0.01 vs. control.
3.5. Dietary inhibitors suppress anti-apoptotic mediators and increase cell death following exposure to chemotherapeutic agents
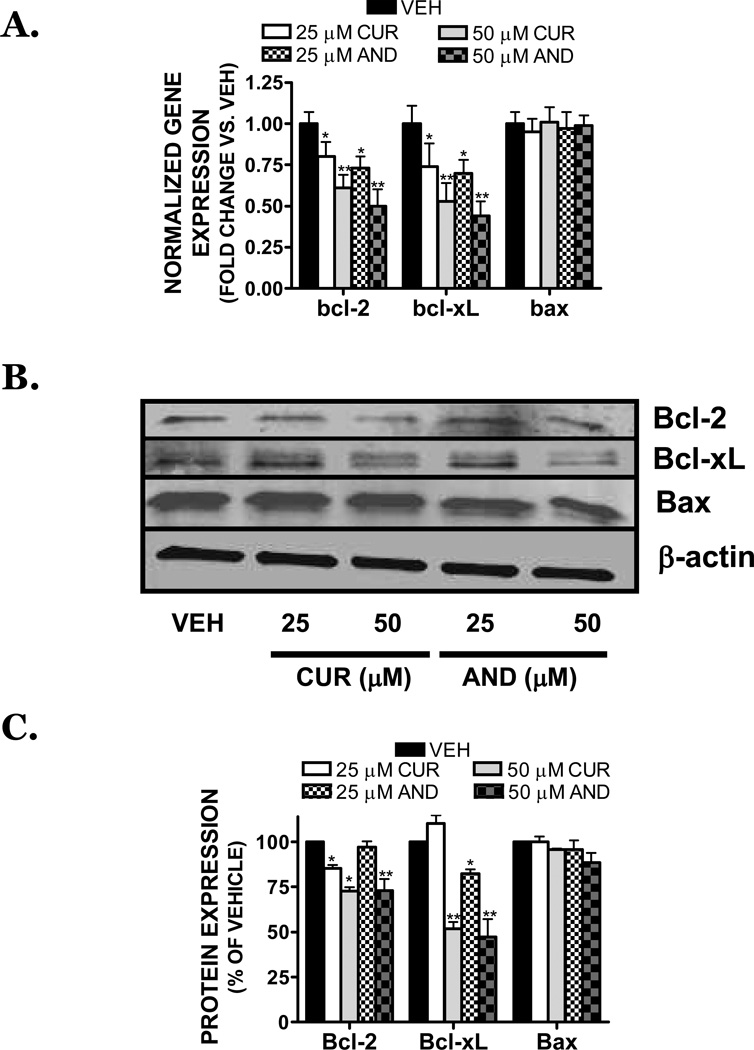
Anti-apoptotic members of the bcl-2 family, including bcl-2 and bcl-xL, are regulated by NFκB. Curcumin (25 µM) reduced the gene expression of bcl-2 and bcl-xL by 20% and 26%, respectively, and by 39% and 47% following treatment with 50 µM (FIGURE 7A). Similarly, 25 µM andrographolide reduced bcl-2 and bcl-xL gene expression by 27% and 30%, whereas 50 µM decreased expression by 50% and 56%, respectively (FIGURE 7A). In contrast, the expression of bax, a pro-apoptotic member of the bcl-2 family, was unaffected by treatment with either compound. These changes in gene expression were mirrored at the protein level, with curcumin and andrographolide significantly reducing Bcl-2 and Bcl-xL protein expression (FIGURE 7B,C).
FIGURE 7. Curcumin and andrographolide attenuate anti-apoptotic gene expression in SK-N-AS cells.
(A) Treatment with vehicle (VEH), curcumin (CUR; 25, 50 µM), or andrographolide (AND; 25, 50 µM) for 6h decreased bcl-2 and bcl-xL gene expression, without influencing the gene expression of pro-apoptotic, bax. Data were normalized to the housekeeping gene, RPS3, and expressed as fold change vs. vehicle-treated cultures. Different subscripts denote significant differences, p<0.05. (B) Representative Western blot of Bcl-2, Bcl-xL, Bax, or β-actin after a 24h treatment with CUR (25, 50 µM) or AND (25, 50 µM). (C) Densitometric quantification of Bcl-2, Bcl-xL, and Bax (as shown in panel (B)). Data are representative of three independent trials (n=5/experiment) and are presented as % vehicle, which was normalized to 100%. *p <0.05, **p<0.01 vs. control.
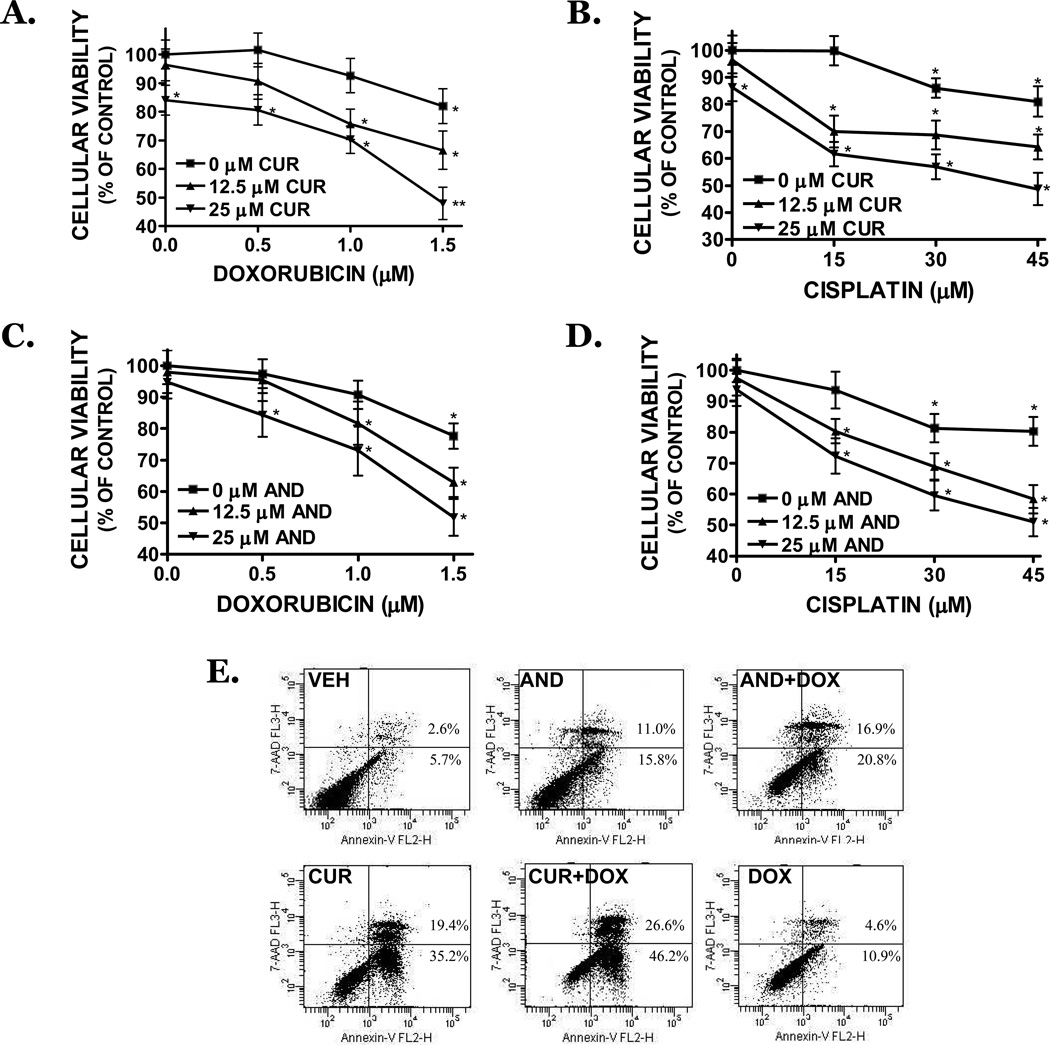
Bcl-2 and Bcl-xL promote tumor chemoresistance; thus, down regulation of these factors may increase the responsiveness of NB cells to chemotherapeutic agents. Pretreatment with either curcumin or andrographolide (15–25 µM), followed by a 1.5h exposure to clinically-relevant concentrations of either cisplatin or doxorubicin, potentiated cytotoxicity by 72h (FIGURE 8A–D). Whereas a 1.5h exposure to curcumin alone reduced cellular viability by ∼16% and 1.5 µM doxorubicin reduced viability by 18%, curcumin and doxorubicin together elicited a 52% reduction in cell viability. Similarly, 30 µM cisplatin reduced cellular viability by ∼11%; however, in conjunction with curcumin, reduced cellular viability by 53%. Andrographolide (25 µM) induced ∼7% cell death alone, yet when added in combination with either cisplatin or doxorubicin, attenuated cellular viability by ∼50%. Consistent with these data, both curcumin and andrographolide strongly increased the percentage of Annexin V+/7-AAD− cells after treatment with doxorubicin (250 nM), indicative of an apoptotic mechanism of action (FIGURE 8E). As was observed in the cellular viability assays, a maximal apoptotic effect (72.8%) was observed following co-treatment with curcumin and doxorubicin.
FIGURE 8. Curcumin and andrographolide increase cisplatin and doxorubicin toxicity in NB cells.
SK-N-AS cells were pretreated for 1.5h with (A,B) curcumin (CUR; 15, 25 µM) or (C,D) andrographolide (AND; 15, 25 µM), then exposed to (A,C) doxorubicin (0.5–1.5 µM) or (B,D) cisplatin (10–30 µM) or for 1.5h. Media was then removed and cells were cultured for 72h further in complete culture media prior to the assessment of cell viability by MTT assay. Data are representative of three independent trials (n=8/experiment). * p<0.05 vs. vehicle-treated cultures. (E) Cells were pre-treated for 24h with curcumin (CUR; 25 µM) or andrographolide (ANDRO; 25 µM), then co-treated with doxorubicin (DOX; 250 nM) for 24h. Following treatments, cells were stained with 7-AAD (y-axis), a dead cell marker, and Annexin V (x-axis), a marker of early apoptotic cell death, and analyzed by flow cytometry. The percentage of early (lower right quadrant) and late (upper right quadrant) apoptotic cell death are provided. Data are representative of three independent experiments.
4. DISCUSSION
In the present investigation, clinically-useful dietary phytochemicals reduced the growth of human S-type NB cells. These effects may be functionally independent from caspase- and p53-activation and were associated with inhibition of the Akt-NFκB signaling pathway. Curcumin and andrographolide, the two most potent compounds investigated, also potentiated cell death in response to treatment with cisplatin and doxorubicin. Given the support for these phytochemicals in promoting neuronal survival and plasticity in non-transformed cells, dietary compounds may represent a novel adjunct therapeutic for the treatment of NB.
The p53 tumor suppressor mediates the cellular response to radiation and chemotherapeutic drugs, inducing cell cycle arrest and apoptosis in NB (Cui et al., 2002). Pharmacological (PFT-α) or genetic (SK-N-BE(2) cells, shRNA-mediated p53 knockdown) inhibition of p53 did not reduce the cytotoxicity of dietary compounds, suggesting p53 transactivation was not causally linked with NB cell death; however, we cannot exclude the possibility that p53 may exert divergent effects in different cellular subtypes. In support of this notion and consistent with the differential response of N-type and S-type NB cells to cytotoxic drugs, nuclear p53 translocation correlated with growth arrest and apoptosis in curcumin-treated N-myc amplified NB cells (N-type cells) (Liontas and Yeger, 2004).
Similar to the observed p53-independent mechanism in S-type NB cells, both curcumin and andrographolide induced apoptotic cell death in NB cells via a mechanism that did not require caspase activity and that was associated with a reduction in the bcl-2:bax ratio. Our findings are in line with previous reports in human glioma cells, which suggest both curcumin and another phytochemical, flavopiridol, promoted p53- and caspase-independent cytotoxicity via a mechanism involving apoptosis-inducing factor (AIF) translocation and a reduction in the bcl-2:bax ratio (Alonso et al., 2003; Karmakar et al., 2006). The potential involvement of AIF in phytochemical-mediated NB cell death could explain the p53- and caspase-independent apoptotic mechanism of cell death observed in this report and will be subject of future studies by our laboratory.
Activation of the NFκB transcription factor, a molecular target for both curcumin and andrographolide, is implicated in the survival of S-type NB cells (Bian et al., 2002). Consistent with this assertion, molecular inhibition of IκB phosphorylation or treatment with wedelolactone, a compound useful in the treatment of septic shock, reduced SK-N-AS viability. Wedelolactone, a furanocoumarin isolated from Eclipta prostrata, directly inhibits the IKK complex, decreasing IκB phosphorylation and subsequent NFκB activity (Kobori et al., 2004). Notably, constitutive activation of Akt, a kinase implicated in IKK activation (Ouyang et al., 2006), directly correlates with chemoresistance and a poor prognosis in NB patients (Opel et al., 2007) and was associated with cellular survival in SK-N-AS cells (this report). Similarly, molecular (dnAkt) or pharmacological (LY294002) Akt inhibition reduced basal NFκB activation, supporting a functional link between Akt activation and downstream NFκB activity in NB cells; however, the possibility that the reduction in Akt activity is secondary to NFκB inhibition cannot be fully excluded (Meng et al., 2002). Nonetheless, overexpression of either activated Akt or Bcl-2 reversed caspase-independent, ceramide-induced cytotoxicity in human NB cells (Kim et al., 2007), suggesting the regulation of bcl-2 genes may be an important determinant of NB viability. In line with this possibility, concentrations of curcumin or andrographolide that inhibited NFκB and elicited cell death, attenuated Akt activation and reduced the expression of the anti-apoptotic mediators, Bcl-2 and Bcl-xL, without influencing the pro-apoptotic mediator, Bax. Future work by our laboratory will assess the functional role for Bcl-2 family members in the induction of cell death following exposure to curcumin and andrographolide.
Curcumin, a clinically-well tolerated compound isolated from Curcuma longa, limited the progression of several high-risk cancers in humans (Cheng et al., 2001; Gabrielian et al., 2002; Scapagnini et al., 2006) and was the most potent compound for reducing NB cellular viability in this study. In contrast to the divergent effects of traditional chemotherapeutic drugs in NB (Bian et al., 2001), curcumin dramatically reduced the viability of both N-type (Liontas and Yeger, 2004) and S-type NB cells (present study). Notably, we previously demonstrated that curcumin, at concentrations that were equal or above those utilized in this study, were non-toxic in cultured neurons (Dhandapani et al., 2007; Lavoie et al., 2009; Scapagnini et al., 2006), astrocytes (Dhandapani et al., 2007; Lavoie et al., 2009), and microvessel cells (Sukumari-Ramesh et al., 2010). Furthermore, we and others determine that curcumin was neuroprotective and neurotrophic in pre-clinical models of brain injury (King et al., 2011; Laird et al., 2010; Thiyagarajan and Sharma, 2004; Wakade et al., 2009; Wu et al., 2006). Together, these findings suggest that curcumin may exhibit a broader cytotoxic effect than some drugs currently in clinical practice to treat NB without the associated detrimental effects on the normal cells of the developing nervous system.
Despite the potent toxicity observed in human S-type NB cells, curcumin is rapidly metabolized in vivo. The curcumin metabolites, ferulic acid and vanillic acid (Holder et al., 1978; Wang et al., 1997), failed to influence SK-N-AS viability, whereas dibenzoylmethane, a curcumin-related β-diketone analogue derived from licorice (Singletary et al., 1998), reduced cellular viability in a manner similar to curcumin. These data suggest the β-diketone structure of curcumin likely contributes to the observed beneficial effects in S-type NB cells. This possibility further is supported by work from our laboratory and others demonstrating NFκB transcriptional activity is attenuated by curcumin and dibenzoylmethane, but not by ferulic acid or vanillic acid (Dhandapani et al., 2007; Murakami et al., 2005; Ronchetti et al., 2006). Given the documented pharmacological safety of curcumin and scarcity of adverse side effects, curcumin (or curcumin analogues) may represent tumor-specific alternatives for the treatment of NB.
Andrographolide, a diterpenoid lactone derived from the leaves of Andrographis paniculata, is an anti-inflammatory and anti-viral compound used to treat digestive disorders and liver disease in traditional Asian medicine (Negi et al., 2008). Andrographolide is also a major component of Kan Jang tablets, a non-toxic herbal formulation to limit the symptoms of influenza (Kulichenko et al., 2003). Similar to curcumin and consistent with a report in human hepatoma cells (Scapagnini et al., 2006), andrographolide induced caspase-independent apoptosis in NB cells; however, unlike curcumin, andrographolide is rapidly and nearly completely absorbed into the bloodstream following oral administration in rats (Panossian et al., 2000), suggesting the possibility of improved bioavailability in patients.
Despite significant adverse side toxicity, doxorubicin and cisplatin remain front-line chemotherapeutics for the treatment of NB. Thus, it is noteworthy that both curcumin and andrographolide potentiated the apoptotic response of cisplatin and doxorubicin in NB cells. Although the mechanism(s) underlying this response remain to be elucidated, Bcl-2 family members are associated with chemoresistance in NB (Dole et al., 1994; Dole et al., 1995). These data suggest the apoptotic effect of curcumin and andrographolide may be mediated, at least in part, via the regulation of Bcl-2 and Bcl-xL. Future studies to fully demonstrate these effects in a pre-clinical model of NB remain to be demonstrated; however, if proven, dietary NFκB inhibitors may permit the use of lower doses of chemotherapeutics (thereby reducing associated neurotoxicity). Additionally, curcumin limits doxorubicin toxicity in the heart and kidney (Venkatesan, 1998; Venkatesan et al., 2000), suggesting a secondary benefit of limiting end organ damage.
As a whole, these studies identify a novel cytotoxic and chemosensitizing effect of dietary NFκB inhibitors in human S-type NB cells. Given the proven clinical safety of these compounds, dietary NFκB inhibitors, such as curcumin and andrographolide, may represent a potent medical adjunct for the treatment of NB, warranting further investigation.
ACKNOWLEDGEMENTS
The authors’ research was supported in part by grants from the National Institutes of Health (NS065172) and the American Heart Association (BGIA2300135) to KMD, by a fellowship from the American Heart Association (2250690) to MDL, and by a grant from the A.R. Staulcup Foundation.
List of abbreviations
- 7-AAD
7-aminoactinomycin D
- AIF
Apoptosis inducing factor
- IκB
Inhibitor of NFκB
- LDH
Lactate Dehydrogenase
- NB
Neuroblastoma
- NFκB
Nuclear factor-κB
- N-type
Neuroblastic cells
- PFT-α
pifithrin-α
- S-type
Stromal (Schwannian) cells
REFERENCES
- Alonso M, Tamasdan C, Miller DC, Newcomb EW. Flavopiridol induces apoptosis in glioma cell lines independent of retinoblastoma and p53 tumor suppressor pathway alterations by a caspase-independent pathway. Mol Cancer Ther. 2003;2:139–150. [PubMed] [Google Scholar]
- Balachandran P, Govindarajan R. Cancer--an ayurvedic perspective. Pharmacol Res. 2005;51:19–30. doi: 10.1016/j.phrs.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Bian X, McAllister-Lucas LM, Shao F, Schumacher KR, Feng Z, Porter AG, Castle VP, Opipari AW., Jr NF-kappa B activation mediates doxorubicin-induced cell death in N-type neuroblastoma cells. J Biol Chem. 2001;276:48921–48929. doi: 10.1074/jbc.M108674200. [DOI] [PubMed] [Google Scholar]
- Bian X, Opipari AW, Jr, Ratanaproeksa AB, Boitano AE, Lucas PC, Castle VP. Constitutively active NFkappa B is required for the survival of S-type neuroblastoma. J Biol Chem. 2002;277:42144–42150. doi: 10.1074/jbc.M203891200. [DOI] [PubMed] [Google Scholar]
- Biedler JL, Helson L, Spengler BA. Morphology and growth, tumorigenicity, and cytogenetics of human neuroblastoma cells in continuous culture. Cancer Res. 1973;33:2643–2652. [PubMed] [Google Scholar]
- Biedler JL, Spengler BA, Chang TD, Ross RA. Transdifferentiation of human neuroblastoma cells results in coordinate loss of neuronal and malignant properties. Prog Clin Biol Res. 1988;271:265–276. [PubMed] [Google Scholar]
- Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, Yu HS, Jee SH, Chen GS, Chen TM, Chen CA, Lai MK, Pu YS, Pan MH, Wang YJ, Tsai CC, Hsieh CY. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]
- Cragg GM, Newman DJ, Snader KM. Natural products in drug discovery and development. J Nat Prod. 1997;60:52–60. doi: 10.1021/np9604893. [DOI] [PubMed] [Google Scholar]
- Cui H, Schroering A, Ding HF. p53 mediates DNA damaging drug-induced apoptosis through a caspase-9-dependent pathway in SH-SY5Y neuroblastoma cells. Mol Cancer Ther. 2002;1:679–686. [PubMed] [Google Scholar]
- Dhandapani KM, Mahesh VB, Brann DW. Curcumin suppresses growth and chemoresistance of human glioblastoma cells via AP-1 and NFkappaB transcription factors. J Neurochem. 2007;102:522–538. doi: 10.1111/j.1471-4159.2007.04633.x. [DOI] [PubMed] [Google Scholar]
- Dole M, Nunez G, Merchant AK, Maybaum J, Rode CK, Bloch CA, Castle VP. Bcl-2 inhibits chemotherapy-induced apoptosis in neuroblastoma. Cancer Res. 1994;54:3253–3259. [PubMed] [Google Scholar]
- Dole MG, Jasty R, Cooper MJ, Thompson CB, Nunez G, Castle VP. Bcl-xL is expressed in neuroblastoma cells and modulates chemotherapy-induced apoptosis. Cancer Res. 1995;55:2576–2582. [PubMed] [Google Scholar]
- Eisenberg HM, Gary HE, Jr, Aldrich EF, Saydjari C, Turner B, Foulkes MA, Jane JA, Marmarou A, Marshall LF, Young HF. Initial CT findings in 753 patients with severe head injury. A report from the NIH Traumatic Coma Data Bank. J Neurosurg. 1990;73:688–698. doi: 10.3171/jns.1990.73.5.0688. [DOI] [PubMed] [Google Scholar]
- Gabrielian ES, Shukarian AK, Goukasova GI, Chandanian GL, Panossian AG, Wikman G, Wagner H. A double blind, placebo-controlled study of Andrographis paniculata fixed combination Kan Jang in the treatment of acute upper respiratory tract infections including sinusitis. Phytomedicine. 2002;9:589–597. doi: 10.1078/094471102321616391. [DOI] [PubMed] [Google Scholar]
- Holder GM, Plummer JL, Ryan AJ. The metabolism and excretion of curcumin (1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) in the rat. Xenobiotica. 1978;8:761–768. doi: 10.3109/00498257809069589. [DOI] [PubMed] [Google Scholar]
- Humpl T. Neuroblastoma. World J Urol. 1995;13:233–239. doi: 10.1007/BF00182969. [DOI] [PubMed] [Google Scholar]
- Karacay B, Sanlioglu S, Griffith TS, Sandler A, Bonthius DJ. Inhibition of the NF-kappaB pathway enhances TRAIL-mediated apoptosis in neuroblastoma cells. Cancer Gene Ther. 2004;11:681–690. doi: 10.1038/sj.cgt.7700749. [DOI] [PubMed] [Google Scholar]
- Karmakar S, Banik NL, Patel SJ, Ray SK. Curcumin activated both receptor-mediated and mitochondria-mediated proteolytic pathways for apoptosis in human glioblastoma T98G cells. Neurosci Lett. 2006;407:53–58. doi: 10.1016/j.neulet.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Kelloff GJ, Crowell JA, Steele VE, Lubet RA, Malone WA, Boone CW, Kopelovich L, Hawk ET, Lieberman R, Lawrence JA, Ali I, Viner JL, Sigman CC. Progress in cancer chemoprevention: development of diet-derived chemopreventive agents. J Nutr. 2000;130:467S–471S. doi: 10.1093/jn/130.2.467S. [DOI] [PubMed] [Google Scholar]
- Kim NH, Kim K, Park WS, Son HS, Bae Y. PKB/Akt inhibits ceramide-induced apoptosis in neuroblastoma cells by blocking apoptosis-inducing factor (AIF) translocation. J Cell Biochem. 2007;102:1160–1170. doi: 10.1002/jcb.21344. [DOI] [PubMed] [Google Scholar]
- King MD, McCracken DJ, Wade FM, Meiler SE, Alleyne CH, Dhandapani KM. Attenuation of hematoma size and neurological injury with curcumin following intracerebral hemorrhage in mice. J Neurosurg. 2011 doi: 10.3171/2011.2.JNS10784. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobori M, Yang Z, Gong D, Heissmeyer V, Zhu H, Jung YK, Gakidis MA, Rao A, Sekine T, Ikegami F, Yuan C, Yuan J. Wedelolactone suppresses LPS-induced caspase-11 expression by directly inhibiting the IKK complex. Cell Death Differ. 2004;11:123–130. doi: 10.1038/sj.cdd.4401325. [DOI] [PubMed] [Google Scholar]
- Kulichenko LL, Kireyeva LV, Malyshkina EN, Wikman G. A randomized, controlled study of Kan Jang versus amantadine in the treatment of influenza in Volgograd. J Herb Pharmacother. 2003;3:77–93. [PubMed] [Google Scholar]
- Laird MD, Sukumari-Ramesh S, Swift AE, Meiler SE, Vender JR, Dhandapani KM. Curcumin attenuates cerebral edema following traumatic brain injury in mice: a possible role for aquaporin-4? 2010;J Neurochem113:637–648. doi: 10.1111/j.1471-4159.2010.06630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie S, Chen Y, Dalton TP, Gysin R, Cuenod M, Steullet P, Do KQ. Curcumin, quercetin, and tBHQ modulate glutathione levels in astrocytes and neurons: importance of the glutamate cysteine ligase modifier subunit. J Neurochem. 2009;108:1410–1422. doi: 10.1111/j.1471-4159.2009.05908.x. [DOI] [PubMed] [Google Scholar]
- Liontas A, Yeger H. Curcumin and resveratrol induce apoptosis and nuclear translocation and activation of p53 in human neuroblastoma. Anticancer Res. 2004;24:987–998. [PubMed] [Google Scholar]
- Meng F, Liu L, Chin PC, D’Mello SR. Akt is a downstream target of NF-kappa B. J Biol Chem. 2002;277:29674–29680. doi: 10.1074/jbc.M112464200. [DOI] [PubMed] [Google Scholar]
- Mora J, Cheung NK, Juan G, Illei P, Cheung I, Akram M, Chi S, Ladanyi M, Cordon-Cardo C, Gerald WL. Neuroblastic and Schwannian stromal cells of neuroblastoma are derived from a tumoral progenitor cell. Cancer Res. 2001;61:6892–6898. [PubMed] [Google Scholar]
- Murakami Y, Ito S, Atsumi T, Fujisawa S. Theoretical prediction of the relationship between phenol function and COX-2/AP-1 inhibition for ferulic acid-related compounds. In Vivo. 2005;19:1039–1043. [PubMed] [Google Scholar]
- Negi AS, Kumar JK, Luqman S, Shanker K, Gupta MM, Khanuja SP. Recent advances in plant hepatoprotectives: a chemical and biological profile of some important leads. Med Res Rev. 2008;28:746–772. doi: 10.1002/med.20115. [DOI] [PubMed] [Google Scholar]
- Opel D, Poremba C, Simon T, Debatin KM, Fulda S. Activation of Akt predicts poor outcome in neuroblastoma. Cancer Res. 2007;67:735–745. doi: 10.1158/0008-5472.CAN-06-2201. [DOI] [PubMed] [Google Scholar]
- Ouyang W, Li J, Ma Q, Huang C. Essential roles of PI-3K/Akt/IKKbeta/NFkappaB pathway in cyclin D1 induction by arsenite in JB6 Cl41 cells. Carcinogenesis. 2006;27:864–873. doi: 10.1093/carcin/bgi321. [DOI] [PubMed] [Google Scholar]
- Panossian A, Hovhannisyan A, Mamikonyan G, Abrahamian H, Hambardzumyan E, Gabrielian E, Goukasova G, Wikman G, Wagner H. Pharmacokinetic and oral bioavailability of andrographolide from Andrographis paniculata fixed combination Kan Jang in rats and human. Phytomedicine. 2000;7:351–364. doi: 10.1016/S0944-7113(00)80054-9. [DOI] [PubMed] [Google Scholar]
- Rajeevan MS, Ranamukhaarachchi DG, Vernon SD, Unger ER. Use of real-time quantitative PCR to validate the results of cDNA array and differential display PCR technologies. Methods. 2001;25:443–451. doi: 10.1006/meth.2001.1266. [DOI] [PubMed] [Google Scholar]
- Ronchetti D, Impagnatiello F, Guzzetta M, Gasparini L, Borgatti M, Gambari R, Ongini E. Modulation of iNOS expression by a nitric oxide-releasing derivative of the natural antioxidant ferulic acid in activated RAW 264.7 macrophages. Eur J Pharmacol. 2006;532:162–169. doi: 10.1016/j.ejphar.2005.12.034. [DOI] [PubMed] [Google Scholar]
- Ross RA, Spengler BA, Biedler JL. Coordinate morphological and biochemical interconversion of human neuroblastoma cells. J Natl Cancer Inst. 1983;71:741–747. [PubMed] [Google Scholar]
- Scapagnini G, Colombrita C, Amadio M, D’Agata V, Arcelli E, Sapienza M, Quattrone A, Calabrese V. Curcumin activates defensive genes and protects neurons against oxidative stress. Antioxid Redox Signal. 2006;8:395–403. doi: 10.1089/ars.2006.8.395. [DOI] [PubMed] [Google Scholar]
- Schmidt ML, Lukens JN, Seeger RC, Brodeur GM, Shimada H, Gerbing RB, Stram DO, Perez C, Haase GM, Matthay KK. Biologic factors determine prognosis in infants with stage IV neuroblastoma: A prospective Children's Cancer Group study. J Clin Oncol. 2000;18:1260–1268. doi: 10.1200/JCO.2000.18.6.1260. [DOI] [PubMed] [Google Scholar]
- Singletary K, MacDonald C, Iovinelli M, Fisher C, Wallig M. Effect of the beta-diketones diferuloylmethane (curcumin) and dibenzoylmethane on rat mammary DNA adducts and tumors induced by 7,12-dimethylbenz[a]anthracene. Carcinogenesis. 1998;19:1039–1043. doi: 10.1093/carcin/19.6.1039. [DOI] [PubMed] [Google Scholar]
- Stiller CA, Parkin DM. International variations in the incidence of neuroblastoma. Int J Cancer. 1992;52:538–543. doi: 10.1002/ijc.2910520407. [DOI] [PubMed] [Google Scholar]
- Sukumari-Ramesh S, Laird MD, Singh N, Vender JR, Alleyne CH, Jr, Dhandapani KM. Astrocyte-derived glutathione attenuates hemin-induced apoptosis in cerebral microvascular cells. Glia. 2010;58:1858–1870. doi: 10.1002/glia.21055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiyagarajan M, Sharma SS. Neuroprotective effect of curcumin in middle cerebral artery occlusion induced focal cerebral ischemia in rats. Life Sci. 2004;74:969–985. doi: 10.1016/j.lfs.2003.06.042. [DOI] [PubMed] [Google Scholar]
- Venkatesan N. Curcumin attenuation of acute adriamycin myocardial toxicity in rats. Br J Pharmacol. 1998;124:425–427. doi: 10.1038/sj.bjp.0701877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan N, Punithavathi D, Arumugam V. Curcumin prevents adriamycin nephrotoxicity in rats. Br J Pharmacol. 2000;129:231–234. doi: 10.1038/sj.bjp.0703067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakade C, King MD, Laird MD, Alleyne CH, Jr, Dhandapani KM. Curcumin attenuates vascular inflammation and cerebral vasospasm after subarachnoid hemorrhage in mice. Antioxid Redox Signal. 2009;11:35–45. doi: 10.1089/ars.2008.2056. [DOI] [PubMed] [Google Scholar]
- Wang YJ, Pan MH, Cheng AL, Lin LI, Ho YS, Hsieh CY, Lin JK. Stability of curcumin in buffer solutions and characterization of its degradation products. J Pharm Biomed Anal. 1997;15:1867–1876. doi: 10.1016/s0731-7085(96)02024-9. [DOI] [PubMed] [Google Scholar]
- Wu A, Ying Z, Gomez-Pinilla F. Dietary curcumin counteracts the outcome of traumatic brain injury on oxidative stress, synaptic plasticity, and cognition. Exp Neurol. 2006;197:309–317. doi: 10.1016/j.expneurol.2005.09.004. [DOI] [PubMed] [Google Scholar]