Abstract
Dendritic cells are arguably the most potent antigen-presenting cells and may be the only cells capable of initiating the adaptive immune response. The epithelial residents of dendritic cells are Langerhans cells, which serve as the “sentinels” of the mucosa, altering the immune system not only to pathogen entry but also of tolerance to self antigen and commensal microbes. Oral mucosal Langerhans cells are capable of engaging and internalizing a wide variety of pathogens and have been found responsive to nickel in patients with nickel allergies, oral Candida species, oral lichen planus, lichenoid drug eruptions, graft versus host diseases, periodontal diseases median rhomboid glossitis, human immunodeficiency virus infection, hairy leukoplakia of the tongue, and oral squamous cell carcinoma. Review focuses on the role of antigen-presenting cells in particular Langerhans cells to better understand the mechanisms underlying immune responses. In this review, comprehensive detail about mucosal diseases has been compiled using the PubMed database and through textbooks.
Keywords: Antigen-presenting cells, Dendritic cells, Langerhans cells, Oral mucosal diseases
Introduction
The term “Dendritic cell” (DC), which now refers to a family of antigen-presenting cells, including Langerhans cells (LCs), was coined by Steinman and Cohn.[1] The DCs represent a large family of antigen-presenting cells that circulates through the bloodstream and are scattered in nearly all tissues of the body. DCs occupy a unique niche in the innate immune system by serving as a “bridge” to the adaptive immune response, and have a powerful capacity to activate immunologically naïve T-cells in an antigen specific way.[2] In the peripheral tissues of humans, three major DC subsets have been described, including two in the myeloid lineage-LCs and interstitial DCs (also known as dermal DCs), and the third being lymphoid or plasmacytoid DCs.[3]
Although first discovered in 1868,[4] the epidermal dendritic LCs remained enigmatic for over a century, until they were identified as the most peripheral outpost of the immune system.[5] And for many years these were regarded as ectodermal cells, artifacts, melanocytes or neural elements such as Schwann cells. As sentinels of immune system, LCs survey the epithelial surface for antigenic pathogens and by initiating immune response against them, secure the integrity and homeostasis of the body's most peripheral organ. LCs were first described in human oral mucosa.[6] Further, recent studies have highlighted mucosal LCs as important arbiters of mucosal immunity, in response to antigen of microbial or tumor origin, but also of tolerance to self antigen and commensal microbes.[3] Oral mucosal LCs have been found responsive to nickel in patients with nickel allergies, oral Candida species, oral lichen planus (OLP), lichenoid drug eruptions, rhomboid median glossitis, human immunodeficiency virus (HIV) infection, hairy leukoplakia of the tongue, oral squamous cell carcinoma, and several other diseases.[3,7,8,9,10,11,12]
In this review, LCs and their immune responses are discussed. The section focuses the role of LCs in oral mucosal diseases, and updates the recent advances in immunologic role of LCs.
Microanatomy of LCs
A detailed ultrastructural analysis of oral mucosal LCs within gingival epithelium has been studied.-[13] LCs in situ possess between 5 and 9 dendrites that extend out in the same horizontal plane and cover about 25% of the surface area of the skin and mucosa [Figure 1].[14] Desmosomes and tonofilaments are absent, the nuclei have clefts, and lysosomes, centrioles, Golgi vesicles, small amount of endoplasmic reticulum, and moderate numbers of mitochondria are seen. Immunohistochemistry later confirmed the presence of vimentin in skin and oral mucosal LCs. Characteristic feature is the presence of Birbeck granules (100 nm to 1 mm in size), some of which are continuous with the cell membrane, appear either as rod-shaped bodies or, if the terminal vesicle is present, as the classic tennis-racket shape.[15] These granules were first described in LCs by Birbeck et al.[16] and are often referred to as “Langerhans cell granules.” Labeling techniques revealed that Birbeck granule form as a consequence of endocytic, clathrin-associated invaginations of the cell membrane. The function of Birbeck granule is unknown and it has often been associated to antigen-trapping and antigen-presenting function and the development of Birbeck granule is thought to be linked to the epidermal micromilieu. However, few support the view that Birbeck granules are not a prerequisite for function to stimulate T-cells and instead represented a peculiar form of endocytosis.[17]
Figure 1.
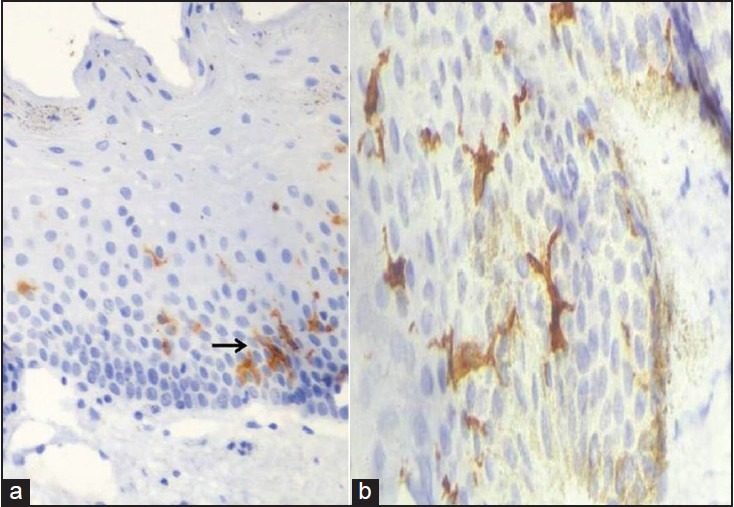
Showing CD1a positive Langerhans cells with dendritic processes within the oral mucosa (a) At 40× (b) At 100× magnification
Two types of LCs have been described based on their electron microscopic appearance: Type 1: is highly dendritic with an electronlucent cytoplasm, numerous granules and is usually found in the suprabasal layers; Type 2: shows fewer dendrites, a more electron-dense cytoplasm with fewer Birbeck granules and is usually located in the basal layer.[18]
Six electron-microscopic criteria of specificity for LCs identification has been put forth as: (a) Indented or lobulated nucleus, (b) Birbeck granules, (c) Absence of tonofilaments, (d) Well-developed Golgi apparatus with a clear cytoplasm, (e) Absence of desmosomes; and (f) Absence of melanosomes and premelanosomes.[19]
Origin
LCs originate from bone marrow precursors, which upon circulation in the peripheral blood, populate in the skin. CD34+ haematopoietic progenitor cells have now been identified as the cells committed to the LC lineage. However, it is well established that, the lineage committed to differentiation of LC is less restricted, and under specific environmental conditions, common precursors can give rise to multiple DC subtypes [Figure 2].[20] Factors like granulocyte/macrophage colony stimulating factor (GM-CSF) and tumor necrosis factor (TNF)-α have been identified, governing the development of LCs.[21] Other stimuli include interleukin-3 (IL-3), stem cell factor (SCF), and Flt3-ligand and transforming growth factor-b1(TGF-b1).[22,23]
Figure 2.
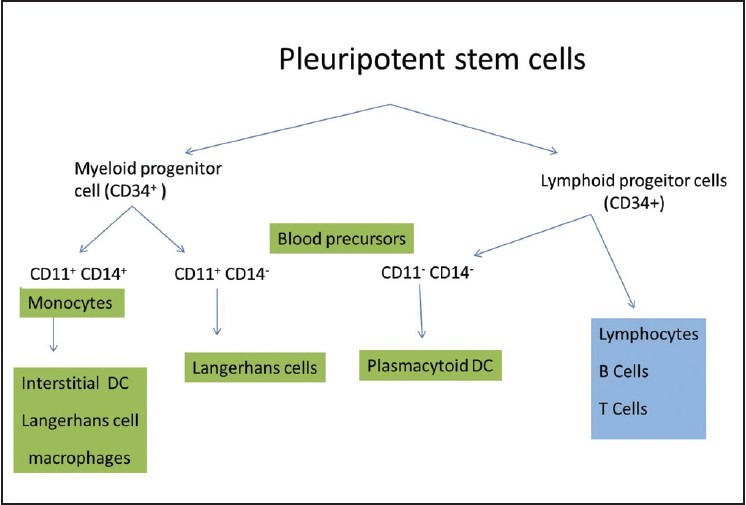
Origin of Langerhans cells from the bone marrow precursor cells
Life cycle
The exact maturational stage at which LCs enter the epidermis/mucosa is still unknown. HLA-DR/ATPase+ DCs can be identified in the human epidermis by 6-7 weeks estimated gestational age. Until 12th week of intrauterine life LCs are CD1a-negative and lack Birbeck granules. Thereafter, there occurs a dramatic increase in CD1a expression.[24]
It is well established that the LCs are chemotactically responsive to the chemokine RANTES, macrophage inflammatory protein (MIP)-1, monocyte chemotactic protein (MCP)-1, and macrophage derived chemokine (MDC), and these chemokines may play an important role in epithelial migation of LCs. CCL20/macrophage inflammatory protein 3-α/MIP3-α is an important chemokine that selectively attracts LC precursors into the epidermis, which is produced by epithelial cells, albeit predominantly under inflammatory conditions.[25]
It is a well-established concept that LCs constitute a mobile cell population with epidermal residence only one step in their life cycle. The migrating pathway is explained in [Figure 3]. LCs migrating away from the epidermis could either be replenished, by circulating LC precursors, or form a pool of self generating cells with a relatively low turnover.[26] Although several studies have suggested a relatively slow turnover of LCs, their actual lifespan remains elusive in humans, the half-life of epidermal LCs ranges from 53 to 78 days (i.e., 2-3 months), in murine skin.[27] However, it is yet unknown whether LCs die by random or by senility. The pool of LCs migrating out of the cutaneous compartment are always present in the afferent lymph that access the T-cell area, but not in efferent lymph, thus indicating that most of the migrating DCs die after their arrival in lymphoid tissues.[28]
Figure 3.
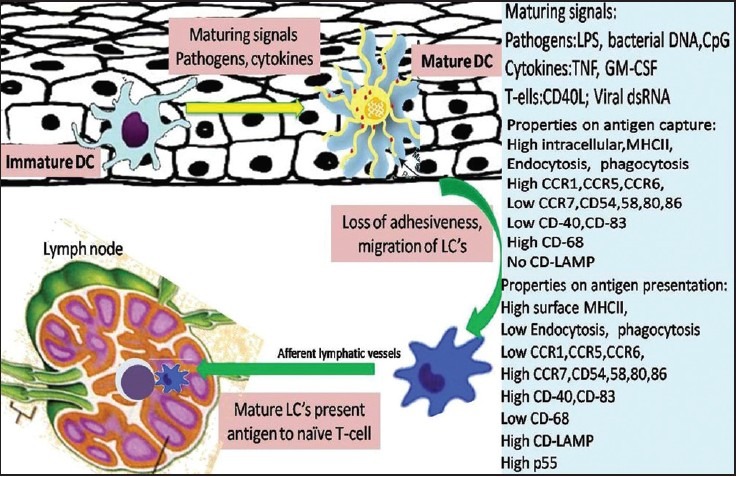
Schematic representation of LCs migration from the oral mucosa to the regional lymph node. The right side of the scheme shows the functional properties of mature and immature LCs
Functional properties
Among the earliest reports it has been found that LCs can present antigen to both helper and cytotoxic T-cells, and the removal of LCs population eliminates this stimulatory effect. The understanding of LCs interaction and immunologic function has tremendously multiplied over the time.[29]
LCs primarily present exogenous antigen-derived peptides via the MHC class II presentation to CD4+helper T-cells. LCs have also been implicated in generation of the MHC class I restricted cytotoxic (CD8+) T-cell responses, which depend on the expression of costimulatory molecules B7-1. In addition, LCs stimulate NK cells facilitated by the production of cytokines, including IL-12.[30]
In most tissues, LCs are present in a so-called “immature” state unable to stimulate T-cells. Immature LCs express an extensive array of surface receptors that can recognize various antigens. These include toll-like receptors, C-type lectin receptors, Fc-g, and complement receptors.[2] Antigens are captured by LCs in peripheral tissues and processed to form MHC-peptide complexes.[31] Mostly, although not exclusively, endogenously derived antigens are processed via the HLA class I pathway in antigen-presenting cells, including tumor cells. As a consequence LCs are mobilized and matured in response to inflammatory cytokines and pathogen associated molecular patterns from oral mucosal pathogens. LCs migrate into the lamina propria and become CD83+ mature DCs [Figure 3].[2] The maturation and migratory activity of LCs is tightly regulated by expression of chemokine receptors, selectins, chemoattractants/inflammatory chemokines, and integrins.[2] Whole bacteria, the microbial cell-wall component (Lipopolysaccharides [LPSs]), and cytokines like IL-1β, GM-CSF, and TNF-α, all stimulate DC maturation and mobilization, whereas IL-10 blocks it. Important family of proteins involved include the signal transducer and activator of transcription (STAT) family and Janus activated kinase (JAK) family of tyrosine kinases.[32]
Maturation entails a down-regulation of phagocytosis activity, and an up-regulation of migratory activity and of costimulatory molecule expression en route to the secondary lymphoid organs.[2] Activated LCs lose their adhesiveness for epithelial and begin to express the surface receptor CCR7 that is specific for chemotactating cytokine produced in the T-cell zone of lymph nodes.[33] An important factor for emigrating LC is the basement membrane. LCs probably attach to it via a6-containg integrin receptors and produce proteolytic enzymes such as type IV collagenase (matrix metalloproteinase 9) to penetrate and to pave their way through the dense dermal network into the lymphatic system.[34] Recently, osteopontin has also been described as an important chemotactic protein that is essential for emigration and attracts LCs to draining lymph nodes by interacting with an N-terminal epitope of the CD44 molecule.[35]
There, LCs may complete their maturation, attract T- and B-cells by releasing chemokines and maintain the viability of recruiting T lymphocytes.[36] If the antigen has also been bound by B-cells, then both B- and T-cells can cluster with mature LCs, which upon activation leave the T-cell area leading to immune responses. B-cell elicits humoral immune response and some form antibody-secreting plasma cells. While T-cells migrate to the original site of antigen deposition, and mediate a cellular response.[37]
In an electron microscopic analysis of the contact area produced between LCs and T-lymphocyte showed two types of physical association between these cells. Type I, which corresponds to small areas of glycocalyx-glycocalyx interaction between protrution and microvilli of LCs and lymphocytes, and type II, characterized by wide and tight areas of close apposition in which there were bridged zones of contact and small septilaminar junctions (that characterize gap junctions) between the plasma membranes of both cells.[16] In addition, the antigen molecules were localized in relation to the type I binding.[37] An additional requirement is the interaction of costimulatory molecules. Costimulatory molecules, CD4 and CD8 bind to invariable regions of MHC further stabilizing the TCR – peptide – MHC complex, and enhancing the level, duration, or both, of TCR-mediated signaling. Furthermore, antigen-presenting cells also provides costimulatory signals via the production of cytokines, such as IL-1, IL-6, IL-12, and IL-15. IL-12 is essential for up-regulation of IFN-g production and generation of type-I helper cells, associated with cell mediated immunity.[38]
Convincing evidence exists that the superior immune-stimulatory activity of LCs is not only mediated by their surface molecules but also by their secretory activity. LCs were found to spontaneously produce mRNA encoding IL-α, IL-β, IL-6, IL-7, IL-12, IL-15, IL-18, TNF-α, TGF-β, M-CSF, and GM-CSF. Prostaglandlin (PG) D2 has been identified as the main cyclooxygenase product of LCs, further product of 5-lipooxygenase pathway include, 5-hydroxyeicosatetraenoic acid (HETE), 12-HETE, 15-HETE and small amounts of LTC4 and LTB4.[39] Furthermore, DCs are a significant source of IL-2, which is essential for T-cell priming and NK-cell activation.[40]
Identification of Langerhans Cells
LCs are not evident in the epithelium by routine hematoxylin and eosin staining. The criteria have been established that now allow the recognition and identification for LCs. These criteria include a clear cytoplasm devoid of tonofilaments, desmosomes, or melanosomes; a lobulated, frequently convoluted nucleus; and the presence of distinctive trilaminar cytoplasmic organelle, termed LC granule or Birbeck granule.[16]
One of the first techniques employed was the metal impregnation staining technique using heavy metals such as gold chloride, although tedious, this technique is rather specific. Impregnation with osmium-zinc-iodide yield good results, but also stains melanocytes and occasionally keratinocytes.[41] The availability of biochemical markers such as adenosinetriphospahtase (ATPase) and 5′-nucleotidase facilitated the identification of LCs in oral mucosa prior to the development of specific immunochemical markers.[3] The demonstration of a membrane bound, formalin resistant, sulfhydryl-dependent ATPase is an excellent method for identification of LCs, although adenosine diphosphatase (ADPase) was recently shown to be more specific.[42]
Another method for staining LCs in routine fixed and paraffin-embedded tissues is based on the use of antiserum against S-100 protein. In the oral mucosa, limitation of the method arises from the fact that S-100 proteins have been found in melanocytes of normal and pathological tissues. Antigenic moieties uniformly present and easily detectable on/in these cells include: (1) panhematopoietic marker cluster differentiation (CD)-45; (2) class II antigen encoded by the HLA-D region of major histocompatibility complex (MHC); (3) CD1a antigen, that is, MHC class I-related restriction elements (4) S-100 protein; (5) Cytoskeleton antigen vimentin; and a Birbeck granule-associated antigen defined by mAb Lag. Among these markers, anti-CD1a immunolabelling is considered a reliable approach for identifying epidermal human LCs, because of high CD1a expression density on both the surface and in the cytoplasm, even one-step immunolabelling procedures usually suffice for reliable LC labeling, and is further capable of staining immature cells also.[43]
LCs constitutively express MHC class II glycoproteins on DC processes. A recently described feature of LCs is their specific expression of langerin, and are the only cells known to express langerin (CD207). Langerin is a C-type lectin that can bind and mediate the uptake of sugar-containing molecules including mannose, N-acetyl-glucosamine, and fucose. Langerin are now known to distinguish LCs from other DC subsets.[43]
CD83 is a well-recognized marker for mature DCs. It is expressed as a cell surface molecule on thymic, circulating, and monocyte derived DCs, as well as LCs in the skin, interdigitating reticulum cells in the T-cell zones of lymphoid organs, and microglia. Low level expression has also been reported on activated T-and B-cells. In addition, CD83 can be expressed in a soluble form, and is able to down-regulate DC maturation and stimulation of T-cells. When CD83 expression was disrupted in mice, CD4+ T-cell generation was impaired.[44,45]
Langerhans Cells in Oral Mucosa
Apart from occasional occurrences at extraepithelial sites (dermis, dermal lymphatics, aortic wall, lymph nodes, thymus), LCs are essentially confined to stratified sqamous epithelia, ranging an average number of 160-550 cells/mm2. Nonkeratinized mucosa, that is, mucosa of the soft palate, ventral tongue, lip, and floor of the mouth had the highest counts, with a mean count of 508 ± 110 LCs/ mm2, whereas keratinized mucosa of the hard palate reported to have the lowest density. LCs constitute 2-4% of total epidermal cell population.[46]
A marked variation in LCs distribution in the oral mucosa has been observed. LCs in buccal mucosa were predominantly found in a suprabasal location. Although present along the length of the epithelium, they exhibit a tendency to cluster around the tip of the connective tissue papillae. In hard palate the cells were predominantly found in the stratum spinosum parallel to the surface of the epithelium with occasional cells in the basal layers. LCs in lateral border of tongue and floor of mouth has a linear arrangement along the length of epithelium and were found in basal and supra-basal layers. The authors observed highest values of LCs in the dorsum of the tongue and buccal mucosa, and lowest values in the floor of mouth.[47] Within the epithelium, LCs are anchored to the keratinocytes by E-cadherin mediated homotypic adhesion.[48]
Oral LCs phenotypically differ from their skin counterpart especially by the expression of LPS receptor/CD14 and the high affinity receptor for IgE (FceRI) of which the latter might enable oral LCs to display more efficient allergen-binding sites and antigen uptake.[49] Furthermore, oral LCs displayed a higher stimulatory capacity.[50]
Langerhans Cells in Oral Diseases
An increased number of LCs with the development of the oral micro flora has been observed.[51] LCs are capable of engaging and internalizing a wide variety of pathogens, and, upon endocytosis, they can process antigens, prime naïve T-cells, and initiate adaptive immune responses.[28] Oral mucosal LCs appear to be oriented in a manner to efficiently sample the oral fluids and bacteria, with their dendrites extending toward the surface, and often represent a hetrogenous population. LCs mobilize and mature in response to inflammatory cytokines and pathogen associated molecular patterns from oral mucosal pathogens.[52]
Emerging data also suggest a role for DCs in initiating autoimmune attacks. If DCs receive an imbalance of cytokine signals, they can differentiate into potentially harmful forms. This behavior could include altered migration, altered release of cytokines, or altered antigen processing so that cryptic self-determinants and/or self-determinants derived from apoptotic bodies are presented. Secondly defects in genes affecting DC function could lead to immune deviation. Alternatively, if expression of host cytokines is improperly regulated because of polymorphisms in promoter regions, DCs might differentiate into dysfunctional forms that provoke autoimmunity.[53]
Gingivitis and periodontitis
LCs in the gingival epithelium are very responsive to the accumulation of bacterial plaque (i.e., the biofilm), migrating into the site during early gingivitis, and migrating out as the gingivitis becomes more chronic (i.e., after 21 days).[54] Five times more number of LCs in inflamed gingiva in comparison to healthy gingiva from the same patient has been found.[27] They also play an important role in the presentation of antigen during all phases of periodontal diseases and may represent “key” cells in pathogenesis and development of periodontal diseases. Such patterns suggest an important role of LCs in pathogenesis. Whereas, an increase in the number of S-100 + LCs in the gingival epithelium and also in connective tissue of specimens with periodontal pockets of <6 -mm depth, and was decreased after the nonsurgical phase of the periodontal treatment.[55] Elevated levels of IL-1β, TNF-α, and PGE2 in chronic periodontitis stimulate DC maturation and migration.[56]
Contact hypersensivity
The first evidence of specific affinity of LCs for contact allergens, and the uptake of 10 known contact allergens by LCs has been demonstrated. It was also observed that the induction of contact hypersensitivity reaction largely depends on LCs density.[57] An increase in the number of LCs has been observed in contact hypersensitivity.[58] DCs were found closely located near lymphocyte-like cells within 4-6 hours of a topical application of an allergen.[59]
Oral mucosal responses to contact allergens are no different from that of skin. It was found that the number of LCs increased to approximately four times in the connective tissue at the site of nickel application.[60]
Chronic hyperplastic candidiasis
It is speculated that the initial colonization of oral mucosa by Candida takes place in areas devoid of LCs and diminished LC number and/or function contributes to the persistence of fungi in candidal leukoplakia.[61] The role of LCs in host defense against candidiasis has become more evident by showing their phagocytic capacity of candidal yeasts and hyphae as well as processing their antigens.[62] It is found that the infiltration of the epithelium with CD1a positive LCs were particularly intense in heavily candida-infected sections and the localization of the LCs was quite variable in chronic hyperplastic candidiasis between different patients and different areas of the same biopsy.[7] Variation in number and localization of LCs in chronic hyperplastic candidiasis probably reflects the dynamic nature of their involvement in local disease mechanisms. Furthermore, LCs were significantly numerous and richer in dendrites and Birbeck granules in erythematous areas than in areas of pseudomembranous candidiasis.[63]
Lichen planus
LCs appear to play an important role in the initiation of the inflammatory process in OLP. It is possible that as yet unidentified antigens are trapped within epidermis by a plexus of inter digitating LCs, which subsequently present it to T-cells. Although the total number of LCs in OLP is unchanged, an increase of class II major histocompatability antigen expression was demonstrated, suggesting that the immunologic activity and antigen-presenting capability of LCs was increased.[27] However, CD1a+/Langerin+ LCs are significantly increased in the epithelium and within the dermal lymphoid infiltrate, respectively.[64] LCs were especially localized along the epithelial-stromal junction, where epithelial damage predominantly occurs. LCs and T-lymphocytes have often been described in direct contact with each other in OLP.[65] Based on these observations, it is suggested that LCs might have an important role in the afferent arm of the adaptive immune response occurring in OLP, by taking up epithelial antigens from apoptotic cells.
Radicular granuloma and cyst
Among the various cells described in pathogenesis of periapical lesions LCs have also been observed in these lesions. CDla-positive LCs in a few periapical granulomas with well differentiated epithelia has been reported.[66]
The keratocysts with well-differentiated epithelial linings had a greater number of LCs than the other cysts, and thus suggested that the LCs distribution appears to be associated with the degree of differentiation of the epithelia. Few LCs showed the classic dendritic morphology while few were spherical, and were either present singly and/or in groups.
DCs in radicular granulomas were found to be distributed in lymphocyte-rich areas, where their number was significantly greater than that of HLA-DR+ macrophages, and contribute to local ineraction with T-cells within the lesion. DCs in radicular granulomas are associated with local defense reactions as stronger antigen-presenting cells as compared with macrophages. It is convincing to find LCs in periapical lesions owing to the fact that these lesions arise as a result of antigenic stimulation from bacteria and their products, where LCs may be associated with antigen processing function.[67]
Human immunodeficiency virus-1
LCs are known to express HIV-1 receptors⁄coreceptors.[3] LCs count was found to decrease in HIV-1 seropositive patients. Further, the oral mucosal manifestations observed in HIV-1 infected patients may be a direct consequence of HIV-1 infection mediated injury to LCs. It has also been suggested that LCs, being selective targets could constitute a reservoir for the virus.[68] A low expression of class II molecules seems to be a general feature of LCs in HIV-infected patients, and could be envisaged as a sign of a terminal differentiation of these cells.[63]
A significant correlation between the detection of HIV p17, the depletion of LCs and the presence of hairy leukoplakia lesions.[69] CD4-bearing oral mucosal LCs may be among the first cells to be infected following mucosal contact with HIV. Once infected, mucosal LCs carrying the integrated provirus may migrate down to the submucosal area where they emigrate with memory T-cells to form conjugates, thereby contributing to the transmission of HIV to T-cells. Indeed, purified normal epidermal LCs could be infected in vitro with HIV and transmit the infection to lymphoid cells.
Oral Hairy leukoplakia
The relationship between Epstein-Barr virus (EBV) replication and oral LCs counts suggest that productive EBV replication in oral hairy leukoplakia (OHLP) results in decreased oral LCs.[11] The authors observed that this effect of EBV replication on oral LCs counts was reversible. Wherein inhibition of EBV replication lead to an increase in oral LCs counts, and the subsequent return of EBV replication resulted in decrease of oral LCs count. They also observed the association between EBV replication in OHLP and decreased oral LCs in the absence of HIV infection.
These findings thus indicate that EBV replication in OHLP has a detrimental effect on oral LCs, and could potentially kill them, perhaps through an apoptotic mechanism, or EBV may infect Langerhans precursor cells in the peripheral blood.[70,71] In addition, EBV replication may inhibit differentiation and immigration of new oral LCs into OHLP through the expression of virally encoded immunomodulators.[11] Such modulations leading to decrease LCs may represent an intrinsic viral mechanism for evasion of the adaptive immune response, and may further permit super infection.[11,72]
Graft versus host disease
The LCs are scattered throughout the epithelium of skin and mucosa and have been associated with the graft versus host disease (GVHD) in patients who underwent bone marrow transplant (BMT). Studies have demonstrated that the host's LCs persist in the skin following allogenic BMT. These cells appear to continuously activate the donor's T-cells that result in the production of cytokines that probably play a key role in the tissue damage observed in skin GVHD.[73]
A statistically significant increase of CD1a+ cells/mm2 in the buccal mucosa of patients who developed GVHD, when compared with those who did not and non-transplanted subjects has been reported.[74] The authors explained that this significant increase of LCs in GVHD is a response to the production of specific chemokines responsible for the recruitment of precursors of donor LCs from the bone marrow.
However, a significant reduction of LCs in GVHD lesions suggest that probably such reduction would be a direct consequence of GVHD, related to an auto-reactive phenomenon, as in the chronic phase most LCs originate in the donor's bone marrow.[75] Thus, these observations raise the possibility of using LCs as a possible therapeutic target, such as the depletion of host DCs before the conditioning regimen, which has demonstrated to promote the tolerance and reduce GVHD, without the need for prolonged T-cell-targeted immunosuppression.[74]
Other mucosal diseases
A 5-to 6-fold reduction in the density of LCs in lesions of median rhomboid glossitis (MRG) when compared with histologically normal tongue has been reported.[9] A depressed density of intraepithelial LCs in MRG would favor the development of tolerance to antigens (e.g., fungi or their products) and thereby promote chronicity. The significance of LCs has also been discussed relative to Apthous stomatitis and Behcet's syndrome an increase in the number of LCs in areas of skin lesions with Behcets disease, and degenerating LCs have also been observed. [76] However, the nature of this nonspecific mechanism is not known, but a possible disturbance of LCs population and/or its precursor cells could occur.[27] Erythema multiforme is an acute dermatitis of unknown etiology, however, the number of LCs and their distribution vary according to the stage of the lesion. In the initial lesion, CD1a-positive cells increase in number and size. In the presence of bullae and necrosis, the number of LCs diminishes or even disappears.[77]
Oral squamous cell carcinoma
In tumor-bearing sites, LCs uptake, process, and present tumor-associated antigens via MHC class I-restricted presentation to naive or memory T-cells. This leads to the generation of tumor specific effector T-cells capable of recognizing and eliminating tumor cells.[78] Several researchers have investigated the distribution of LCs in squamous cell carcinoma, however, none have yielded consistent results, some observed an increase[12,79] while few observed a decrease[80,81] in LC density, whereas some observed no change at all.[82]
The number of LCs infiltrating the tumor site is regarded as a highly significant prognostic parameter in patients with oral squamous cell carcinoma. Numerous reports in the literature suggest that the presence of LCs is associated with improved prognosis. Also fewer LCs were observed in metastatic lesions than in primary lesions.[78] Studies have concluded that the CD1a-positive peritumoral subpopulation of DCs is functionally distinct and is more important to antitumor immunity than the other subpopulations studied.[83] Repeated sub-cutaneous injection of GM-CSF activated LCs that had been loaded with tumor fragments resulted in host resistance to a subsequent challenge with viable tumor cells. Thus, a defective antigen presentation may play a role in tumor escape.[84] Also the number and activation state of intratumoral DCs are critical factors in host response to tumors.[85]
Herpetic Lesions
Evidence suggests that DCs, the most potent antigen-presenting cells known, play a role in the immunological control of Herpes simplex virus (HSV) infections. HSV infection of DCs induced submaximal maturation, but DCs failed to mature further in response to lipopolysaccharide (LPS). LPS-induced IL-12 secretions and the induction of primary and secondary T-cell responses were impaired by infection. Ultimately, DC infection resulted in delayed, asynchronous apoptotic cell death. However, infected DCs induced HSV recall responses in some individuals. Furthermore, soluble factors secreted by DCs after infection induced DC maturation and primed for IL-12 secretion after LPS stimulation. These data support a pathogenetic model of HSV infection, in which initial delay in the generation of immune responses to HSV at peripheral sites is mediated by disruption of DC function but is overcome by bystander DC maturation and cross-presentation of HSV antigens.[86]
Antigen presentation by DCs is critical for the induction of a specific immune response. The immunotherapeutic potential of antigen-pulsed DCs for the treatment of cancer has been confirmed in a number of experimental tumor models and in several preclinical trials. Recent advances in our understanding of the interaction of microbial pathogens with DCs have provided the basis to explore DCs as vaccine carriers for the induction of protective immune responses to infections. Support for this strategy comes from animal studies demonstrating that DCs, after ex vivo loading with microbial antigens, confer protection against microbial challenges in vivo. This may have important implications for the development of novel strategies for prophylactic or therapeutic immunizations against various microbial pathogens.[87]
Conclusion
Immunology of oral diseases has long been focused on antigens and lymphocytes, whereas the fact remains that the antigen-presenting cells, like the LCs of the epithelium are initiators and modulators of the immune response. LCs as APCs play a significant role in various oral pathologic conditions. We have reviewed LCs and summarized the specific role played by LCs in various oral pathologic conditions. However, several questions still remain unanswered and insights gained from further investigation would have both a wide immunological significance as well as clinical implications. Such concepts may allow us to device specific immunotherapies and treatment modalities.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I Morphology, quantitation, tissue distribution. J Exp Med. 1973;137:1142–62. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cutler CW, Jotwani R. Antigen-presentation and the role of dendritic cells in periodontitis. Periodontology 2000. 2004;35:135–7. doi: 10.1111/j.0906-6713.2004.003560.x. [DOI] [PubMed] [Google Scholar]
- 3.Cutler CW, Jotwani R. Oral mucosal expression of HIV-1 receptors, co-receptors, and alpha-defensins: Tableau of resistance or susceptibility to HIV infection? Adv Dent Res. 2006;19:49–51. doi: 10.1177/154407370601900110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Langerhans P. Nerven der menschlichen Haut. Virchows Arch Abt B Pathol Anat. 1886;44:325–37. [Google Scholar]
- 5.Silberberg I. Apposition of mononuclear cells to Langerhans cells in contact allergic reactions. An ultrastructural study. Acta Derm Venereol. 1973;53:1–12. [PubMed] [Google Scholar]
- 6.Schroeder H, Thelade J. Electron microscopy of normal human gingival epithelium. J Perodont Res. 1986;21:640–52. doi: 10.1111/j.1600-0765.1966.tb01850.x. [DOI] [PubMed] [Google Scholar]
- 7.Ali A, Rautemaa R, Hietanen J, Beklen A, Konttinen Y. A possible CD1a Langerhans cell-mast cell interaction in Chronic Hyperplastic Candidosis. J Oral Pathol Med. 2007;36:329–36. doi: 10.1111/j.1600-0714.2007.00527.x. [DOI] [PubMed] [Google Scholar]
- 8.McCartan BE, Lamey PJ. Expression of CD1 and HLA-DR by Langerhans cells (LC) in oral lichenoid drug eruptions (LDE) and idiopathic oral lichen planus (LP) J Oral Palhol Med. 1997;26:176–80. doi: 10.1111/j.1600-0714.1997.tb00454.x. [DOI] [PubMed] [Google Scholar]
- 9.Walsh LJ, Cleveland DB, Cumming CG. Quantitative evaluation of Langerhans cells in Median Rhomboid Glossitis. J Oral Pathol Med. 1992;21:28–32. doi: 10.1111/j.1600-0714.1992.tb00965.x. [DOI] [PubMed] [Google Scholar]
- 10.Thomas G, Hashibe M, Jacob BJ, Ramadas K, Mathew B, Sankaranarayanan R, et al. Risk factors for multiple oral premalignant lesions. Int J Cancer. 2003;107:285–91. doi: 10.1002/ijc.11383. [DOI] [PubMed] [Google Scholar]
- 11.Walling Dennis M, Flaitz Catherine M, Hosein Fabian G, Montes-Walters M, Mark NC. Effect of epstein-barr virus replication on langerhans cells in pathogenesis of oral hairy leukoplakia. J Infect Dis. 2004;189:1656–63. doi: 10.1086/383132. [DOI] [PubMed] [Google Scholar]
- 12.Kurihara K, Hashimoto N. The pathologic significance of Langerhans cell in oral cancer. J Oral Pathol. 1985;4:289–98. doi: 10.1111/j.1600-0714.1985.tb00496.x. [DOI] [PubMed] [Google Scholar]
- 13.Waterhouse JP, Squier CA. The Langerhans cell in human gingival epithelium. Arch Oral Biol. 1967;12:341–8. doi: 10.1016/0003-9969(67)90217-8. [DOI] [PubMed] [Google Scholar]
- 14.Yu RC, Morris JF, Pritchard J. Defective alloantigen-presenting capacity of Langerhans cell histocytosis cells. Arch Dis Child. 1992;67:1370–2. doi: 10.1136/adc.67.11.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barrett AW, Cruchley AT, Williams DM. Oral mucosal langerhans’ cells. Crit Rev Oral Biol Med. 1996;7:36–58. doi: 10.1177/10454411960070010301. [DOI] [PubMed] [Google Scholar]
- 16.Birbeck MS, Breathnach AS, Everall JD. An electron microscopic study of basal melanocytes and high-level clear cells in vitiligo. J Invest Dermatol. 1961;37:51–63. [Google Scholar]
- 17.Concha M, Figueroa CD, Caorsi I. Ultrastructural characteristic of the contact zones between Langerhans cells and Lymphocytes. J Pathol. 1988;156:29. doi: 10.1002/path.1711560108. [DOI] [PubMed] [Google Scholar]
- 18.Breathnach AS. Variation in ultrastructural appearance of Langerhans cells in normal human epidermis. Br J Dermatol. 1977;97:14. [Google Scholar]
- 19.Berman B. Advances in the characterization of Langerhans cell structure and function. In: Fleishmajer R, editor. Progress in diseases of the skin. New York: Grune ans Stratton; 1983. [Google Scholar]
- 20.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–61. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 21.Strunk D, Rappersberger K, Egger C, Strobl H, Krömer E, Elbe A. Generation of human dendritic cell/Langerhans cells from circulating CD34+ hematopoietic progenitor cells. Blood. 1996;87:1292–302. [PubMed] [Google Scholar]
- 22.Caux C, Vanbervliet B, Masacrier C, Durand I, Banchereau J. Interleukin 3 cooperates with tumor necrosis factor alpha for the development of human dendritic/Langerhans cells from cord blood Cd34+ hematopoietic progenitor cells. Blood. 1996;87:2376–85. [PubMed] [Google Scholar]
- 23.Szabolcs P, Moore MA, Young JW. Expansion of immunostimulatory dendritic cells among the myeloid progeny of human CD34+ bone marrow precursors cultured with c-kit ligand, granulocyte-macrophage colony stimulating factor, and TNF-α. J Immunol. 1995;154:5851–61. [PubMed] [Google Scholar]
- 24.Foster CA, Holbrook KA, Farr AG. Ontogeny of Langerhans cells in Human embryonic and fetal skin: Expression of HLA-DR and OKT-6 determination. J Invest Dermatol. 1986;86:240. doi: 10.1111/1523-1747.ep12285201. [DOI] [PubMed] [Google Scholar]
- 25.Dieu-Nosjea MC, Massacrier C, Homey B, Vanbervliet B, Pin JJ, Vicari A, et al. Macrophage inflammatory protein 3a is expressed at inflamed epithelial surfaces and is the most potent chemokine known in attracting Langerhans cell precursors. J Exp Med. 2000;192:705–17. doi: 10.1084/jem.192.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Czernielewski JM, Dermarchiz M. Epidermal Langerhans cells- a cycling cell population. J Invest Dermatol. 1985;84:424. doi: 10.1111/1523-1747.ep12265523. [DOI] [PubMed] [Google Scholar]
- 27.Vishwanath M, Nishibu A, Saeland S, Ward BR, Mizumoto N, Ploegh HL, et al. Development of intravital intermittent confocal imaging system for studying Langerhans cell turnover. J Invest Dermatol. 2006;126:2452. doi: 10.1038/sj.jid.5700448. [DOI] [PubMed] [Google Scholar]
- 28.Lombardi T, Hauser C, Budtz-Joegensen E. Langerhans cells: Structure, Function and Role in Oral Pathologic Conditions’. J Oral Pathol Med. 1993;22:193–202. doi: 10.1111/j.1600-0714.1993.tb01056.x. [DOI] [PubMed] [Google Scholar]
- 29.Inaba K. Immunologic properties of epidermal langerhans cells. Distinct requirement for stimulation of inprimed and sensitized T-lymphocytes. J Exp Med. 1986;164:605–13. doi: 10.1084/jem.164.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watts C. Capture and processing of exogenous antigens for presentation on MHC molecules. Annu Rev Immunol. 1997;15:821. doi: 10.1146/annurev.immunol.15.1.821. [DOI] [PubMed] [Google Scholar]
- 31.Ferris RL, Whiteside Theresa L, Soldano F. Immune escape associated with functional defects in antigen-processing machinery in head and neck cancer. Clin Cancer Res. 2006;12:3890. doi: 10.1158/1078-0432.CCR-05-2750. [DOI] [PubMed] [Google Scholar]
- 32.Banchereau J, Steinman Ralph M. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 33.Stoitzner P, Holzmann S, McLellan AD, Ivarsson L, Stössel H, Kapp M, et al. Visualization and characterization of migratory Langerhans cells in murine skin and lymph nodes by antibodies against Langerin/CD207. J Invest Dermatol. 2003;120:266–74. doi: 10.1046/j.1523-1747.2003.12042.x. [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi Y. Langerhans cells produce type VII collagenase (MMP-9) following epicutaneous stimulation with haptens. Immunology. 1997;90:496. doi: 10.1046/j.1365-2567.1997.00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weiss JM, Renkl AC, Maier CS, Kimmig M, Liaw L, Ahrens T, et al. Osteopontin is involved in the initiation of cutaneous contact hypersensitivity by inducing Langerhans and dendritic cell migration to lymph nodes. J Exp Med. 2001;194:1219. doi: 10.1084/jem.194.9.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schuler G, Steinman RM. Murine epidermal Langerhans cells mature into potent immunostimulatory dendritic cells in vitro. J Exp Med. 1985;161:526–46. doi: 10.1084/jem.161.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Z, Malhotra PS, Thomas GR, Ondrey FG, Duffey DC, Smith CW, et al. Expression of proinflammatory and proangiogenic cytokines in patients with head and neck cancer. Clin Cancer Res. 1999;5:1369–79. [PubMed] [Google Scholar]
- 38.Bos Jan D. Skin Immune System. 2nd ed. CRC Press; 1997. [DOI] [PubMed] [Google Scholar]
- 39.Wang B, Amerio P, Sauder DN. Role of cytokines in epidermal Langerhans cell migration. J Leukoc Biol. 1999;66:33–9. doi: 10.1002/jlb.66.1.33. [DOI] [PubMed] [Google Scholar]
- 40.Granucci F, Vizzardelli C, Pavelka N, Feau S, Persico M, Virzi E, et al. Inducible IL-2 production by dendritic cells revealed by global gene expression analysis. Nat Immunol. 2001;2:882–8. doi: 10.1038/ni0901-882. [DOI] [PubMed] [Google Scholar]
- 41.Charbit Y, Monteil RA, Hitzig C, Sanget P, Benaiche N, Jasmin JR. S-100 immunolaballing of Langerhans cells in oral epithelium. J Oral Pathol. 1986;15:419–22. doi: 10.1111/j.1600-0714.1986.tb00650.x. [DOI] [PubMed] [Google Scholar]
- 42.Chaker MB, Tharp MD, Bergstresser PR. Rodent epidermal Langerhans cells demonstrate greater histochemical specificity for ADP than for ATP and AMP. J Invest Dermatol. 1984;82:496. doi: 10.1111/1523-1747.ep12261031. [DOI] [PubMed] [Google Scholar]
- 43.Mizumoto N, Takashima A. CD1a and langerin: Acting as more than Langerhans cell markers. J Clin Investig. 2004;113:658–60. doi: 10.1172/JCI21140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou LJ. A novel cell-surface molecule expressed by human interdigitating reticulum cells, Langerhans cells, and activated lymphocyte is a new member of the Ig superfamily. J Immunol. 1992;149:735–42. [PubMed] [Google Scholar]
- 45.Zhou LJ, Tinder TF. Human blood dendritic cells selectively express CD83, a member of the immunoglobulin superfamily. J Immunol. 1995;154:3821–35. [PubMed] [Google Scholar]
- 46.Daniels TE. Human mucosal Langerhans cells: Postmortem identification of regional variation in oral mucosa. J Invest Dermatol. 1984;82:21–4. doi: 10.1111/1523-1747.ep12259038. [DOI] [PubMed] [Google Scholar]
- 47.Cruchley AT, Williams DM, Farthimh PM, Lesch CA, Squier CA. Regional variation in Langerhans cell distribution and density in normal human oral mucosa determined using monoclonal antibodies against CD1, HLADR, HLADQ and HLADP. J Oral Pathol Med. 1989;18:510–6. doi: 10.1111/j.1600-0714.1989.tb01353.x. [DOI] [PubMed] [Google Scholar]
- 48.Tang A, Amagai M, Granger LG, Stanley JR, Udey MC. Adhesion of epidermal Langerhans cells to keratinocytes mediated by E-cadherin. Nature. 1993;361:82–5. doi: 10.1038/361082a0. [DOI] [PubMed] [Google Scholar]
- 49.Allam JP, Niederhagen B, Bucheler M, Appel T, Betten H, Bieber T, et al. Comparative analysis of nasal and oral mucosa dendritic cells. Allergy. 2006;61:166–72. doi: 10.1111/j.1398-9995.2005.00965.x. [DOI] [PubMed] [Google Scholar]
- 50.Hasséus B, Jontell M, Bergenholtz G, Dahlgren UI. Langerhans cells from human oral epithelium are more effective at stimulating allogeneic T cells in vitro than Langerhans cells from skin. Clin Exp Immunol. 2004;136:483–9. doi: 10.1111/j.1365-2249.2004.02469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bos IR, Burkhardt A. Intraepithelial cells of oral mucosa. J Oral Pathol. 1980;9:65–81. doi: 10.1111/j.1600-0714.1980.tb01389.x. [DOI] [PubMed] [Google Scholar]
- 52.Ito H, Takekoshi T, Miyauchi M, Ogawa I, Takata T, Nikai H. Three-dimensional appearance of Langerhans cells in human gingival epithelium as revealed by confocal laser scanning microscopy. Arch Oral Biol. 1998;43:741–4. doi: 10.1016/s0003-9969(98)00066-1. [DOI] [PubMed] [Google Scholar]
- 53.Drakesmith H, Chain B, Beverley P. How can dendritic cells cause autoimmune disease? Immunology Today. 2000;21:214–7. doi: 10.1016/s0167-5699(00)01610-8. [DOI] [PubMed] [Google Scholar]
- 54.Moughal NA, Adonogianaki E, Kinane DF. Langerhans cells dynamics inhuman gingiva during experimentally induced inflammation. J Biol Buccale. 1992;20:163–7. [PubMed] [Google Scholar]
- 55.Dereka XE, Tosios KI, Chrysomali E, Angelopoulou E. Factor XIIIa+ dendritic cells and S-100 protein+ Langerhans’ cells in adult periodontitis. J Periodont Res. 2004;39:447–52. doi: 10.1111/j.1600-0765.2004.00764.x. [DOI] [PubMed] [Google Scholar]
- 56.Heasman PA, Lauffart BL, Preshaw PM. Crevicular fluid prostaglandin E2 evels inperiodontitis-resistent and periodontitis suscepptibe adults. J Clin Periodontol. 1998;25:1003–7. doi: 10.1111/j.1600-051x.1998.tb02405.x. [DOI] [PubMed] [Google Scholar]
- 57.Shelly WB, Juhlin L. Selective uptake of contact allergens by Langerhans cells. Arch Dermatol. 1977;113:187. [PubMed] [Google Scholar]
- 58.Christensesn OB Daniels TE, Maibach HI. Expression of the OKT6 antigen by Langerhans cells in the patch test reaction. Contact Dermatitis. 1986;14:26–31. doi: 10.1111/j.1600-0536.1986.tb01148.x. [DOI] [PubMed] [Google Scholar]
- 59.Silberberg-Sinakin I, Thorbecke J. Contact hypersensitivity and Langerhans cells. J Invest Dermatol. 1980;75:61–7. doi: 10.1111/1523-1747.ep12521144. [DOI] [PubMed] [Google Scholar]
- 60.van Loon LA, van Elsas PW, Bos JD, Harkel-Hagenaar HC, Krieg SR, Davidson CL. T-lymphocyte and Langerhans cell distribution in normal and allergically induced oral mucosa in contact with nickel containing dental alloys. J Oral Pathol. 1988;17:129–37. doi: 10.1111/j.1600-0714.1988.tb01899.x. [DOI] [PubMed] [Google Scholar]
- 61.Daniels TE, Schwartz O, Larsen V, Dahelsteen E, Pindborg J. Langerhans cells in candidal leukoplakia. J Oral Pathol. 1985;14:733–9. doi: 10.1111/j.1600-0714.1985.tb00552.x. [DOI] [PubMed] [Google Scholar]
- 62.Newman SL, Holly A. Candida albicans is phagocytosed, killed, and processed for antigen presentation by human dendritic cells. Infect Immun. 2001;69:6813–22. doi: 10.1128/IAI.69.11.6813-6822.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Romagnoli P, Pimpinelli N, Mori M, Reichart PA, Eversole LR, Ficarra G. Immunocompetent cells in oral candidiasis of HIV- infected patients: An immunohistochemical and electron microscopical study. Oral Dis. 1997;3:99–105. doi: 10.1111/j.1601-0825.1997.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 64.Santoro A, Majorana A, Roversi L, Gentili F, Marrelli S, Vermi W, et al. Recruitment of Langerhans cells in oral lichen planus. J Pathol. 2005;205:426–4. doi: 10.1002/path.1699. [DOI] [PubMed] [Google Scholar]
- 65.El-Labban NG. An ultrastructural study of Langerhans cells crossing the basal complex in oral lichen planus. Arch Oral Biol. 1977;22:629–31. doi: 10.1016/0003-9969(77)90077-2. [DOI] [PubMed] [Google Scholar]
- 66.Gao Z, Mackenzie IC, Rittman BR, Korszun AK, Williams DM, Cruchley AT. Immunocytochemical examination of immune cells in periapical granulomata and odontogenic cysts. J Oral Pathol. 1988;17:84–90. doi: 10.1111/j.1600-0714.1988.tb01512.x. [DOI] [PubMed] [Google Scholar]
- 67.Kaneko T, Okiji T, Kaneko R, Nör JE, Suda H. Antigen-presenting cells in human radicular granulomas. J Dent Res. 2008;87:553–7. doi: 10.1177/154405910808700617. [DOI] [PubMed] [Google Scholar]
- 68.Sporri B, von Overbeck J, Brand CU, Schmidli J, Sanchez ML, Grunow R, et al. Reduced number of Langerhans cells in oral mucosal washings from HIV-1 seropositives. J Oral Pathol Med. 1994;23:399–402. doi: 10.1111/j.1600-0714.1994.tb00084.x. [DOI] [PubMed] [Google Scholar]
- 69.Chou L, Epstein J, Casso SA, West DM, He W, Firth JD. Oral mucosal Langerhans’ cells as target, effector and vector in HIV infection. J Oral Pathol Med. 2000;29:394–402. doi: 10.1034/j.1600-0714.2000.290805.x. [DOI] [PubMed] [Google Scholar]
- 70.Westphal EM, Blackstock W, Feng W, Israel B, Kenney SC. Activation of lytic Epstein-Barr virus (EBV) infection by radiation and sodium butyrate in vitro and in vivo: A potential method for treating EBV positive malignancies. Cancer Res. 2000;60:5781–8. [PubMed] [Google Scholar]
- 71.Masy E, Adriaenssens E, Montpellier C. Human monocytic cell lines transformed in vitro by Epstein-Barr virus display a type II latency and LMP-1-dependent proliferation. J Virol. 2002;76:6460–72. doi: 10.1128/JVI.76.13.6460-6472.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Starzl TE, Zinkernagel RM. Mechanisms of disease: antigen localization and migration in immunity and tolerance. N Engl J Med. 1998;339:1905–13. doi: 10.1056/NEJM199812243392607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Merad M, Hoffmann P, Ranheim E. Depletion of host Langerhans cells before transplantation of donor alloreactive T cells prevents skin graft-versus-host disease. Nat Med. 2004;5:510–7. doi: 10.1038/nm1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Orti-Raduan ES, Nunes AJ, Oliveira DT, Lara VS, Taveira LA. Quantitative analysis of Langerhans’ cells in oral chronic graft-vs.-host disease. J Oral Pathol Med. 2009;38:132–7. doi: 10.1111/j.1600-0714.2008.00712.x. [DOI] [PubMed] [Google Scholar]
- 75.Aractingi S, Gluckman E, Dauge-Geffroy MC. Langerhans’ cells are depleted in chronic graft versus host disease. J Clin Pathol. 1997;50:305–9. doi: 10.1136/jcp.50.4.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Satio T, Honma T. Epidermal langerhans cells, macrophages and lymphocytes after the prick test in patients with Behcets syndrome and recurrent oral ulcerations. Biomed Res. 1981;2:2658–69. [Google Scholar]
- 77.Margolis RJ, Tonnesen MG, Harrist TJ. Lymphocyte subset and Langerhans cells/intermediate cells in Erythema Multiforme. J Invest Dermatol. 1983;81:403–6. doi: 10.1111/1523-1747.ep12522001. [DOI] [PubMed] [Google Scholar]
- 78.Reichart TE, Strauss L, Wagner EM, Gooding W, Whiteside TL. Signaling abnormalities, apoptosis and reduced proliferation of circulating and tumor infiltrating lymphocytes in patients with oral cancer. Clin Cancer Res. 2002;8:3137–45. [PubMed] [Google Scholar]
- 79.McArdle John P, Knight BA, Halliday GM, Muller HK, Rowden G. Quantitative assessment of Langerhans cells in actinic keratosis, Bowen's Disease, Keratoacanthoma, squamous cell carcinoma and basal cell carcinoma. Pathology. 1985;18:212–6. doi: 10.3109/00313028609059461. [DOI] [PubMed] [Google Scholar]
- 80.Lisi P. Investigation on Langerhans’ cells in pathological human epidermis. Acta Derm (Stockholm) 1973;53:425–8. [PubMed] [Google Scholar]
- 81.Fernandez-Bussy R. T cell subsets and Langerhans’ cells in skin tumours. Eur J Cancer Clin Oncol. 1983;19:907–13. doi: 10.1016/0277-5379(83)90056-1. [DOI] [PubMed] [Google Scholar]
- 82.Gatter KC, Morris HB, Roach B. Langerhans’ cells and T-cells in human skin tumors: An immunohistological study. Histopathology. 1984;8:229–44. doi: 10.1111/j.1365-2559.1984.tb02338.x. [DOI] [PubMed] [Google Scholar]
- 83.Goldman SA, Baker E, Weyant RJ, Clarke MR, Myers JN, Lotze MT. Peritumoral CD1a positive dendritic cells are associated with improved survival in patients with tongue carcinoma. Arch Otolaryngol Head Neck Surg. 1998;124:641–6. doi: 10.1001/archotol.124.6.641. [DOI] [PubMed] [Google Scholar]
- 84.Grabbe S, Bruvers S, Gallo RL, Knisely TL, Nazareno R, Granstein RD. Tumor antigen presentation by murine epidermal cells. J Immunol. 1991;146:3656–61. [PubMed] [Google Scholar]
- 85.Furumoto K, Soares L, Engleman EG, Merad M. Induction of potent antitumor immunity by in situ targeting of intra-tumoral dendritic cells. J Clin Invest. 2004;113:774–83. doi: 10.1172/JCI19762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pollara G, Speidel K, Samady L, Rajpopat M, McGrath Y, Ledermann J, et al. Herpes simplex virus infection of dendritic cells: Balance among activation, inhibition, and immunity. J Infect Dis. 2003;187:165–78. doi: 10.1086/367675. [DOI] [PubMed] [Google Scholar]
- 87.Moll H, Berberich C. Dendritic cells as vectors for vaccination against infectious diseases Int. J Med Microbiol. 2001;291:323–9. doi: 10.1078/1438-4221-00138. [DOI] [PubMed] [Google Scholar]


