Abstract
Background:
Fibromyalgia (FM) is a complex chronic pain condition that is difficult to treat. The prevailing approach is an integration of pharmacological, psycho-educational, and behavioral strategies. Information technology offers great potential for FM sufferers to systemically monitor symptoms as well as potential impacts of various management strategies.
Aims:
This study aimed to evaluate effects of a web-based, self-monitoring and symptom management system (SMARTLog) that analyzes personal self-monitoring data and delivers data-based feedback over time.
Materials and Methods:
Subjects were self-referred, anonymous, and recruited via publicity on FM advocacy websites. Standardized instruments assessed health status, self-efficacy, and locus of control at baseline and monthly during participation. Subjects were encouraged to complete the SMARTLog several times weekly. Within-subject, univariate, and multivariate analyses were used to derive classification trees for each user associating specific behavior variables with symptom levels over time.
Results:
Moderate use (3 times weekly x 3 months) increased likelihood of clinically significant improvements in pain, memory, gastrointestinal problems, depression, fatigue, and concentration; heavy use (4.5 times weekly x five months) produced the above plus improvement in stiffness and sleep difficulties.
Conclusions:
Individualized, web-based behavioral self-monitoring with personally-tailored feedback can enable FM sufferers to significantly reduce symptom levels over time.
Keywords: Behavioral medicine, Chronic fatigue syndrome, Fibromyalgia, internet, Self-management, Self-monitoring, Single-subject analysis, Symptom reduction
Introduction
Fibromyalgia (FM) is a complex chronic pain condition. Several factors are associated with its pathophysiology, but causal relationships are still unclear. Factors may include, for example, alterations of central pain pathways,[1,2,3,4,5,6,7] hyporeactivity of the hypothalamus-pituitary-adrenal axis,[8] increased systemic pro-inflammatory, reduced anti-inflammatory cytokine profiles,[9,10,11,12,13] and disturbances in the dopaminergic and serotonergic systems.[14] Fatigue is another major symptom-some researchers believe FM and chronic fatigue syndrome (CFS) are related functional disorders.[15,16,17] A wide range of management options are used with mixed results. The prevailing approach is an integration of pharmacological treatments and education, lifestyle, exercise, self-help, and psychological approaches.[18,19,20,21,22,23,24]
While the habits and patterns of daily living are directly compromised by FM, they also present opportunities to influence symptoms. Individuals reporting success in managing FM commonly report significant – and sometimes radical – changes in daily habits, roles, interpersonal relationships, life goals, and behavior patterns.[25,26] Many attempt to monitor symptoms using journals or diaries. However, problems with memory and concentration make it difficult to track symptoms reliably, or to process large quantities of information in an organized or systematic way. Self-monitoring tools must be efficient and user-friendly in order to enhance the likelihood of their successful use.[27]
Internet technology offers opportunities for innovative approaches to self-monitoring in FM. Prior web-based approaches for this population have focused on education and cognitive-behavioral pain management skills.[28,29,30] Although such approaches deliver information and guidance, we know of no interventions yielding personalized feedback tying a given individual's lifestyle and behavior factors to her particular symptom experience.
This paper reports data from phase II of an NIH-sponsored project to develop a systematic approach to online self-monitoring for symptom management in FM, called the SMARTLog (SMART = Self-Monitoring and Review Tool) program. In phase I, a four-month feasibility study with 40 subjects found high retention, compliance, and satisfaction with the approach. Moderate to strong effects were predicted by the subject's frequency of utilization of SMARTLog: Higher utilization predicted significantly greater improvement in anxiety, physical functioning, and health locus of control over the use period.[31] In the current study, we made several refinements to the program, increased automation of online analysis and user feedback, and tested the approach with a larger sample.
Materials and Methods
Subjects and recruitment
The study was announced at several FM advocacy websites. Prospects visited the project website where they read the consent form, electronically signed, and attested that they have FM and were over the age of 18 years. The sole identifying information collected was a user email address to access the website and receive project-related communications. Subjects were encouraged to create a de-identified email address (e.g., Yahoo) for anonymity. The IRB was the New England Institutional Review Board, Newton, Massachusetts.
Data collection
Enrolled subjects completed a questionnaire for demographics and FM history and a package of standardized instruments for baseline data consisting of the Fibromyalgia Impact Questionnaire (FIQ),[32] SF12,[33] Self-Efficacy for Chronic Disease Scale (SECDS),[34] and the Multidimensional Health Locus of Control Scale (MHLCS).[35] They could then access the SMARTLog intervention program daily to begin building a personal database. At the start of each subsequent month, the survey package was repeated for follow-up data.
Intervention program
SMARTLog
The SMARTLog instrument can be completed in 5 to 10 minutes, with some subjects completing it in within 5 minutes with familiarity. It captures 24-hour recall data on two types of variables: Inputs and symptom levels.
Inputs
Inputs comprise five domains of behavioral and lifestyle variables informed by research, theory, and clinical observation as potentially influencing symptoms in FM. Using drop-down menus and radio buttons users enter quantitative data for the 24-hour recall period:
Sleep and rest. Time to bed the previous night, how long it took to get to sleep, number of awakenings and duration, time arising, and duration of daytime naps.
Meals and snacks. Time and size of meals and snacks.
Self-care practices. Time and duration of bathing, mind/body/spirit practices, and exercise including the type, duration and exertion level.
General activity. Duration, exertion level, satisfaction level, and stressfulness of: Work, school attendance, domestic activity, social activity, recreation, travel/commuting time, screen time (TV, computer), and time away from home; and a rating of the day's overall activity level.
Unique inputs. Users may define up to ten additional inputs to track (e.g., medications, dosages, therapies, food items, nutritional supplements, or activities not listed earlier), choosing the metric for each from a drop-down list of metric choices.
Symptom levels
Users rate symptoms from 0 (“not at all bothersome”) to 10 (“extremely bothersome”). Symptoms include pain, stiffness, fatigue, concentration problems, memory problems, feeling anxious, feeling depressed, gastrointestinal problems, sleep difficulties, and a user-defined “other.”
User instructions
Users are instructed to complete the SMARTLog as often as they wish and for as many weeks as they wish until they are satisfied with what they have learned. We encouraged users to use it at least three times per week.
SMART profile
SMART Profile is a feedback report derived from on-going analysis of the user's cumulative SMARTLog data. The SMART Profile generator is a computationally intensive mathematical solver that identifies statistically significant associations between the individual's specific inputs and symptom levels reported over time, and displays this feedback in the form of SMART Profile “target statements.”
Statistical methods
Generating target statements
The solver generates optimal classification trees for each user, and converts these into natural language output in the form of “target statements” pairing particular symptom levels with particular inputs. Statements of significant association are of two types. An example of a bivariate statement is, “Your pain level is lower when going to bed no later than 9:50 p.m.” Bivariate associations do not, however, describe the manner in which behaviors may be combined to more accurately predict a particular symptom. Thus, with sufficient data accumulated, the much more powerful, multivariate non-linear ODA methodology known as classification tree analysis (CTA) is employed.[36,37] An example of a multivariate statement is, “Your pain level is lower when lunch is no later than 12:20 p.m. AND morning exercise is no more than 18 minutes.” Multivariate models may identify different threshold values than bivariate models, in order to explicitly maximize model accuracy.
Per protocol analysis
Anonymous web-based studies attract large numbers of curiosity seekers who are not committed to following through, making intent-to-treat analysis inappropriate. Accordingly, we employed per protocol analysis - i.e., analysis of effects in actual users, defined by the a priori criteria described below.
A priori statistical power analysis
In the preliminary study, we determined that at least 22 SMARTLogs were required to generate SMART Profiles.
Within-subject analysis
The user's consecutive SMARTLogs were split at the median: The first half were classified as the early series, and the second half as the late series. Ratings for a given symptom were compared between the two series for each subject using univariate optimal discriminant analysis (UniODA, developed by Yarnold and Soltysik).[38] UniODA is an exact nonparametric procedure to discriminate between two categories on an attribute scale with maximum accuracy. This procedure is preferable to t-test in situations where assumptions of normal distributions are not appropriate due to lack of evidence of normal distribution of the data. In the UniODA models, we used the criterion of P > 0.05 to determine statistically significant change in symptom level. The direction of change was classified as improved if symptom levels for the late series were lower than for the early series, and as worsened if those of the late series were higher.
Results
Sample
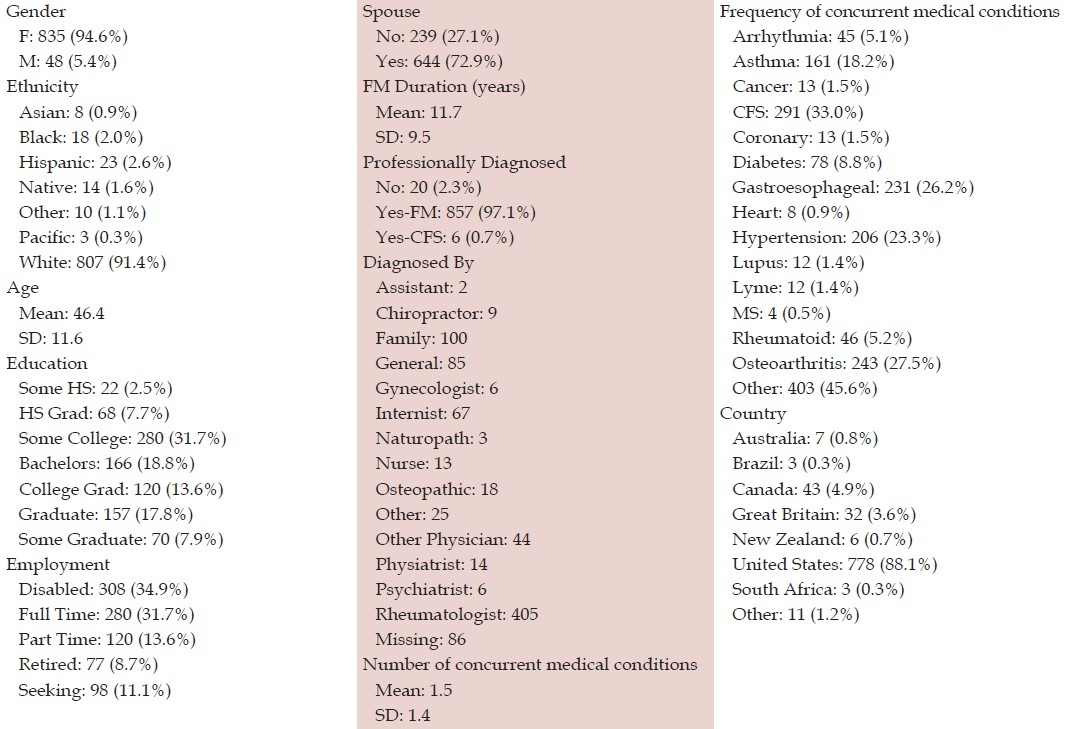
As seen in [Table 1], 883 individuals applied, primarily US-based white females in their mid-forties. Most were professionally diagnosed, had a spouse, were college-educated, and were unemployed, with mean 11.7 years duration of symptoms. A third reported concurrent CFS.
Table 1.
Sample characteristics (N=883)
Utilization
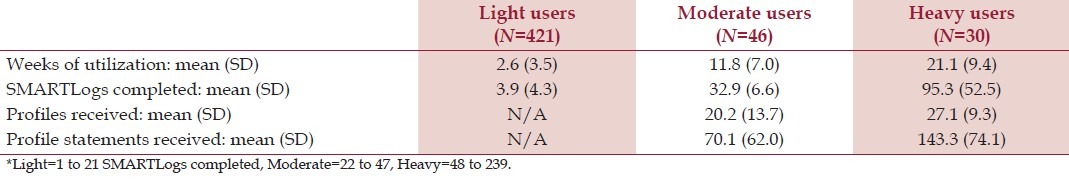
Table 2 reports utilization. Of all, 497 (56% of enrollees) used SMARTLog at least once and are considered “users.” Of these, 76 met the a priori criteria for production of SMART Profile target statements. We classified subjects completing 1 to 21 SMARTLogs as light users, 22-47 as moderate users, and 48+ as heavy users. We found no demographic predictors of study participation versus non-participation within the sample.
Table 2.
Utilization statistics (N=497)*
Change in survey scores
A total of 222 follow-up Monthly Surveys (mean 3.1) were completed. To compare baseline to follow-up, Kendall's tau-b correlations were obtained using the consecutive number of monthly surveys completed as an independent variable [Table 3].
Table 3.
Change in monthly survey scores over time – Kendall's tau (N=222)
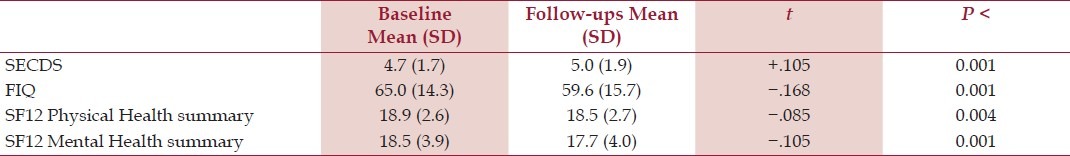
Survey data showed significant though modest 5-point improvement on the FIQ, a statistically though not clinically significant improvement in the SECDS, and statistically though not clinically significant declines in the SF12 Physical and Mental scales, as the number of monthly surveys progressed. For the MHLCS, no significant changes were seen: Baseline mean (SD) values were Internal, 21.3 (6.2); Chance, 15.6 (6.0); Doctors, 9.4 (3.6), and Other People, 7.7 (3.0).
Change in daily symptom levels
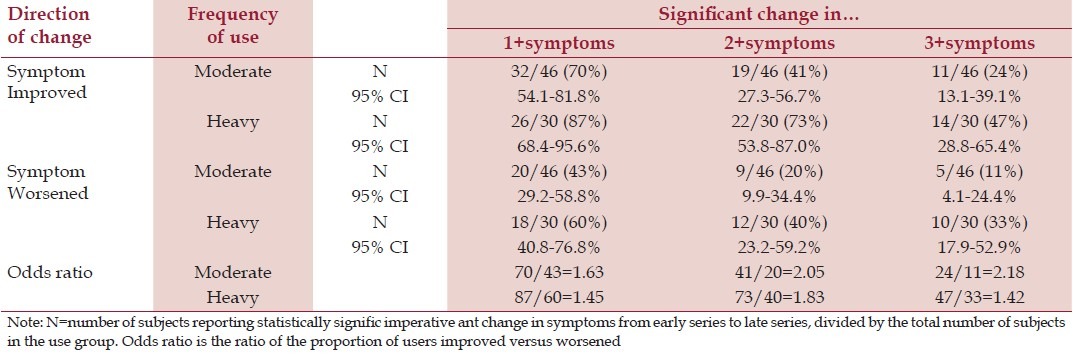
For subjects meeting the a priori criteria for analysis (moderate and heavy users, N = 76), we evaluated change in daily symptom levels across the early and late series of SMARTLogs. We limited analysis to 100 SMARTLogs per subject to ensure both a consistent series start point and comparable prior experience using the system.
As seen in Table 4, for both moderate and heavy users, the proportions with improved symptoms exceeded those with worsened symptoms. We note that the 95% confidence interval (CI) for prevalence of improved symptoms among heavy users exceeded that for prevalence of worsened symptoms among moderate users. Also, the 95% CI was greater for heavy users who experienced improvement in one vs three or more symptoms, and for moderate users who experienced worsening in one vs three or more symptoms. Since all other 95% CIs overlapped, this suggests that the observed high prevalence of improvement among heavy users and low prevalence of worsening among moderates are statistical outliers, particularly for improvement in one symptom.
Table 4.
Change in daily symptom levels (moderate and heavy users)
Detailed analysis of symptom change
Table 5 reports on the percentage of users with significant change in specific symptoms, as determined by UniODA applied to the users’ early and late series data. Analyses first focused on results with basic usage of the system, i.e., completing SMARTLogs as a self-monitoring activity without regard to whether the individual received any Profile target statements. These “basic use” data are reported in the “Log” column listing the percent of users with significant improvement (+) or worsening (−) by symptom. The ratio of these two percentages is also shown (+/−); this odds ratio (OR) is reported as a measure of effect size of the intervention.
Table 5.
Significant change and odds ratio (OR) for specific symptoms from early series to late series:†
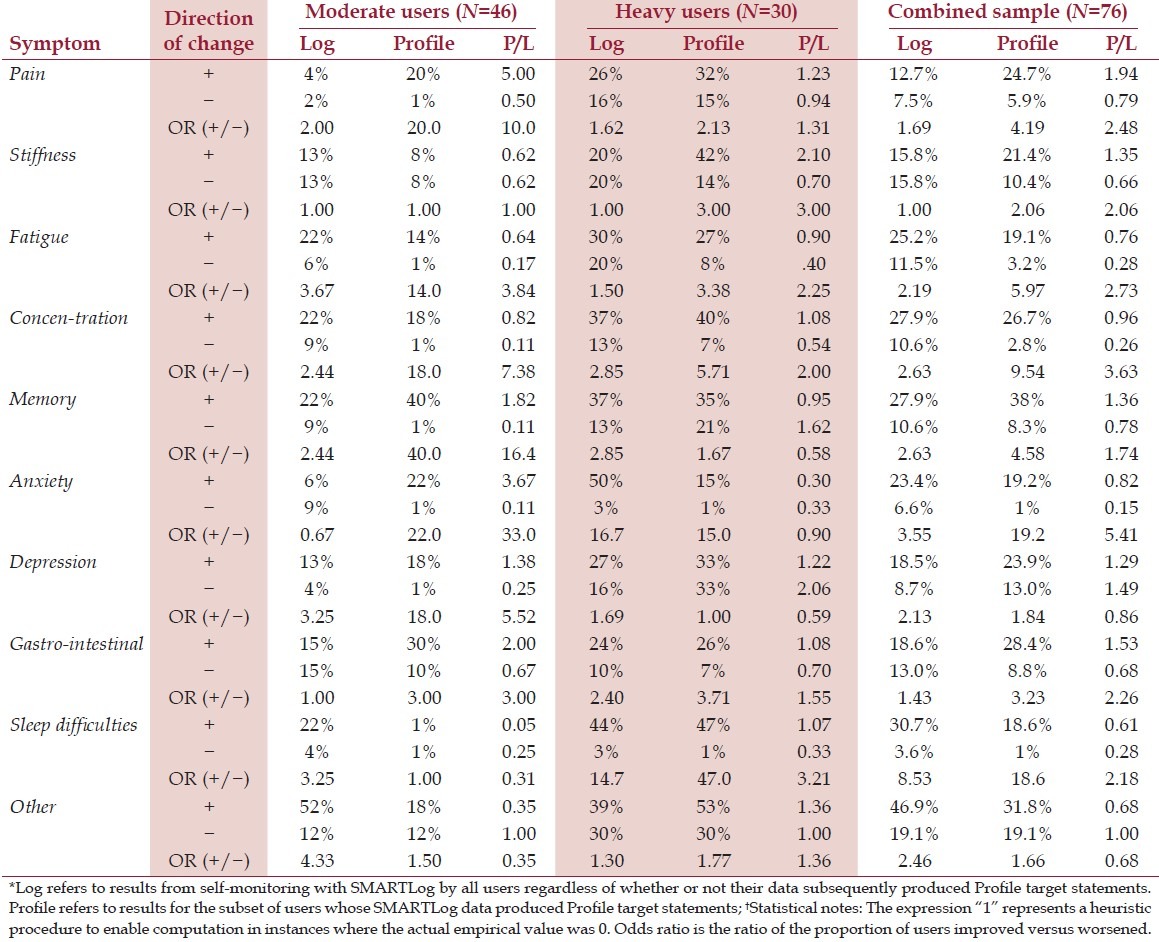
Next, results are presented for the subset of users whose data produced one or more Profiled target statements. These data are shown in the “Profile” column.
The “P/L” column indicates the ratio of the percent of users with significant symptom change in the Profile column divided by that in the Log column.
Discussion
A common axiom in reliability analysis is that a greater number of observations are more reliable than a smaller number. In this study, analyses were based on two sets of data: 222 Monthly Surveys and 6,014 SMARTLogs. The monthly survey data showed statistically, but generally not clinically, significant changes. The SMARTLog data may be a more reliable source for assessing overall impact of the intervention. We also note that the baseline period for the surveys was autumn, and that FM patients typically experience a decline in well-being from autumn into winter. This may help explain the slight declines in SF12 Physical and Mental scores during the early study period, though not the improving FIQ scores.
In terms of effects of use of SMARTLog alone vs the experience of receiving Profile target statements, we note that in phase I of this project (discussed in the Introduction), qualitative data from follow-up focus groups suggested that the usefulness of SMARTLog is not dependent on receiving SMART Profile target statements. This was supported by the reports of subjects that their regular use of SMARTLog raised their awareness of the impacts of daily behaviors independently from any feedback they received from Profiles. Completing SMARTLogs helped them become aware of health impacts and changed behavior even though they had not received a Profile statement addressing that issue. We interpret this as suggesting that one mechanism of benefit of SMARTLog is heightened vigilance of behavioral impacts on symptoms. This is important, as subjects are not guaranteed that significant associations will be discovered; there may not always be enough submissions or variability within their data to reveal significant associations, yet they may still benefit from using the system due to the heightened self-awareness it generates.
This study should be considered in light of other recent efforts underscoring the value of self-activation and individualized, self-directed approaches in FM. This point of view has gained consensus in the field. For example, the authors of the 2012 Canadian Guidelines for the Diagnosis and Management of Fibromyalgia Syndrome concluded that patients must be active participants in their healthcare and non-pharmacologic strategies are imperative. Further, they recommend symptom-based, patient-tailored management.[39]
In terms of non-pharmacologic strategies, a recent systematic review of 58 studies in psychological treatment of FM found cognitive-behavioral approaches to be most commonly used.[40] While not a “cognitive-behavioral therapy” per se, the SMARTLog approach is both cognitive and behavioral in the sense that it generates individually-relevant information to raise self-awareness of behavior and symptom patterns. And, of course, behavioral variables are quantified and monitored to further reinforce cognitive understanding.
As for patient-tailored management, one key area - and one that is easily addressed in the SMARTLog approach - is that of exercise. A recent review of studies on this issue reported that fear of movement and avoidance of physical activity is highly prevalent in FM, is related to symptom severity and self-reported quality of life and disability, and that an individually tailored approach is the most promising strategy for encouraging or restoring physical activity.[41] With SMARTLog, users can modulate and directly track their exercise activity and symptom levels in response, since exercise is a major category included in the SMARTLog query system. The Profile statements are capable of providing direct data-based guidance to the individual on this issue, which can potentially have important implications for self-management of exercise.
Finally, we note that no intervention is completely without risk, including online self-monitoring and automated feedback interventions. Particularly, in interventions designed for autonomous use, the role of personal judgment should be emphasized. Though we had no reports of adverse effects, it is possible that users could experience feelings of disappointment or failure if their expectations related to using the program do not materialize. Thus, an important aspect of implementation is instructions to potential users to understand that their personal data may not necessarily yield targeted Profile statements. Also, they should exercise their best personal judgment about whether to implement any Profile target statements that are offered, as the data on which they are based could be incomplete, or the guidance could contradict other strategies the user needs to implement.
Conclusions
The data on utilization patterns and integrated analysis of symptom change over time together support the following conclusions:
Moderate use - completing the SMARTLog at least three times per week for three months - increases the likelihood of clinically significant improvements in pain, memory, gastrointestinal problems, depression, fatigue, and concentration.
Heavy use - completing the SMARTLog an average of 4.5 times weekly over a five month period - increases the likelihood of clinically significant improvements in all of the above plus stiffness and sleep difficulties.
These outcomes are seen with basic utilization of the SMARTLog system regardless of whether the user's data led to specific SMART Profile target statements. When the user's data produce tailored SMART Profile target statements, the impact of the intervention on specific symptoms is further amplified.
This project has shown that an individualized, web-based behavioral self-monitoring program with personally-tailored feedback can enable FM patients to significantly reduce symptom levels over time. The intervention can be used without limitation due to geographic location, physical mobility, or access to professionals or formal healthcare services. Future development will focus on confirming the results of this study with a larger sample, determining predictors of likelihood of benefit, and identifying obstacles to utilization. Also needed is further refinement and development of the web-based technology toward fuller automation that can serve unlimited numbers of users. Updated information on the status of the program is available at www.collinge.org/FMProject.html.
Footnotes
Source of Support: Sponsored by National Institute of Arthritis, Musculoskeletal and Skin Diseases, grant #R44AR052640.
Conflict of Interest: None declared.
References
- 1.Yunus MB. Fibromyalgia and overlapping disorders: The unifying concept of central sensitivity syndromes. Semin Arthritis Rheum. 2007;36:339–56. doi: 10.1016/j.semarthrit.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 2.Winfield JB. Fibromyalgia and related central sensitivity syndromes twenty-five years of progress. Semin Arthritis Rheum. 2007;36:335–8. doi: 10.1016/j.semarthrit.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Yunus MB. Central sensitivity syndromes: A new paradigm and group nosology for fibromyalgia and overlapping conditions, and the related issue of disease versus illness. Semin Arthritis Rheum. 2008;37:339–52. doi: 10.1016/j.semarthrit.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Kindler LL, Bennett RM, Jones KD. Central sensitivity syndromes: Mounting pathophysiologic evidence to link fibromyalgia with other common chronic pain disorders. Pain Manag Nurs. 2011;12:15–24. doi: 10.1016/j.pmn.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woolf CJ. Central sensitization: Implications for the diagnosis and treatment of pain. Pain. 2011;152:S2–15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wood PB, Schweinhardt P, Jaeger E, Dagher A, Hakyemez H, Rabiner EA, et al. Fibromyalgia patients show an abnormal dopamine response to pain. Eur J Neurosci. 2007;25:3576–82. doi: 10.1111/j.1460-9568.2007.05623.x. [DOI] [PubMed] [Google Scholar]
- 7.Wood PB, Patterson JC, 2nd, Sunderland JJ, Tainter KH, Glabus MF, Lilien DL. Reduced presynaptic dopamine activity in fibromyalgia syndrome demonstrated with positron emission tomography: A pilot study. J Pain. 2007;8:51–8. doi: 10.1016/j.jpain.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 8.Riva R, Mork PJ, Westgaard RH, Rø M, Lundberg U. Fibromyalgia syndrome is associated with hypocortisolism. Int J Behav Med. 2010;17:223–33. doi: 10.1007/s12529-010-9097-6. [DOI] [PubMed] [Google Scholar]
- 9.Behm FG, Gavin IM, Karpenko O, Lindgren V, Gaitonde S, Gashkoff PA, et al. Unique immunologic patterns in fibromyalgia. BMC Clin Pathol. 2012;12:25. doi: 10.1186/1472-6890-12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muzammil S, Cooper HC. Acute pancreatitis and fibromyalgia: Cytokine link. N Am J Med Sci. 2011;3:206–8. doi: 10.4297/najms.2011.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Omoigui S. The biochemical origin of pain: The origin of all pain is inflammation and the inflammatory response. Part 2 of 3 – Inflammatory profile of pain syndromes. Med Hypotheses. 2007;69:1169–78. doi: 10.1016/j.mehy.2007.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bazzichi L, Rossi A, Massimetti G, Giannaccini G, Giuliano T, De Feo F, et al. Cytokine patterns in fibromyalgia and their correlation with clinical manifestations. Clin Exp Rheumatol. 2007;25:225–30. [PubMed] [Google Scholar]
- 13.Üçeyler N, Häuser W, Sommer C. Systematic review with meta-analysis: Cytokines in fibromyalgia syndrome. BMC Musculoskelet Disord. 2011;12:245. doi: 10.1186/1471-2474-12-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ablin JN, Buskila D. Fibromyalgia syndrome - Novel therapeutic targets. Maturitas. 2013;75:335–40. doi: 10.1016/j.maturitas.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Brown MM, Jason LA. Functioning in individuals with chronic fatigue syndrome: Increased impairment with co-occurring multiple chemical sensitivity and fibromyalgia. Dyn Med. 2007;6:6. doi: 10.1186/1476-5918-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedberg F, Jason LA. Chronic fatigue syndrome and fibromyalgia: Clinical assessment and treatment. J Clin Psychol. 2001;57:433–55. doi: 10.1002/jclp.1040. [DOI] [PubMed] [Google Scholar]
- 17.Moldofsky H. Fibromyalgia, sleep disorder and chronic fatigue syndrome. Ciba Found Symp. 1993;173:262–79. doi: 10.1002/9780470514382.ch15. [DOI] [PubMed] [Google Scholar]
- 18.Niazi AK, Niazi SK. Mindfulness-based stress reduction: A non-pharmacological approach for chronic illnesses. N Am J Med Sci. 2011;3:20–3. doi: 10.4297/najms.2011.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedberg F, Williams DA, Collinge W. Lifestyle-oriented non-pharmacological treatments for fibromyalgia: A clinical overview and applications with home-based technologies. J Pain Res. 2012;5:425–35. doi: 10.2147/JPR.S35199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American College of Rheumatology. [Accessed July 9, 2013]. at http://www.rheumatology.org/Practice/Clinical/Patients/Diseases_And_Conditions/Fibromyalgia .
- 21.Leslie M. Fibromyalgia syndrome: A comprehensive approach to identification and management. Clin Excell Nurse Pract. 1999;3:165–71. [PubMed] [Google Scholar]
- 22.Glombiewski JA, Sawyer AT, Gutermann J, Koenig K, Rief W, Hofmann SG. Psychological treatments for fibromyalgia: A meta-analysis. Pain. 2010;151:280–95. doi: 10.1016/j.pain.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 23.Bernardy K, Füber N, Köllner V, Häuser W. Efficacy of cognitive-behavioral therapies in fibromyalgia syndrome: A systematic review and meta-analysis of randomized controlled trials. J Rheumatol. 2010;37:1991–2005. doi: 10.3899/jrheum.100104. [DOI] [PubMed] [Google Scholar]
- 24.Häuser W, Arnold B, Eich W, Felde E, Flügge C, Henningsen P, et al. Management of fibromyalgia syndrome - an interdisciplinary evidence-based guideline. Ger Med Sci. 2008;6:Doc14. [PMC free article] [PubMed] [Google Scholar]
- 25.Mengshoel AM, Heggen K. Recovery from fibromyalgia - previous patients’ own experiences. Disabil Rehabil. 2004;26:46–53. doi: 10.1080/09638280410001645085. [DOI] [PubMed] [Google Scholar]
- 26.Henriksson CM. Living with continuous muscular pain - patient perspectives. Part II: Strategies for daily life. Scand J Caring Sci. 1995;9:77–86. doi: 10.1111/j.1471-6712.1995.tb00391.x. [DOI] [PubMed] [Google Scholar]
- 27.Mossavar-Rahmani Y, Henry H, Rodabough R, Bragg C, Brewer A, Freed T, et al. Additional self-monitoring tools in the dietary modification component of The Women's Health Initiative. J Am Diet Assoc. 2004;104:76–85. doi: 10.1016/j.jada.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 28.Lorig KR, Ritter PL, Laurent DD, Plant K. The internet-based arthritis self-management program: A one-year randomized trial for patients with arthritis or fibromyalgia. Arthritis Rheum. 2008;59:1009–17. doi: 10.1002/art.23817. [DOI] [PubMed] [Google Scholar]
- 29.Williams DA, Kuper D, Segar M, Mohan N, Sheth M, Clauw DJ. Internet-enhanced management of fibromyalgia: A randomized controlled trial. Pain. 2010;151:694–702. doi: 10.1016/j.pain.2010.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Menga G, Dupre BJ, Gauthier C, Davis WE, Webb-Detiege TA, Scopelitis E. Fibromyalgia: Can online cognitive behavioral therapy help? Arthritis Rheum. 2011;63(Suppl10):937. [Google Scholar]
- 31.Collinge W, Soltysik RC, Yarnold PR. An internet-based intervention for fibromyalgia self-management: Initial design and alpha test. Optim Data Anal. 2010;1:163–75. [Google Scholar]
- 32.Dunkl PR, Taylor AG, McConnell GG, Alfano AP, Conaway MR. Responsiveness of fibromyalgia clinical trial outcome measures. J Rheumatol. 2000;27:2683–91. [PubMed] [Google Scholar]
- 33.Ware J, Turner-Bowker DM, Kosinski M, Gandek B. SF-12v2 Health Survey User's Manual. Lincoln, RI: Quality Metric, Inc; 2003. p. 250. [Google Scholar]
- 34.Lorig K, Stewart A, Ritter P, González V, Laurent D, Lynch J . Outcome Measures for Health Education and other Health Care Interventions. Thousand Oaks, CA: Sage Publications; 1996. .Thousand Oaks, CA: Sage Publications; 1996; pp. 24–45. [Google Scholar]
- 35.Wallston KA, Stein MJ, Smith CA. Form C of the MHLC scales: A condition-specific measure of locus of control. J Pers Assess. 1994;63:534–53. doi: 10.1207/s15327752jpa6303_10. [DOI] [PubMed] [Google Scholar]
- 36.Yarnold PR, Soltysik RC, Bennett CL. Predicting in-hospital mortality of patients with AIDS-related pneumocystis carinii pneumonia: An example of hierarchically optimal classification tree analysis. Stat Med. 1997;16:1451–63. doi: 10.1002/(sici)1097-0258(19970715)16:13<1451::aid-sim571>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 37.Optimal Data Analysis. [Accessed June 23, 2013]. at http://www.optimaldataanalysis.com/softwarecta.htm .
- 38.Yarnold PR, Soltysik RC. Optimal Data Analysis: Guidebook with Software for Windows. Washington, DC: APA Books; 2005. [Google Scholar]
- 39.Fitzcharles MA, Ste-Marie PA, Goldenberg DL, Pereira JX, Abbey S, Choinière M, et al. National Fibromyalgia Guideline Advisory Panel.2012 Canadian guidelines for the diagnosis and management of fibromyalgia syndrome: Executive summary. Pain Res Manag. 2013;18:119–26. doi: 10.1155/2013/918216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lami MJ, Martinez MP, Sanchez AI. Systematic review of psychological treatment in fibromyalgia. Curr Pain Headache Rep. 2013;17:345. doi: 10.1007/s11916-013-0345-8. [DOI] [PubMed] [Google Scholar]
- 41.Nijs J, Roussel N, Van Oosterwijck J, De Kooning M, Ickmans K, Struyf F, et al. Fear of movement and avoidance behaviour toward physical activity in chronic-fatigue syndrome and fibromyalgia: State of the art and implications for clinical practice. Clin Rheumatol. 2013;32:1121–9. doi: 10.1007/s10067-013-2277-4. [DOI] [PubMed] [Google Scholar]


