Abstract
Background:
In diabetic patients, persistent hyperglycemia and poor glycemic control cause disturbances of lipid profiles, especially an increased production of oxygen free radicals. Lipid peroxidation has been considered to be a pathogenic factor of diabetic complications in Type-1 Diabetes mellitus.
Aims:
The aim of the present study was to investigate the effect of glycemic control on blood viscosity, lipid profile, and lipid peroxidation in Type-1 Diabetic subjects.
Materials and Methods:
The study included three groups; Group-I (age-matched healthy control subjects, n = 50), Group-II (Type-1 Diabetics with good glycemic control, n = 10), and Group-III (Type-1 Diabetics with poor glycemic control, n = 15). The Type 1 diabetic patients with duration of diabetes for more than 5 years were taken. Blood samples of all subjects were analyzed for all biochemical, hematological, and oxidative stress parameters.
Results:
The Erythrocyte malondialdehyde level was non-significantly changed (P = NS) in group–II patients but significantly increased (P < 0.001) in group-III patients, and no significant changes were found (P = NS) in Blood viscosity of both the groups (group-II and group-III), as compared to healthy control subjects (group-I).
Conclusions:
Our findings suggest that the monitoring of Oxidative stress and Blood Viscosity in poorly controlled Type-1 diabetic patients may be very useful marker of diabetic complications.
Keywords: Blood viscosity, Glycemic control, Lipid peroxidation, Lipid profile, Type-1 diabetes mellitus
Introduction
Diabetes mellitus is a common metabolic disorder resulting from defects in iInsulin secretion or action or both, which is characterized by hyperglycemia often accompanied by glycosuria, polydipsia, and polyuria[1] resulting from an absolute or relative deficiency of insulin secretion or action.[2] Insulin is the key hormone in blood glucose homeostasis.[3] Dyslipidaemia is a common feature of diabetes even with good glycemic control.[4] The key features of this diabetic dyslipidaemia are elevated level of triglyceride and LDL-c.[5] Improved metabolic control may decrease very-low-density lipoprotein cholesterol (VLDL-Cholesterol) and low-density lipoprotein cholesterol (LDL-Cholesterol).[6] The hyperglycemia or dyslipidaemia easily induce serious oxidative stress that causes serious cellular dysfunction as well as hematic and vascular complications in diabetic patients.[7],[8] Persistent hyperglycemia causes increased production of free radicals, especially reactive oxygen species (ROS), for all tissues from glucose auto-oxidation and protein glycosylation.[9] Noberasco et al.[10] and Gallon et al.[11] reported that MDA is the most commonly used marker of lipid peroxidation in diabetes mellitus. Jenkins et al. (1996) reported a normal susceptibility of LDL to in vitro oxidation in well-controlled Insulin dependent Diabetes Mellitus (IDDM) patients.[12]
Blood and plasma viscosity have emerged as independent risk factors for atherothromobtic vascular disease.[13] Fibrinogen is a risk factor for atherothrombiotic disease.[14] Fibrinogen is an important determinant of blood viscosity that results from Fibrinogen-induced erythrocyte aggregation and its contribution to plasma viscosity.[15] Defeo et al.[16] reported that fibrinogen synthesis is inhibited by the administration of insulin, hyper fibrinogenemia associated with insulinopemia.
Materials and Methods
The present study included 25 Type-1 diabetic patients (without complication) and 50 age-matched normal healthy controls who volunteered. The patients were recruited from the wards and OPDs of the Department of Medicine, Surgery, G.R. medical College and J.A. Hospital, Gwalior, M.P., India. The study was approved by the institutional ethical committee and written consent was also taken from the patients. These subjects were divided into three groups:
Group I: This group consisted of age-matched healthy control (n = 50).
Group II: This group consisted of good glycemic control (n = 10) Type-1 subjects.
Group III: This group consisted of poor glycemic control (n = 15) Type-1 subjects.
Controls were defined as not having a major medical illness, no hospital admissions, no current medication, and a subjective perception of good health. None of the healthy subjects received any medication and trace element supplement in the previous 2-3 months.
All diabetics were on insulin therapy since the last 5 to 15 years. The blood sample was taken from diabetic patients and healthy controls after an overnight fast under all aseptic precautions for analysis. All the samples were analyzed on the same day of collection. This sample was distributed in the following vials:
Ethylene di-amine tetra acetic acid (EDTA) sample for estimation of Fasting plasma glucose (FPG), plasma fibrinogen, Hematocrit, and Erythrocyte malondialdehyde (E-MDA). Citrated sample for estimation of Glycosylated hemoglobin (HbA1 c), Plain vial (Serum) for estimation of Total cholesterol (TC), Triglycerides (TG), VLDL-c, and HDL-c.
FPG was estimated by the method of Glucose Oxidase-Peroxidase (GOD-POD) by Trinder,[17] Glycosylated hemoglobin was measured by spectrophotometric method,[18] Triglyceride estimated by Von handle method,[19] Total cholesterol done by Ferric chloride method,[20] HDL cholesterol by Bernstein method,[21] LDL and VLDL cholesterol were calculated by Friedwal's formula.[22]
Friedwal's formula for LDL Cholecterol: Total cholesterol minus high-density lipoprotein (HDL) cholesterol minus VLDL cholesterol (estimated as triglyceride multiplied by 0.46).
LDL cholesterol (mmol/L) = Total cholesterol – HDL Cholesterol - VLDL cholesterol
Friedwal's formula for VLDL Cholesterol:
VLDL cholesterol (mmol/L) = 0.46 × Triglyceride
E-MDA for lipid peroxidation was done by Bidder and Jaeger Method,[23] Plasma fibrinogen was determined by Tyrosine (Lempert) method,[24] Hematocrit was determined by Wintrobe's method,[25] and Blood viscosity was calculated by Merril's formula.[26]
The blood viscosity was calculated by the formula as described by Merril.
Blood viscosity (YSS) = 13.5 × 10−6 × (Fibrinogen Concentration in gm%)2 × (Hct-6)3
Where: YSS = Yield shear stress, gm = Gram, Hct = Haematocrit
Statistical study
All results were expressed in Mean ± SD. The statistical analysis was performed using Statistical Product and Service Solutions (SPSS) version 7.0. Pearson correlation coefficients were calculated to analyze relationships between biochemical parameters. The level of significance was considered as P value > 0.05.
Results
Table 1 shows the levels of fasting blood sugar, glycosylated hemoglobin, lipid profile, and E-MDA in Type-1 diabetic patients and healthy control subjects. There were significant increases in Group-III (n = 15) patients in the levels of FPG and (P > 0.001) and TC (P > 0.001), TG (P > 0.05), and HDL (P > 0.001), while Group-II (n = 10) patients had non-significant changes in total cholesterol, VLDL-c, and LDL-c when compared with normal healthy subjects.
Table 1.
FBG, HbA1c, Lipid profile, and E-MDA in Group-I, Group-II, and Group-III subjects
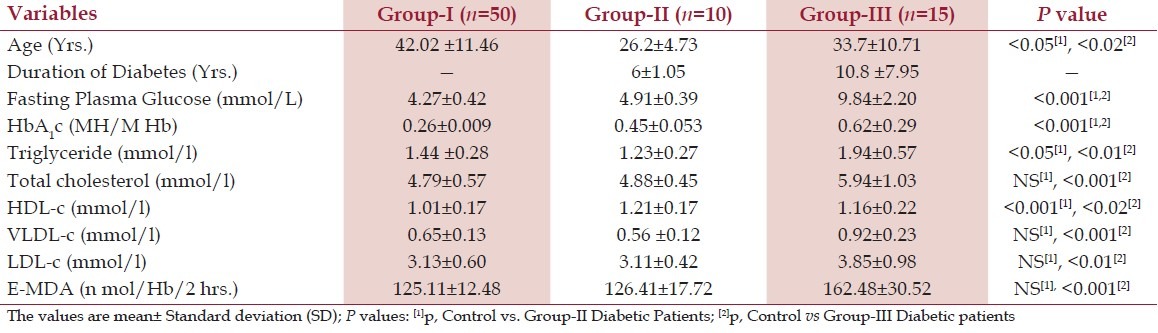
Table 2 shows the levels of plasma fibrinogen, hematocrit, and blood viscosity in Group-I, Group-II, and Group-III. Plasma fibrinogen, hematocrit, and blood viscosity (P = NS) were non-significantly changed as compared with those of healthy control subjects [Table 2]. These results indicate that in Group-II (n = 10), good glycemic control helps in lowering the level of lipid profile and blood viscosity, which may contribute to the prevention of onset of diabetic complications.
Table 2.
Levels of plasma fibrinogen, hematocrit, and blood viscosity in Group-I, Group-II, and Group-III subjects (Mean±SD)
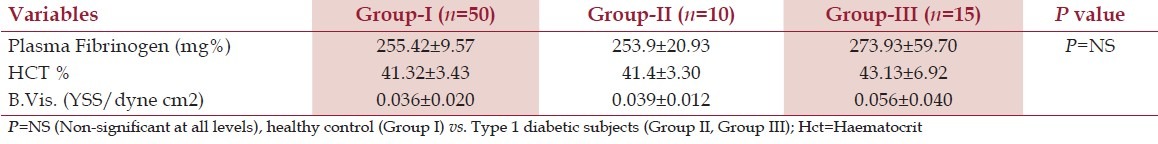
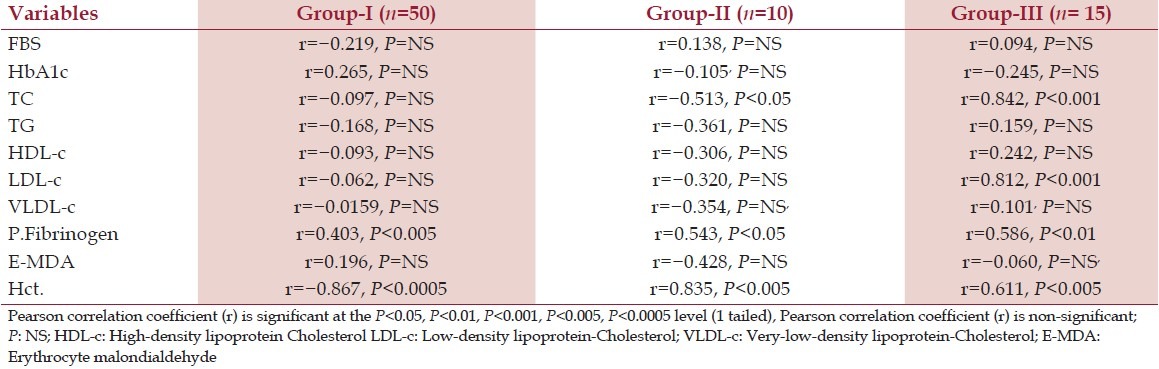
Table 3 shows the correlation between blood viscosity vs fasting blood sugar, glycosylated hemoglobin, lipid profile, plasma fibrinogen, hematocrit, and E-MDA. There was significant correlation found between blood viscosity vs TC (P > 0.001), LDL-C (P > 0.001), plasma fibrinogen (P > 0.05), and hematocrit (P > 0.001), whereas non-significant correlation have been found between blood viscosity vs FBS, TG, HDL-c, VLDL-c, and E- MDA (P = NS).
Table 3.
Correlation of blood viscosity with FPG, HbA1c, lipid profile, plasma fibrinogen, E-MDA, and hematocrit in Group-I, Group-II, and Group-III subjects
Discussion
Blood viscosity is a basic biological parameter that affects blood flow both at large arteries and in the microcirculation. There is a sufficient evidence that the elevated blood viscosity is a pathogenic factor of diabetic microangiopathy, altering microcirculation and leading to insufficient tissue nutrition.[27] A number of researchers found that the blood viscosity was altered in diabetes.[28],[29],[30] As the osmolarity of the blood increases due to increased sugar level, the capillary permeability increases, thus increasing hematocrit and subsequently the blood viscosity.[31] Similar findings have been observed by Lowe et al.[32] and Langer et al.[33] that osmotic diuresis is caused by hyperglycemia, leading to decreaaed plasma volume and increased hematocrit. Barnes et al.[34] reported that both hematocrit and blood viscosity decreased after institution of good diabetic control. Diabetes patients had a higher blood viscosity than healthy people.[35],[36]
There are convincing experimental and clinical evidences that the generation of ROS is increasing in both types of diabetes and that the onset of diabetes is closely associated with oxidative stress.[37],[38] Free radicals are formed disproportionately in diabetes by glucose autoxidation, polyol pathway, and non-enzymatic glycation of proteins.[39] The increased production and/or ineffective scavenging of such free radicals may play a crucial role in determining tissue injury, and increased lipid peroxidation which leads to complications of diabetes mellitus.[40] The increased level of glycosylated hemoglobin was observed in the diabetic patients and this increase is directly proportional to the blood glucose level.[41] This suggests the increase in oxidative stress due to hyperglycemia. Increased lipid peroxidation impairs membrane function by decreasing membrane fluidity and changing the activity of membrane-bound enzymes and receptors. The products of lipid peroxidation are harmful to most cells in the body and are associated with a variety of diseases, such as atherosclerosis and brain damage.[42]
In our study, a significant increase of MDA was observed in the Group-III diabetic patients. We have found that after taking insulin, some diabetic subjects had poor glycemic control and some had good glycemic control. A study was carried out in these two groups and observed that glycemic control plays a very important role and could help in reducing the onset of diabetic complications. An increase in plasma TG concentration signifies and elevation of the VLDL and /or chylomicrone concentrations as suggested previously.[43] Patients with IDDM who are in extremely poor metabolic control develop severe hypertriglyceridemia, primarily due to accumulation of chylomicrones.
Patients have taken insulin therapy, and fibrinogen synthesis is inhibited by the administration of insulin.[44],[45] Lower fibrinogen and hematocrit level can help to affect the blood viscosity level.
A further research is needed on large sample size to enhance our present understanding statistically, especially on Group-I and Group-II of Type-1 Diabetic patients.
Conclusions
The increased efficiency of oxidative stress in Type-1 diabetic patients with poor glycemic control constitute the pathogenic link between hyperglycemia and development of endothelial dysfunction. Moreover, blood viscosity was not increased due to insulin administration in both the groups of Type-1 Diabetes mellitus patients.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.American Diabetes Association (ADA) Diagnosis and classification of diabetes mellitus. Diabetes Care. 2005;28(suppl 1):S37–42. doi: 10.2337/diacare.28.suppl_1.s37. [DOI] [PubMed] [Google Scholar]
- 2.Le Devehat C, Khodabandehlou T, Vimeux M. Impaired hemorheological properties in diabetic patients with lower limb arterial ischaemia. Clin Hemorheol Microcirc. 2001;25:43–8. [PubMed] [Google Scholar]
- 3.Kumar V, Abbas A, Fausto N. Robbins and Cotran Pathologic Basis of Disease. 7th ed. Philadelphia: Elsevier Saunders; 2004. [Google Scholar]
- 4.Kuusisto J, Mykkänen L, Pyörälä K, Laakso M. Cardiovascular risk factors as predictors of tyhpe-2 (NIDDM) in elderly subjects. Diabetologia. 1993;36:553–9. doi: 10.1007/BF02743273. [DOI] [PubMed] [Google Scholar]
- 5.Feingold KR, Grunfeld C, Pang M, Doerrler W, Krauss RM. LDL subclass phenotypes and triglyceride metabolism in non-insulin-dependent disease. Arterioscler Thromb. 1992;12:1496–502. doi: 10.1161/01.atv.12.12.1496. [DOI] [PubMed] [Google Scholar]
- 6.Dunn FL, Pietri A, Raskin P. Plasma lipid and lipoprotein levels with continuous subcutaneous insulin infusion in Type-1 diabetes mellitus. Ann Intern Med. 1981;95:426–30. doi: 10.7326/0003-4819-95-4-426. [DOI] [PubMed] [Google Scholar]
- 7.Khera PK, Joiner CH, Carruthers A, Lindsell CJ, Smith EP, Franco RS, et al. Evidence for interindividual heterogeneity in the glucose gradient across the human red blood cell membrane and its relationship to hemoglobin glycation. Diabetes. 2008;57:2445–52. doi: 10.2337/db07-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kröger J, Zietemann V, Enzenbach C, Weikert C, Jansen EH, Döring F, et al. Erythrocyte membrane phospholipid fatty acids, desaturase activity, and dietary fatty acids in relation to risk of type 2 diabetes in the European Prospective Investigation into Cancr and Nutrition (EPIC)-Potsdam Study. Am J Clin Nutr. 2011;93:127–42. doi: 10.3945/ajcn.110.005447. [DOI] [PubMed] [Google Scholar]
- 9.Robertson RP. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. J Biol Chem. 2004;279:42351–4. doi: 10.1074/jbc.R400019200. [DOI] [PubMed] [Google Scholar]
- 10.Noberaseo G, Odetti P, Boeri D, Maiello M, Adezati L. Malandialdehyde (MDA) level in diabetic subjects. Relationship with blood glucose and glycated hemoglobin. Biomed Pahrmacother. 1991;45:193–6. doi: 10.1016/0753-3322(91)90107-5. [DOI] [PubMed] [Google Scholar]
- 11.Gallou G, Ruelland A, Legras B, Mangendre D, Allannic H, Cloarr L. Plasma malondialdehyde in type 1 and 2 diabetic patients. Clin Chim Acta. 1993;214:227–34. doi: 10.1016/0009-8981(93)90114-j. [DOI] [PubMed] [Google Scholar]
- 12.Jenkins AJ, Klevin RI, Chassereau CN, Hermayer KL, Lopes-Vierlla MF. LDL from patients with well-controlled IDDM is not more susceptible to invitro oxidation. Diabetes. 1996;45:762–7. doi: 10.2337/diab.45.6.762. [DOI] [PubMed] [Google Scholar]
- 13.Koenig W, Ernst E. The possible role of haemorrheology in Atherothrombogenesis. Atherosclerosis. 1992;94:93–107. doi: 10.1016/0021-9150(92)90234-8. [DOI] [PubMed] [Google Scholar]
- 14.Vague P, Juhan-vague I. Fibrinogen, Fibrinolysis and diabetes mellitus: A comment. Diabetologia. 1997;40:738–40. doi: 10.1007/s001250050743. [DOI] [PubMed] [Google Scholar]
- 15.Ernst E, Resch KL. Fibrinogen as a cardiovascular risk factor: A meta-analysis and review of literature. Ann Intern Med. 1993;118:956–63. doi: 10.7326/0003-4819-118-12-199306150-00008. [DOI] [PubMed] [Google Scholar]
- 16.De Feo P, Volpi E, Lucioi P, Cruciani G, Reboldi G, Siepi D, et al. Physiological increments in plasma insulin concentrations have selective and different effects on synthesis of hepatic proteins in normal humans. Diabetes. 1993;42:995–1002. doi: 10.2337/diab.42.7.995. [DOI] [PubMed] [Google Scholar]
- 17.Trinder P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann Clin Biochem. 1969;6:24–7. [Google Scholar]
- 18.Beena Rai K, Krishna Sharma K, Pattabiraman TN. Colorimetric method for the estimation of glycosylated haemoglobin. Biochem Med. 1984;31:65–72. doi: 10.1016/0006-2944(84)90060-7. [DOI] [PubMed] [Google Scholar]
- 19.van handle E, Zilversmit DB. Micromethod for the direct determination of serum triglycerides. J Lab Clin Med. 1957;50:152–7. [PubMed] [Google Scholar]
- 20.Zlatkis A, Zak B, Boyle AJ. A new method for the direct determination of the serum cholesterol. J Lab Clin Med. 1953;41:486–92. [PubMed] [Google Scholar]
- 21.Burnstein M, Scholnick HR, Morfin R. Clinical practical Biochemistry by Harvold varley. 5th ed. Vol. 1. New Delhi, India: CBS Publisher; 1991. pp. 665–7. [Google Scholar]
- 22.Friedwald WT, Levy RI. Fredrickson DS: Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 23.Bidder TG, Jaeger PD. Malondialdehyde production by erythrocyte from alcoholic and non-alcoholic subjects. Life Sci. 1982;30:1021–7. doi: 10.1016/0024-3205(82)90520-3. [DOI] [PubMed] [Google Scholar]
- 24.Lampert Varley Harvold. Practical clinical Biochemistry. 4th ed. New Delhi, India: CBS Publisher; 1988. Plasma fibrinogen estimation by Tyrosine method; pp. 244–5. [Google Scholar]
- 25.Wintrobe MM. A simple and accurate hematocrit. J Lab Clin Med. 1929;15:287–9. [Google Scholar]
- 26.Merill LW, Cheng CS, Pelletier GA. Yield shear stress on normal human blood as function of endogenous fibrinogen. J Appl Physiol. 1969;26:1–3. doi: 10.1152/jappl.1969.26.1.1. [DOI] [PubMed] [Google Scholar]
- 27.Müller R. Diabetic angiopathy and blood viscosity. Acta Diabet Lat. 1973;10:1311–24. doi: 10.1007/BF02590717. [DOI] [PubMed] [Google Scholar]
- 28.Skovborg F, Nielsen AV, Schlichtkrull J, Ditzel J. Blood-viscosity in diabetic patients. Lancet. 1966;1:129–31. doi: 10.1016/s0140-6736(66)91264-5. [DOI] [PubMed] [Google Scholar]
- 29.Dintenfass L. Blood viscosity factors in severe nondiabetic and diabetic retinopathy. Biorheology. 1977;14:151–7. doi: 10.3233/bir-1977-14401. [DOI] [PubMed] [Google Scholar]
- 30.Schmid-Schonbein H, Volger E. Red-cell aggregation and red-cell deformability in diabetes. Diabetes. 1796;25(2 Suppl):897–902. [PubMed] [Google Scholar]
- 31.Meiselman HJ, Merrill EW, Gilliland ER, Pelletier GA, Salzman EW. Influence of plasma osmolarity on the rheology of human blood. J Appl Physiol. 1967;22:772–81. doi: 10.1152/jappl.1967.22.4.772. [DOI] [PubMed] [Google Scholar]
- 32.Lowe GD, Lowe JM, Drummond MM, Reith S, Belch JJ, Kesson CM, et al. Blood viscosity in young male diabetics with and without retinopathy. Diabetologia. 1980;18:359–63. doi: 10.1007/BF00276814. [DOI] [PubMed] [Google Scholar]
- 33.Langer L, Bergentz SE, Bjure J, Fagerberg SE. The effect of exercise on haematocrit, plasma volume and viscosity in diabetes mellitus. Diabetologia. 1971;7:29–33. doi: 10.1007/BF02346251. [DOI] [PubMed] [Google Scholar]
- 34.Barnes AJ, Locke P, Dormandy TL, Dormandy JA. Blood viscosity and metabolic control in diabetes mellitus. Clin Sci Mol Med. 1977;52:24–25. [Google Scholar]
- 35.Peduzzi M, Melli M, Fonda S, Codeluppi L, Guerrieri F. Comparative evaluation of blood viscosity in diabetic retinopathy. Int Ophthalmol. 1984;7:15–9. doi: 10.1007/BF00138264. [DOI] [PubMed] [Google Scholar]
- 36.Isogai Y, Iida A, Michizuki K, Abe M. Haemorheological study on the pathogenesis of diabetic microangiopathy. Thromb Res. 1971;81(Suppl 2):17–24. doi: 10.1016/0049-3848(76)90043-8. [DOI] [PubMed] [Google Scholar]
- 37.Johansen JS, Harris AK, Rychly DJ, Ergul A. Oxidative stress and the use of antioxidants in diabetes: Linking basic science to clinical practice. Cardiovasc Diabetol. 2005;4:5. doi: 10.1186/1475-2840-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rösen P, Nawroth PP, King G, Möller W, Tritschler HJ, Packer L. The role of oxidative stress in the onset and progression of diabetes and its complication. Diabetes Metab Res Rev. 2001;17:189–212. doi: 10.1002/dmrr.196. [DOI] [PubMed] [Google Scholar]
- 39.Obrosova IG, Vanlteysen C, Fathallah L, Cao XC, Greene DA, Stevens MJ. An aldose reductase inhibitor reverses early diabetes-induced changes in peripheral nerve function. FASEB J. 2002;16:123–5. doi: 10.1096/fj.01-0603fje. [DOI] [PubMed] [Google Scholar]
- 40.Maritim AC, Sanders RA, Watkins JB., 3rd Diabetes oxidative stress and antioxidants: A review. J Biochem Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 41.Yassind D Al, Ibrahim KA. Minor haemoglobin fraction and the level of fasting blood glucose. J Fac Med Univ Baghdad. 1981;23:373–80. [Google Scholar]
- 42.Acworth IN, Mccabe DR, Maher T. The analysis of free radicals, their reaction products, and antioxidants. In: Baskin SI, Salem H, editors. Oxidants, Antioxidants and Free Radicals. Chapter 2. Washington DC: Taylor and Francis; 1997. [Google Scholar]
- 43.Albrink MJ. Dietary and drug treatment of hyper lipidemia in diabetes. Diabetes. 1974;23:913–8. doi: 10.2337/diab.23.11.913. [DOI] [PubMed] [Google Scholar]
- 44.Defeo P, Gaisano MG, Haymard MW. Different effects on insulin deficiency on albumin and fibrinogen synthesis in humans. J Clin Invest. 1991;88:833–40. doi: 10.1172/JCI115384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Feo P, Volpi E, Lucidi P, Cruciani G, Reboldi G, Siepi D, et al. Physiological increments in plasma insulin concentrations have selective and different effects on synthesis of hepatic proteins in normal humans. Diabetes. 1993;42:995–1002. doi: 10.2337/diab.42.7.995. [DOI] [PubMed] [Google Scholar]


