Abstract
With current pharmacological treatments, preventing the remodeling of the left ventricle and the progression to heart failure is a difficult task. Gene therapy is considered to provide a direct treatment to the long-term complications of ischemic heart diseases. Although current gene therapies that use single molecular targets seem potentially possible, they have not achieved a success in the treatment of ischemic diseases. With an efficient polymeric gene carrier, PAM-ABP, we designed a synergistically combined gene delivery strategy to enhance vascular endothelial growth factor (VEGF) secretion and prolong anti-apoptotic effects. A hypoxia-inducible plasmid expressing both hypoxia-inducible heme oxygenase-1 (HO-1) and the Src homology domain-2 containing tyrosine phosphatase-1 microRNA (miSHP 1) and a hypoxia-responsive VEGF plasmid were combined in this study. The positive feedback circuit between HO-1 and VEGF, and the negative regulatory role of SHP-1 in angiogenesis enhance VEGF secretion synergistically. The synergy in VEGF secretion as a consequence of the gene combination and the prolonged HO-1 activity was confirmed in hypoxic cardiomyocytes and cardiomyocyte apoptosis under hypoxia, and was decreased synergistically. These results suggest that the synergistic combination of VEGF, HO-1, and miSHP-1 may be promising for the clinical treatment of ischemic diseases.
Keywords: Gene delivery, Heme oxygenase-1, SHP-1, VEGF, microRNA, PAM-ABP
1. Introduction
Gene therapy is an attractive alternative to current pharmacological treatments for ischemic diseases due to its direct effects in treating and reversing the pathophysiology underlying the long-term complications of ischemic heart diseases 1, 2. Gene therapy can change the function and fate of the cells in ischemic tissue 3. Plasmid DNA that contains promoters/enhancers, stabilizing domains, and targeting sequences is able to regulate gene expression in response to the intracellular environment, and minimizes unwanted side effects caused by unrestricted protein synthesis. Small interfering RNA (siRNA) is capable of silencing its target mRNA, resulting in a decrease in protein production. Since both plasmid DNA and siRNA are not able to penetrate cell membrane due to their high molecular weight and negative charge, gene carriers are essential for successful gene therapy 4.
Novel biodegradable cationic polymers as non-viral gene carriers have been used to deliver plasmid DNA or siRNA 5. Arginine-grafted bioreducible poly(disulfide amine) (ABP), a recently developed cationic polymer for gene delivery, showed low cytotoxicity and good transfection efficiency; however, a large amount of polymer is required for high transfection 6. This is due, in part, to the low molecular weight and the low charge density of ABP. Polymeric gene carriers with low molecular weight may be less stable, because the weak electrostatic interaction between polymer and gene results in the formation of a loose nano-structure. High molecular weight ABP therefore needs to be developed. To increase the molecular weight and the charge density of ABP, we have developed PAM-ABP composed of one poly(amido amine) (PAMAM) G0 dendrimer and four ABP molecules 7. PAM-ABP reduces the minimum amount of polymer that is required to form a compact polyplex and to increase gene transfection even at a low ratio of PAM-ABP to DNA. In addition, the disulfide bond between ABP and PAMAM is cleaved in the cytoplasm, facilitating DNA release and reducing cytotoxicity.
Sequentially combined gene therapy is a promising treatment for ischemic diseases because the single gene therapies are effective for either preventing apoptosis of ischemic cells or inducing angiogenesis. Gene therapy for ischemic diseases necessitates induction of neovascularization and inhibition of apoptosis. In addition, successful ischemic disease gene therapy via the above approaches needs sufficient genetic interventions based on precise basic understanding of the mechanisms of heart failure. The genetic intervention includes: 1) overexpression of a target molecule by introduction of plasmid DNA; 2) loss-of-function approach by introduction of RNA interference (RNAi); and 3) correcting deleterious gene mutations/deletions at the genome or primary mRNA level. Neovascularization and inhibition of apoptosis are considered as good approaches for the sequentially combined gene therapy for ischemic disease. In the early stage of myocardial infarct, reduced oxygen supply and increased ROS occur in ischemic cardiomyocytes followed by apoptosis. Protecting the cells from apoptosis is the first step and the second step is to reestablish vasculature through angiogenesis that returns hypoxic condition back to normoxic state.
SiRNA is widely used for anti-apoptosis of cells under ischemia; however, this knockdown approach takes long time to exert its activity compared to knock-in gene therapy (plasmid DNA). The efficacy of siRNA-based anti-apoptotic gene therapy is limited because it is inadequate to inhibit cardiomyocyte apoptosis in the early stage. Plasmid DNA that expresses anti-apoptotic protein can compensate for the shortcomings of siRNA-mediated approach. To combine knock-in and knockdown genes, we developed a hypoxia-inducible plasmid that expresses dual genes of heme oxygenase-1 (HO-1; knock-in) and Src homology domain 2 containing tyrosine phosphatase-1 microRNA (miSHP-1; knockdown), both of which have anti-apoptotic effect. HO-1 is a stress inducible anti-inflammatory, anti-apoptotic, anti oxidant enzyme that protects cardiomyocytes under ischemia 8. HO-1 can prevent cardiac remodeling upon ischemia/reperfusion injury by suppressing the early inflammation and inhibiting cardiomyocyte apoptosis 9. SHP-1 negatively regulates anti-apoptotic signaling pathways, including extracellular signal-regulated kinase (ERK1/2) and BCL-2. SHP-1 binding to TNFR-1, and FAS-R promotes de-phosphorylation in signal transduction pathways resulting in apoptosis 10, 11. Inhibiting SHP-1 decreased cardiomyocyte apoptosis and increased cardio-protection through Akt activation 12, 13. In addition, both HO-1 and miSHP-1 can synergistically enhance the secretion of vascular endothelial growth factor (VEGF). Several studies have reported that both HO-1 overexpression and SHP-1 silencing accelerate angiogenesis in ischemic areas 14,16. In the present synergistically combined gene delivery, HO-1, miSHP-1, and VEGF are capable of protecting cardiomyocytes, while VEGF induces angiogenesis.
A plasmid cocktail composed of the Dual plasmid and the VEGF plasmid was prepared at different mixing ratios, and the gene expression was determined after transfecting the cocktails to ischemic cardiomyocytes. The best condition to prepare the plasmid cocktail was found and the condition showed synergistically increased HO-1 expression and VEGF secretion. Finally, the current sequentially combined gene delivery showed a synergy in anti-apoptotic effects.
2. Materials and Methods
2.1 Materials
All cell culture products including fetal bovine serum (FBS), Dulbecco's phosphate buffered saline (DPBS), and Dulbecco's modified Eagle's medium (DMEM) were obtained from Invitrogen (GibcoBRL, Carlsbad, CA). Furin inhibitor II was obtained from EMD chemicals Inc. (Gibbstown, NJ). HumanVEGF ELISA kit was purchased from Thermo Scientific (Rockford, IL). HumanHO-1 and ratVEGF ELISA development kits were obtained from R & D Systems (Minneapolis, MN). RatHO-1 ELISA development kit, zinc (II) protoporphyrin IX (ZnPP), and cobalt (III) protoporphyrin IX chloride (CoPP) were from Enzo Life Sciences (Farmingdale, NY). Caspase-Glo® 3/7 Assay was from Promega (Madison, WI).
2.2 Plasmid preparation
The pβ-SP-ODD-VEGF (SP: signal peptide; ODD: oxygen-dependent degradation domatin) plasmid was prepared as described previously 17. In brief, the human VEGF (hVEGF) cDNA was amplified by PCR using pSV-VEGF as a template, and the furin recognition site was inserted at the upstream of the start codon of the VEGF cDNA. The ODD amplified by PCR was inserted upstream of the furin-VEGF cDNA, resulting in construction of the pβ-ODD-VEGF. Finally, the SP cDNA was inserted into pβ-ODD-VEGF, producing the pβ-SP-ODD-VEGF. The plasmid expressing both human HO-1 (hHO-1) gene and rat SHP-1 miRNA was prepared according to the original paper published by our group 18. In brief, the HO-1 cDNA amplified by PCR using pSV HO-1 was inserted downstream of the Epo enhancer and SV40 promoter of pEpo-SV, resulting in the construction of pEpo-SV-HO-1. The SHP-1 siRNA sequence (5’-GGACAUUUCUUGUGC-GUGA-3’) was inserted into the miR-30 backbone (miSHP-1). As a negative control, luciferase miRNA (miLuci) was also constructed. The miRNA was inserted into the synthetic intron sequence in the GeneSwitchTM vector (Invitrogen; Carlsbad, CA). The intron containing miRNA was amplified by PCR and inserted into the pEpo-SV-HO-1, resulting in the construction of pEpo-SV-miSHP-1-HO-1.
2.3 PAM-ABP synthesis
PAM-ABP was synthesized as previously detailed 19. In brief, ABP was first synthesized as described previously 6. The mixture of SPDP and ABP was reacted for 1 hour, and then dialyzed using a dialysis membrane (MWCO = 1 kDa, Spectrum Laboratories, Inc., Rancho Dominguez, CA). PAMAM dissolved in phosphate buffered saline was reacted with Traut's reagent to introduce sulfhydryl groups for 2 h. The PAMAM-SH was purified and lyophilized. The ABP-SPDP was mixed with the PAMAM-SH, and the reaction was terminated by dialysis when no release of pyridine-2-thione was observed under UV.
2.4 Gel retardation
The pβ-SP-ODD-VEGF (referred to as VEGF) and the pEpo-SV-miSHP-1-HO-1 (referred to as Dual) were mixed at different weight ratios from 1:9 to 9:1 (VEGF:Dual). Five microgram of PAM-ABP was added to 1μg of pDNAs (PAM-ABP:DNA=5:1, w/w). This weight ratio was used in all transfection experiments. After 20 minutes of incubation, the samples including pDNA-only groups were run in 0.8% agarose gel at 100 V for 20 minutes.
2.5 Cell culture and transfection
H9C2 cells, rat cardiomyocytes, were cultured in DMEM containing 10% FBS and 1% antibiotics at 37°C under 5% CO2. The cells were seeded on 24-well plates at a density of 2.0 × 104 cells/well. After 24 hours of incubation, the culture media was replaced with plain media containing PAM-ABP/pDNA polyplexes prepared by mixing 1 μg pDNA and 5 μg PAM-ABP. After 4 hours, the cells were washed with PBS and cultured with DMEM. The culture plates were placed in a hypoxia chamber filled with 5% CO2, 94% N2, and 1% O2, and this hypoxia condition was used for all subsequent experiments.
2.6 VEGF and HO-1 ELISA
Cells were cultured and transfected with polyplexes as described above. The cell culture media was collected and centrifuged for 30 seconds at 13,000 rpm to remove impurities at the time of interest after transfection for VEGF ELISA. At same time points, the cells were washed with PBS and lysed with 150 μl of reporter lysis buffer for 20 minutes. To determine HO-1 expression, the cells were harvested and centrifuged for 30 seconds at 13,000 rpm. The amount of VEGF secretion and HO-1 expression were quantified and normalized as pg VEGF or HO-1/mg total protein using ELISA kits and BCA protein assay kit (Promega; Madison, WI) according to the manufacturer's protocol.
2.7 Caspase assay
Cells lysates were prepared as described above. Caspase-Glo 3/7-assay reagent was mixed with the cell lysates at a ratio of 1:1, and then incubated for 40 minutes. The luminescence of each sample in a 96-well plate was measured using a luminometer (Tecan; San Jose, CA). The caspase activity was normalized as activity/mg total protein.
3. Results
3.1 DNA condensation
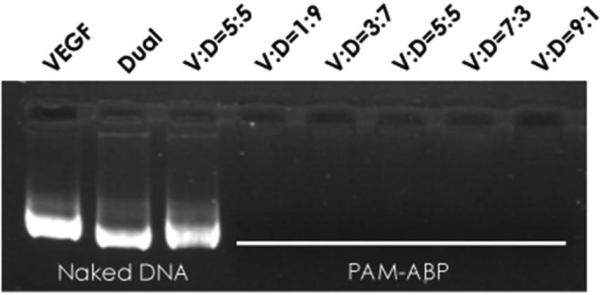
In order to confirm that PAM-ABP is capable of condensing the plasmid cocktail prepared at different ratios of the Dual and the VEGF, gel retardation assay was performed as shown in Fig. 1. The VEGF and the Dual plasmids mixed at ratios from 1:9 to 9:1 (VEGF:Dual) were condensed by PAM-ABP, and then loaded into agarose gel. The VEGF only, the Dual only, and the mixture of VEGF and Dual at 5:5 were electrophoresed without PAM-ABP. PAM-ABP condensed the plasmid cocktails prepared at different conditions completely.
Figure 1.
DNA condensation using PAM-ABP. Gel retardation of the VEGF, the Dual, and the mixture of VEGF and Dual using PAM-ABP. The V:D indicates the ratio between VEGF:Dual. Five mixtures of VEGF and Dual were prepared at different ratios and condensed by PAM-ABP.
3.2 Optimum mixing condition of the plasmid cocktail
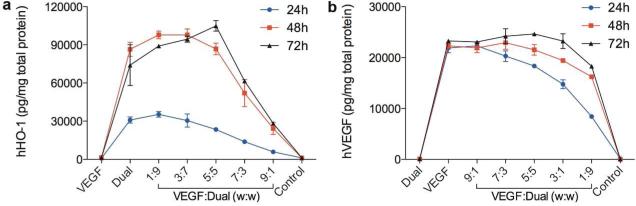
The cocktails of two plasmids were transfected to H9C2 cells using PAM-ABP, and hHO-1 expression and hVEGF secretion were determined in a time-dependent manner. The plasmid cocktails composed of a different amount of VEGF plasmid and the Dual plasmid were prepared, and the same amount of the mixed plasmid was transfected. The cells transfected with the VEGF only, expressed no hHO-1 (Fig. 2a), and the Dual-only treated cells showed no secretion of hVEGF (Fig. 2b). Expression of hHO-1 in the Dual group was increased over time and reached a peak at 48 hours. As the ratio of the Dual plasmid in the cocktail decreased, the overall hHO-1 expression decreased at 24 hours post transfection. Interestingly, the level of hHO-1 increased up to the ratio 3:7 and 5:5 at 48 hours and 72 hours, respectively, and highest hHO-1 expression was observed at the ratio 5:5 at 72 hours incubation. Secretion of hVEGF reduced with increasing portion of the Dual plasmid in the cocktail at 24 hours post-transfection. Similar to hHO-1 expression, hVEGE secretion increased up to the ratio 7:3 and 5:5 at 48 hours and 72 hours, respectively. Also, the peak VEGF secretion was found at the ratio 5:5 72 hours after transfection.
Figure 2.
Time- and ratio-dependent hHO-1 expression and hVEGF secretion. (a) HO-1 expression and (b) VEGF secretion after transfection of the plasmid cocktail prepared at five different ratios were determined at 24, 48, and 72 hours post-transfection using ELISA kits. The mixture of the VEGF plasmid and the Dual plasmid was transfected to hypoxic H9C2 cells using PAM-ABP. Data represent mean ± SD.
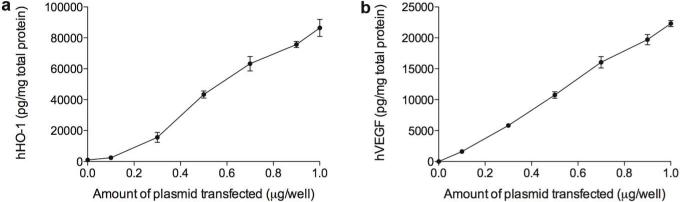
It may be possible that the production of hHO-1 and hVEGF in the cells was already saturated at 1 μg DNA transfected, suggesting that the increased gene expression when half amount of DNA was used for transfection might be an artifact. To prove that the data shown in Fig. 2 are not due to the saturation, we observed a correlation between the gene expression and the amount of plasmid transfected. Fig. 3 shows that hHO-1 expression or hVEGF secretion was in direct proportion to the amount of the Dual or the VEGF, respectively. When half amount of the plasmid was transfected to the cells, the gene expression also reduced by 50% as seen in Fig. 3a,b. This demonstrates that if the plasmid cocktail mixed at 5:5 is transfected to the cells, the gene expression of each plasmid is expected to be ~50%. Thus, the similar or the higher hHO-1 expression or hVEGF secretion in the cells treated with the plasmid cocktail prepared at 5:5 is, in part, due to the positive feedback between HO-1 and VEGF.
Figure 3.
Correlation between the amount of plasmid transfected and the protein production. (a) hHO-1 expression and (b) hVEGF secretion at 48 hours post-transfection. Different amounts of the plasmids were transfected to H9C2 cells using PAM-ABP. Data represent mean ± SD.
3.3 Confirmation of the positive feedback
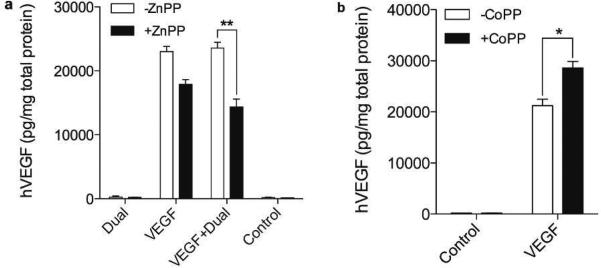
According to our hypothesis, HO-1 plays a crucial role to improve VEGF secretion. In order to verify whether the increased hVEGF secretion is due to HO-1 activity or not, we observed hVEGF secretion in the presence or the absence of the HO-1 inhibitor or the HO-1 activator. ZnPP, a HO-1 inhibitor,20 and CoPP, a HO-1 activator,21 are known to reduce and to enhance, respectively, the activity of HO-1. The cells transfected with VEGF-only showed a ~30% decrease in hVEGF secretion in the presence of ZnPP, compared to that in the absence of ZnPP, and the VEGF + Dual treated group exhibited ~40% less hVEGF secretion when incubated with ZnPP (Fig. 4a). Incubation with CoPP led to an ~25% increase in hVEGF secretion as shown in Fig. 4b. This confirms that HO-1 positively regulates VEGF secretion.
Figure 4.
Effects of HO-1 inhibitor and activator on hVEGF secretion. (a) hVEGF secretion with or without HO-1 inhibitor. H9C2 cells were transfected with Dual-only, VEGF only, and the cocktail of the Dual plasmid and the VEGF plasmid (VEGF+Dual mixed at 5:5 ratio) and incubated in the presence or absence of ZnPP (10 μM). (b) hVEGF secretion with or without HO-1 activator. H9C2 cells were transfected with VEGF-only and incubated in the presence or absence of CoPP (10 μM). Secretion of hVEGF was measured 48 hours after transfection using ELISA kit. Data represent mean ± SD (*p<0.05 and **p<0.01).
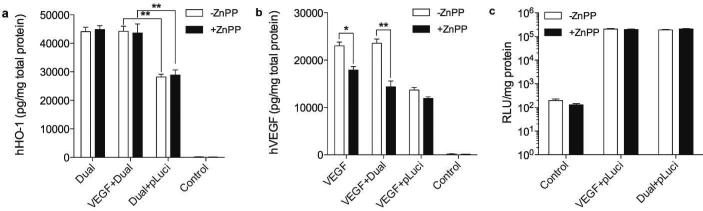
The positive feedback between HO-1 and VEGF was further investigated by co-transfection with an empty plasmid, pLuciferase (pLuci). No increase in hHO-1 expression, hVEGF secretion, and luciferase expression was expected because both HO-1 and VEGF have no effect on luciferase activity or vice versa. Fig. 5a shows that transfection of the Dual + pLuci led to no enhancement of hHO-1 expression, while the VEGF + Dual treated group demonstrated a very similar level of hHO-1 expression compared to the Dual-only group. Also, hVEGF secretion was not improved when transfected with pLuci (Fig. 5b). This reveals that there were no artifacts that increase hHO-1 expression and hVEGF secretion when they were co-transfected. In addition, no difference in luciferase activity was observed in all transfection groups, demonstrating that hHO-1 and hVEGF positively affect each other rather than luciferase (Fig. 5c).
Figure 5.
Effects of empty vector on hHO-1 expression and hVEGF secretion. (a) hHO-1 expression, (b) VEGF secretion, and (c) luciferase expression after co-transfection with pLuci. The Dual plasmid and the VEGF plasmid were mixed with the same amount of the luciferase plasmid. The mixtures were transfected to H9C2 cells, and hHO-1 expression, hVEGF secretion, and luciferase activity were determined. Data represent mean ± SD (*p<0.05 and **p<0.01).
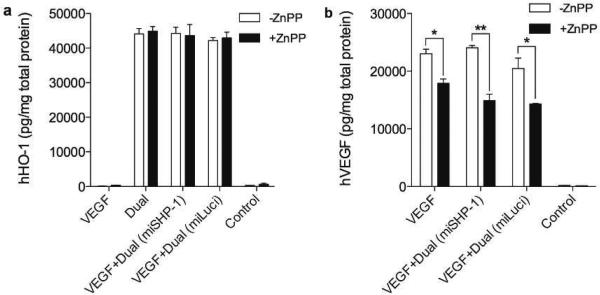
Effect of miSHP-1 on hVEGF secretion was investigated because down-regulation of SHP-1 is known to enhance VEGF secretion. A Dual plasmid composed of hHO-1 and microRNA for luciferase (miLuci) was used as a negative control. No difference in hHO-1 expression was observed regardless of the HO-1 inhibitor as shown in Fig. 6a, while a slight decrease in hVEGF secretion was seen in the VEGF + Dual (miLuci)-transfected group (-ZnPP, Fig. 6b). This reveals that down regulation of SHP-1 may enhance hVEGF secretion, but the effect is not dramatic compared to the effect of HO-1. It is possible that the miSHP-1 could further increase hVEGF secretion in the long term because gene down-regulation takes longer time than gene expression. This means that the 48 hours incubation might be sufficient to silence SHP-1 mRNA, but not enough to lead to SHP-1 protein down regulation. Also, the enhanced VEGF secretion controlled by SHP-1 is expected to occur after SHP-1 is down regulated. With this experimental setting, it is hard to prove how further SHP-1 down regulation by miSHP-1 up-regulates hVEGF secretion in the long-term.
Figure 6.
Effect of miSHP-1 on hHO-1 expression and hVEGF secretion. (a) hHO-1 expression and (b) VEGF secretion with or without miSHP-1 in the presence or absence of HO-1 inhibitor. The dual vector containing miLuci was used as a negative control. After 48 hours of transfection, hHO-1 and hVEGF level was determined using ELISA kit. Data represent mean ± SD (*p<0.05 and **p<0.01).
3.4 Effects of the co-transfection on endogenous gene expression
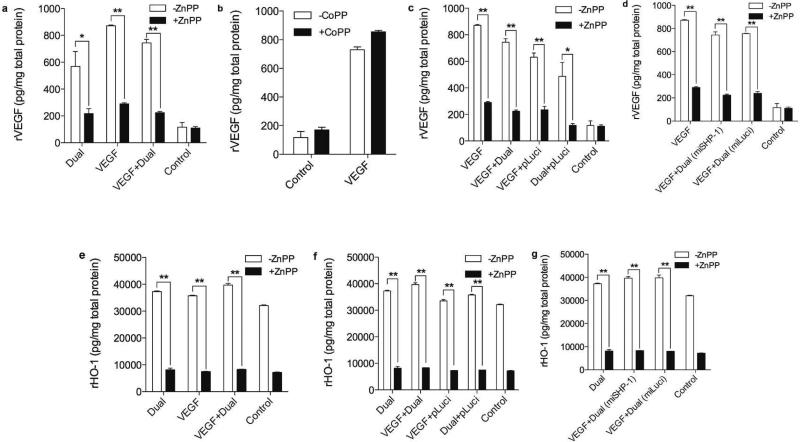
Despite the genes used in this study being of human origins; they may influence endogenous gene expression. The rat HO-1 (rHO-1) expression and the rat VEGF (rVEGF) secretion were monitored under the same test conditions. The transfection of Dual plasmid led to an increase in rVEGF secretion, and the VEGF + Dual treatment further enhanced rVEGF secretion (Fig. 7a). The cells incubated with or without the HO-1 inhibitor or activator proved that HO-1 positively regulates rVEGF secretion as seen Fig. 7a,b. Co-transfection with the pLuci exhibited a similar pattern of rVEGF secretion as shown in hVEGF secretion data (Fig. 7c) and the effect of miSHP-1 on rVEGF was found to be not significant (Fig. 7d). Transfection of the VEGF + Dual slightly increased rHO-1 expression (Fig. 7e), and no increase was observed in the VEGF + Dual (pLuci) treated group (Fig. 7f). Fig. 7g shows no correlation between SHP-1 and rHO-1 expression. These results demonstrate that the overall effect of the human gene transfection on the endogenous gene is moderate compared to their counterpart human genes. This is likely due to the interaction between the human HO-1 and VEGF being stronger than that between human protein and rat protein.
Figure 7.
Endogenous rVEGF secretion and rHO-1 expression. Secretion of rVEGF with or without (a) HO-1 inhibitor and (b) HO-1 activator, (c) rVEGF secretion after co transfection with pLuci, and (d) effect of miSHP-1 on rVEGF secretion. (e) rHO-1 expression in the presence or the absence of HO-1 inhibitor, (f) rHO-1 expression transfected with pLuci, and (g) effect of miSHP-1 on rHO-1 expression. Data represent mean ± SD (*p<0.05 and **p<0.01).
3.5 Anti-apoptotic effect
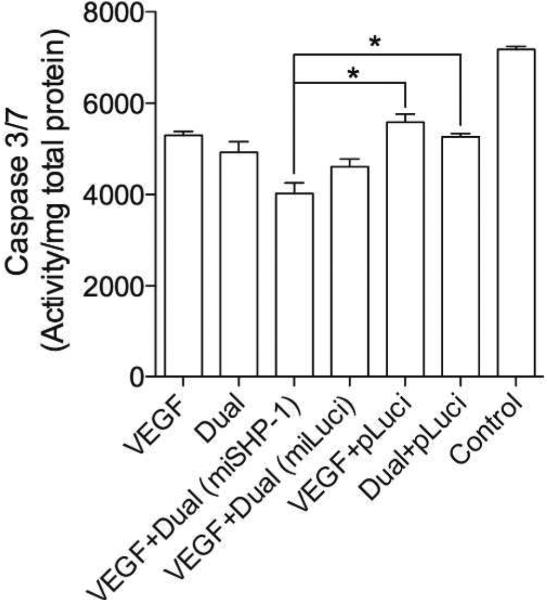
Anti-apoptotic effect of the Dual plasmid has been proved, and VEGF is also known to reduce apoptosis of cells under ischemic condition. This suggests that HO-1, miSHP-1 and VEGF can protect cells against apoptosis under hypoxia. Apoptosis of H9C2 cells, with or without transfection under hypoxia, was determined by caspase 3/7 assay (Fig. 8). The VEGF, the Dual, the VEGF + pLuci, and the Dual + pLuci treated cells demonstrated ~25% decrease in the caspase 3/7 activity. The VEGF + Dual (miSHP-1) and the VEGF + Dual (miLuci) transfection groups showed ~50% and ~40% decrease in apoptosis, respectively. This reveals that combination of three genes reduced apoptosis synergistically.
Figure 8.
Caspase 3/7 activity. Antiapoptotic effects of the combined treatment of VEGF, HO-1, and miSHP-1 were investigated by measuring caspase activity in H9C2 cells under hypoxia. Data represent mean ± SD (*p<0.05)
4. Discussion
For a success in combined gene therapy, an approach containing both knock-in genes and knockdown genes is promising due to their totally different mechanisms. The use of two target genes, which have the same mechanism of expression, may reduce the therapeutic efficacy. Cells have very complicated signaling pathways, and one protein is usually involved in several cellular functions. This means that unexpected problems may happen if two knock-in genes are used. On the other hand, induction of two RNAi machineries and introduction of too much siRNA may lead to severe off-target effects. Hypoxia-responsive plasmid that encodes miRNA can regulate miRNA production in response to hypoxia, thereby RNAi processes occur mostly in hypoxic cells depending on degree of hypoxia 22. The hypoxia-inducible plasmid producing miSHP-1 would therefore minimize the side effects caused by unrestricted RNAi machineries. We believe that the ideal dual gene expression system is the combination of knock-in and knockdown. Thus, the Dual plasmid that expressed both HO-1 as a knock-in and miSHP-1 as a knockdown was used in the present study.
HO-1, a stress-inducible antioxidant enzyme, protects hypoxic cells through anti-inflammation, anti-apoptosis, and anti-oxidation 23,26. HO-1 overexpression induced by hypoxia is associated with the down-regulation of pro-apoptotic Bax, Bak, and caspase 3/7, and up-regulation of the anti-apoptotic protein, Bcl-2 27. It was reported that HO-1 gene delivery inhibited cardiomyocyte apoptosis and early inflammatory responses caused by ischemia/reperfusion damage, resulting in protecting the heart 9, 28. These studies demonstrate the therapeutic potential of HO-1 gene therapy for ischemic diseases. SHP-1, a key molecular mediator in apoptosis and reduction in Akt phosphorylation, binds to TNFR-1 and FAS-R. The binding of SHP-1 to the death receptors promotes apoptosis 29, 30, suggesting that the inhibition of SHP-1 decreases apoptosis. Silencing of SHP-1 using SHP-1 siRNA reduced cardiomyocyte apoptosis and protected cardiomyocytes under hypoxia through Akt activation 12, 13. It was therefore demonstrated that a therapeutic strategy designed to inhibit expression of SHP-1 by miRNA would be effective in ischemic diseases 18.
The mechanism of VEGF gene therapy in ischemic tissues is not only inducing angiogenesis but also protecting cells against apoptosis 31, 32. Although VEGF is over expressed in response to hypoxia, the level of VEGF produced spontaneously is not enough to completely restore myocardial function 33. This suggests that VEGF needs to be introduced exogenously for sufficient angiogenesis. These three target genes, HO-1, miSHP-1, and VEGF, have the possible synergy that is associated with their positive feedback in ischemic cardiomyocytes. In addition to the anti-apoptotic effects of HO-1 and miSHP-1, many studies have reported that either HO-1 over expression or SHP-1 silencing accelerates angiogenesis through enhancing VEGF secretion in ischemic cells 14,16. In contrast, it has been well known that VEGF prolongs HO-1 activity; however, the exact underlying mechanism in the HO-1 induction by VEGF is unclear 34.
Although the molecular mechanisms, such as the relative contributions of carbon monoxide (CO), biliverdin, and bilirubin, underlying the enhanced VEGF secretion by HO-1 remain unclear, it is envisioned that HO-1 and the angiogenic factors can activate a positive-feedback circuit to amplify neovascularization in adult tissues 14, 35. The increased rVEGF secretion could be through the following steps; 1) HO-1 over expression, 2) HIF-1 activation (heterodimerization), and 3) HIF-1 mediated enhanced VEGF synthesis 36. The VEGF plasmid used in this study contains no promoter that is sensitive to HIF-1. Thus, the potential mechanism underlying the enhanced hVEGF secretion as a result of HO-1 over expression is different from the rVEGF secretion. In the VEGF plasmid used here, VEGF secretion is mainly regulated in the post-translational stage. The oxygen-dependent degradation (ODD) domain stabilizes VEGF under hypoxia and degrades VEGF as oxygen levels increases. Oxidative metabolism of heme by HO-1 requires three molecules of molecular oxygen (O2) per heme molecule oxidized 8. This process maintains the oxygen at quite a low level in the cells, possibly resulting in the increased hVEGF stability and secretion.
SHP-1 plays a negative regulatory role in signal transduction by dephosphorylation of the receptors, to which activated cytokines, growth factors, and antigen receptors bind 29, 37. SHP-1 is involved in reducing angiogenesis through the inactivation of KDR/flk-1, a substrate for SHP-1 38, 39. KDR/flk-1 inactivation by SHP-1 is involved in reducing angiogenesis through inhibiting the binding of VEGF to KDR/flk-1 that triggers dimerization and auto-phosphorylation of tyrosine residues—a key step in angiogenesis 40. The suppression of SHP-1 by using miSHP-1 is expected to stimulate angiogenesis in ischemic cardiomyocytes. The SHP-1 mechanism associated with angiogenesis explains why SHP-1 down-regulation did not lead to a significant increase in VEGF secretion. Unlike HO-1, SHP-1 controls angiogenesis indirectly without a direct interaction with VEGF; hence, the effect of miSHP-1 on VEGF secretion was moderate as shown in Fig. 6.
The combined gene delivery demonstrated in this study is promising for ischemic diseases in terms of not only synergistic efficacy, but also sequential activities in the order of preventing cardiomyocyte apoptosis and inducting angiogenesis. Anti-apoptotic genes typically protect cells in ischemic tissues for up to 2 weeks, and neovascularization requires more than 1 week upon VEGF secretion 41. The present sequentially and synergistically combined gene therapy provides double effects of cardiomyocyte protection in the early stage of ischemia and vascular regeneration in the late stage. We described here, for the first time, the actual synergistically combined sequential gene therapy that bases on consecutive activities of anti-apoptosis and angiogenesis for the treatment of ischemic diseases. This is different from sequential efficacy resulting from serial administration. Although this gene delivery strategy needs to be examined in an animal model with ischemia/reperfusion injury, this study provides a direction of future cardiovascular gene therapy.
Acknowledgment
This work was supported by the NIH grants HL065477 (SW Kim). The work of Minhyung Lee was supported by the Ministry of Education, Science and Technology, Korea (2012K001394).
References
- 1.Lavu M, Gundewar S, Lefer DJ. Gene therapy for ischemic heart disease. J Mol Cell Cardiol. 2011;50(5):742–50. doi: 10.1016/j.yjmcc.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vinge LE, Raake PW, Koch WJ. Gene therapy in heart failure. Circ Res. 2008;102(12):1458–70. doi: 10.1161/CIRCRESAHA.108.173195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rissanen TT, Yla-Herttuala S. Current status of cardiovascular gene therapy. Mol Ther. 2007;15(7):1233–47. doi: 10.1038/sj.mt.6300175. [DOI] [PubMed] [Google Scholar]
- 4.Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov. 2009;8(2):129–38. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luten J, van Nostrum CF, De Smedt SC, Hennink WE. Biodegradable polymers as non-viral carriers for plasmid DNA delivery. J Control Release. 2008;126(2):97–110. doi: 10.1016/j.jconrel.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 6.Kim TI, Ou M, Lee M, Kim SW. Arginine-grafted bioreducible poly(disulfide amine) for gene delivery systems. Biomaterials. 2009;30(4):658–64. doi: 10.1016/j.biomaterials.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nam HY, Nam K, Lee M, Kim SW, Bull DA. Dendrimer type bio-reducible polymer for efficient gene delivery. J Control Release. 2012 doi: 10.1016/j.jconrel.2012.04.025. [DOI] [PubMed] [Google Scholar]
- 8.Stocker R, Perrella MA. Heme oxygenase-1: a novel drug target for atherosclerotic diseases? Circulation. 2006;114(20):2178–89. doi: 10.1161/CIRCULATIONAHA.105.598698. [DOI] [PubMed] [Google Scholar]
- 9.Tang YL, Tang Y, Zhang YC, Qian K, Shen L, Phillips MI. Protection from ischemic heart injury by a vigilant heme oxygenase-1 plasmid system. Hypertension. 2004;43(4):746–51. doi: 10.1161/01.HYP.0000120152.27263.87. [DOI] [PubMed] [Google Scholar]
- 10.Chong ZZ, Maiese K. The Src homology 2 domain tyrosine phosphatases SHP-1 and SHP-2: diversified control of cell growth, inflammation, and injury. Histol Histopathol. 2007;22(11):1251–67. doi: 10.14670/hh-22.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forget G, Gregory DJ, Whitcombe LA, Olivier M. Role of host protein tyrosine phosphatase SHP-1 in Leishmania donovani induced inhibition of nitric oxide production. Infect Immun. 2006;74(11):6272–9. doi: 10.1128/IAI.00853-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsui T, Tao J, del Monte F, Lee KH, Li L, Picard M, Force TL, Franke TF, Hajjar RJ, Rosenzweig A. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation. 2001;104(3):330–5. doi: 10.1161/01.cir.104.3.330. [DOI] [PubMed] [Google Scholar]
- 13.Nam HY, Kim J, Kim S, Yockman JW, Kim SW, Bull DA. Cell penetrating peptide conjugated bioreducible polymer for siRNA delivery. Biomaterials. 2011;32(22):5213–22. doi: 10.1016/j.biomaterials.2011.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin HH, Chen YH, Chang PF, Lee YT, Yet SF, Chau LY. Heme oxygenase-1 promotes neovascularization in ischemic heart by coinduction of VEGF and SDF-1. J Mol Cell Cardiol. 2008;45(1):44–55. doi: 10.1016/j.yjmcc.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki M, Iso-o N, Takeshita S, Tsukamoto K, Mori I, Sato T, Ohno M, Nagai R, Ishizaka N. Facilitated angiogenesis induced by heme oxygenase-1 gene transfer in a rat model of hindlimb ischemia. Biochem Biophys Res Commun. 2003;302(1):138–43. doi: 10.1016/s0006-291x(03)00114-1. [DOI] [PubMed] [Google Scholar]
- 16.Sugano M, Tsuchida K, Maeda T, Makino N. SiRNA targeting SHP-1 accelerates angiogenesis in a rat model of hindlimb ischemia. Atherosclerosis. 2007;191(1):33–9. doi: 10.1016/j.atherosclerosis.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 17.Won YW, Lee M, Kim HA, Bull DA, Kim SW. Post-translational regulated and hypoxia-responsible VEGF plasmid for efficient secretion. J Control Release. 2012;160(3):525–31. doi: 10.1016/j.jconrel.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Won YW, Lee M, Kim HA, Bull DA, Kim SW. Hypoxia inducible plasmid expressing both miSHP-1 and HO-1 for the treatment of ischemic disease. J Control Release. 2013;165(1):22–8. doi: 10.1016/j.jconrel.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nam HY, Nam K, Lee M, Kim SW, Bull DA. Dendrimer type bio reducible polymer for efficient gene delivery. Journal of controlled release : official journal of the Controlled Release Society. 2012;160(3):592–600. doi: 10.1016/j.jconrel.2012.04.025. [DOI] [PubMed] [Google Scholar]
- 20.Hirai K, Sasahira T, Ohmori H, Fujii K, Kuniyasu H. Inhibition of heme oxygenase-1 by zinc protoporphyr in IX reduces tumor growth of LL/2 lung cancer in C57BL mice. Int J Cancer. 2007;120(3):500–5. doi: 10.1002/ijc.22287. [DOI] [PubMed] [Google Scholar]
- 21.Loboda A, Jazwa A, Wegiel B, Jozkowicz A, Dulak J. Heme oxygenase-1 dependent and -independent regulation of angiogenic genes expression: effect of cobalt protoporphyrin and cobalt chloride on VEGF and IL-8 synthesis in human microvascular endothelial cells. Cell Mol Biol (Noisy-le-grand) 2005;51(4):347–55. [PMC free article] [PubMed] [Google Scholar]
- 22.Lee Y, Jeon K, Lee JT, Kim S, Kim VN. MicroRNA maturation: stepwise processing and subcellular localization. Embo J. 2002;21(17):4663–70. doi: 10.1093/emboj/cdf476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Otterbein LE, Soares MP, Yamashita K, Bach FH. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol. 2003;24(8):449–55. doi: 10.1016/s1471-4906(03)00181-9. [DOI] [PubMed] [Google Scholar]
- 24.Clark JE, Foresti R, Sarathchandra P, Kaur H, Green CJ, Motterlini R. Heme oxygenase-1 derived bilirubin ameliorates postischemic myocardial dysfunction. Am J Physiol Heart Circ Physiol. 2000;278(2):H643–51. doi: 10.1152/ajpheart.2000.278.2.H643. [DOI] [PubMed] [Google Scholar]
- 25.Yet SF, Tian R, Layne MD, Wang ZY, Maemura K, Solovyeva M, Ith B, Melo LG, Zhang L, Ingwall JS, Dzau VJ, Lee ME, Perrella MA. Cardiac specific expression of heme oxygenase-1 protects against ischemia and reperfusion injury in transgenic mice. Circ Res. 2001;89(2):168–73. doi: 10.1161/hh1401.093314. [DOI] [PubMed] [Google Scholar]
- 26.Vulapalli SR, Chen Z, Chua BH, Wang T, Liang CS. Cardioselective overexpression of HO-1 prevents I/R-induced cardiac dysfunction and apoptosis. Am J Physiol Heart Circ Physiol. 2002;283(2):H688–94. doi: 10.1152/ajpheart.00133.2002. [DOI] [PubMed] [Google Scholar]
- 27.Dulak J, Zagorska A, Wegiel B, Loboda A, Jozkowicz A. New strategies for cardiovascular gene therapy: regulatable pre-emptive expression of pro-angiogenic and antioxidant genes. Cell Biochem Biophys. 2006;44(1):31–42. doi: 10.1385/CBB:44:1:031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pachori AS, Melo LG, Hart ML, Noiseux N, Zhang L, Morello F, Solomon SD, Stahl GL, Pratt RE, Dzau VJ. Hypoxia-regulated therapeutic gene as a preemptive treatment strategy against ischemia/reperfusion tissue injury. Proc Natl Acad Sci U S A. 2004;101(33):12282–7. doi: 10.1073/pnas.0404616101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen HE, Chang S, Trub T, Neel BG. Regulation of colony-stimulating factor 1 receptor signaling by the SH2 domain-containing tyrosine phosphatase SHPTP1. Mol Cell Biol. 1996;16(7):3685–97. doi: 10.1128/mcb.16.7.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daigle I, Yousefi S, Colonna M, Green DR, Simon HU. Death receptors bind SHP-1 and block cytokine induced anti-apoptotic signaling in neutrophils. Nat Med. 2002;8(1):61–7. doi: 10.1038/nm0102-61. [DOI] [PubMed] [Google Scholar]
- 31.Dai Y, Xu M, Wang Y, Pasha Z, Li T, Ashraf M. HIF-1alpha induced-VEGF overexpression in bone marrow stem cells protects cardiomyocytes against ischemia. J Mol Cell Cardiol. 2007;42(6):1036–44. doi: 10.1016/j.yjmcc.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta K, Kshirsagar S, Li W, Gui L, Ramakrishnan S, Gupta P, Law PY, Hebbel RP. VEGF prevents apoptosis of human microvascular endothelial cells via opposing effects on MAPK/ERK and SAPK/JNK signaling. Exp Cell Res. 1999;247(2):495–504. doi: 10.1006/excr.1998.4359. [DOI] [PubMed] [Google Scholar]
- 33.Brogi E, Schatteman G, Wu T, Kim EA, Varticovski L, Keyt B, Isner JM. Hypoxia-induced paracrine regulation of vascular endothelial growth factor receptor expression. J Clin Invest. 1996;97(2):469–76. doi: 10.1172/JCI118437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bussolati B, Ahmed A, Pemberton H, Landis RC, Di Carlo F, Haskard DO, Mason JC. Bifunctional role for VEGF-induced heme oxygenase-1 in vivo: induction of angiogenesis and inhibition of leukocytic infiltration. Blood. 2004;103(3):761–6. doi: 10.1182/blood-2003-06-1974. [DOI] [PubMed] [Google Scholar]
- 35.Bussolati B, Mason JC. Dual role of VEGF-induced heme-oxygenase-1 in angiogenesis. Antioxid Redox Signal. 2006;8(7-8):1153–63. doi: 10.1089/ars.2006.8.1153. [DOI] [PubMed] [Google Scholar]
- 36.Deramaudt BM, Braunstein S, Remy P, Abraham NG. Gene transfer of human heme oxygenase into coronary endothelial cells potentially promotes angiogenesis. J Cell Biochem. 1998;68(1):121–7. doi: 10.1002/(sici)1097-4644(19980101)68:1<121::aid-jcb12>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 37.Klingmuller U, Lorenz U, Cantley LC, Neel BG, Lodish HF. Specific recruitment of SH-PTP1 to the erythropoietin receptor causes inactivation of JAK2 and termination of proliferative signals. Cell. 1995;80(5):729–38. doi: 10.1016/0092-8674(95)90351-8. [DOI] [PubMed] [Google Scholar]
- 38.Wo YY, McCormack AL, Shabanowitz J, Hunt DF, Davis JP, Mitchell GL, Van Etten RL. Sequencing, cloning, and expression of human red cell-type acid phosphatase, a cytoplasmic phosphotyrosyl protein phosphatase. J Biol Chem. 1992;267(15):10856–65. [PubMed] [Google Scholar]
- 39.Guo DQ, Wu LW, Dunbar JD, Ozes ON, Mayo LD, Kessler KM, Gustin JA, Baerwald MR, Jaffe EA, Warren RS, Donner DB. Tumor necrosis factor employs a protein-tyrosine phosphatase to inhibit activation of KDR and vascular endothelial cell growth factor-induced endothelial cell proliferation. J Biol Chem. 2000;275(15):11216–21. doi: 10.1074/jbc.275.15.11216. [DOI] [PubMed] [Google Scholar]
- 40.Waltenberger J, Claesson-Welsh L, Siegbahn A, Shibuya M, Heldin CH. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem. 1994;269(43):26988–95. [PubMed] [Google Scholar]
- 41.May D, Gilon D, Djonov V, Itin A, Lazarus A, Gordon O, Rosenberger C, Keshet E. Transgenic system for conditional induction and rescue of chronic myocardial hibernation provides insights into genomic programs of hibernation. Proc Natl Acad Sci U S A. 2008;105(1):282–7. doi: 10.1073/pnas.0707778105. [DOI] [PMC free article] [PubMed] [Google Scholar]