Abstract
The ginseng plant (Panax ginseng Meyer) has a large number of active ingredients including steroidal saponins with a dammarane skeleton as well as protopanaxadiol and protopanaxatriol, commonly known as ginsenosides, which have antioxidant, anticancer, antidiabetic, anti-adipocyte, and sexual enhancing effects. Though several discoveries have demonstrated that ginseng saponins (ginsenosides) as the most important therapeutic agent for the treatment of osteoporosis, yet the molecular mechanism of its active metabolites is unknown. In this review, we summarize the evidence supporting the therapeutic properties of ginsenosides both in vivo and in vitro, with an emphasis on the different molecular agents comprising receptor activator of nuclear factor kappa-B ligand, receptor activator of nuclear factor kappa-B, and matrix metallopeptidase-9, as well as the bone morphogenetic protein-2 and Smad signaling pathways.
Keywords: Panax ginseng, Ginsenosides, Osteoporosis, Receptor activator of nuclear factor kappa-B ligand, Bone morphogenetic protein-2
INTRODUCTION
Bone remodeling occurs throughout life via synthesis of bone matrix through the action of two major bone cells: osteoblasts and osteoclasts [1-3]. Osteoclasts are responsible for bone resorption, while osteoblasts are responsible for bone formation [4,5]. The proper functioning of these cells is necessary for the maintenance of bone mass as well as bone mineral density (BMD). During old age and especially postmenopause there is excessive bone resorption relative to bone formation due hormone deficiencies, which reduces bone mass and ultimately causes bone diseases and osteoporosis [6,7].
Osteoporosis is a widespread health dilemma and its occurrence is projected to rise in the upcoming decades due to the aging of many societies [8]. It is a bone disease that is thought to cause stumpy bone mass and micro-architectural weakening of bone materials, enhance bone brittleness, decrease bone strength, and subsequently increase the threat of fracture [9]. Osteoporosis causes distress throughout the United States, Europe, China, Japan and the rest of the world [10]. According to the World Health Organization, almost 75 million people in the United States, Europe, and Japan are affected by osteoporosis [11].
TYPES OF OSTEOPOROSIS
There are two main types of osteoporosis: primary osteoporosis and secondary osteoporosis. Primary osteoporosis is usually associated with aging and low levels of reproductive hormones, especially estrogen, which leads to reduced gonadal activity in the elderly. Secondary osteoporosis has other causes, including deficiencies of calcium, vitamin D, and parathyroid hormone [12].
PATHOPHYSIOLOGY OF OSTEOPOROSIS
An understanding of the molecular mechanisms and anabolic and catabolic activity of bone is important in the development of new drugs for treating bone diseases, especially osteoporosis. For osteoporosis, the search for new therapeutic agents is mostly concerned with the control of bone resorption and the induction of bone formation by osteoclasts and osteoblasts, respectively [12]. The main molecular factors involved in osteoporosis are the receptor activator of nuclear factor kappa-B ligand (RANKL), osteoprotegerin (OPG) and the receptor activator of nuclear factor kappa-B (RANK).
OSTEOCLASTOGENESIS/OSTEOCLAST DIFFERENTIATION
Osteoclasts are multinucleate and highly specialized giant cells that have the ability to work in perfect synchronization with osteoblasts to retain the skeletal system. They are produced from monocytic precursors. On completion of bone formation, they migrate into the bone marrow [13,14].
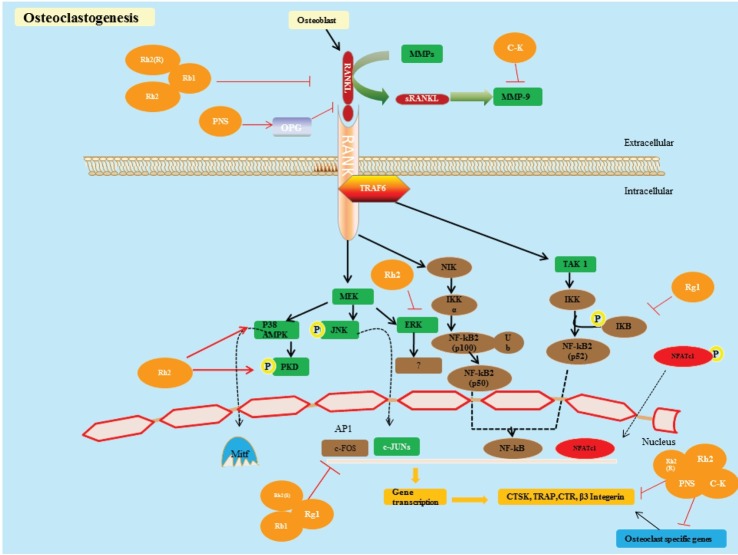
The discoveries of the RANKL and OPG have altered our understanding of the primary mechanisms involved in osteoclast differentiation and activity. RANKL, a transmembrane protein, belongs to the tumor necrosis factor superfamily member 11, and a ligand for OPG is produced by osteoblast and stromal cells [15-17]. RANKL binds with the membrane bound protein RANK that is expressed by osteoclasts (OC) with the combination of tumor necrosis factor (TNF)-receptor-associated factor-6 (TRAF-6) and activates different downstream signaling pathways (Fig. 1) [18]. Consequently, it plays an important role in osteoclast differentiation and bone resorption, which leads to osteoporosis [19].
Fig. 1. Osteoclastogenesis. Proposed model of a ginsenoside inhibiting the binding of receptor activator of nuclear factor kappa-B ligand (RANKL) to receptor activator of nuclear factor kappa-B (RANK) and melatonin receptor type 1A, matrix metallopeptidase (MMP) by binding with RANK or RANKL. This decreases the response of RANKL, reduces the induction of MMP-9, and blocks the RANKL, RANK signaling pathway. RANKL binds to RANK, so tumor necrosis factor-receptor-associated factor-6 (TRAF6) binds to RANK and plays a key role in osteoclast differentiation by regulating and activating downstream signaling pathways, such as the nuclear factor-kappa B (NF-κB) pathway, the inhibitor of NF-κB kinase (INK) pathway, the c-Jun N-terminal kinase (JNK) pathway and the p38 pathway. These pathways ultimately prop up osteoclast differentiation and bone resorption by stimulating different transcriptional factors such as activator protein-1 (AP1) and NF-κB pathways. It is not clear how these factors are activated by TRAF6 and cause bone resorption by activating osteoclast specific markers, such as tartrate-resistant acid phosphatase (TRAP), cathepsin (CTSK), β3 integrin and calcitonin receptors (CTR). MMPs, and in particular MMP-9, are responsible for bone resorption, which is extremely articulated in osteoclasts, are stimulated by the action of RANKL signaling pathways and influence osteoclast differentiation and bone resorption [18]. C-K, compound K; PNS, Panax notoginseng saponins; OPG, osteoprotegerin; NIK, NF-kappa-B-inducing kinase; MEK, mitogen-activated protein kinase kinase; AMPK, AMP-activated protein kinase; IKK, inhibitor of nuclear factor kappa-B kinase; PKD, protein kinase D; NFAT, nuclear factor of activated T-cell.
Osteoblasts secrete a natural soluble decoy receptor for the RANKL. OPG belongs to tumor necrosis factor receptor superfamily member 11B, which has the ability to restrain osteoclast differentiation/formation by preventing RANKL from binding to RANK. Hence, stability and balance between RANKL and OPG is necessary for osteoclast activity [20,21]. The most important factor in osteoclast activation is a protein known as RANKL, which brings these osteoclasts and osteoblasts in contact by inserting itself into the external membrane of osteoclasts, thereby triggering its receptor RANK, which is then responsible for bone resorption. The coupling of RANKL to RANK is facilitated by the nuclear factor (NF)-κB signaling pathway [19].
The TRAF family of proteins (TRAF, 2-6) comprises the most significant factors involved in osteoclastogenesis when RANKL binds to RANK. A pivotal role is played by TRAF6 via the regulation and activation of downstream signaling pathways, such as the NF-κB pathway, the inhibitor of NF-κB kinase pathway, the c-Jun N-terminal kinase (JNK) pathway, and the p38 pathway. These pathways ultimately prop-up osteoclast differentiation and bone resorption by stimulating different transcriptional factors such as activator protein-1 and the NF-κB pathways. However, it is not clear how these factors are activated by TRAF6 or how they lead to bone resorption by activating osteoclast-specific markers such as tartrate-resistant acid phosphatase (TRAP), cathepsin, β3 integrin, and calcitonin receptors [21-23]. Recently it has been suggested that, among the proteases, matrix metallopeptidases (MMPs) are responsible for the deprivation of bone extracellular matrix. In particular, MMP-9 is strongly expressed in osteoclasts since it is stimulated by the action of the RANKL signaling pathway and it plays a vital role in bone resorption. At the molecular level, however, little is known about how RANKL induces MMP-9 gene stimulation [24].
OSTEOBLASTOGENESIS/OSTEOBLAST DIFFERENTIATION
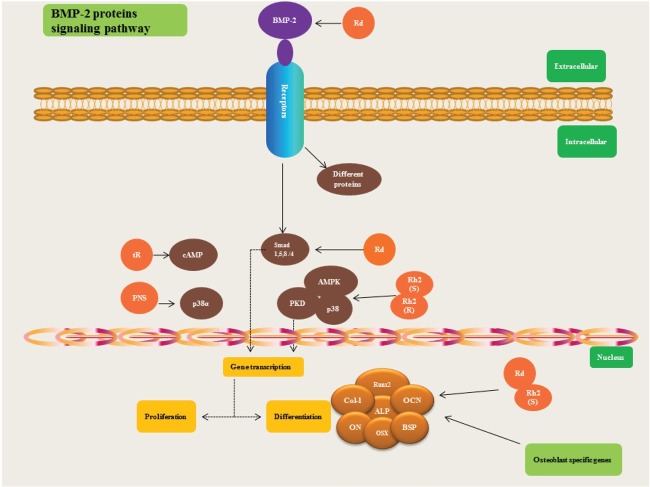
Osteoblasts are mononucleate cells that are derived from mesenchymal stem cells, which have the ability to suppress osteoclast activity and increase bone formation by secreting a bone mineralization organic matrix such as collagen-I (Col-I) in the osteoid [25]. In osteoblast differentiation, the Wnt canonical signaling pathway and the bone morphogenetic protein (BMPs) pathways are the most pivotal. The BMPs belong to the transforming growth factor (TGF)-β superfamily and BMP-2 plays an especially significant role in osteoblastogenesis. When BMP-2 binds to a transmembrane protein such as a bone morphogenetic protein receptor II, it phosphorylates type I receptor, and hence activates the Smad complex (Smad 1, 4, 5, and 8) signaling pathways [26-29], which can aid in the activation of osteoblast-specific transcriptional regulation genes involved in bone formation, such as osteocalcin (OCN), collagen type I, osteonectin, osteopontin, osterix, and bone sialoprotein (BSP) (Fig. 2). Recently it has been suggested that retinoblastoma binding protein 1 may be the co-activator of runt-related transcription factor 2 (Runx2) [30,31].
Fig. 2. Osteoblastogenesis. Upon the binding of bone morphogenetic protein 2 (BMP-2) to transmembrane proteins such as bone morphogenetic protein receptor II, it phosphorylate type I receptor, and hence activates the Smad complex (Smad 1, 4, 5, and 8) signaling pathways [26-29], which can help in the activation of osteoblast specific transcriptional regulation genes such as osteocalcin (OCN), collagen type I (Col-I), osteonectin (ON), osterix (OSX), and bone sialoprotein (BSP). Recently it has been suggested that retinoblastoma binding protein 1 (RBP1) may be the co-activator of Runx2 [30,31]. cAMP, cyclic adenosine monophosphate; PNS, Panax notoginseng saponins; AMPK, AMP-activated protein kinase; PKD, protein kinase D; Runx2, runt-related transcription factor 2; ALP, alkaline phosphatase; BSP, bone sialoprotein.
Different therapeutic agents have been used for the treatment, management, and prevention of osteoporosis, including denosumab, bisphosphonate, raloxifene, calcitonin, teriparatide, strontium ranelate, and hormone replacement therapy, but these drugs may have side effects. Recently, it has been found that bisphosphonate causes esophagitis, esophageal cancer, and atypical femur fractures when it is used for more than five years. Similarly, it has been suggested that hormone replacement therapy may cause breast cancer, coronary heart disease, stroke, and venous thromboembolism [32-35].
Modern pharmacological therapy is costly and produces many side effects, resulting in significant patient non-compliance. Thus, there is a strong need to explore alternative therapies, particularly from herbal sources as these are cost-effective and have minimal side effects. Over the past decades, herbal medicine has become a topic of global importance, making an impact on both world health and international trade. Medicinal plants continue to play a central role in the healthcare of much of the world population.
Panax ginseng Meyer commonly known as Korean ginseng belongs to the family Araliaceae. The name, ginseng, is derived from the Chinese word “rénshēn,” which means “man root.” The plant is native to Asia and North America. As much as 2000 years ago the roots of American ginseng (P. quinquefolius) and Korean ginseng (P. ginseng Meyer) were traditionally used for the treatment of many diseases [53,54]. Among the 15 Panax species, 4 different species include P. ginseng, P. notoginseng, P. japonicus, P. quinquefolius were used commercially. The Korean ginseng can be divided into nine cultivars and three lines.
The ginseng plant contains many active ingredients, including steroidal saponins with a dammarane skeleton as well as protopanaxadiol and protopanaxatriol, commonly known as ginsenosides, which is the energetic part of glycoside plus an aglycone. Recently 128 ginsenosides have been identified from P. ginseng [55].
Although ginsenosides have been reported to possess antioxidant, anti-cancer, anti-diabetic, anti-adipocyte, and sexual enhancement properties, very few studies have been conducted on their anti-osteoporotic activity [56-60]. Cell and animal studies of the anti-osteoporotic activities of different ginsenosides have been conducted. Protopanaxadiol-type saponins (Rb1, Rg3, Rd, and Rh2), protopanaxatriol-type saponins (Re and Rg1), P. notoginseng saponins (PNS; Rg1, Rb1, and R1), and ginseng mixtures have been reported to show anti-osteoporotic activity in these studies (Table 1).
Table 1.
Effects of ginseng on different molecular pathways related to osteoporosis in cell line and animal studies
| Ginsenosides | In vitro/in vivo | Molecular mechanism | Reference |
|---|---|---|---|
|
| |||
| Rh2 (S) | MC3T3-E1 | ↑ mRNA expression ALP, OCN, OPN | [36] |
| ↑ Osx and Col-I, PKD/AMPK phosphorylation % bone formation | |||
| Rh2 (R) | RAW264.7 | ↓ OC activity and bone resorption | [37] |
| RAW264.7 | ↓ RANKL, NF-kB, JNK and p38 MAPK, specific transcription factors (c-Fos and NFATc1), reduce OCs and bone resorption | [38] | |
| Rd | MC3T3-E1 | ↑ BMP-2 secretion, AMPK phosphorylation, ALP, OCN % Col-I | [39] |
| Rh2(S) | MC3T3-E1 | ↑ mRNA expression ALP, OCN, Osx % Col-I | [40] |
| ↑ PKD/p38 phosphorylation | |||
| Ginsenoside Rh2 | Mouse bone marrow cells | ↓ c-Fos, NFATc1, Bone resorption | [41] |
| ↓ Osteoclastogenesis by blocking RANKL activity | |||
| PNS | Bone marrow stromal cells | ↑ ALP, OPG, BSN, cbfα1 | [42] |
| ↓ PPARγ2 and RANKL and osteoclast activity | |||
| PNS | Bone marrow stromal cells | ↑ mRNA level of ALP, BSN, cbfa1,ERK, p38 phosphorylation | [43] |
| ↓ mRNA level PPARγ2 | |||
| Ginsenoside (tR) | OVX (lumbar vertebrae, Tibia) | ↑ BMD, cAMP | [44] |
| ↓ Bone loss | |||
| Rg1 | RAW 264.7 | ↓ TNF-α, IL-6 and LPS, Inhibition of NF-kB | [45] |
| ↓ JNK % ERK | |||
| ↓ Phosphorylation of IkB | |||
| Red ginseng acidic polysaccharide | OVX | ↑ Tumoricidal activity of NK cells, iNOS | [46] |
| Red ginseng acidic polysaccharide | Peritoneal macrophages | ↑ TNF-α, NO, IL-1 | [47,48] |
| Liquid extract from | Male Wistar rats | ↓ Calcium and hydroxyproline in urine, steroidal effect | [49] |
| Siberian ginseng | ↑ Breaking strength of femoral diaphyses and vertebrae | ||
| Ginseng mixture (HER-S)+17β-estradiol | Female Sprague-Dawley rats (OVX) MC3T3-E1 cells | ↓ Body weight, bone mineral loss/resorption in OVX, TRAP activity | [50] |
| Line/osteoclast (IRC mice) | ↑ Femoral trabecular width, BMDs, estrogen levels | ||
| PNS | Rats | ↓ Losses of BMD, microstructure. deterioration, in trabecular, DPD/Cr, while | [51] |
| ↑ BV/TV, Conn.D, Tb.N, Tb.Th, ALP | |||
| PNS | Bone marrow stromal cells | ↑ BMSCs proliferation, ALP, Runx2, OC, and BSP | [52] |
| ↓ Secretion of PPARγ2 | |||
ALP, alkaline phosphatase; OCN, osteocalcin; OPN, osteopontin; Osx, ostrex; Col-I, collagen I; OC, osteoclast; PKD, protein kinase D; RANKL, receptor activator of nuclear factor kappa-B ligand; NF-κB, nuclear factor kappa-B; JNK, c-Jun N-terminal kinases; AMPK, AMP-activated protein kinase; NFATc1, nuclear factor of activated T-cells, cytoplasmic 1; BMP-2, bone morphogenetic protein 2; PNS, Panax notoginseng saponins; OPG, osteoprotegerin; cbfa1, core binding factor alpha-1; PPAR γ2, peroxisome proliferator-activated receptors γ2; ERK, extracellular-signal-regulated kinases; BMD, bone mineral density; cAMP, cyclic adenosine monophosphate; TNF-α, tumor necrosis factor-alpha; IL-6, interleukin-6; TRAP, tartrate-resistant acid phosphatase; NK, natural killer cells; iNOS, isoform nitric oxide synthases; NO, nitric oxide; OVX, ovariectomised rats; DPD/Cr, urinary deoxypyridinoline/creatinine; BV/TV, trabecular bone volume over total bone volume; Conn.D, connectivity density; Tb.N, trabecular number; Tb.Th, trabecular thickness; BMSCs, bone marrow stromal cells; Runx 2, runt-related transcription factor 2; BSP, bone sialoprotein.
Even though the exact mechanisms of ginseng’s anti-osteoporotic effects are not fully understood, in vitro and in vivo data suggest three possibilities: 1) modulation of osteoblastogenesis and bone formation, 2) modulation of osteoclastogenesis and bone resorption, and 3) modulation of osteoporosis. It is obvious from the Table 1 that Rh2, Rd, Rh2(S), and (tR) ginsenosides stimulate the secretion and phosphorylation of alkaline phosphatase (ALP), OCN, osteopontin, ostrex, Col-I, BMP-2, protein kinase D (PKD)/AMP-activated protein kinase, PKD/p38, and cAMP and increase bone mineral density [36,39,40,44]. Similarly, He et al. [42] and Li et al. [43] demonstrated that P. notoginseng has the ability to affect the mRNA level of ALP, BSP, core-binding factor subunit alpha-1, and OPG, increases phosphorylation of extracellular-signal-regulated kinases and p38, and inhibits the secretion of peroxisome proliferator-activated receptors γ 2 (PPARγ2) and RANKL. On the other hand, ginsenosides Rh2(R), Rb1, Rh2, and Rg1 may have the ability to suppress the secretion and activity of osteoclasts, RANKL, NF-kB, JNK, c-Fos, NFATc1, and TNF-α, interleukin (IL)-6, and lipopolysaccharide [37,38,41,45]. Monroe et al. [46], Kim et al. [47], and Yovel et al. [48] suggest that red ginseng acidic polysaccharide can increase the tumoricidal activity of isoform nitric oxide synthases of natural killer cells in ovariectomised rats (OVX), while it may increase TNF-α, nitric oxide, and IL-1 expression by peritoneal macrophages. However, calcium and hydroxyproline secretion in the urine of male Wistar rats was significantly suppressed by treatment with a liquid extract from Siberian ginseng (Eleutherococcus senticosus), while also increasing the strength of femoral diaphyses and vertebrae [49]. According to Kim et al. [50], the ginseng mixture (HER-S) has the ability to induce the deposition of BMD, increase femoral trabecular width and estrogen levels, and decrease TRAP activity in OVX. Similarly Shen et al. [51] and Li et al. [52] reported that PNS decreased the loss of BMD, PPARγ2, microstructure corrosion in trabecular bone, and urinary deoxypyridinoline/creatinine, while increasing trabecular bone volume over total bone volume, connectivity density, trabecular number, trabecular thickness, alkaline phosphatase, Runx2, OC, and BSP levels in bone marrow stromal cells. Kim et al. [61] found clinically that treatment of postmenopausal osteoporosis patients with Korean red ginseng together with maltose capsules had no significant consequences.
CONCLUSION
Ginseng that is used as a folk and conventional medicine for the treatment of different diseases for decades has been recognized as one of the valuable source in drug discovery and development. The fundamental of ginseng’s ability is its pharmacological active ingredients called ginsenosides. Ginseng has widespread pharmacological beneficial activities and mechanism of action. Many studies demonstrate that ginsenosides can decrease osteoporosis by inhibiting production of NF-kB, stimulate ALP, Col-I, Runx2, increase blood circulation, and enhance memory [42]. Furthermore many studies indicate that ginsenosides have a huge number of activities in both pathological and physiological circumstances concerning with bone disease. However the effects and mechanisms of action of ginsenosides are still not yet entirely understood.
Data from in vitro and in vivo studies have revealed that ginseng saponins (ginsenosides) have beneficial effects in the treatment of osteoporosis and may increase the osteogenesis of bone marrow stromal cells and pre-osteoblast cells. Even though some ginsenosides have been studied for the treatment of osteoporosis, their functions at the pharmacokinetic and pharmacodynamic levels are not well understood, and are important for understanding the inhibition of bone resorption and osteoclastogenesis. Future studies about osteoporosis with ginsenosides should include detailed mode of action and mechanisms both in vitro and in vivo.
Acknowledgments
The research funding was fully supported by the Korea Institute of Planning & Evaluation for Technology in Food, Agriculture, Forestry & Fisheries (KIPET 309019-03-3-SB010). The ginseng sample used in this study was provided by Kyung Hee University.
References
- 1.Theill LE, Boyle WJ, Penninger JM. RANK-L and RANK: T cells, bone loss, and mammalian evolution. Annu Rev Immunol. 2002;20:795–823. doi: 10.1146/annurev.immunol.20.100301.064753. [DOI] [PubMed] [Google Scholar]
- 2.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 3.Karsenty G, Wagner EF. Reaching a genetic and molecular understanding of skeletal development. Dev Cell. 2002;2:389–406. doi: 10.1016/S1534-5807(02)00157-0. [DOI] [PubMed] [Google Scholar]
- 4.Soltanoff CS, Yang S, Chen W, Li YP. Signaling networks that control the lineage commitment and differentiation of bone cells. Crit Rev Eukaryot Gene Expr. 2009;19:1–46. doi: 10.1615/CritRevEukarGeneExpr.v19.i1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang W, Yang S, Shao J, Li YP. Signaling and transcriptional regulation in osteoblast commitment and differentiation. Front Biosci. 2007;12:3068–3092. doi: 10.2741/2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kogianni G, Noble BS. The biology of osteocytes. Curr Osteoporos Rep. 2007;5:81–86. doi: 10.1007/s11914-007-0007-z. [DOI] [PubMed] [Google Scholar]
- 7.Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest. 2005;115:3318–3325. doi: 10.1172/JCI27071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ng KW. Future developments in osteoporosis therapy. Endocr Metab Immune Disord Drug Targets. 2009;9:371–384. doi: 10.2174/187153009789839192. [DOI] [PubMed] [Google Scholar]
- 9.Report of a WHO Study Group. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. World Health Organ Tech Rep Ser. 1994;843:1–129. [PubMed] [Google Scholar]
- 10.National Osteoporosis Foundation. Available from: http://www.nof.org/node/40. Learn about osteoporosis.
- 11.World Health Organization. Available from: http://www.who.int/chp/topics/Osteoporosis.pdf. WHO scientific group on the assessment of osteoporosis at primary health care level.
- 12.Lau RY, Guo X. A review on current osteoporosis research: with special focus on disuse bone loss. J Osteoporos. 2011;2011:293808. doi: 10.4061/2011/293808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Narducci P, Nicolin V. Differentiation of activated monocytes into osteoclast-like cells on a hydroxyapatite substrate: an in vitro study. Ann Anat. 2009;191:349–355. doi: 10.1016/j.aanat.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 14.Baron R, Neff L, Vignery A. Differentiation and functional characteristics of osteoclasts. Bone. 1985;6:414. doi: 10.1016/8756-3282(85)90385-0. [DOI] [Google Scholar]
- 15.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/S0092-8674(00)81569-X. [DOI] [PubMed] [Google Scholar]
- 16.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett T, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/S0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 17.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du J, Cheng B, Zhu X, Ling C. Ginsenoside Rg1, a novel glucocorticoid receptor agonist of plant origin, maintains glucocorticoid efficacy with reduced side effects. J Immunol. 2011;187:942–950. doi: 10.4049/jimmunol.1002579. [DOI] [PubMed] [Google Scholar]
- 19.Koga T, Inui M, Inoue K, Kim S, Suematsu A, Kobayashi E, Iwata T, Ohnishi H, Matozaki T, Kodama T, et al. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature. 2004;428:758–763. doi: 10.1038/nature02444. [DOI] [PubMed] [Google Scholar]
- 20.Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology. 2001;142:5050–5055. doi: 10.1210/en.142.12.5050. [DOI] [PubMed] [Google Scholar]
- 21.Hsu H, Lacey DL, Dunstan CR, Solovyev I, Colombero A, Timms E, Tan HL, Elliott G, Kelley MJ, Sarosi I, et al. Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc Natl Acad Sci U S A. 1999;96:3540–3545. doi: 10.1073/pnas.96.7.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lomaga MA, Yeh WC, Sarosi I, Duncan GS, Furlonger C, Ho A, Morony S, Capparelli C, Van G, Kaufman S, et al. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 1999;13:1015–1024. doi: 10.1101/gad.13.8.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyce BF, Xing L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res Ther. 2007;9(Suppl 1):S1. doi: 10.1186/ar2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sundaram K, Nishimura R, Senn J, Youssef RF, London SD, Reddy SV. RANK ligand signaling modulates the matrix metalloproteinase-9 gene expression during osteoclast differentiation. Exp Cell Res. 2007;313:168–178. doi: 10.1016/j.yexcr.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Jayakumar P, Di Silvio L. Osteoblasts in bone tissue engineering. Proc Inst Mech Eng H. 2010;224:1415–1440. doi: 10.1243/09544119JEIM821. [DOI] [PubMed] [Google Scholar]
- 26.Derynck R, Zhang Y. Intracellular signalling: the mad way to do it. Curr Biol. 1996;6:1226–1229. doi: 10.1016/S0960-9822(96)00702-6. [DOI] [PubMed] [Google Scholar]
- 27.Kingsley DM. The TGF-beta superfamily: new members, new receptors, and new genetic tests of function in different organisms. Genes Dev. 1994;8:133–146. doi: 10.1101/gad.8.2.133. [DOI] [PubMed] [Google Scholar]
- 28.Massague J. TGFbeta signaling: receptors, transducers, and Mad proteins. Cell. 1996;85:947–950. doi: 10.1016/S0092-8674(00)81296-9. [DOI] [PubMed] [Google Scholar]
- 29.Reddi AH. Bone and cartilage differentiation. Curr Opin Genet Dev. 1994;4:737–744. doi: 10.1016/0959-437X(94)90141-O. [DOI] [PubMed] [Google Scholar]
- 30.Berridge M. Available from: http://www.biochemj.org/csb. Cell signaling biology.
- 31.Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233–241. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 32.Yamashita H, Ten Dijke P, Heldin CH, Miyazono K. Bone morphogenetic protein receptors. Bone. 1996;19:569–574. doi: 10.1016/S8756-3282(96)00259-1. [DOI] [PubMed] [Google Scholar]
- 33.Merck. Fosamax (alendronate sodium) tablets and oral solution prescribing information. Merck; Whitehouse Station: 2011. [Google Scholar]
- 34.Novartis Pharmaceuticals Corporation. Reclast (zoledronic acid) injection prescribing information. Novartis Pharmaceuticals Corporation; East Hanover: 2011. [Google Scholar]
- 35.Wysowski DK. Reports of esophageal cancer with oral bisphosphonate use. N Engl J Med. 2009;360:89–90. doi: 10.1056/NEJMc0808738. [DOI] [PubMed] [Google Scholar]
- 36.Hwang JT, Kim SH, Lee MS, Kim SH, Yang HJ, Kim MJ, Kim HS, Ha J, Kim MS, Kwon DY. Anti-obesity effects of ginsenoside Rh2 are associated with the activation of AMPK signaling pathway in 3T3-L1 adipocyte. Biochem Biophys Res Commun. 2007;364:1002–1008. doi: 10.1016/j.bbrc.2007.10.125. [DOI] [PubMed] [Google Scholar]
- 37.Kim DY, Park KH, Jung MS, Huang B, Yuan HD, Quan HY, Chung SH. Ginsenoside Rh2(S) induces differentiation and mineralization of MC3T3-E1 cells through activation of the PKD/AMPK signaling pathways. Int J Mol Med. 2011;28:753–759. doi: 10.3892/ijmm.2011.750. [DOI] [PubMed] [Google Scholar]
- 38.Liu J, Shiono J, Shimizu K, Yu H, Zhang C, Jin F, Kondo R. 20(R)-ginsenoside Rh2, not 20(S), is a selective osteoclastgenesis inhibitor without any cytotoxicity. Bioorg Med Chem Lett. 2009;19:3320–3323. doi: 10.1016/j.bmcl.2009.04.054. [DOI] [PubMed] [Google Scholar]
- 39.Cheng B, Li J, Du J, Lv X, Weng L, Ling C. Ginsenoside Rb1 inhibits osteoclastogenesis by modulating NF-κB and MAPKs pathways. Food Chem Toxicol. 2012;50:1610–1615. doi: 10.1016/j.fct.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 40.Kim DY, Park YG, Quan HY, Kim SJ, Jung MS, Chung SH. Ginsenoside Rd stimulates the differentiation and mineralization of osteoblastic MC3T3-E1 cells by activating AMP-activated protein kinase via the BMP-2 signaling pathway. Fitoterapia. 2012;83:215–222. doi: 10.1016/j.fitote.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 41.Kim DY, Jung MS, Park YG, Yuan HD, Quan HY, Chung SH. Ginsenoside Rh2(S) induces the differentiation and mineralization of osteoblastic MC3T3-E1 cells through activation of PKD and p38 MAPK pathways. BMB Rep. 2011;44:659–664. doi: 10.5483/BMBRep.2011.44.10.659. [DOI] [PubMed] [Google Scholar]
- 42.He L, Lee J, Jang JH, Lee SH, Nan MH, Oh BC, Lee SG, Kim HH, Soung NK, Ahn JS, et al. Ginsenoside Rh2 inhibits osteoclastogenesis through down-regulation of NF-κB, NFATc1 and c-Fos. Bone. 2012;50:1207–1213. doi: 10.1016/j.bone.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 43.Li XD, Chang B, Chen B, Liu ZY, Liu DX, Wang JS, Hou GQ, Huang DY, Du SX. Panax notoginseng saponins potentiate osteogenesis of bone marrow stromal cells by modulating gap junction intercellular communication activities. Cell Physiol Biochem. 2010;26:1081–1092. doi: 10.1159/000323986. [DOI] [PubMed] [Google Scholar]
- 44.Li XD, Liu ZY, Chang B, Liu DX, Chen B, Guo C, Wang YG, Xu JK, Huang DY, Du SX. Panax notoginseng saponins promote osteogenic differentiation of bone marrow stromal cells through the ERK and P38 MAPK signaling pathways. Cell Physiol Biochem. 2011;28:367–376. doi: 10.1159/000331753. [DOI] [PubMed] [Google Scholar]
- 45.Gong YS, Chen J, Zhang QZ, Zhang JT. Effect of 17beta-oestradiol and ginsenoside on osteoporosis in ovariectomised rats. J Asian Nat Prod Res. 2006;8:649–656. doi: 10.1080/10286020500246063. [DOI] [PubMed] [Google Scholar]
- 46.Monroe DG, Hawse JR, Subramaniam M, Spelsberg TC. Retinoblastoma binding protein-1 (RBP1) is a Runx2 coactivator and promotes osteoblastic differentiation. BMC Musculoskelet Disord. 2010;11:104. doi: 10.1186/1471-2474-11-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim KS, Pyo SK, Sohn EH. Immunomodulation of NK cell activity by red ginseng acidic polysaccharide (RGAP) in ovariectomized rats. J Ginseng Res. 2009;33:99–103. doi: 10.5142/JGR.2009.33.2.099. [DOI] [Google Scholar]
- 48.Yovel G, Shakhar K, Ben-Eliyahu S. The effects of sex, menstrual cycle, and oral contraceptives on the number and activity of natural killer cells. Gynecol Oncol. 2001;81:254–262. doi: 10.1006/gyno.2001.6153. [DOI] [PubMed] [Google Scholar]
- 49.Kropotov AV, Kolodnyak OL, Koldaev VM. Effects of Siberian ginseng extract and ipriflavone on the development of glucocorticoid-induced osteoporosis. Bull Exp Biol Med. 2002;133:252–254. doi: 10.1023/A:1015834717178. [DOI] [PubMed] [Google Scholar]
- 50.Kim HR, Cui Y, Hong SJ, Shin SJ, Kim DS, Kim NM, So SH, Lee SK, Kim EC, Chae SW, et al. Effect of ginseng mixture on osteoporosis in ovariectomized rats. Immunopharmacol Immunotoxicol. 2008;30:333–345. doi: 10.1080/08923970801949125. [DOI] [PubMed] [Google Scholar]
- 51.Shen Y, Li YQ, Li SP, Ma L, Ding LJ, Ji H. Alleviation of ovariectomy-induced osteoporosis in rats by Panax notoginseng saponins. J Nat Med. 2010;64:336–345. doi: 10.1007/s11418-010-0416-7. [DOI] [PubMed] [Google Scholar]
- 52.Li XD, Wang JS, Chang B, Chen B, Guo C, Hou GQ, Huang DY, Du SX. Panax notoginseng saponins promotes proliferation and osteogenic differentiation of rat bone marrow stromal cells. J Ethnopharmacol. 2011;134:268–274. doi: 10.1016/j.jep.2010.11.075. [DOI] [PubMed] [Google Scholar]
- 53.Brewer L, Williams D, Moore A. Current and future treatment options in osteoporosis. Eur J Clin Pharmacol. 2011;67:321–331. doi: 10.1007/s00228-011-0999-2. [DOI] [PubMed] [Google Scholar]
- 54.Yuan HD, Kim JT, Kim SH, Chung SH. Ginseng and diabetes: the evidences from in vitro, animal and human studies. J Ginseng Res. 2012;361:27–39. doi: 10.5142/jgr.2012.36.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang KC, Williams WM. The pharmacology of Chinese herbs. CRC Press; Boca Raton: 1999. [Google Scholar]
- 56.Kwak YS, Park JD, Yang JW. Present and its prospect of red ginseng efficacy research. Food Ind Nutr. 2003;8:30–37. [Google Scholar]
- 57.Quan LH, Cheng LQ, Kim HB, Kim JH, Son NR, Kim SY, Jin HO, Yang DC. Bioconversion of ginsenoside Rd into compound K by Lactobacillus pentosus DC101 isolated from kimchi. J Ginseng Res. 2010;34:288–295. doi: 10.5142/jgr.2010.34.4.288. [DOI] [Google Scholar]
- 58.Shin HY, Jeong HJ, An HJ, Hong SH, Um JY, Shin TY, Kwon SJ, Jee SY, Seo BI, Shin SS, et al. The effect of Panax ginseng on forced immobility time & immune function in mice. Indian J Med Res. 2006;124:199–206. [PubMed] [Google Scholar]
- 59.Wang W, Zhao Y, Rayburn ER, Hill DL, Wang H, Zhang R. In vitro anti-cancer activity and structure-activity relationships of natural products isolated from fruits of Panax ginseng. Cancer Chemother Pharmacol. 2007;59:589–601. doi: 10.1007/s00280-006-0300-z. [DOI] [PubMed] [Google Scholar]
- 60.Polan ML, Hochberg RB, Trant AS, Wuh HC. Estrogen bioassay of ginseng extract and ArginMax, a nutritional supplement for the enhancement of female sexual function. J Womens Health (Larchmt) 2004;13:427–430. doi: 10.1089/154099904323087114. [DOI] [PubMed] [Google Scholar]
- 61.Kim NH, Lee HM, Choi CH, Lim SK. Clinical effect of Korean red ginseng on osteoporosis. J Ginseng Res. 1998;22:114–121. [Google Scholar]