Abstract
MGB-20 findings show that the ginseng berry extracts that had been processed with microwave and vinegar for 20 min peaked in the level of ginsenoside Rg2 (2.28%) and Rh1 (1.28%). MGB-1 peaked in the level of ginsenoside Rg3 (1.13%) in the ginseng berry extract processed with microwave and vinegar for 1 min.
Keywords: Panax ginseng, Berry, Microwave, Vinegar, Rg2
It was confirmed that Korean ginseng berries, a flesh part of the berries, contain ginseng saponins such as Rb1, Rb2, Rd, Re, Rg1, Rg2, and Rh1 [1]. It was also reported that other effects of ginseng berries include such physiologically activating functions as antidiabetic [2], antiobese [2], Antisenility [3], antistress [4], antiallergic [5], and anticancer effects [6].
Among others, it was particularly confirmed that ginsenoside Re is contained as much as approximately 6%, supposedly making it possible to transform to ginsenoside Rg2 by means of biological conversion, hydrolysis and so forth [1].
Accordingly, the current study proposes to probe into changes in the ginseng saponin composition of ginseng berries through microwave treatment for the purpose of developing ginseng berry products containing high-concentrated ginsenoside Rg2.
The current study proposes to examine differences with a focus on the pattern of saponin contents by comparing and analyzing the distribution of contents of individual ginsenoside contained in ginseng berries added with vinegar, and treated and processed with microwave, to develop a preparation containing high-concentrated ginseng-activated prosapogenins such as ginsenoside Rg2, Rg3, Rg5, Rg6, Rh1, and F4, and to provide basic physiochemical information on the same proposed preparation.
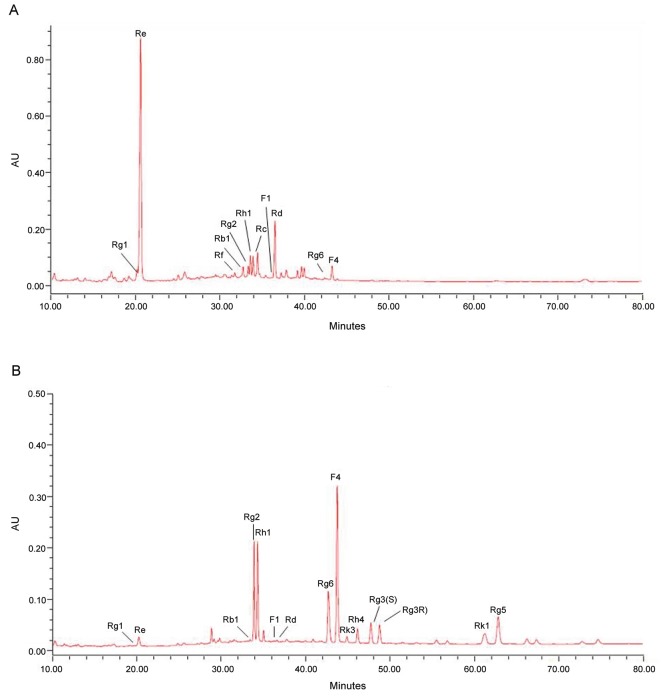
For experiments, 4-year-old Korean ginseng berries were collected at Eumseong (Korea) on August 20, 2010 (Fig.1). The specimens were stored at the Oriental Medical Food Research Laboratory, Semyung University.
Fig. 1. HPLC chromatogram of ginsenosides in the ginseng berry processed with microwave and vinegar. (A) Ginseng berry extract. (B) Ginseng berry extract processed with microwave and vinegar for 20 min.
Ginseng berries were selected, dried, and powdered. Exactly 500g of powdered samples were refluxed four times with 2.5 L of 95% ethyl alcohol for 2 h in water bath. The extracts were filtered through the filter paper (Nylon membrane filters 7404-004; Whatman, Dassel, Germany) and concentrated by the vacuum evaporator.
The ethanol extract was added with 200 mL vinegar (twice vinegar [pH 2.30, acidity 13% to 14%]; Ottugi, Seoul, Korea) per 1 g extract, put in the microwave oven (RE-C20DB; Samsung Electronics, Seoul, Korea) with an oscillation frequency of 2,450 MHz and a rated high frequency output of 700 W, and treat at 1, 5, 10, 20, 30, and 40 min each. Precisely 2 g each was extracted with ethylether three times by using a sonicator (4020P; KODO, Hwaseong, Korea), after removing lipid soluble materials in ethylether phase. The residue was treated with water saturated-n-butanol three times again. n-Butanol fraction that built up in the sonicator was filtered and concentrated by a vacuum evaporator. All the process was performed quantitatively. The amount of the concentrate was equivalent to that of crude saponin [7].
Ginsenoside composition of the concentrate was analyzed with HPLC according to the method of Lee et al. [7]. The total ginsenoside content and ginsenoside composition of each sample were analyzed three times. The pure ginsenoside standards (99% purity) used in this experiment were purchased from Chromadex (St. Santa Ana, CA, USA).
The HPLC instrument model used was Waters 1525 binary HPLC system (Waters, Milford, MA, USA), with Eurospher100-5 C18P column (250x3 mm; Knauer, Berlin, Germany). The mobile phase was the mixture of acetonitrile (HPLC grade; Sigma-Aldrich, St. Louis, MO, USA) and distilled water (HPLC grade; JT Baker, Phillipsburg, NJ, USA). The content of acetonitrile was sequentially increased from 17% to 25% (20 min), 25% to 42% (38 min), 42% to 60% (85 min), 60% to 80% (95 min) and adjusted from 80% to 17% again lastly. Operating temperature was set at room temperature, and the flow rate at 0.8 mL/min. An elution profile on chromatogram was obtained by using a UV/VIS detector at 203 nm (2487 dual λabsorbance detector, Waters).
The current study proposes to develop a preparation containing high-concentrated prosapogenin, a ginseng activated ingredient [8], such as ginsenoside Rg2, Rg3, Rg5, Rg6, Rh1, and F4 and examine differences with a focus on saponin content patterns by comparing and analyzing distribution of contents of individual ginsenoside for the aerial parts of ginseng samples which were added with vinegar, and treated and processed with microwave, and provide their basic physiochemical information.
Ginseng saponins that were subject to our analysis included ginsenosides Rb1, Rb2, Rc, Rd, Re, Rf, Rg1, Rg2, Rg3, Rg5, Rg6, Rh1, Rh4, Rk1, Rk3, F1, and F4, which were directly compared with the samples and confirmed through the HPLC as shown in Fig. 1, and the average was statistically treated and calculated. Samples were collected at Eumseong, Choongbuk province, Korea, a major ginseng cultivation area. A comparative analysis of saponin contents of the samples indicates that ginseng berry extract processed with microwave and vinegar (MGB)-30 reached 40.22%, MGB-20 34.58%, and MGB-1 39.88%, respectively, in terms of the quantity of crude saponins in the preparation of ginseng berries processed with microwave and vinegar as shown in Table 1, where crude saponin contents of ginseng berry preparations processed with microwave and vinegar for 30 min were measured as relatively high.
Table 1.
Ginsenoside composition of the ginseng berry extracts processed with microwave and vinegar over time
| Ginsenosides | Ginseng berry processed with microwave and vinegar (%, w/w) | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| GB | MGB-11) | MGB-51) | MGB-101) | MGB-201) | MGB-301) | MGB-401) | |
|
| |||||||
| Rb1 | 0.77±0.18 | 0.18±0.04 | 0.01±0.01 | 0.04±0.04 | 0.07±0.05 | 0.04±0.02 | 0.02±0.01 |
| Rb2 | 0.60±0.11 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rd | 1.53±0.18 | 0.41±0.01 | 0.01±0.00 | 0.02±0.01 | 0.03±0.01 | 0.01±0.00 | 0.01±0.01 |
| Re | 11.17±0.16 | 3.59±0.1 | 0.17±0.01 | 0.18±0.00 | 0.29±0.04 | 0.20±0.03 | 0.21±0.02 |
| Rf | 0.33±0.12 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rg1 | 0.57±0.01 | 0.17±0.02 | 0.01±0.01 | 0.01±0.00 | 0.03±0.00 | 0.02±0.01 | 0.01±0.01 |
| Rg2 | 0.80±0.22 | 1.040±0.04 | 1.76±0.03 | 1.39±0.04 | 2.28±0.37 | 1.57±0.17 | 1.09±0.04 |
| 20S-Rg3 | 0 | 0.15±0.01 | 0.38±0.01 | 0.26±0.00 | 0.43±0.06 | 0.34±0.03 | 0.24±0.01 |
| 20R-Rg3 | 0 | 0.97±0.00 | 0.33±0.02 | 0.23±0.00 | 0.40±0.06 | 0.33±0.03 | 0.24±0.00 |
| Rg6 | 0.04±0.03 | 0.162±0.00 | 0.28±0.00 | 0.26±0.00 | 0.45±0.06 | 0.38±0.04 | 0.28±0.00 |
| Rh1 | 0.63±0.10 | 0.447±0.02 | 0.95±0.02 | 0.71±0.03 | 1.28±0.21 | 0.96±0.11 | 0.71±0.03 |
| Rh4 | 0 | 0.02±0.00 | 0.06±0.00 | 0.04±0.00 | 0.08±0.01 | 0.08±0.01 | 0.08±0.00 |
| Rk1 | 0 | 0.06±0.00 | 0.16±0.00 | 0.12±0.00 | 0.21±0.03 | 0.18±0.02 | 0.133±0.00 |
| Rk3 | 0 | 0.01±0.00 | 0.02±0.00 | 0.02±0.00 | 0.04±0.01 | 0.04±0.00 | 0.03±0.00 |
| F1 | 0.19±0.15 | 0.02±0.01 | 0.01±0.01 | 0.01±0.01 | 0.04±0.02 | 0.01±0.00 | 0.01±0.01 |
| F4 | 0.19±0.03 | 0.35±0.01 | 0.78±0.01 | 0.59±0.00 | 1.01±0.14 | 0.90±0.09 | 0.66±0.01 |
| Prosapogenin2) | 1.86 | 3.23 | 4.72 | 3.62 | 6.21 | 4.77 | 3.48 |
| Total saponin3) | 16.79 | 7.57 | 4.92 | 3.87 | 6.63 | 5.03 | 3.73 |
Values represent the mean±SE (n=3).
GB, ginseng berry extract; MGB-1, ginseng berry extract processed with microwave and vinegar for 1 min.
1) Minute.
2) Rg2 + 20S-Rg3 + 20R-Rg3 + Rg6 + Rh1 +Rh4 + Rk1 + Rk3 + F1 + F4.
3) Sum of individual ginsenosides content.
The total saponin content, a sum of each ginsenoside, showed that MGB-1, MGB-20, and MGB-30 stood at 7.57%, 6.63%, and 5.03%, respectively as shown in Table 1, where the total saponin of ginseng berries processed with microwave and vinegar for one minute showed a high saponin content.
It is known that prosapogenin, an ingredient generated as a result of hydrolysis by heat or acid, has a absorption level better than ginseng saponin glycoside found in the wild, with pharmacological effects reinforced.
MGB-20, which stood at 6.21%, peaked in the total amount of prosapogenin (ginsenoside Rg2, Rg3, Rg5, Rg6, Rh1, Rh4, Rk1, Rk3, F1, and F4), followed by MGB-30 (4.77%) and MGB-5 (4.72%).
In the content of ginsenoside Rg2, featuring wrinkle improving effects in particular [9], MGB-20 peaked with 2.28%, followed by MGB-5 (1.76%) and MGB-30 (1.57%). MGB-20 was found to contain 2.8 times as high as ginseng berry extracts (MGB, 0.80%).
On the other hand, when it comes to ginsenoside Rg3, which displays cancer prevention, cancer cell growth-resistant [10], hypotensive [11], brain cell protection [12], antithrombotic [13] and antioxidant actions [14], MGB-1 peaked with 1.13%, followed by MGB-20 (0.83%) and MGF-B (0.71%). However, no ginsenoside Rg3 was found in ginseng berry extracts.
As for ginsenoside Rg6 [7], another product of thermo-hydrolysis, MGB-20 topped the list of contents with 0.45%, followed by MGB-30 (0.38%) and MGB-5 (0.29%). On the other hand, ginseng berry extracts (MGB) showed a ginsenoside Rg6 content of 0.04%, a very low level.
In the case of ginsenoside Rh1, which can be generated as a result of ginsenoside Rg2 being hydrolized, a physiologically activated ingredient which is reported to have allergy-resistant actions [15], MGB-20 peaked in content with 1.28%, followed by MGB-30 (0.96%) and MGB-5 (0.95%). The content of MGB-20 of this level is equivalent to twice as much as ginseng berry extracts (MGB, 0.63%). At the same time, in ginsenoside F4, MGB-20 peaked in content with 1.01%, followed by MGB-30 (0.90%) and MGB-5 (0.78%).
Acknowledgments
This study was carried out with the support of ‘Forest Science & Technology Projects (Project No. S211012L030100)’ provided by Korea Forest Service.
References
- 1.Ko SK, Bae HM, Cho OS, Im BO, Chung SH, Lee BY. Analysis of ginsenoside composition of ginseng berry and seed. Food Sci Biotechnol. 2008;17:1379–1382. [Google Scholar]
- 2.Dey L, Zhang L, Yuan CS. Anti-diabetic and anti-obese effects of ginseng berry extract: comparison between intraperitoneal and oral administrations. Am J Chin Med. 2002;30:645–647. doi: 10.1142/S0192415X02000648. [DOI] [PubMed] [Google Scholar]
- 3.Huo YS. Anti-senility action of saponin in Panax ginseng fruit in 327 cases. Zhong Xi Yi Jie He Za Zhi. 1984;4:593–596,578.. [PubMed] [Google Scholar]
- 4.Zhang SC, Jiang XL. The anti-stress effect of saponins extracted from Panax ginseng fruit and the hypophyseal-adrenal system. Yao Xue Xue Bao. 1981;16:860–863. [PubMed] [Google Scholar]
- 5.Bae HM, Cho OS, Kim SJ, Im BO, Cho SH, Lee S, Kim MG, Kim KT, Leem KH, Ko SK. Inhibitory effects of ginsenoside Re isolated from ginseng berry on histamine and cytokine release in human mast cells and human alveolar epithelial cells. J Ginseng Res. 2012;36:369–374. doi: 10.5142/jgr.2012.36.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang W, Zhao Y, Rayburn ER, Hill DL, Wang H, Zhang R. In vitro anti-cancer activity and structure-activity relationships of natural products isolated from fruits of Panax ginseng. Cancer Chemother Pharmacol. 2007;59:589–601. doi: 10.1007/s00280-006-0300-z. [DOI] [PubMed] [Google Scholar]
- 7.Lee SA, Jo HK, Im BO, Kim S, Whang WK, Ko SK. Changes in the contents of prosapogenin in the red ginseng (Panax ginseng) depending on steaming batches. J Ginseng Res. 2012;36:102–106. doi: 10.5142/jgr.2012.36.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ko SK, Lee KH, Hong JK, Kang SA, Sohn UD, Im BO, Han ST, Yang BW, Chung SH, Lee BY. Change of ginsenoside composition in ginseng extract by the vinegar process. Food Sci Biotechnol. 2005;14:509–513. [Google Scholar]
- 9.Jeong SJ, Han SH, Kim DY, Lee JC, Kim HS, Kim BH, Lee JS, Hwang EH, Park JK. Effects of mRg2, a mixture of ginsenosides containing 60% Rg2, on the ultraviolet B-induced DNA repair synthesis and apoptosis in NIH3T3 cells. Int J Toxicol. 2007;26:151–158. doi: 10.1080/10915810701226370. [DOI] [PubMed] [Google Scholar]
- 10.Keum YS, Han SS, Chun KS, Park KK, Park JH, Lee SK, Surh YJ. Inhibitory effects of the ginsenoside Rg3 on phorbol ester-induced cyclooxygenase-2 expression, NF-kappaB activation and tumor promotion. Mutat Res. 2003;523-524:75–85. doi: 10.1016/S0027-5107(02)00323-8. [DOI] [PubMed] [Google Scholar]
- 11.Kim ND, Kang SY, Park JH, Schini-Kerth VB. Ginsenoside Rg3 mediates endothelium-dependent relaxation in response to ginsenosides in rat aorta: role of K+ channels. Eur J Pharmacol. 1999;367:41–49. doi: 10.1016/s0014-2999(98)00898-x. [DOI] [PubMed] [Google Scholar]
- 12.Yang L, Hao J, Zhang J, Xia W, Dong X, Hu X, Kong F, Cui X. Ginsenoside Rg3 promotes beta-amyloid peptide degradation by enhancing gene expression of neprilysin. J Pharm Pharmacol. 2009;61:375–380. doi: 10.1211/jpp.61.03.0013. [DOI] [PubMed] [Google Scholar]
- 13.Lee WM, Kim SD, Park MH, Cho JY, Park HJ, Seo GS, Rhee MH. Inhibitory mechanisms of dihydroginsenoside Rg3 in platelet aggregation: critical roles of ERK2 and cAMP. J Pharm Pharmacol. 2008;60:1531–1536. doi: 10.1211/jpp.60.11.0015. [DOI] [PubMed] [Google Scholar]
- 14.Keum YS, Park KK, Lee JM, Chun KS, Park JH, Lee SK, Kwon H, Surh YJ. Antioxidant and anti-tumor promoting activities of the methanol extract of heat-processed ginseng. Cancer Lett. 2000;150:41–48. doi: 10.1016/S0304-3835(99)00369-9. [DOI] [PubMed] [Google Scholar]
- 15.Zheng H, Jeong Y, Song J, Ji GE. Oral administration of ginsenoside Rh1 inhibits the development of atopic dermatitis-like skin lesions induced by oxazolone in hairless mice. Int Immunopharmacol. 2011;11:511–518. doi: 10.1016/j.intimp.2010.12.022. [DOI] [PubMed] [Google Scholar]