Abstract
20S-dihydroprotopanaxadiol (2H-PPD) is a derivative of protopanaxadiol, a glycone of ginsenosides prepared from Panax ginseng. Although ginsenosides and acidic polysaccharides are known to be major active ingredients in ginseng, the immunopharmacological activities of their metabolites and derivatives have not been fully explored. In this study, we aimed to elucidate the regulatory action of 2H-PPD on the function of monocytes and macrophages in innate immune responses. 2H-PPD was able to boost the phagocytic uptake of fluorescein isothiocyanate-dextran in macrophages and enhance the generation of radicals (reactive oxygen species) in sodium nitroprusside-treated RAW264.7 cells. The surface levels of the costimulatory molecules such as CD80 and CD86 were also increased during 2H-PPD treatment. In addition, this compound boosted U937 cellcell aggregation induced by CD29 and CD43 antibodies, but not by cell-extracellular matrix (fibronectin) adhesion. Similarly, the surface levels of CD29 and CD43 were increased by 2H-PPD exposure. Therefore, our results strongly suggest that 2H-PPD has the pharmacological capability to upregulate the functional role of macrophages/monocytes in innate immunity.
Keywords: Panax ginseng, 20S-dihydroprotopanaxadiol, Phagocytosis, Reactive oxygen species generation, Adhesion
INTRODUCTION
Innate immunity is one of the major responses against pathogenic infections by bacteria, virus, and fungi. Macrophages are the cells representative of innate immune functions and are therefore regarded as important cells in managing anticancer, antibacterial, antifungal, and antiviral responses [1-3]. Macrophages are a fully differentiated form of monocyte derived from hematopoietic stem cells. Major functions carried out by these cells include phagocytic uptake of infective pathogens, the release of toxic radical molecules such as reactive oxygen species (ROS), the stimulation of T cells by antigen presentation and by producing costimulatory signals via CD80 and CD86, and adhesion to target cells or tissues via functional activation of adhesion molecules such as CD29 (β1-integrins), CD43, CD98, and CD147 [1-5]. Through these cellular responses, innate immunity plays a central role in responses to various infectious conditions [6].
Panax ginseng, a perennial plant of Family Araiaceae, is a representative herbal medicine traditionally prescribed for supporting vitality and long life and for supplementing spirits [7]. Different species of Genus Panax have been widely used in many different countries. Nonetheless, the pharmacological and phytochemical values of Korean ginseng (P. ginseng) are still under investigation [8]. Ginsenosides and acidic polysaccharides from Korean ginseng are regarded as key components acting in regulatory processes in cardiovascular disease, diabetes mellitus, cancer, stress, and immunostimulation [9-11]. Owing to the improvement of numerous chemical and biological techniques, different types of ginseng-derived metabolites or derivatives such as compound K, ginsenoside F1, and 20S-dihydroprotopanaxadiol (2H-PPD) have been identified and developed as herbal remedies [12-14].
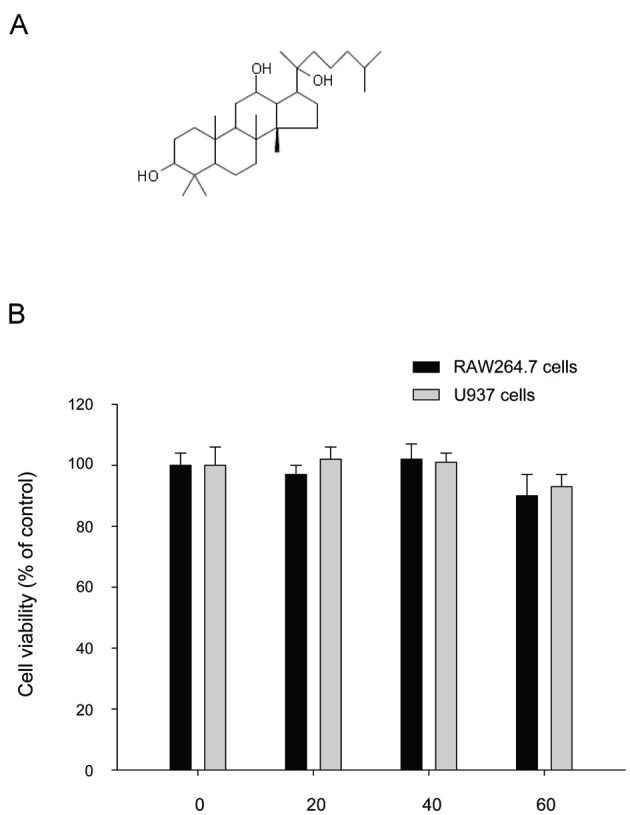
Thus far, no papers have reported the pharmacological action of 2H-PPD (Fig. 1A), a protopanaxadiol (PPD) derivative. Because its source compound, PPD, is known to exhibit various biological activities, including anti-cancer, pro-apototic, and anti-inflammatory effects [15-17], exploring the pharmacological actions of 2H-PPD could be beneficial for developing novel ginseng-derived compounds. Therefore, in this study, we investigated the immunomodulatory role of 2H-PPD on the innate immune responses of macrophages and monocytes.
Fig. 1. Chemical structure of 20S-dihydroprotopanaxadiol (2H-PPD) and its effect on the viability of RAW264.7 and U937 cells. (A) Chemical structure of 2H-PPD. (B) RAW264.7 or U937 cells (1×106 cells/mL) were incubated with 2H-PPD (0 to 60 mM) for 24 h. Cell viability was determined by conventional MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolum bromide) assay as described in Materials and Methods section.
MATERIALS AND METHODS
Materials
2H-PPD was obtained from Ambo Institute (Daejeon, Korea). The purity of the compound was greater than 98% according to HPLC analysis. Lipopolysaccharide (LPS) and sodium nitroprusside (SNP) were obtained from Sigma (St. Louis, MO, USA). RAW264.7 and U937 cells were purchased from American Type Culture Collection (Manassas, VA, USA). Cell–cell-adhesion-inducing antibodies to CD43 and CD29 were used as reported previously [18,19]. Antibodies to CD82 and CD86 were purchased from BD Bioscience (San Diego, CA, USA).
Cell culture
RAW264.7 and U937 cells were cultured in RPMI1640 with 10% fetal bovine serum and 100 U/mL penicillin/streptomycin at 37℃ in a humidified 5% CO2 atmosphere.
Cell viability assay
Cell viability and the extent of proliferation were assessed by a conventional 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolum bromide (MTT) assay [4]. RAW264.7 and U937 cells (5×104 cells/well) were incubated with various concentrations of 2H-PPD (0 to 60 μM) for 24 h and were further incubated with MTT solution (0.5 mg/mL) for an additional 4 h at 37℃. The absorbance of the samples was measured at 490 nm using a microplate reader (Molecular Devices, Menlo Park, CA, USA).
Determination of phagocytotic uptake
To measure the phagocytic activity of RAW264.7 cells, we modified a method reported previously [20]. RAW264.7 (5×104) cells treated with 2H-PPD (0 to 40 μM) were resuspended in 100 μL PBS containing 1% human AB serum and incubated with fluorescein isothiocyanate (FITC)-dextran (1 mg/mL) at 37℃ for 2 h. The incubations were stopped by adding 2 mL ice-cold phosphate-buffered saline (PBS) containing 1% human serum and 0.02% sodium azide. The cells were then washed three times with cold PBS-azide and analyzed on a FACScan flow cytometer, as reported previously [21].
Determination of reactive oxygen species generation
The level of intracellular ROS was determined by a change in fluorescence resulting from the oxidation of the fluorescent probe, dihydrorhodamine 123 (DHR123). Briefly, 5×105 RAW264.7 cells were exposed to 2H-PPD (0 to 60 μM) for 30 min. After incubation, cells were then incubated with SNP (0.25 mM), an inducer of ROS production, at 37℃ for 2 h. Cells were incubated with 20 μM of the fluorescent probe DHR123 for 1 h at 37℃. The degree of fluorescence, corresponding to intracellular ROS, was determined using a FACScan flow cytometer (Beckton-Dikinson, San Jose, CA, USA), as reported previously [21].
Flow cytometric analysis
The expression of co-stimulatory molecules (CD80 [16-10A1, 1:50] and CD86 [GL1, 1:50]) in RAW264.7 cells was determined by flow cytometric analysis [4,5]. The RAW264.7 cells (2×106 cells/mL) treated with 2H-PPD (0 to 40 μM) for 12 h were washed with a staining buffer (containing 2% rabbit serum and 1% sodium azide in PBS) and incubated with directly labeled antibodies for a further 45 min on ice. After washing three times with staining buffer, stained cells were analyzed on a FACScan flow cytometer (Becton-Dickinson).
Cell-cell or cell-extracellular matrix protein (fibronectin) adhesion assay
A U937 cell-cell adhesion assay was performed as reported previously [4,22]. Briefly, U937 cells were pre-incubated with 2H-PPD (0 to 40 μM) for 1 h at 37℃, and they were further incubated with function-activating (agonistic) anti-CD29 or anti-CD43 antibodies (1 μg/mL each) in a 96-well plate. After a 30 min incubation, cell-cell clusters were identified by a homotypic cell-cell adhesion assay using a hemocytometer [4,5] and photographed with an inverted light microscope equipped with a COHU high-performance CCD (Diavert) video camera. For a cell-fibronection adhesion assay, U937 cells (5×105 cells/well) were seeded on a fibronectin (50 mg/mL)-coated plate and incubated for 3 h [23]. After removing unbound cells with PBS, the attached cells were treated with 0.1% of crystal violet for 15 min. The optical denisity value at 570 nm was measured by a Spectramax 250 microplate reader.
Statistical analysis
Student’s t-test and one-way ANOVA were used to determine the statistical significance of the differences between values for the various experimental and control groups. Data are expressed as mean±SEM, and the results were obtained from at least three independent experiments performed in triplicate. A p-value of 0.05 or less was considered statistically significant.
RESULTS AND DISCUSSION
Numerous studies have reported that ginseng and its ingredients can modulate immune functions [10,24]. However, the specific compounds from ginseng carry out regulation of various immunological responses are not fully understood. Here, we aimed to develop a synthesizable compound from ginseng-derived components with immunomodulatory properties. 2H-PPD is a representative derivative of PPD, which can now be produced by synthetic methods [8]. Therefore, we investigated the regulatory function of 2H-PPD (Fig. 1A) on the innate immune responses of macrophages and monocytes, such as phagocytic uptake, ROS generation, costimulatory molecule expression, cell-cell adhesion, and regulation of surface adhesion molecules in monocytic and macrophage-like cells such as RAW 264.7 murine macrophages and U937 human monocytic cells, respectively.
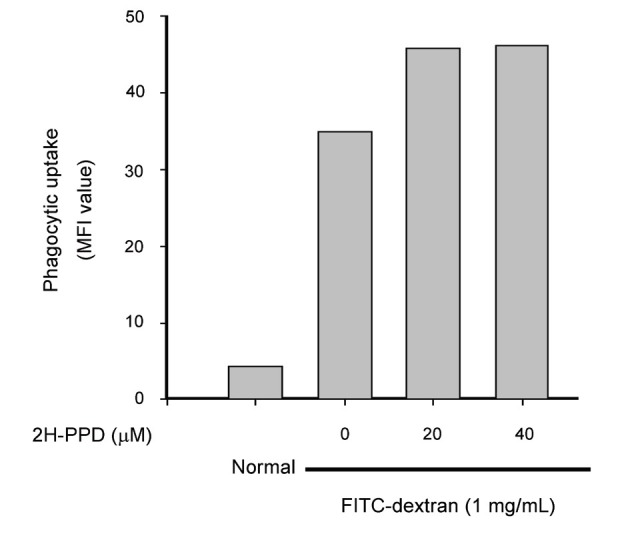
Interestingly, without altering the viability of RAW264.7 and U937 cells (Fig. 1B), 2H-PPD significantly enhanced the phagocytic uptake of FITC-dextran in RAW264.7 cells (Fig. 2), indicating that this compound could stimulate innate immune responses by macrophage cells, including engulfment of bacterial, virus, or fungi.
Fig. 2. Effect of 20S-dihydroprotopanaxadiol (2H-PPD) on phagocytic uptake of RAW264.7 cells. RAW264.7 cells pre-incubated with 2H-PPD were treated with fluorescein isothiocyanate (FITC)-dextran (1 mg/mL) for 2 h. The level of dextran uptake was determined by flow cytometric analysis as described in Materials and Methods section. MFI, mean fluorescence intensity.
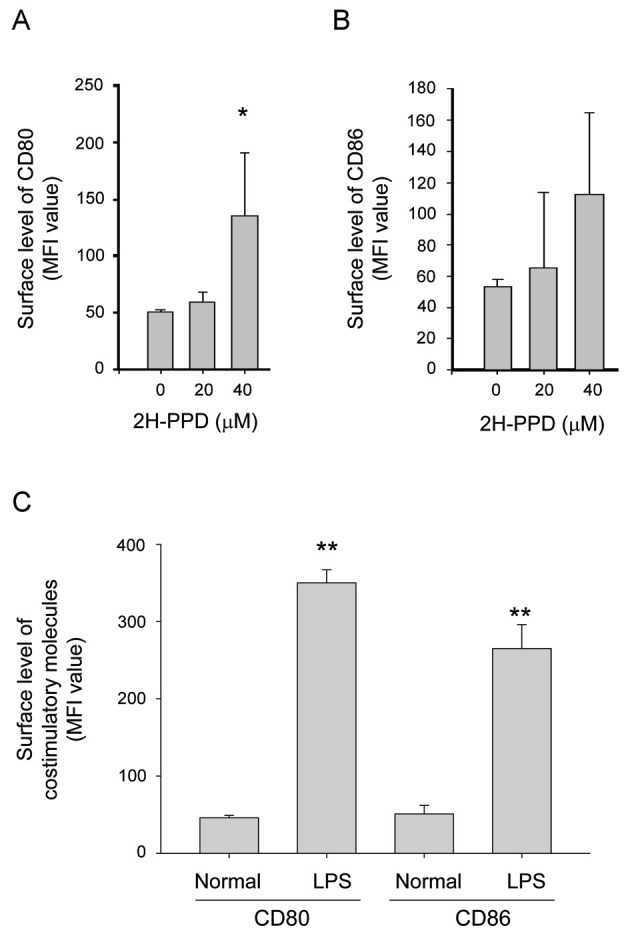
Moreover, this compound enhanced the release of SNP-derived radicals (Fig. 3), suggesting a putative role of indirect anti-bacterial activity via toxic molecule generation, as has been reported with other radical generating compounds [25]. In addition, 2H-PPD also stimulated an increase in surface levels of costimulatory molecules such as CD80 (Fig. 4A) and CD86 (Fig. 4B), essential molecules giving signal 2 for boosting the interactions of MHC class II/T cell receptor [26] in RAW264.7 cells. Although LPS displayed higher induction activity of costimulatory molecules (Fig. 4C) as reported previously [26,27], these results indicate that macrophages can be functionally stimulated by 2H-PPD treatment.
Fig. 3. Effect of 20S-dihydroprotopanaxadiol (2H-PPD) on reactive oxygen species (ROS) generation in sodium nitroprusside (SNP)-treated RAW264.7 cells. RAW264.7 cells pre-incubated with 2H-PPD were treated with dihydrorhodamine 123 (DHR123, 20 μM) in the presence or absence of SNP (0.25 mM) for 2 h. The level of radicals was determined by flow cytometric analysis as described in Materials and Methods. MFI, mean fluorescence intensity.
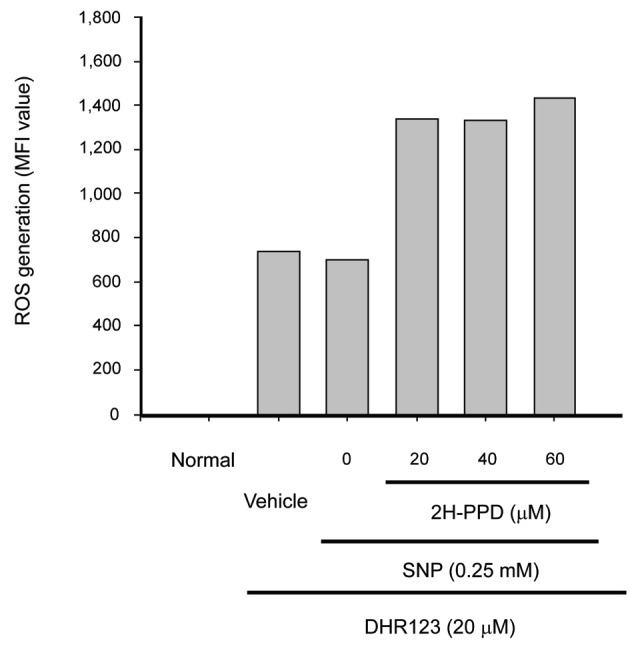
Fig. 4. Effect of 20S-dihydroprotopanaxadiol (2H-PPD) on the expression of costimulatory molecules in RAW264.7 cells. (A-C) RAW264.7 cells (2×106 cells/mL) were incubated with 2H-PPD or lipopolysaccharide (LPS) for indicated time. After 12 h, the surface levels of CD80 and CD86 in RAW264.7 cells were determined by flow cytometry. MFI, mean fluorescence intensity. *p<0.05 and **p<0.01 compared with normal or control.
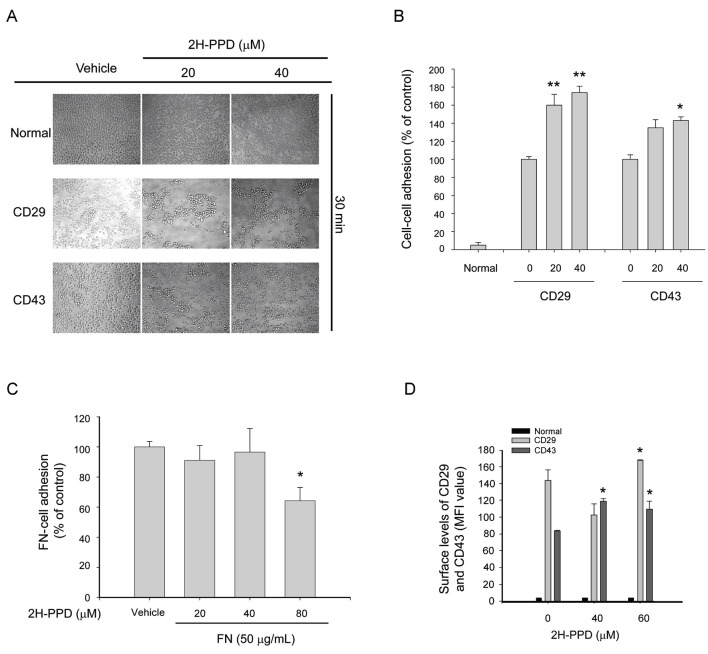
To remove infected pathogens, monocytes/macrophages must migrate into inflamed tissue. For this process, the functional activation of adhesion molecules is essential [28]. Representative adhesion molecules of macrophages/monocytes are CD29, CD98, CD147, and CD43 [4,5]. Therefore, whether 2H-PPD can modulate the adhesion events mediated by CD29 and CD43 was examined using function-activating antibodies against CD29 and CD43. Interestingly, this compound boosted cluster formation between U937 cells (Fig. 5A, B), while it blocked the adhesion of U937 cell to the extracellular matrix protein fibronectin (Fig. 5C), which is mediated by β1-integrins [5]. Furthermore, at 60 μM, 2H-PPD significantly increased surface levels of CD29 and CD43 (Fig. 5D), suggesting that this compound has a predominant role in accelerating CD29- and CD43-mediated cell-cell interactions, but not cell-tissue interactions.
Fig. 5. Effect of 20S-dihydroprotopanaxadiol (2H-PPD) on U937 cell adhesion. (A,B) U937 cells (1×106 cells/ml) pretreated with 2H-PPD were incubated in the presence or absence of pro-aggregative antibodies (1 mg/mL each) to CD43 (161-46) and CD29 (MEM-101A) for 30 min. Images of the cells in culture were obtained using an inverted phase contrast microscope attached to a video camera. Quantitative evaluation of U937 cell-cell clusters was performed by a quantitative cell-cell aggregation assay. (C) Effects of 2H-PPD on cell-fibronectin (FN) adhesion was examined with U937 cells pretreated with 2H-PPD by seeding on FN (50 μg/mL)-coated plates for 3 h. Attached cells were visualized by crystal violet assay, as described in Materials and Methods section. (D) The surface levels of CD29 and CD43 in U937 cells for 6 h were determined by flow cytometry. Data represent mean±SEM of three independent observations performed in triplicate. MFI, mean fluorescence intensity. *p<0.05 and **p<0.01 compared to control.
The innate immune functions of macrophages, such as phagocytosis, migration, and adhesion to other cells, require changes in membrane structures via actin cytoskeleton rearrangement [29,30]. As such, inhibition of changes in the actin cytoskeleton by actin cytoskeleton disruptors such as cytochalasin B has been found to diminish phagocytic uptakes, morphological changes, and cell adhesion [31,32]. Recently, we reported that ginsenoside Rp1, a ginsenoside derivative, is able to directly control the activation of the actin cytoskeleton regulatory protein, vasodilator-stimulated phosphoprotein [33]. Whether 2H-PPD is also able to regulate the actin cytoskeleton machinery in macrophages/monocytes has not yet been determined. Since phagocytosis and cell-cell adhesion are regulated by actin cytoskeleton changes [4,34,35], the mechanism by which 2H-PPD might control these events in terms of actin cytoskeleton rearrangement will be further evaluated in future studies.
In conclusion, we found that 2H-PPD can boost the cellular functions of macrophages/monocytes such as phagocytic uptake, ROS generation, expression of costimulatory molecules, and cell-cell adhesion. Since these are important events in the innate immune responses mediated by macrophages/monocytes, our results strongly suggest that 2H-PPD might serve as a novel immunomodulator targeted to the functional activation of macrophages and monocytes in order to enhance innate immunity. Related mechanistic studies between cellular responses and in vivo innate immunity conditions will be carried out in future studies.
References
- 1.Lee YG, Lee J, Byeon SE, Yoo DS, Kim MH, Lee SY, Cho JY. Functional role of Akt in macrophage-mediated innate immunity. Front Biosci. 2011;16:517–530. doi: 10.2741/3702. [DOI] [PubMed] [Google Scholar]
- 2.Yu T, Yi YS, Yang Y, Oh J, Jeong D, Cho JY. The pivotal role of TBK1 in inflammatory responses mediated by macrophages. Mediators Inflamm. 2012;2012:979105. doi: 10.1155/2012/979105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byeon SE, Yi YS, Oh J, Yoo BC, Hong S, Cho JY. The role of Src kinase in macrophage-mediated inflammatory responses. Mediators Inflamm. 2012;2012:512926. doi: 10.1155/2012/512926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho JY, Fox DA, Horejsi V, Sagawa K, Skubitz KM, Katz DR, Chain B. The functional interactions between CD98, beta1-integrins, and CD147 in the induction of U937 homotypic aggregation. Blood. 2001;98:374–382. doi: 10.1182/blood.V98.2.374. [DOI] [PubMed] [Google Scholar]
- 5.Lee YG, Lee J, Cho JY. Cell-permeable ceramides act as novel regulators of U937 cell-cell adhesion mediated by CD29, CD98, and CD147. Immunobiology. 2010;215:294–303. doi: 10.1016/j.imbio.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Jeannin P, Duluc D, Delneste Y. IL-6 and leukemia-inhibitory factor are involved in the generation of tumor-associated macrophage: regulation by IFN-γ. Immunotherapy. 2011;3(4 Suppl):23–26. doi: 10.2217/imt.11.30. [DOI] [PubMed] [Google Scholar]
- 7.Hasani-Ranjbar S, Nayebi N, Larijani B, Abdollahi M. A systematic review of the efficacy and safety of herbal medicines used in the treatment of obesity. World J Gastroenterol. 2009;15:3073–3085. doi: 10.3748/wjg.15.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim DH. Chemical diversity of Panax ginseng, Panax quinquifolium, and Panax notoginseng. J Ginseng Res. 2012;36:1–15. doi: 10.5142/jgr.2012.36.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hasegawa H. Proof of the mysterious efficacy of ginseng: basic and clinical trials: metabolic activation of ginsenoside: deglycosylation by intestinal bacteria and esterification with fatty acid. J Pharmacol Sci. 2004;95:153–157. doi: 10.1254/jphs.FMJ04001X4. [DOI] [PubMed] [Google Scholar]
- 10.Byeon SE, Lee J, Kim JH, Yang WS, Kwak YS, Kim SY, Choung ES, Rhee MH, Cho JY. Molecular mechanism of macrophage activation by red ginseng acidic polysaccharide from Korean red ginseng. Mediators Inflamm. 2012;2012:732860. doi: 10.1155/2012/732860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yayeh T, Jung KH, Jeong HY, Park JH, Song YB, Kwak YS, Kang HS, Cho JY, Oh JW, Kim SK, et al. Korean red ginseng saponin fraction downregulates proinflammatory mediators in LPS stimulated RAW264.7 cells and protects mice against endotoxic shock. J Ginseng Res. 2012;36:263–269. doi: 10.5142/jgr.2012.36.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wakabayashi C, Murakami K, Hasegawa H, Murata J, Saiki I. An intestinal bacterial metabolite of ginseng protopanaxadiol saponins has the ability to induce apoptosis in tumor cells. Biochem Biophys Res Commun. 1998;246:725–730. doi: 10.1006/bbrc.1998.8690. [DOI] [PubMed] [Google Scholar]
- 13.Choo MK, Sakurai H, Kim DH, Saiki I. A ginseng saponin metabolite suppresses tumor necrosis factor-alpha-promoted metastasis by suppressing nuclear factor-kappaB signaling in murine colon cancer cells. Oncol Rep. 2008;19:595–600. [PubMed] [Google Scholar]
- 14.Kim YS, Yoo MH, Lee GW, Choi JG, Kim KR, Oh DK. Ginsenoside F1 production from ginsenoside Rg1 by a purified β-glucosidase from Fusarium moniliforme var. subglutinans. Biotechnol Lett. 2011;33:2457–2461. doi: 10.1007/s10529-011-0719-0. [DOI] [PubMed] [Google Scholar]
- 15.Chen G, Yang X, Nong S, Yang M, Xu B, Zhang W. Two novel hydroperoxylated products of 20(S)-protopanaxadiol produced by Mucor racemosus and their cytotoxic activities against human prostate cancer cells. Biotechnol Lett. 2013;35:439–443. doi: 10.1007/s10529-012-1098-x. [DOI] [PubMed] [Google Scholar]
- 16.Zhu GY, Li YW, Tse AK, Hau DK, Leung CH, Yu ZL, Fong WF. 20(S)-protopanaxadiol, a metabolite of ginsenosides, induced cell apoptosis through endoplasmic reticulum stress in human hepatocarcinoma HepG2 cells. Eur J Pharmacol. 2011;668:88–98. doi: 10.1016/j.ejphar.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Lee SH, Seo GS, Ko G, Kim JB, Sohn DH. Anti-inflammatory activity of 20(S)-protopanaxadiol: enhanced heme oxygenase 1 expression in RAW 264.7 cells. Planta Med. 2005;71:1167–1170. doi: 10.1055/s-2005-873147. [DOI] [PubMed] [Google Scholar]
- 18.Cho JY, Kim AR, Joo HG, Kim BH, Rhee MH, Yoo ES, Katz DR, Chain BM, Jung JH. Cynaropicrin, a sesquiterpene lactone, as a new strong regulator of CD29 and CD98 functions. Biochem Biophys Res Commun. 2004;313:954–961. doi: 10.1016/j.bbrc.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 19.Kim BH, Lee YG, Lee J, Lee JY, Cho JY. Regulatory effect of cinnamaldehyde on monocyte/macrophage-mediated inflammatory responses. Mediators Inflamm. 2010;2010:529359. doi: 10.1155/2010/529359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duperrier K, Eljaafari A, Dezutter-Dambuyant C, Bardin C, Jacquet C, Yoneda K, Schmitt D, Gebuhrer L, Rigal D. Distinct subsets of dendritic cells resembling dermal DCs can be generated in vitro from monocytes, in the presence of different serum supplements. J Immunol Methods. 2000;238:119–131. doi: 10.1016/S0022-1759(00)00147-2. [DOI] [PubMed] [Google Scholar]
- 21.Park PH, Hur J, Lee DS, Kim CH, Jeong GS, Sohn DH. Inhibition of nitric oxide production by ethyl digallates isolated from Galla Rhois in RAW 264.7 macrophages. Biomol Ther. 2011;19:419–424. doi: 10.4062/biomolther.2011.19.4.419. [DOI] [Google Scholar]
- 22.Cho JY, Skubitz KM, Katz DR, Chain BM. CD98-dependent homotypic aggregation is associated with translocation of protein kinase Cdelta and activation of mitogen-activated protein kinases. Exp Cell Res. 2003;286:1–11. doi: 10.1016/S0014-4827(03)00106-X. [DOI] [PubMed] [Google Scholar]
- 23.Larrucea S, Gonzalez-Rubio C, Cambronero R, Ballou B, Bonay P, Lopez-Granados E, Bouvet P, Fontan G, Fresno M, Lopez-Trascasa M. Cellular adhesion mediated by factor J, a complement inhibitor. Evidence for nucleolin involvement. J Biol Chem. 1998;273:31718–31725. doi: 10.1074/jbc.273.48.31718. [DOI] [PubMed] [Google Scholar]
- 24.Vo VA, Lee JW, Chang JE, Kim JY, Kim NH, Lee HJ, Kim SS, Chun W, Kwon YS. Avicularin inhibits lipopolysaccharide-induced inflammatory response by suppressing ERK phosphorylation in RAW 264.7 macrophages. Biomol Ther. 2012;20:532–537. doi: 10.4062/biomolther.2012.20.6.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okai Y, Ishizaka S. A possible immunomodulating activity of arbekacin (ABK), a newly synthesized antibiotic against methicillin-resistant Staphylococcus aureus (MRSA). Int J Immunopharmacol. 1994;16:321–327. doi: 10.1016/0192-0561(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 26.Lee YG, Byeon SE, Kim JY, Lee JY, Rhee MH, Hong S, Wu JC, Lee HS, Kim MJ, Cho DH, et al. Immunomodulatory effect of Hibiscus cannabinus extract on macrophage functions. J Ethnopharmacol. 2007;113:62–71. doi: 10.1016/j.jep.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 27.Lee YG, Kim JY, Lee JY, Byeon SE, Hong EK, Lee J, Rhee MH, Park HJ, Cho JY. Regulatory effects of Codonopsis lanceolata on macrophage-mediated immune responses. J Ethnopharmacol. 2007;112:180–188. doi: 10.1016/j.jep.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 28.Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol. 2009;78:539–552. doi: 10.1016/j.bcp.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mork T, Hancock RE. Mechanisms of nonopsonic phagocytosis of Pseudomonas aeruginosa. Infect Immun. 1993;61:3287–3293. doi: 10.1128/iai.61.8.3287-3293.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamaguchi H, Haranaga S, Widen R, Friedman H, Yamamoto Y. Chlamydia pneumoniae infection induces differentiation of monocytes into macrophages. Infect Immun. 2002;70:2392–2398. doi: 10.1128/IAI.70.5.2392-2398.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Syme RM, Spurrell JC, Ma LL, Green FH, Mody CH. Phagocytosis and protein processing are required for presentation of Cryptococcus neoformans mitogen to T lymphocytes. Infect Immun. 2000;68:6147–6153. doi: 10.1128/IAI.68.11.6147-6153.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim JY, Lee YG, Kim MY, Byeon SE, Rhee MH, Park J, Katz DR, Chain BM, Cho JY. Src-mediated regulation of inflammatory responses by actin polymerization. Biochem Pharmacol. 2010;79:431–443. doi: 10.1016/j.bcp.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 33.Kim BH, Cho JY. Regulatory role of ginsenoside Rp1, a novel ginsenoside derivative, on CD29-mediated cell adhesion. Planta Med. 2009;75:316–320. doi: 10.1055/s-0028-1112213. [DOI] [PubMed] [Google Scholar]
- 34.Cho JY, Katz DR, Chain BM. Staurosporine induces rapid homotypic intercellular adhesion of U937 cells via multiple kinase activation. Br J Pharmacol. 2003;140:269–276. doi: 10.1038/sj.bjp.0705436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cho JY, Chain BM, Vives J, Horejsi V, Katz DR. Regulation of CD43-induced U937 homotypic aggregation. Exp Cell Res. 2003;290:155–167. doi: 10.1016/S0014-4827(03)00322-7. [DOI] [PubMed] [Google Scholar]