Abstract
In this study, gamma rays were used to irradiate embryogenic calli induced from cotyledon explants of Panax ginseng Meyer. After the embryogenic calli were irradiated, they were transferred to adventitious roots using an induction medium; next, mutated adventitious root (MAR) lines with a high frequency of adventitious root formations were selected. Two MAR lines (MAR 5-2 and MAR 5-9) from the calli treated with 50 Gy of gamma rays were cultured on an NH4NO3-free Murashige and Skoog medium with indole-3-butyric acid 3 mg/L. The expression of genes related to ginsenoside biosynthesis was analyzed using reverse transcription polymerase chain reaction with RNA prepared from native ginseng (NG), non-irradiated adventitious root (NAR) and 2 MAR lines. The expression of the squalene epoxidase and dammarenediol synthase genes was increased in the MAR 5-2 line, whereas the phytosterol synthase was increased in the MAR 5-9 line. The content and pattern of major ginsenosides (Rb1, Rb2, Rc, Rd, Re, Rf, and Rg1) were analyzed in the NG, NAR, and 2 MAR lines (MAR 5-2 and MAR 5-9) using TLC and HPLC. In the TLC analysis, the ginsenoside patterns in the NG, NAR, and 2 MAR lines were similar; in contrast, the MAR 5-9 line showed strong bands of primary ginsenosides. In the HPLC analysis, compared with the NG, one new type of ginsenoside was observed in the NAR and 2 MAR lines, and another new type of ginsenoside was observed in the 2 MAR lines irradiated with gamma rays. The ginsenoside content of the MAR 5-9 line was significantly greater in comparison to the NG.
Keywords: Panax ginseng, Gamma irradiation, Ginsenoside, HPLC, TLC
INTRODUCTION
Panax ginseng Meyer is a perennial herb that belongs to the family Araliceae. Ginseng root is a well-known oriental herb that has been widely used for health food and traditional medicine since ancient times. Various pharmacological effects of ginseng have been reported, such as improved immunity, stamina, health and enhanced resistance to stress [1-4]. Recent research has confirmed the medicinal properties and pharmacological potential of the ginseng root [5-8], thereby increasing global interest in its use. The primary pharmacologically active ingredients in ginseng are ginsenosides, which are triterpene saponins [9]. Ginsenosides are a secondary metabolite of ginseng and are classified into two groups based on their aglycone structure, namely, the dammarane and oleanane types. The most common ginsenoside is the dammarane type, which can be divided into Rb (Rb1, Rb2, Rc, and Rd) and Rg (Re, Rf, and Rg1) groups by the difference in the 20(S)-protopanaxadiol and 20(S)- protopanaxatriol structure, respectively [10-12]. The oleanane-type ginsenosides are the only R0 in which the fundamental skeletons are pentacyclic. More than 30 kinds of ginsenosides, such as Rg2, Rf1 and Rh2, have been isolated and studied [6,7].
Although ginseng has many beneficial pharmacological properties, native ginseng takes a long time to reach maturity (4 to 6 years), cultivation is risky, and ginsenoside production is minimal. Therefore, mass production of ginseng is needed in order to meet demand. As a result of these challenges, many reports have been published concerning ginsenoside production using transformed roots, adding biotic or abiotic elicitors in cell cultures, and producing large-scale cultures with bioreactors [13-16]. Tissue culture is particularly important for the mass production of ginseng, and the quality of culture is highly dependent on the culture media and hormone composition. In numerous other studies, ginseng culture techniques, such as callus induction, cell suspension, adventitious and hairy root induction, and optical culture conditions, such as the addition of elicitors and hormones for mass production, have been evaluated [17-20].
The term ‘somaclonal variation’ characterizes the process of induced genetic variation by in vitro culture. Somaclonal variations are attributable to point mutation, chromosomal rearrangements, and DNA methylation [21,22]. Somaclonal mutants have provided valuable genetic resources for plant breeding. However, although it is possible to select mutants by somaclonal variation, mutant frequencies are often low compared with artificial mutagenesis employing such methods as ionizing radiation and chemical treatments [23].
Ionizing radiation can affect plants in several ways, such as by damaging DNA, altering bases and sugars, forming DNA-DNA and DNA-protein crosslinks, and by causing single strand breaks and double-strand breaks [24,25]. Numerous researchers have recently performed mutation breeding by ionizing radiation. Kim et al. [26] reported that high levels of amino acids were obtained from mutant rice lines. The transcript levels of the putative OASA2 mutant gene in these mutant lines were higher than in the control. Also, according to Lee et al. [27], the radiation-induced gene mutations within the anthocyanin pathway are associated with variations in the color of chrysanthemum flowers. In addition, new and improved varieties of many crops, such as cocoa, potato, banana, and sugarcane, have been developed [28-31].
In this study, we induced adventitious roots from the cotyledon of ginseng that had been mutagenized with gamma rays. Next, we selected mutant lines showing high-growth performance. We analyzed the expression patterns of genes related to triterpene biosynthesis in the mutated adventitious root (MAR) lines compared with the native ginseng (NG) and non-irradiated adventitious root (NAR) lines. In addition, we profiled and qualified ginsenosides using TLC and HPLC systems.
MATERIALS AND METHODS
Plant materials
Callus cell cultures were induced on a Murashige and Skoog (MS) medium (pH 5.8); the cultures were subsequently supplemented with 1 mg/L of 2,4-dichlorophenoxyacetic acid (2,4-D) and 0.1 mg/L of kinetin at 25℃ in a dark environment. The calli of P. ginseng were irradiated with 50 Gy of a cobalt-60 gamma-irradiator (150 TBq of capacity; ACEL, Ottawa, ON, Canada) at the Korea Atomic Energy Research Institute.
Adventitious roots were formed on a solid NH4NO3-free MS medium and supplemented with 3 mg/L of inodole-3-butyric acid (IBA) at 23℃ in a dark environment [32]. The fresh mass of adventitious roots was measured after 4 wk. The liquid culture was maintained in an NH4NO3-free MS medium supplemented with 3 mg/L of IBA on a gyratory shaker (100 rpm) under the same conditions.
Reverse transcription polymerase chain reaction analysis
After 4 wk of culture, the total RNA from 0.1 g of P. ginseng hairy roots was isolated using an easy-spin Plant RNA Extraction Kit (Intron Biotechnology, Seoul, Korea). For reverse transcription, 1 μg of RNA was subjected to cDNA synthesis in 10 μL of reaction mixture. Reverse transcription (RT) reactions were performed using 0.25 U of avian myeloblastosis virus (AMV) reverse transcriptase in 1 U of RNase inhibitor, 1 mM dNTP, 5 mM MgCl2, 1×RT buffer, and oligo (dT) 15 primers at 42℃ for 60 min. The reaction was terminated by heating the mixture at 70℃ for 10 min followed by cooling at 4℃. The resulting cDNA served as a template for subsequent polymerase chain reaction (PCR) amplification using primers for the ginsenoside synthesis genes (Table 1).
Table 1.
The primers of ginsenoside synthetic genes for the reverse transcription polymerase chain reaction analysis
| Oligo name | Sequence | Size (bp) |
|---|---|---|
|
| ||
| PgSE (1) | F: ATGGGAAGTTTGGGGGCAATTCT | 1,252 |
| R: GTTCTCACTGTTTGTTCAGTAGTAGGTT | ||
| PgSE (2) | F: AGCAGCAGTTGACAAAGG | 506 |
| R: GCCACATTCGTTTTGGTGAAGG | ||
| PNX (3) | F: TCATCAGATGGCTCATGGTACG | 361 |
| R: TCTCCTCCTGTGGGAAATCACC | ||
| PNY (4) | F: TATCCTGGACACCGAAAGAAGG | 445 |
| R: CTCCACTTATTTCCTGTTGGGG | ||
| PNY2 (5) | F: TGGATTTTCTAATAAAATCGCAACGCAG | 425 |
| R: TTTCATTTGAGTATTGGCAGGCCG | ||
| PgDDS (6) | F: ATGTGGAAGCTGAAGGTTGCTCAAGGA | 2,310 |
| R: TTAAATTTTGAGCTGCTGGTGCTTAGGC | ||
PgSE, squalene epoxidase; F, forward; R, reverse; PNX, cycloartenol synthase; PNY, oxidosqualene cyclase; PNY2, oxidosqualene cyclase 2; PgDDS, dammarenediol synthase.
The PCR amplification conditions involved 3 mM at 94℃ for the initial denaturation followed by 25 cycles of the following: 30 s at 94℃, 30 s each at 58℃, and 30 s at 72℃ followed by 5 min at 72℃ for the final extension. The sizes of the PCR products were determined using electrophoresis in an agarose gel (1.2%).
Extraction of ginsenosides
The extraction of ginsenosides was performed using the methods described in Woo et al. [15]. Milled powder (0.5 g) from freeze-dried adventitious roots was soaked in 80% MeOH at 40℃. After the liquid evaporated, the residue was dissolved in H2O and washed with (C2H5)2O. Then, the residue was extracted with H2O-saturated n-butanol and washed twice with H2O. The n-butanol layer was subsequently evaporated to produce the ginsenoside fraction. Next, each sample was dissolved in MeOH (HPLC-grade) and filtered once through a 0.2-μm nylon filter.
TLC identification of individual ginsenosides
The ginsenoside fraction was analyzed using a TLC system. The TLC analysis was conducted using automated multiple development (AMD; Camag, Muttenz, Switzerland). The ginseng extracts (25 mg) in methanol were sprayed onto a TLC plate (silica gel, 10 cm×20 cm). The mobile phase was conducted using a solvent system (chloroform:methanol:water=65:35:10) and a migration distance of 80 mm. The ginsenosides were subsequently imaged with brown bands by applying 10% H2SO4 and heating at 120℃.
HPLC analysis
The HPLC conditions were based on those described in Court et al. [33], which provided satisfactory resolution of the seven major ginsenosides, Rb1, Rb2, Rc, Rd, Re, Rf, and Rg1. The ginsenoside separation was conducted on a Zorbax 300SB-C18 analytical fractions column (particle size 5 μm, 4.6 mm×150 mm; Agilent, Santa Clara, CA, USA) using the following gradient system: 0 to 10 min, 10% acetonitrile and 90% distilled water (DW); 10 to 45 min, 50% acetonitrile and 50% DW; 45 to 55 min, and 90% acetonitrile and 10% DW. The flow rate was 1 mL/min, and the ginsenosides were monitored at 203 nm. The sample injection quantity was 15 μL, and the temperature of the column was sustained at 30℃. The ginsenoside peaks were monitored, with the peak areas corresponding to samples matching authentic ginsenoside standards (Rb1, Rb2, Rc, Rd, Re, Rf, and Rg1) purchased from ChromaDex (Santa Anna, CA, USA). The data were compared with an external standard calibration curve.
RESULTS AND DISCUSSION
Selection of mutated adventitious root lines
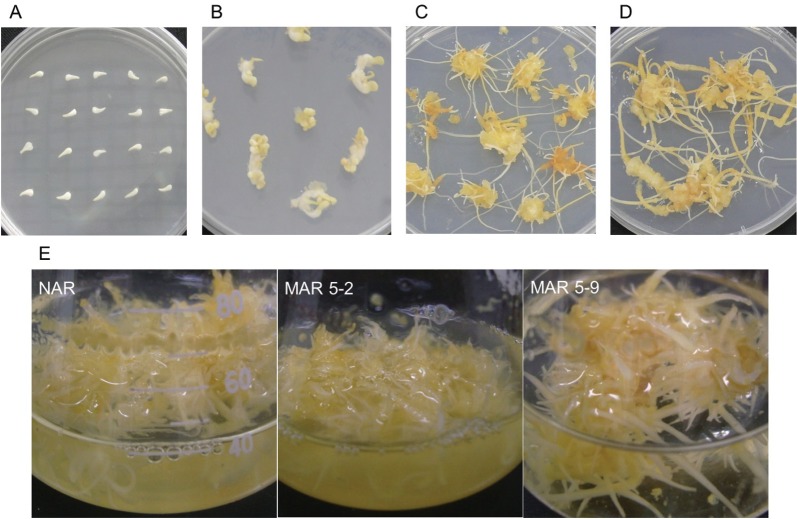
To obtain the MAR lines, the ginseng seed was germinated on a MS media supplemented with 1 mg/L 2,4-D and 0.1 mg/L Kinetin. An amorphous embryogenic callus was induced from ginseng cotyledons and was subcultured on the same medium (Fig. 1A, B). The embryogenic callus was irradiated with 50 Gy of gamma rays (from a 60Co source). After irradiation, the embryogenic callus was transferred to an adventitious root induction solid from an NH4NO3-free medium supplemented with IBA 3 mg/L; next, mutated adventitious root lines showing high growth performance were selected (Fig. 1C, D).
Fig. 1. Adventitious root production from gamma irradiated mutant lines: (A) ginseng seed was germinated by in vitro culture, (B) amorphous embryogenic calli were induced from ginseng cotyledons, (C) embryogenic callus formation on the callus induction medium, (D) selected high frequency of adventitious root formation, (E) proliferation of the adventitious root in the liquid adventitious root induction medium. NAR, non-irradiated adventitious root; MAR, mutated adventitious root (5-line).
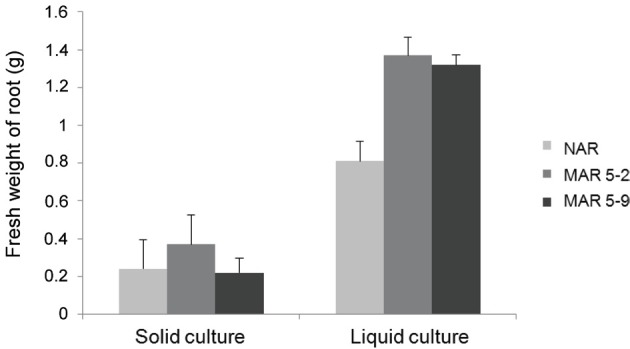
In our previous report, the highest frequency of adventurous root formation was observed on the NH4NO3-free MS medium with IBA 3 mg/L when compared with the medium from hormones 2,4-D and naphthalene acetic acid [34,35]. The fresh mass was confirmed to be greater from a 50 Gy treatment compared with the fresh mass resulting from the non-irradiated callus [32]. We selected 2 MAR lines (MAR 5-2 and MAR 5-9) from the calli treated with 50 Gy of gamma rays. These lines were propagated on the NH4NO3-free MS medium with IBA 3 mg/L (Fig. 1E). The fresh mass of the 2 MAR lines (MAR 5-2 and MAR 5-9) was compared with the NAR line after the liquid and solid cultures were incubated for 4 wk. The fresh weight of the MAR 5-9 line was similar to that of the NAR line, whereas the MAR 5-2 line was greater than the NAR line in the solid culture. In contrast, the growth of the 2 MAR lines was much greater than that of the NAR line in the liquid culture (Fig. 2).
Fig. 2. The fresh mass of adventitious roots of the non-irradiated adventitious root (NAR) and 2 mutated adventitious root (MAR) lines after 4 wk.
Tissue culture techniques influence the growth of plant mass production. Also, there can be various outcomes from mutation breeding by irradiation. Each spectrum of γ-irradiation has a different effect on plant growth [29,32].
Expression of ginsenoside biosynthesis
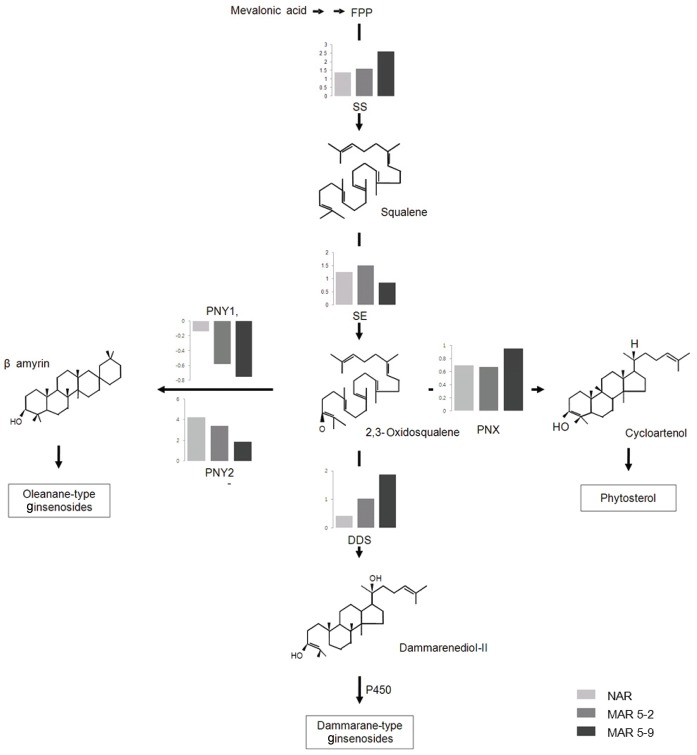
Ginsenosides, which are glycosylated triterpenes and triterpenes, are biosynthesized by the mevalonate pathway in the cytosol (Fig. 3). The expression of genes related to ginsenoside biosynthesis (Table 1) was examined by RT-PCR analysis with RNA prepared from a 6-year-old native ginseng plant (naturally grown ginseng, NG) and from an NAR and 2 MAR lines (Figs. 3 and 4).
Fig. 3. The biosynthetic pathway of ginsenoside in Panax ginseng. FPP, farnesyl diphosphate, SS, squalene synthase; SE, squalene epoxidase; PNX, cycloartenol synthase; PNY1, oxidosqualene cyclase 1; PNY2, oxidosqualene cyclase 2; DDS, dammarenediol synthase; NAR, non-irradiated adventitious root; MAR, mutated adventitious root.
Fig. 4. The expression of genes related to ginsenoside biosynthesis in the mutated adventitious root (MAR). NG, native ginseng; NAR, non-irradiated adventitious root; SS, squalene synthase; SE, squalene epoxidase; PNX, cycloartenol synthase; PNY1, oxidosqualene cyclase 1; PNY2, oxidosqualene cyclase 2; DDS, dammarenediol synthase.
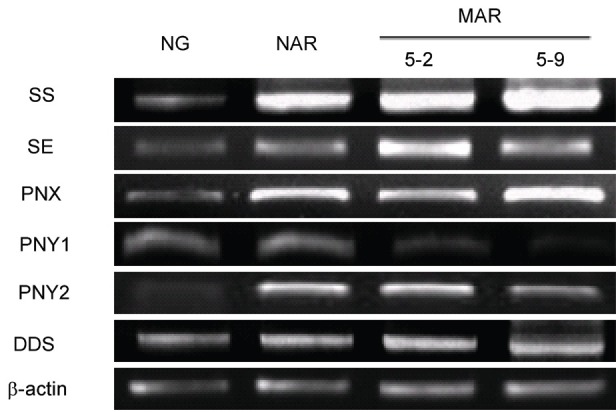
The transcription levels of PgSE and PgSE genes were increased in the NAR and 2 MAR lines compared with the NG line. The PgSE gene was highly expressive in the MAR 5-9 line, whereas the PgSE gene was highly expressive in the MAR 5-2 line. The PgSE and PgSE genes catalyzed the first step at creating ginsenoside biosynthesis pathways that regulate the production of phytosterols and triterpenoids in ginseng [36,37]. Three types of ginsenosides were synthesized from oxidosqualene, by cylization of oxidosqualene cyclases (OSC) genes: dammarenediol synthase (DDS), phytosterol synthase (PNX), and oleanane-type synthase (PNY1, PNY2). The expression of OSC genes, except for PNY1, was increased, whereas the expression of PNY1 was decreased slightly compared with the NG. The PNX and DDS genes were the most highly expressive in the MAR 5-9 line. The OSC genes are involved in the biosynthesis of phytosterol synthase, oleanane-type synthase, and dammarenediol synthase [38,39]. The DDS gene was involved in the synthesis of ginsenoside, indicating that the DDS gene was highly expressive in the MAR 5-9 line [37]. These results indicate that the ginsenosides are more expressive in the MAR 5-9 line than the other samples.
Ginsenoside analysis by TLC and HPLC
Quantitative and qualitative variation of the seven major ginsenosides of the Rb1, Rb2, Rc, Rd, Re, Rf, and Rg types have been compared with the NG, NAR, and 2 MAR lines. To analyze the ginsenoside pattern, the extract of the NG, NAR, and 2 MAR lines were loaded on preparative silica gel TLC plates (Fig. 5A). The TLC revealed that the ginsenoside pattern was similar in the NG, NAR, and 2 MAR lines, although the bands of the NAR line and the MAR 5-2 line were smears. In contrast, the MAR 5-9 line was strongly represented in most of the bands compared with the NG. Also, the MAR 5-9 showed a new band under the Rd (Fig. 5A).
Fig. 5. A ginsenoside analysis using TLC and HPLC. (A) TLC analysis of ginsenosides. The star indicates a new type of ginsenoside. (B) Ginsenoside contents in the native ginseng (NG), non-irradiated adventitious root (NAR) and 2 mutated adventitious root (MAR) lines. Black arrow was a new band.
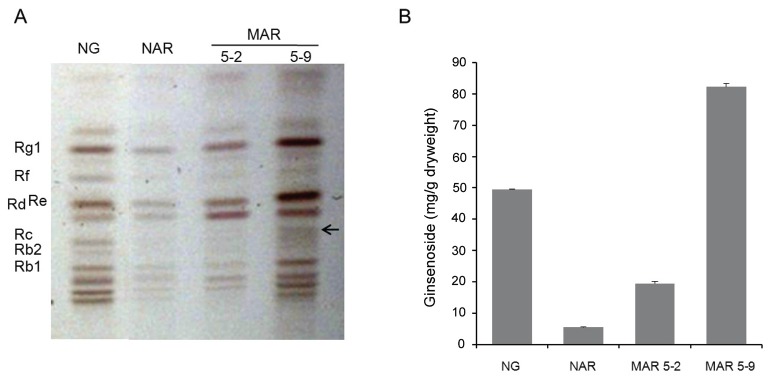
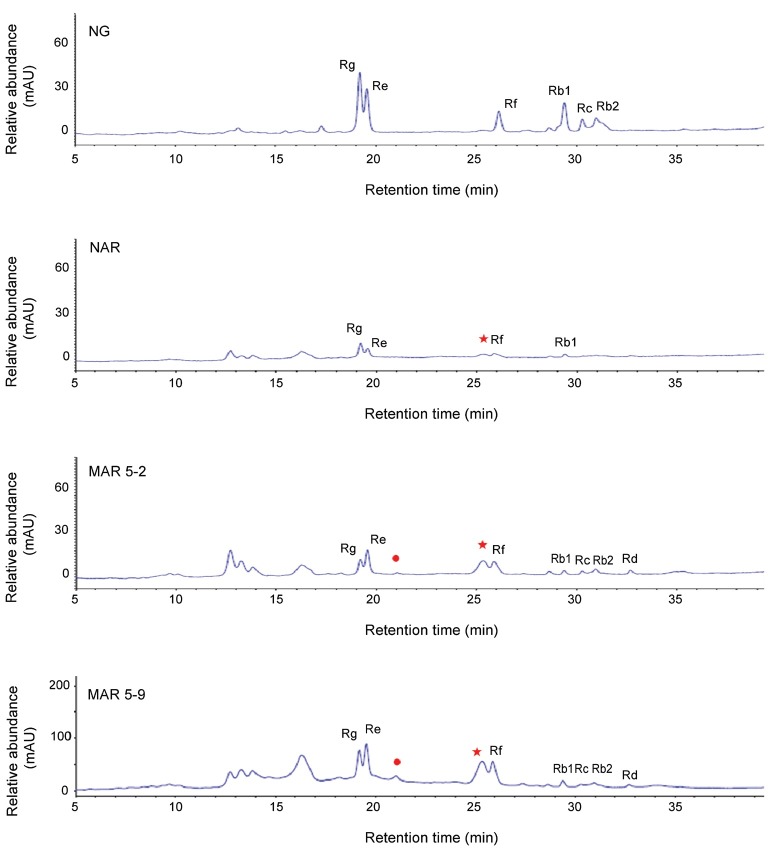
The ginsenoside content was determined from the roots of the NG, NAR, and 2 MAR lines. Compared with the NG, the amount of 7 ginsenosides of the MAR 5-9 line was 1.7-fold greater (Fig. 5B). The peaks were determined based on the retention times of the primary ginsenoside standard (Fig. 6). Among the 2 MAR lines, the MAR 5-9 line showed 4 times the ginsenoside content. The HPLC chromatogram revealed that the pattern of HPLC peaks was similar in the NG, NAR, and 2 MAR lines, although there were remarkable quantitative differences in each sample. The MAR 5-9 line showed greater quantities overall compared with the other samples. More ginsenosides were detected form the NG than form the NAR and MAR -5-2 lines.; however, the Rd ginsenoside from the NG line and the Rb2, Rc and Rd ginsenosides from the NAR line were undetectable in the HPLC. The MAR 5-9 line and the NG line were compared: relative to the NG, the Rb1 and Rc ginsenosides of the protopanxadiol group decreased in 2-fold, whereas Re, Rf, and Rg1 ginsenosides of the protopanaxatirol group increased in 2.5-fold, respectively (Table 2).
Fig. 6. The HPLC chromatograms of ginseng extracts in the non-irradiated adventitious root (NAR) and the 2 mutated adventitious root (MAR) lines. Seven ginsenosides including the Rb1, Rb2, Rc, and Rd of the Rb groups and the Re, Rf and Rg1 of the Rg group, were marked at each peak area. The star indicates a new type ginsenoside. NG, native ginseng.
Table 2.
Contents of ginsenosides in the NG, NAR, and 2 MAR lines
| Line | Protopanaxadiol | Protopanaxatriol | Sum | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Rb1 | Rb2 | Rc | Rd | Re | Rf | Rg1 | ||
|
| ||||||||
| NG | 8.81±0.04 | 14.01±0.06 | 4.15±0.01 | - | 9.11±0.05 | 4.14±0.01 | 9.29±0.01 | 49.50±0.02 |
| NAR | 0.77±0.02 | - | - | - | 1.71±0.00 | 0.80±0.03 | 2.20±0.01 | 5.48±0.06 |
| MAR 5-2 | 0.92±0.03 | 4.74±0.01 | 1.80±0.62 | 0.59±0.44 | 5.49±0.03 | 3.55±0.01 | 2.37±0.01 | 19.46±0.58 |
| MAR 5-9 | 4.05±0.02 | 15.64±0.05 | 2.79±0.03 | 1.74±1.31 | 26.08±0.13 | 16.48±0.05 | 15.43±0.05 | 82.22±1.12 |
NG, native ginseng; NAR, non-irradiated adventitious root; MAR, mutated adventitious root.
Furthermore, all of the ginsenoside content detected in the NAR and MAR 5-2 lines was low. In the previous report, the amount of 7 ginsenosides was higher in the NG callus culture [40,41]. In this paper, the ginsenoside content in the NG appeared to be greater than that of all of the samples, except for the MAR 5-2 lines. The enhanced accumulation of the ginsenoside content in the MAR 5-9 line suggests a significant effect of γ-irradiation on ginsenoside biosynthesis. The ginsenoside analysis of the NAR and 2 MAR lines showed different ginsenoside profiles compared to the NG. Also, the 2 MAR lines showed different ginsenoside patterns with those of the NAR (Fig. 6).
We suggested that the differentiation of ginsenoside content among NAR and 2 MAR lines was caused by differential expression of genes related to the ginsenoside biosynthesis. The synthetic genes of dammarane-type ginsenosides in 2 MAR lines showed higher expression level than NAR line, expect PgSE in MAR 5-9 (Fig. 3). Kim et al. [42] reported that the change of tryptophan synthesis key enzyme in mutant rice caused the change of tryptophan content. We seemed that the ginsenoside content in MAR 5-9 line was the highest than MAR 5-2 and NAR because of the highest level of PgDDS (Table 2 and Fig. 3).
The pattern and content of ginsenosides appeared differently in each γ-irradiated sample, suggesting that there was different enzyme activity involved in the ginsenoside pathways and culture conditions of each sample [10,34].
Acknowledgments
This work was conducted as part of project granted by the Korea Atomic Energy Research Institute.
References
- 1.Gillis CN. Panax ginseng pharmacology: a nitric oxide link? Biochem Pharmacol. 1997;54:1–8. doi: 10.1016/S0006-2952(97)00193-7. [DOI] [PubMed] [Google Scholar]
- 2.Keum YS, Park KK, Lee JM, Chun KS, Park JH, Lee SK, Kwon H, Surh YJ. Antioxidant and anti-tumor promoting activities of the methanol extract of heat-processed ginseng. Cancer Lett. 2000;150:41–48. doi: 10.1016/S0304-3835(99)00369-9. [DOI] [PubMed] [Google Scholar]
- 3.Sticher O. Getting to the root of ginseng. Chemtech. 1998;28:26–32. [Google Scholar]
- 4.Kim YK, Guo Q, Packer L. Free radical scavenging activity of red ginseng aqueous extracts. Toxicology. 2002;172:149–156. doi: 10.1016/S0300-483X(01)00585-6. [DOI] [PubMed] [Google Scholar]
- 5.Vogler BK, Pittler MH, Ernst E. The efficacy of ginseng: a systematic review of randomised clinical trials. Eur J Clin Pharmacol. 1999;55:567–575. doi: 10.1007/s002280050674. [DOI] [PubMed] [Google Scholar]
- 6.Shibata S. Chemistry and cancer preventing activities of ginseng saponins and some related triterpenoid compounds. J Korean Med Sci. 2001;16 Suppl:S28–S37. doi: 10.3346/jkms.2001.16.S.S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kiefer D, Pantuso T. Panax ginseng. Am Fam Physician. 2003;68:1539–1542. [PubMed] [Google Scholar]
- 8.Kalinowska M, Zimowski J, Paczkowski C, Wojciechowski ZA. The formation of sugar chains triterpenoid saponins and glycoalkaloids. Phytochem Rev. 2005;4:237–257. doi: 10.1007/s11101-005-1422-3. [DOI] [Google Scholar]
- 9.Shibata S, Tanaka O, Soma K, Ando T, Iida Y, Nakamura H. Studies on saponins and sapogenins of ginseng. The structure of panaxatriol. Tetrahedron Lett. 1965;42:207–213. doi: 10.1016/S0040-4039(01)99595-4. [DOI] [PubMed] [Google Scholar]
- 10.Palazon J, Cusido RM, Bonfill M, Mallol A, Moyano E, Morales C, Pinol MT. Elicitation of different Panax ginseng transformed root phenotypes for an improved ginsenoside production. Plant Physiol Biochem. 2003;41:1019–1025. doi: 10.1016/j.plaphy.2003.09.002. [DOI] [Google Scholar]
- 11.Kim MK, Lee BS, In JG, Sun H, Yoon JH, Yang DC. Comparative analysis of expressed sequence tags (ESTs) of ginseng leaf. Plant Cell Rep. 2006;25:599–606. doi: 10.1007/s00299-005-0095-0. [DOI] [PubMed] [Google Scholar]
- 12.Shi W, Wang Y, Li J, Zhang H, Ding L. Investigation of ginsenosides in different parts and ages of Panx ginseng. Food Chem. 2007;102:664–668. doi: 10.1016/j.foodchem.2006.05.053. [DOI] [Google Scholar]
- 13.Furuya Y, Yoshikawa T, Orihar Y, Oda H. Studies of the culture conditions for Panax ginseng cells in jar fermentors. J Nat Prod. 1984;47:70–75. doi: 10.1021/np50031a008. [DOI] [Google Scholar]
- 14.Yu KW, Gao W, Hahn EJ, Paek KY. Jasmonic acid improves ginsenoside accumulation in adventitious root culture of Panax ginseng C.A. Meyer. Biochem Eng J. 2002;11:211–215. doi: 10.1016/S1369-703X(02)00029-3. [DOI] [Google Scholar]
- 15.Woo SS, Song JS, Lee JY, In DS, Chung HJ, Liu JR, Choi DW. Selection of high ginsenoside producing ginseng hairy root lines using targeted metabolic analysis. Phytochemistry. 2004;65:2751–2761. doi: 10.1016/j.phytochem.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 16.Ali MB, Yu KW, Hahn EJ, Paek KY. Methyl jasmonate and salicylic acid elicitation induces ginsenosides accumulation, enzymatic and non-enzymatic antioxidant in suspension culture Panax ginseng roots in bioreactors. Plant Cell Rep. 2006;25:613–620. doi: 10.1007/s00299-005-0065-6. [DOI] [PubMed] [Google Scholar]
- 17.Asaka I, Li I, Hirotani M, Asada Y, Furuya T. Production of ginsenoside saponins by culturing ginseng (Panax ginseng) embryogenic tissues in bioreactors. Biotechnol Lett. 1993;15:1259–1264. doi: 10.1007/BF00130308. [DOI] [Google Scholar]
- 18.Choi SM, Son SH, Yun SR, Kwon OW, Seon JH, Paek KY. Pilot-scale culture of adventitious roots of ginseng in a bioreactor system. Plant Cell Tissue Organ Cult. 2000;62:187–193. doi: 10.1023/A:1006412203197. [DOI] [Google Scholar]
- 19.Sivakumar G, Yu KW, Hahn EJ, Paek KY. Optimization of organic nutrients for ginseng hairy roots production in large-scale bioreactors. Curr Sci. 2005;89:641–649. [Google Scholar]
- 20.Thanh NT, Murthy HN, Yu KW, Hahn EJ, Paek KY. Methyl jasmonate elicitation enhanced synthesis of ginsenoside by cell suspension cultures of Panax ginseng in 5-l balloon type bubble bioreactors. Appl Microbiol Biotechnol. 2005;67:197–201. doi: 10.1007/s00253-004-1759-3. [DOI] [PubMed] [Google Scholar]
- 21.Larkin PJ, Scowcroft WR. Somaclonal variation: a novel source of variability from cell cultures for plant improvement. Theor Appl Genet. 1981;60:197–214. doi: 10.1007/BF02342540. [DOI] [PubMed] [Google Scholar]
- 22.Kaeppler SM, Kaeppler HF, Rhee Y. Epigenetic aspects of somaclonal variation in plants. Plant Mol Biol. 2000;43:179–188. doi: 10.1023/A:1006423110134. [DOI] [PubMed] [Google Scholar]
- 23.Nakano M, Amano J, Watanabe Y, Nomizu T, Suzuki M, Mizunashi K, Mori S, Kuwayama S, Han DS, Saito H, et al. Morphological variation in Tricyrtis hirta plants regenerated from heavy ion beam-irradiated embryogenic calluses. Plant Biotechnol. 2010;27:155–160. doi: 10.5511/plantbiotechnology.27.155. [DOI] [Google Scholar]
- 24.Kovalchuk I, Molinier J, Yao Y, Arkhipov A, Kovalchuk O. Transcriptome analysis reveals fundamental differences in plant response to acute and chronic exposure to ionizing radiation. Mutat Res. 2007;624:101–113. doi: 10.1016/j.mrfmmm.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 25.Rakwal R, Agrawal GK, Shibato J, Imanaka T, Fukutani S, Tamogami S, Endo S, Sahoo SK, Masuo Y, Kimura S. Ultra low-dose radiation: stress responses and impacts using rice as a grass model. Int J Mol Sci. 2009;10:1215–1225. doi: 10.3390/ijms10031215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim DS, Lee IS, Jang CS, Kang SY, Seo YW. Characterization of the altered anthranilate synthase in 5-methyltryptophan-resistant rice mutants. Plant Cell Rep. 2005;24:357–365. doi: 10.1007/s00299-005-0943-y. [DOI] [PubMed] [Google Scholar]
- 27.Lee GJ, Chung SJ, Park IS, Lee JS, Kim JB, Kim DS, Kang SY. Variation in the phenotypic features and transcripts of color mutants of chrysanthemum (Dendranthema grandiflorum) derived from gamma ray mutagenesis. J Plant Biol. 2008;51:418–423. doi: 10.1007/BF03036063. [DOI] [Google Scholar]
- 28.Arnold NP, Barthakur NN, Tanguay M. Mutagenic effects of acute gamma irradiation on miniature roses: target theory approach. HortScience. 1998;33:127–129. [Google Scholar]
- 29.Das A, Gosal SS, Sidhu JS, Dhaliwal HS. Induction of mutations for heat tolerance in potato by using in vitro culture and radiation. Euphytica. 2000;114:205–209. doi: 10.1023/A:1003965724880. [DOI] [Google Scholar]
- 30.Patade VY, Suprasanna P. Radiation induced in vitro mutagenesis for sugarcane improvement. Sugar Tech. 2008;10:14–19. doi: 10.1007/s12355-008-0002-4. [DOI] [Google Scholar]
- 31.Bermudez-Caraballoso I, Garcia LR, Veitia N, Torres D, Pardon Y, Romero C, Orellana P. Mutant plantains (Musa spp.) with height reduction obtained by in vitro mutagenesis. Euphytica. 2010;176:105–112. doi: 10.1007/s10681-010-0233-9. [DOI] [Google Scholar]
- 32.Kim DS, Kim SY, Jeong IY, Kim JB, Lee GJ, Kang SY, Kim W. Improvement of ginsenoside production by Panax ginseng adventitious roots induced by γ-irradiation. Biol Plant. 2009;53:408–414. doi: 10.1007/s10535-009-0079-y. [DOI] [Google Scholar]
- 33.Court WA, Hendel GJ, Elmi J. Reversed-phase high-performance liquid chromatography determination of ginsenosides of Panax quinquefolium. J Chromatogr A. 1996;775:11–17. [Google Scholar]
- 34.Bonfill M, Cusido RM, Palazon J, Teresa Pinol M, Morales C. Influence of auxins on organogenesis and ginsenoside production in Panax ginseng calluses. Plant Cell Tissue Organ Cult. 2002;68:73–78. doi: 10.1023/A:1012996116836. [DOI] [Google Scholar]
- 35.Han JY, Kwon YS, Yang DC, Jung YR, Choi YE. Expression and RNA interference-induced silencing of the dammarenediol synthase gene in Panax ginseng. Plant Cell Physiol. 2006;47:1653–1662. doi: 10.1093/pcp/pcl032. [DOI] [PubMed] [Google Scholar]
- 36.Abe I, Rohmer M, Prestwich GD. Enzymatic cyclization of squalene and oxidosqualene to sterols and triterpenes. Chem Rev. 1993;93:2189–2206. doi: 10.1021/cr00022a009. [DOI] [Google Scholar]
- 37.Han JY, In JG, Kwon YS, Choi YE. Regulation of ginsenoside and phytosterol biosynthesis by RNA interferences of squalene epoxidase gene in Panax ginseng. Phytochemistry. 2010;71:36–46. doi: 10.1016/j.phytochem.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 38.Han JY, Jung SJ, Kim SW, Kwon KS, Yi MJ, Yi JS, Cho YE. Induction of adventitious roots and analysis of ginsenoside content and the genes involved in triterpene biosynthesis in Panax ginseng. J Plant Biol. 2006;49:26–33. doi: 10.1007/BF03030785. [DOI] [Google Scholar]
- 39.Han JY, Choi YE. Rapid induction of Agrobacterium tumefaciens-mediated transgenic roots directly from adventitious roots in Panax ginseng. Plant Cell Tissue Organ Cult. 2009;96:143–149. doi: 10.1007/s11240-008-9470-1. [DOI] [Google Scholar]
- 40.Wu J, Zhong JJ. Production of ginseng and its bioactive components in plant cell culture: current technological and applied aspects. J Biotechnol. 1999;68:89–99. doi: 10.1016/s0168-1656(98)00195-3. [DOI] [PubMed] [Google Scholar]
- 41.Langhansova L, Marsik P, Vanek T. Production of saponins from Panax ginseng suspension and adventitious root cultures. Biol Plant. 2005;49:463–465. doi: 10.1007/s10535-005-0030-9. [DOI] [Google Scholar]
- 42.Kim DS, Lee KJ, Yim WC, Kim JB, Ha BK, Kim SH, Kang SY. Transcriptional network analysis of the tryptophan-accumulating rice mutant during grain filling. Mol Genet Genomics. 2012;287:699–709. doi: 10.1007/s00438-012-0712-x. [DOI] [PubMed] [Google Scholar]