Abstract
Serum and liver metabolites in rats fed red ginseng (RG) were analyzed by ultra-performance liquid chromatography-quadrupole time-of-flight mass spectrometry. The mass data were analyzed by partial least squares-discriminant analysis (PLS-DA) to discriminate between control and RG groups and identify metabolites contributing to this discrimination. The RG group was clearly separated from the control group on PLS-DA scores plot for serum samples, but not liver samples. The major metabolites contributing to the discrimination included lipid metabolites (lysophosphatidylcholine, acyl-carnitine, and sphingosine), isoleucine, nicotinamide, and corticosterone in the serum; the blood levels of all but isoleucine were reduced by RG administration. Not all metabolites were positively correlated with the health benefits of RG. However, the blood levels of lysophosphatidylcholine, which stimulate various diseases, and long-chain acylcarnitines and corticosterone, which activate the stress response, were reduced by RG, suggesting long-term RG might relieve stress and prevent physiological and biological problems.
Keywords: Corticoterone, Lysophosphatidylcholines, Metabolomics, Red ginseng, Ultra-performance liquid chromatographyquadrupole time-of-flight mass spectrometry
INTRODUCTION
With increasing scientific evidence of the health benefits of ginseng (Panax ginseng Meyer), widely used in traditional medicine in Asia, its global market is growing. Ginseng’s health benefits are associated with many compounds including polyacetylenes, polysaccharides, sesquiterpenes, peptidoglycans, nitrogen-containing compounds, phenolic compounds, and vitamins [1], but its therapeutic effects have primarily been associated with more than 30 triterpenoid saponins, also known as ginsenosides [2]. However, the profiles of these compounds vary by ginseng type (fresh, white, and red) and cultivation environment. Red ginseng (RG), which is steamed and dried without peeling unlike white ginseng, which is dried after peeling, is the center of attention in functional health foods because many pharmacological and biological effects of RG suggest it is more beneficial than other types of ginseng. However, there is limited information on the health benefits of RG [3-6].
To understand the functional properties of foods including RG, comprehensive investigations of their health effects using high-throughput analyses such as genomics, proteomics, and metabolomics will be necessary. In particular, metabolomics, a newly emerging technology for comprehensive quantitative and qualitative analysis of small metabolites in organs or biofluids [7], is suitable to distinguish phenotypes and find the metabolites contributing to the phenotypic differences. LC-MS or NMR-based metabolomics were recently applied to estimate variations in ginseng property relative to processing, cultivation time, and origin [8-15], and to evaluate the effect of ginseng on myocardial infarction and cold stress in animal models [16,17]. However, changing metabolomic profiles in serum and liver after RG administration has been not investigated. In this study, metabolomic profiles of the serum and liver of rats given oral RG was analyzed by ultra-performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UPLC-Q-TOF MS) to improve our understanding of the effect of RG, in which the data were subjected to multivariate analysis to identify metabolites that differ in serum and liver between control and RG groups.
MATERIALS AND METHODS
Materials and chemicals
RG extract was purchased from Green Bio Co. Ltd. (Icheon, Korea) and the ginsenosides Rb1, Rb2, Rb3, Rc, Rd, Re, Rf, Rg1, Rg2, Rg3, Rh1, and Rh2 were purchased from Fleton Reference Substance (Chengdu, China). All HPLC or MS analytical-grade solvents were obtained from JT Baker (Phillipsburg, NJ, USA) or Sigma Chemical (St. Louis, MO, USA).
HPLC analysis of ginsenosides
Ginsenoside analysis was performed on a SunFire (Waters, Milford, MA, USA) C18 column (4.6×250 mm, 5 μm) connected to a Jasco (Tokyo, Japan) HPLC system. Ginseng extract diluted with water (1 g/25 mL of water) was injected into the column and eluted in a gradient of solvent A (water) and B (acetonitrile) at 1.6 mL/min for 82 min: solvent B was maintained at 20% for the first 10 min, increased from 20% to 32% for the next 30 min, 32% to 50% for 15 min, 50% to 65% for 15 min, and 65% to 90% for 2 min, and then maintained at 10% for 10 min. The eluted sample was detected at 203 nm by UV-detector. The ginsenoside standards mixture containing Rb1, Rb2, Rb3, Rc, Rd, Re, Rf, Rg1, Rg2, Rg3, Rh1, and Rh2 was prepared for qualitative and quantitative analysis.
Animals and red ginseng administration
Six week-old male Sprague-Dawley rats (control, 4 rats; RG, 8 rats) were housed in the Korea Food Research Institute at a constant temperature (22℃ to 26℃) under light/dark cycles of 12 h per d. Rats had access to water and a normal pellet diet ad libitum for 6 wk. Rats were given oral doses of RG (3 mL, 300 mg/kg) every day for 6 wk. Body weight was measured weekly. Serum was prepared from blood collected from the eyes of the rats just before sacrifice, and after weighing, the harvested liver was immediately snap-frozen in liquid nitrogen. All samples were stored at -70℃ until analysis.
Triglyceride and cholesterol
Triglyceride (TG) and cholesterol in the blood and liver were extracted by a solvent mixture of chloroform: methanol (2:1) and measured with a TG assay kit (Asan Pharmaceutical, Seoul, Korea) and a cholesterol/cholesteryl ester quantitation kit (Asan Pharmaceutical).
Sample preparation
Serum protein was precipitated by addition of cold acetonitrile (ACN). After centrifugation, the dried supernatant was dissolved in 20% aqueous methanol containing external standards (caffeine, sulfadimethoxine, terfenadine, 4-acetoamidophenol, and reserpine) for UPLC-Q-TOF MS analysis. The freeze-dried liver powder (50 mg) was extracted with 1 mL cold ACN in a homogenizer. After centrifugation, the dried supernatant was dissolved in 40% aqueous methanol containing external standards for UPLC-Q-TOF analysis.
Ultra-performance liquid chromatography-quadrupole time-of-flight mass spectrometry analysis of serum and liver extracts
A UPLC-Q-TOF MS system (Waters) was used to analyze the serum and liver extracts. The extracts were injected on an Acquity UPLC BEH C18 column (2.1×50 mm, 1.7 μm; Waters) equilibrated with water containing 0.1% formic acid (FA). Sample was eluted in a gradient of ACN containing 0.1% FA at a flow rate of 0.35 mL/min for 15 min; eluted metabolites were detected by Q-TOF MS. The Q-TOF was operated in electrospray ionization (ESI) positive mode. Voltages of the capillary and sampling cone were set at 3 kV and 30 V, respectively. The desolvation flow was set to 700 L/h at 300℃ and the source temperature was set to 110℃. The Q-TOF MS data were collected in the range of m/z 100 to 1,000 with a scan time of 0.2 s and interscan delay time of 0.02 s. To ensure accuracy and reproducibility, leucine-enkephalin (556.2771 Da in ESI positive mode, 200 ρmole) was used as the lock spray for all analyses with a flow rate of 3 μL/min and a frequency of 10 s. MS/MS spectra were obtained by a collision energy ramp of 10 to 30 eV. MassLynx (Waters) was used to calculate and sequence the accurate mass and composition of the precursor and fragment ions.
Data processing
All LC/MS data including retention time, m/z, and ion intensity were extracted by MarkerLynx software (Waters). Peaks were collected using a peak width at 5% height of 12 s, a noise elimination of 6, and intensity threshold of 100. All mass spectra were aligned with mass tolerance of 0.02 Da and retention time window of 0.20, and normalized to the intensity of an external standard by MarkerLynx (Waters). Assignment of metabolites was carried out using the Human metabolome database (http://www.hmdb.ca). Authentic standards were used to confirm the assignments and their quantitative analysis.
Statistical analysis
The aligned and normalized LC/MS data sets were analyzed by multivariate statistical analysis of SIMCA-P+ ver. 12.0.1 (Umetrics, Umea, Sweden). Partial least-squares discriminant analysis (PLS-DA) was used to visualize discrimination between RG and control groups. Hotelling's T2 test was used to exclude outliers from the 95% confidence region. The quality of PLS-DA models was assessed by 3 parameters: R2X, R2Y, and Q2Y. The goodness of fit was quantified by R2X and R2Y and the predictive ability was indicated by Q2Y. To validate models, a 6-fold cross validation was applied to PLSDA models and reliability was rigorously validated by permutation testing (n=200). The S-plot showing the combined covariance p(1) and correlation p(corr) from the PLS-DA model was used to visualize the metabolites contributing to the discrimination. Independent t-test was applied to identify metabolites contributing to the discrimination. Identified metabolites with significant differences (p<0.05) were also visualized in a heat map drawn by R with gplots. Metabolomic analyses of serum and liver were duplicated. Statistical analyses of body, liver, and facial weight, and TG and total cholesterol (TC) contents were performed by independent t-test.
RESULTS
Animal characteristics
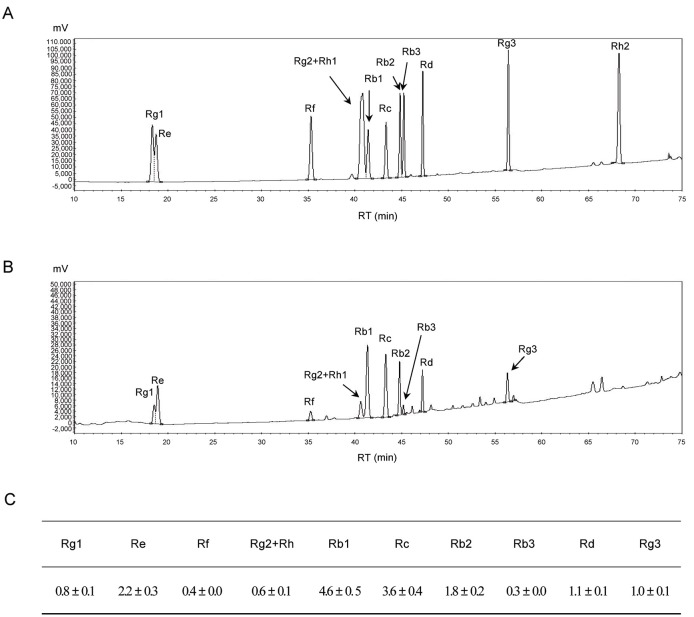
The effect of oral administration of RG containing 16.4±0.2 g of ginsenosides (Rb1, Rb2, Rb3, Rc, Rd, Re, Rf, Rg1, Rg2, Rg3, Rh1, and Rh2) (Fig. 1) on rat characteristics was investigated. Body weight (512.46±46.76 g), food intake (28.66±0.07 g/d), liver weight (17.32±1.79 g), and blood and liver TC level (99.75±16.33 mg/mL and 2.52±0.82 mg/g) were not significantly changed by 6-weeks RG administration (Table 1). However, RG-treated rats had lower TG in blood (109.46±30.70 mg/mL vs. 140.50±29.54 mg/mL in controls) and liver (13.35±1.21 mg/g vs. 14.90±1.69 mg/mg in controls).
Fig. 1. HPLC chromatograms of the ginsenoside standards (A), red ginseng extract (B), and the ginsenoside content in red ginseng (mg/g) (C). RT, retention time.
Table 1.
Characteristics of RG-administrated rats
| Control | RG | ||
|---|---|---|---|
|
| |||
| Body weight (g) | 512.46±46.76 | 505.64±36.22 | |
| Food intake (g/d) | 28.66±0.07 | 28.57±1.55 | |
| Liver weight (g) | 17.32±1. 79 | 17.32±1.79 | |
| TG | Blood (mg/mL) | 140.50±29.54 | 109.46±30.70* |
| Liver (mg/g) | 14.90±1.69 | 13.35±1.21** | |
| TC | Blood (mg/mL) | 99.75±16.33 | 91.13±18.56 |
| Liver (mg/g) | 2.52±0.82 | 1.93±0.66 | |
Animal characteristic data were statistically analyzed by independent t-test and expressed as mean±standard deviation.
Blood and liver TG contents of control and RG groups were significantly different at p<0.058 (*) and p<0.054 (**), respectively.
RG, red ginseng; TG, triglyceride; TC, total cholesterol.
Multivariate statistical analysis of serum and liver metabolites
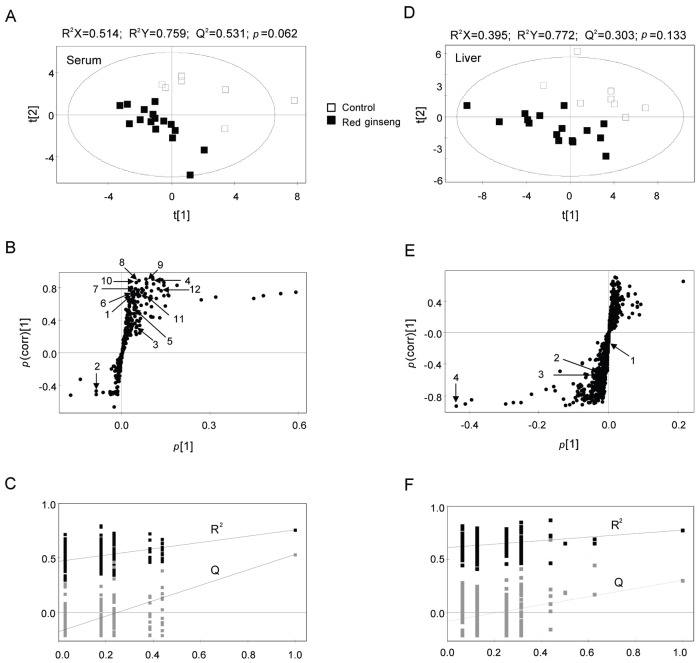
MS data for serum and liver metabolites from RG-treated and control rats were analyzed by UPLC-Q-TOF MS and the mass data were applied to a PLS-DA score plot (Fig. 2A, D). Both groups were distinguished from each other on the first 2-component PLS-DA score plots. The quality parameters of PLS-DA models showed R2X=0.514, R2Y=0.759, Q2=0.531 for serum samples and R2X=0.395, R2Y=0.772, Q2=0.303 for liver samples. Moreover, the cross validation with permutation test (Fig. 2C, F) for the PLS-DA models indicated both groups were separated with p-values of 0.062 for serum and 0.133 for liver.
Fig. 2. Partial least-squares discriminant analysis (PLS-DA) scores (A,D) and loading plots (B,E) obtained from ultra-performance liquid chromatography-quadrupole time-of-flight mass spectrometry data from serum (A,B) and liver (B,E) samples. Outliers from the elliptical region of the 95% confidence interval were excluded by Hotelling’s T2 test. The scores plots showed significant separation between samples based on the model quality parameters: R2X, R2Y, and Q2Y. The PLS-DA model was validated by a permutation test (n=200): p-values and intercepts of R2 (Ri) and Q2 (Qi) (C,F). The numbered metabolites are listed in Table 2.
To identify the metabolites contributing to the discrimination, the S-plots of p(1) and p(corr)(1) were generated using Par-scaling (Fig. 2B, E). The S-plots revealed that metabolites far from the cross section of p(1) and p(corr) were the more relevant ions for discrimination between groups. In serum and liver samples, the metabolites in the positive p(1) range were reduced by RG, whereas those with negative values were increased.
Qualitative and quantitative analysis of serum and liver metabolites
UPLC-Q-TOF detected 465 serum metabolites and 1,131 liver metabolites (including ion fragments). Of these, 46 serum metabolites and 84 liver metabolites had variable importance in the projection values of >0.5, meaning they are highly relevant to the differences between sample groups, and were statistically affected (p<0.05) by RG. However, only 12 metabolites (nicotinamide, isoleucine, uric acid, sphingosine, corticosterone, 3 acyl carnitines, and 4 lysophosphatidylcholines [lysoPCs]) from serum and 4 metabolites (cytidine and 3 lysoPCs) from liver could be identified (Table 2).
Table 2.
Identification of serum and hepatic metabolites from red ginseng-administrated-rats analyzed using ultra-performance liquid chromatography-quadrupole time-of-flight mass spectrometry
| No.1) | Identity | Actual mass (M+H) | Exact mass (M+H) | Mass error (mDa) | Mass fragments | p-valueb) | VIP3) | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Serum | 1 | Nicotinamide | 123.0569 | 123.0558 | 1.1 | 106, 96, 80 | 1.01E-06 | 1.03 |
| 2 | Isoleucine | 132.1032 | 132.1025 | 0.7 | 86 | 0.0493 | 1.65 | |
| 3 | Uric acid | 169.0374 | 169.0350 | 2.4 | 152, 141, 126, 98 | 0.0307 | 1.10 | |
| 4 | Sphingosine | 300.2909 | 300.2903 | 0.1 | 282, 252 | 0.0207 | 1.87 | |
| 5 | Corticosterone | 347.2229 | 347.2222 | 0.7 | 329, 121, 97 | 0.0431 | 0.61 | |
| 6 | Tetradecanoylcarnitine | 372.3093 | 372.3114 | -2.1 | 313, 211, 144, 85 | 0.0003 | 0.70 | |
| 7 | Linolenylcarnitine | 424.3431 | 424.3427 | 0.4 | 343, 245, 144, 85 | 0.0070 | 0.63 | |
| 8 | Vaccenylcarnitine | 426.3581 | 426.3583 | -0.2 | 367, 309, 144, 85 | 0.0355 | 0.77 | |
| 9 | lysoPC (C15:0) | 482.3246 | 482.3232 | 1.4 | 464, 184, 104, 86 | 0.0164 | 0.96 | |
| 10 | lysoPC (C18:1) | 506.3618 | 506.3550 | 6.8 | 488, 184, 104, 86 | 0.0025 | 0.66 | |
| 11 | lysoPC (C18:3) | 518.3245 | 518.3247 | -0.2 | 500, 184, 104, 86 | 0.0023 | 1.46 | |
| 12 | lysoPC (C20:5) | 542.3212 | 542.3232 | -2.0 | 524, 184, 104, 86 | 0.0319 | 2.77 | |
| Liver | 1 | Cytidine | 244.0947 | 244.0933 | 1.4 | 112 | 0.0116 | 0.80 |
| 2 | lysoPc (C15:0) | 482.3586 | 482.3232 | 35.4 | 464, 184, 104, 86 | 0.0500 | 0.62 | |
| 3 | lysoPc (C17:1) | 508.3769 | 508.3457 | 31.2 | 490, 184, 104, 86 | 0.0506 | 0.78 | |
| 4 | lysoPc (C18:0) | 524.3701 | 524.3716 | -1.5 | 506, 184, 104, 86 | 0.03362 | 2.94 | |
M, mass; VIP, variable importance in the projection; lysoPC, lysophosphatidylcholine.
1) The number of metabolites marked in Fig. 2
2)p-value was obtained from t-test.
3)Its value of above 1.0 showing high relevance for explaining the differences of sample groups.
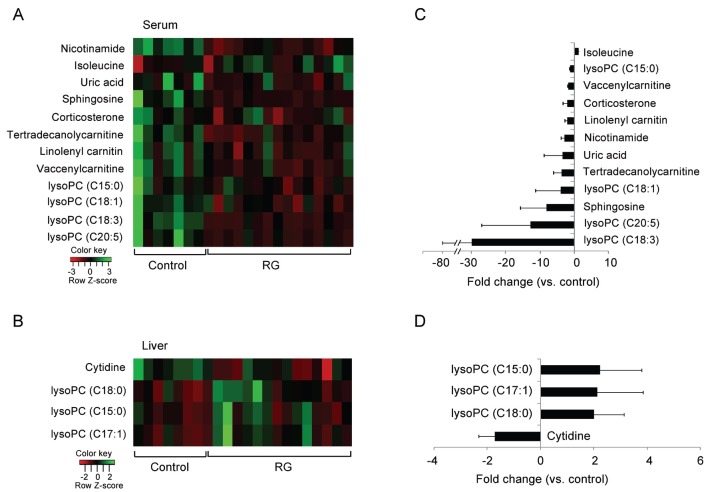
Most metabolites except isoleucine in serum and lysoPCs in liver were significantly reduced by RG administration (Fig. 3). In particular, lipid metabolites including lysoPCs (C18:3 and C20:5) and sphingosine in serum were assigned as major metabolites contributing to the difference between groups with 8-fold lower levels in the RG group than in the controls.
Fig. 3. Heat map of serum and liver metabolites showing significant differences between samples (A); fold changes (B). The heat map was drawn by R with gplots. The quantitative fold changes of serum and liver metabolites in the red ginseng (RG) group were calculated against that of control rats. lysoPC, lysophosphatidylcholines.
DISCUSSION
Many clinical trials and in vivo and in vitro studies on the pharmacological and biological effects of ginseng have been suggested that ginseng (including RG) is effective for treatment and/or prevention of various diseases including cancer, diabetes, cardiovascular diseases, and erectile dysfunction, improving immune function and central nervous system function, and relieving stress [18]. However, more evidence is needed to understand the health benefits of ginseng and settle controversies in the field.
Therefore, we investigated the change in serum and liver metabolites in rats given oral RG to improve our understanding of the relationship between ginseng and health. The metabolic profiles obtained using UPLC-Q-TOF were statistically analyzed and visualized by PLS-DA. The control and RG groups were clearly separated on PLS-DA scores plots of serum samples without significant differences in group characteristics except for blood and liver TG contents. These results except for TG contents are in contrast to the results of previous studies in which RG or ginseng reduced the level of blood lipids and/or weight in human and animal models on normal and high-fat diets [19,20]. We identified serum metabolites contributed to the difference and were mainly associated with lipid metabolism and stress response.
The levels of 4 lysoPCs, 3 long-chain acylcarnitines, and sphingosine were reduced by RG administration in serum, but not in the liver. Recent studies have clearly implicated that along with other lysophospholipids including sphingosine 1-phosphate, lysoPC, a circulating blood lipid comprised of phospholipase A2 on phosphatidylcholine [21], is an important cell signaling molecule regulating a variety of biological processes including cell proliferation, cardiovascular system, wound healing, tumor cell invasion, inflammation, and atherosclerosis [21-23]. These biological processes are regulated by G protein-coupled receptors (GPRs) including G2A and GPR4 activated by lysoPC [21,22]. In particular, the biological processes stimulated by lysoPC are associated with the health benefits of ginseng. Although we did not measure GPRs in response to RG and lysoPC-related physiological activities, our findings suggest the benefits of RG might be associated with reduced lysoPC levels. An in vivo study on very long-chain acyl-CoA dehydrogenase knockout mice showed that long-chain acylcarnitines, which mediate fatty acid transfer into mitochondria to produce energy through β-oxidation, increased in response to stresses such as fasting, exercise, or exposure to cold [24]. The accumulation of long-chain acylcarnitines might enhance energy production to overcome stress. In contrast, blood levels of long-chain acylcarnitines in normal unstressed rats decreased with RG administration.
In addition to long-chain acylcarnitines, a variety of steroid hormones is associated with stress. Corticosterone, a corticosteroid produced primarily in the adrenal cortex, is a well-known stress hormone [25]. Corticosterone is involved in a variety of behavioral and neurochemical responses to stress [26]. Emotional arousal activates the hypothalamic-pituitary-adrenocortical axis, resulting in increased corticosterone blood levels [27], which influence long-term memory consolidation and increase the incidence of cardiovascular disease including hypertension [28]. However, we found that the blood level of corticosterone decreased with long-term RG administration. The opposite results were observed in unanesthetized, pentobarbital-anesthetized, or alloxan-diabetes rats [29] and non-stressed male ICR mice [30] with oral or intraperitoneal administration of ginseng saponin. However, studies of the relationship between stress and corticosterone or corticosteroids using acute and chronic stressed rat models [31], morphine-induced thymic apoptosis mice models [32], and immobilization-stressed male Mongolian gerbils [33] produced findings similar to ours, implicating RG as an anti-stress reagent. However, the blood level of uric acid as a marker of oxidative stress [34] was reduced by RG administration.
In conclusion, serum and liver metabolites from oral RG and control rats were analyzed by UPLC-Q-TOF and their metabolomic profiles were compared by multivariate statistical analysis. Few serum metabolites associated with lipid metabolism and stress response were altered by RG administration, contributing to the difference between the control and RG groups, but not in liver. The blood levels of disease-stimulating lysoPCs and stress response-associated long-chain acylcarnitines and corticosterone were significantly reduced by RG. However, not all metabolites were positively correlated with the health benefits of RG. Although further studies on the relationship between the physiological and biological effects of RG and metabolites are necessary, our findings in normal healthy rats suggest long-term RG administration might relieve stress and prevent a variety of physiological and biological problems.
Acknowledgments
This work was supported by research grants from the Small and Medium Business Administration and the Korea Food Research Institute, Korea.
References
- 1.Yang SY, Kim HK, Lefeber AW, Erkelens C, Angelova N, Choi YH, Verpoorte R. Application of two-dimensional nuclear magnetic resonance spectroscopy to quality control of ginseng commercial products. Planta Med. 2006;72:364–369. doi: 10.1055/s-2005-916240. [DOI] [PubMed] [Google Scholar]
- 2.Lee EJ, Shaykhutdinov R, Weljie AM, Vogel HJ, Facchini PJ, Park SU, Kim YK, Yang TJ. Quality assessment of ginseng by (1)H NMR metabolite fingerprinting and profiling analysis. J Agric Food Chem. 2009;57:7513–7522. doi: 10.1021/jf901675y. [DOI] [PubMed] [Google Scholar]
- 3.Jang DJ, Lee MS, Shin BC, Lee YC, Ernst E. Red ginseng for treating erectile dysfunction: a systematic review. Br J Clin Pharmacol. 2008;66:444–450. doi: 10.1111/j.1365-2125.2008.03236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishijo H, Uwano T, Zhong YM, Ono T. Proof of the mysterious efficacy of ginseng: basic and clinical trials: effects of red ginseng on learning and memory deficits in an animal model of amnesia. J Pharmacol Sci. 2004;95:145–152. doi: 10.1254/jphs.FMJ04001X3. [DOI] [PubMed] [Google Scholar]
- 5.Won I, Kim YJ, Kim SJ, Kim EH, Hahm KB. Nutrigenomic approach to tackle the unpleasant journey to Helicobacter pylori-associated gastric carcinogenesis. J Dig Dis. 2011;12:157–164. doi: 10.1111/j.1751-2980.2011.00492.x. [DOI] [PubMed] [Google Scholar]
- 6.Yun TK. Experimental and epidemiological evidence on non-organ specific cancer preventive effect of Korean ginseng and identification of active compounds. Mutat Res. 2003;523-524:63–74. doi: 10.1016/S0027-5107(02)00322-6. [DOI] [PubMed] [Google Scholar]
- 7.Nicholson JK, Lindon JC, Holmes E. ‘Metabonomics’: understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica. 1999;29:1181–1189. doi: 10.1080/004982599238047. [DOI] [PubMed] [Google Scholar]
- 8.Toh DF, New LS, Koh HL, Chan EC. Ultra-high performance liquid chromatography/time-of-flight mass spectrometry (UHPLC/TOFMS) for time-dependent profiling of raw and steamed Panax notoginseng. J Pharm Biomed Anal. 2010;52:43–50. doi: 10.1016/j.jpba.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Kang J, Lee S, Kang S, Kwon HN, Park JH, Kwon SW, Park S. NMR-based metabolomics approach for the differentiation of ginseng (Panax ginseng) roots from different origins. Arch Pharm Res. 2008;31:330–336. doi: 10.1007/s12272-001-1160-2. [DOI] [PubMed] [Google Scholar]
- 10.Zhang HM, Li SL, Zhang H, Wang Y, Zhao ZL, Chen SL, Xu HX. Holistic quality evaluation of commercial white and red ginseng using a UPLC-QTOF-MS/MS-based metabolomics approach. J Pharm Biomed Anal. 2012;62:258–273. doi: 10.1016/j.jpba.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 11.Angelova N, Kong HW, van der Heijden R, Yang SY, Choi YH, Kim HK, Wang M, Hankemeier T, van der Greef J, Xu G, et al. Recent methodology in the phytochemical analysis of ginseng. Phytochem Anal. 2008;19:2–16. doi: 10.1002/pca.1049. [DOI] [PubMed] [Google Scholar]
- 12.Kim SH, Hyun SH, Yang SO, Choi HK, Lee BY. (1)H-NMR-based discrimination of thermal and vinegar treated ginseng roots. J Food Sci. 2010;75:C577–C581. doi: 10.1111/j.1750-3841.2010.01685.x. [DOI] [PubMed] [Google Scholar]
- 13.Kim N, Kim K, Choi BY, Lee D, Shin YS, Bang KH, Cha SW, Lee JW, Choi HK, Jang DS, et al. Metabolomic approach for age discrimination of Panax ginseng using UPLC-Q-Tof MS. J Agric Food Chem. 2011;59:10435–10441. doi: 10.1021/jf201718r. [DOI] [PubMed] [Google Scholar]
- 14.Yang SO, Shin YS, Hyun SH, Cho S, Bang KH, Lee D, Choi SP, Choi HK. NMR-based metabolic profiling and differentiation of ginseng roots according to cultivation ages. J Pharm Biomed Anal. 2012;58:19–26. doi: 10.1016/j.jpba.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 15.Xie G, Plumb R, Su M, Xu Z, Zhao A, Qiu M, Long X, Liu Z, Jia W. Ultra-performance LC/TOF MS analysis of medicinal Panax herbs for metabolomics research. J Sep Sci. 2008;31:1015–1026. doi: 10.1002/jssc.200700650. [DOI] [PubMed] [Google Scholar]
- 16.Liang X, Chen X, Liang Q, Zhang H, Hu P, Wang Y, Luo G. Metabonomic study of Chinese medicine Shuanglong formula as an effective treatment for myocardial infarction in rats. J Proteome Res. 2011;10:790–799. doi: 10.1021/pr1009299. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Su M, Qiu Y, Ni Y, Zhao T, Zhou M, Zhao A, Yang S, Zhao L, Jia W. Metabolic regulatory network alterations in response to acute cold stress and ginsenoside intervention. J Proteome Res. 2007;6:3449–3455. doi: 10.1021/pr070051w. [DOI] [PubMed] [Google Scholar]
- 18.Wee JJ, Park KM, Chung AS. Biological activities of ginseng and its application to human health. In: Benzie IF, Wachtel-Galor S, eds. Herbal medicine: biomolecular and clinical aspects. 2nd ed. CRC Press; Boca Raton: 2011. pp. 157–174. [Google Scholar]
- 19.Song YB, An YR, Kim SJ, Park HW, Jung JW, Kyung JS, Hwang SY, Kim YS. Lipid metabolic effect of Korean red ginseng extract in mice fed on a high-fat diet. J Sci Food Agric. 2012;92:388–396. doi: 10.1002/jsfa.4589. [DOI] [PubMed] [Google Scholar]
- 20.Kim SH, Park KS. Effects of Panax ginseng extract on lipid metabolism in humans. Pharmacol Res. 2003;48:511–513. doi: 10.1016/S1043-6618(03)00189-0. [DOI] [PubMed] [Google Scholar]
- 21.Schmitz G, Ruebsaamen K. Metabolism and atherogenic disease association of lysophosphatidylcholine. Atherosclerosis. 2010;208:10–18. doi: 10.1016/j.atherosclerosis.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 22.Meyer zu Heringdorf D, Jakobs KH. Lysophospholipid receptors: signalling, pharmacology and regulation by lysophospholipid metabolism. Biochim Biophys Acta. 2007;1768:923–940. doi: 10.1016/j.bbamem.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 23.Kabarowski JH. G2A and LPC: regulatory functions in immunity. Prostaglandins Other Lipid Mediat. 2009;89:73–81. doi: 10.1016/j.prostaglandins.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spiekerkoetter U, Tokunaga C, Wendel U, Mayatepek E, Exil V, Duran M, Wijburg FA, Wanders RJ, Strauss AW. Changes in blood carnitine and acylcarnitine profiles of very long-chain acyl-CoA dehydrogenase-deficient mice subjected to stress. Eur J Clin Invest. 2004;34:191–196. doi: 10.1111/j.1365-2362.2004.01308.x. [DOI] [PubMed] [Google Scholar]
- 25.Schoenfeld TJ, Gould E. Stress, stress hormones, and adult neurogenesis. Exp Neurol. 2012;233:12–21. doi: 10.1016/j.expneurol.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev. 2000;33:13–33. doi: 10.1016/S0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- 27.McGaugh JL, Roozendaal B. Role of adrenal stress hormones in forming lasting memories in the brain. Curr Opin Neurobiol. 2002;12:205–210. doi: 10.1016/S0959-4388(02)00306-9. [DOI] [PubMed] [Google Scholar]
- 28.Scheuer DA. Regulation of the stress response in rats by central actions of glucocorticoids. Exp Physiol. 2010;95:26–31. doi: 10.1113/expphysiol.2008.045971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hiai S, Yokoyama H, Oura H. Features of ginseng saponin-induced corticosterone secretion. Endocrinol Jpn. 1979;26:737–740. doi: 10.1507/endocrj1954.26.737. [DOI] [PubMed] [Google Scholar]
- 30.Kim DH, Moon YS, Jung JS, Min SK, Son BK, Suh HW, Song DK. Effects of ginseng saponin administered intraperitoneally on the hypothalamo-pituitary-adrenal axis in mice. Neurosci Lett. 2003;343:62–66. doi: 10.1016/S0304-3940(03)00300-8. [DOI] [PubMed] [Google Scholar]
- 31.Rai D, Bhatia G, Sen T, Palit G. Anti-stress effects of Ginkgo biloba and Panax ginseng: a comparative study. J Pharmacol Sci. 2003;93:458–464. doi: 10.1254/jphs.93.458. [DOI] [PubMed] [Google Scholar]
- 32.Kim YR, Lee SY, Shin BA, Kim KM. Panax ginseng blocks morphine-induced thymic apoptosis by lowering plasma corticosterone level. Gen Pharmacol. 1999;32:647–652. doi: 10.1016/S0306-3623(98)00240-7. [DOI] [PubMed] [Google Scholar]
- 33.Lee SH, Jung BH, Kim SY, Lee EH, Chung BC. The antistress effect of ginseng total saponin and ginsenoside Rg3 and Rb1 evaluated by brain polyamine level under immobilization stress. Pharmacol Res. 2006;54:46–49. doi: 10.1016/j.phrs.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Glantzounis GK, Tsimoyiannis EC, Kappas AM, Galaris DA. Uric acid and oxidative stress. Curr Pharm Des. 2005;11:4145–4151. doi: 10.2174/138161205774913255. [DOI] [PubMed] [Google Scholar]