Abstract
Background
Meningioma was the first solid tumor shown to contain a recurrent genetic alteration e.g. monosomy 22/del(22q), NF2 being the most relevant gene involved. Although monosomy 22/del(22q) is present in half of all meningiomas, and meningiomas frequently carry NF2 mutations, no study has been reported so far in which both alterations are simultaneously assessed and correlated with the features of the disease.
Methods
Here, we analyzed the frequency of both copy number changes involving chromosome 22 and NF2 mutations in 20 sporadic meningiomas using high-density SNP-arrays, interphase-FISH and PCR techniques.
Results
Our results show a significant frequency of NF2 mutations (6/20 patients, 30%), most of which (5/6) had not been previously reported in sporadic meningiomas. NF2 mutations involved five different exons and led to a truncated protein (p.Leu163CysfsX46, p.Phe62LeufsX61, p.Asp281MetfsX15, p.Phe285LeufsX11, p.Gln389ArgfsX37) and an in frame deletion of Phe119. Interestingly, all NF2 mutated cases were menopausal women with monosomy 22 but not del(22q).
Conclusions
These results confirm and extend on previous observations about the high frequency and heterogeneity of NF2 mutations in sporadic meningiomas and indicate they could be restricted to a well-defined cytogenetic and clinical subgroup of menopausal women. Further studies in large series of patients are required to confirm our observations.
Keywords: Mutation, NF2 gene, Sporadic meningiomas, Monosomy 22, Menopausal women
Background
Meningioma was the first type of solid tumor which already in 1967, was shown to contain a specific recurrent genetic alteration consisting of loss of a chromosome 22 in around half of the cases [1]. The association observed between the dominantly-inherited neurofibromatosis type-2 syndrome and central nervous system (CNS) tumors such as meningiomas and schwannomas, has rapidly led to the identification of the NF2 gene located in chromosome 22q as a candidate predisposing gene in both familial and sporadic meningiomas [2,3]. However, loss of chromosome 22 and/or del(22q) are only found in a fraction of all meningiomas suggesting that molecular and chromosomal changes other than those targeting the NF2 gene may also be involved in the development of meningiomas [4,5]. Since the earliest reports till 1994, more than 400 different NF2 mutations have been described in meningiomas [6-27]; however, the precise significance of the NF2 gene status in sporadic meningiomas remains unclear. Based on sequencing data from the literature, NF2 mutations may involve all exons of the gene except exon 16. Interestingly, an association between NF2 gene mutations and specific subtypes of meningiomas such as fibroblastic and transitional tumors has been observed [7-9,24], although such data could not be always confirmed in other series [28].
In this study, we report on the frequency of NF2 mutations in a group of 20 sporadic WHO grade I/benign meningiomas and its association with the distinct genetic and histopathological subtypes of meningiomas. At the same time, we also review the literature reported so far on NF2 mutations in meningiomas. Overall, six distinct NF2 mutations were found (6/20 cases; 30%) five of which had not been previously reported in sporadic meningiomas [10,24,29]. Noteworthy, NF2-mutated tumors were systematically associated with complete loss of chromosome 22 (monosomy 22) but not del(22q), usually in the absence of additional genetic alterations involving other chromosomes as assessed by both interphase fluorescence in situ hybridization (iFISH) and single nucleotide polymorphism (SNP) arrays. These results, together with the higher median age and the slightly greater frequency of transitional meningiomas, among the mutated cases, suggest that NF2-mutated tumors may represent a uniquely well-defined subgroup of sporadic meningiomas.
Methods
Patients and samples
A total of 20 adult WHO grade I (sporadic) meningioma patients (3 males and 17 females; mean age of 60 ± 16 years) were included in this study. Prior to entering the study, informed consent was given by each individual and the study was approved by the ethics committee of the University Hospital of Salamanca (Salamanca, Spain). Classification of meningioma was established according to the WHO criteria, with the following distribution: meningothelial meningiomas, 10 cases; transitional, 7, and; fibroblastic meningiomas, 3 tumors. In each individual patient, paired fresh EDTA-anticoagulated peripheral blood (PB) and tumoral samples were obtained in parallel and stored in liquid nitrogen (−150°C), until analysed.
DNA extraction
Both tumoral and PB cells’ DNA was extracted using the QIAamp DNA mini kit (Qiagen, Hilden, Germany) following the instructions of the manufacturer. A NanoDrop-1000 Spectrophotometer (Nano-Drop Technologies, Wilmington, DE, USA) was used to quantify the amount of DNA obtained and assess its quality.
Copy number alterations and loss of heterozygosity (LOH) of chromosome 22 by iFISH and SNP-arrays
In order to confirm the presence of numerical changes involving chromosome 22, iFISH studies were performed with commercially available probes for chromosome 9 and 22q (LSI bcr/abl ES DC Probe). In addition, in 15/20 patients copy number alterations and LOH were analyzed with the GeneChip Human Mapping 250 K Nsp and 250 K Sty-arrays (Affymetrix), as previously reported [30].
Identification of NF2 mutations
DNA from the 20 meningioma samples was amplified by conventional PCR [20]. In order to identify mutations in the NF2 gene sequence, 32 customized primers were used. Oligonucleotide primer sequences were obtained from the UniSTS database at NCBI (http://www.ncbi.nlm.nih.gov).
PCR products were analysed by capillary electrophoresis using an ABI 3130xl instrument (Applied Biosystems, Foster City, CA, USA) and the Chromas (Technelysium Pty Ltd,http://technelysium.com.au) software was used to analyze the DNA sequences obtained.
Statistical analyses
The statistical significance of differences observed between groups was assessed by the Student T and the Mann–Whitney U tests, for parametric and non-parametric (continuous) variables, respectively; for qualitative variables, the X 2 test was used (SPSS software, SPSS 15.0, SPSS Inc, Chicago, IL, USA). P-values <0.05 were considered to be associated with statistical significance.
Results
Frequency, localization and type of NF2 mutations in sporadic meningiomas
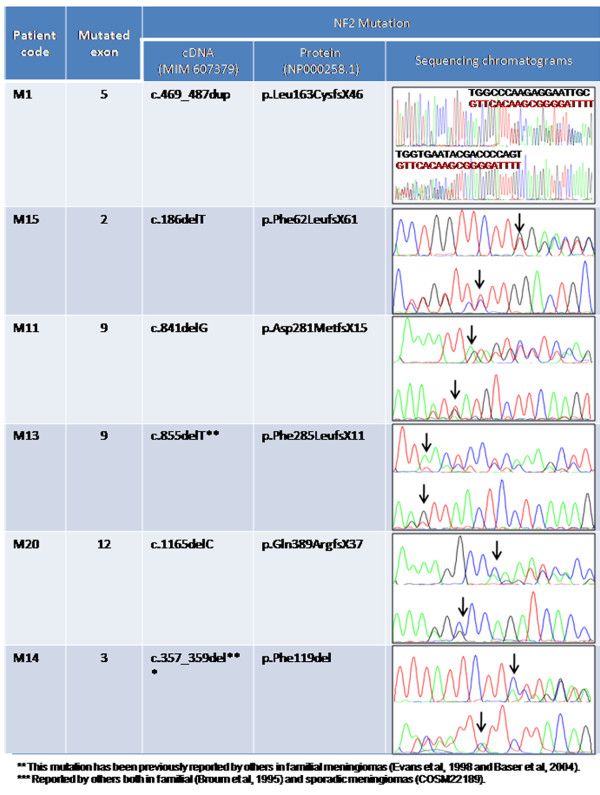
NF2 gene mutations were found in 6/20 meningiomas studied (30%) (Table 1). Specific NF2 mutations identified consisted of four deletions of a single base, one deletion of 3 bp and a duplication of 19 bp, (Figure 1). Therefore, changes in cDNA consisting of a c.186delT, c.841delG, c.855delT and c.1165delC were found in those four cases with single nucleotide deletions, such mutations generating the synthesis of the p.Phe62LeufsX61, p.Gln389ArgfsX37, p.Asp281MetfsX15 and p.Phe285LeufsX11 truncated proteins, respectively (Figure 1). The remaining deletion identified involved three consecutive bp (“CTT”) at exon 3 (c.357_359del) also leading to an in frame deletion of Phe119, and the duplication of 19 bp involved positions 469 to 487 of the NF2 gene, leading to a p.Leu163Cys mutated nf2 protein with a stop after 46 codons (Figure 1).
Table 1.
Clinical, histopathological and genetic characteristics of meningioma patients grouped according to the presence vs absence of NF2 gene mutations (n = 20)
| Patient features |
NF2-mutated meningiomas |
Non-mutated meningiomas |
p-value | |
|---|---|---|---|---|
| (n = 6) | (n = 14) | |||
| Age (median in years) |
73 (56–82) |
53 (26–80) |
0.03 |
|
| Gender (Male/Female) |
0/6 |
3/11 |
0.32 |
|
| (0%/100%) |
(21%/79%) |
|||
| |
Skull base |
1 (17%) |
7 (50%) |
|
| Tumor localization |
Convexity |
1 (17%) |
2 (14%) |
0.37 |
| Tentorium |
4 (66%) |
4 (29%) |
|
|
| Spinal |
0 (0%) |
1 (7%) |
|
|
| Histological subtype |
Meningothelial |
2 (33%) |
8 (57%) |
|
| Transitional |
3 (50%) |
4 (29%) |
NS |
|
| Fibroblastic |
1 (17%) |
2 (14%) |
|
|
| Chromosome 22 status by iFISH |
Diploid |
0 (0%) |
9 (64%) |
0.005 |
| del(22q) |
0 (0%) |
3 (22%) |
||
| Monosomy 22 |
6 (100%) |
2 (14%) |
||
| Cytogenetic subgroups | Diploid |
0 (0%) |
7 (50%) |
0.03 |
| Isolated monosomy 22 |
4 (67%) |
2 (14%) |
||
| Complex-karyotype: with monosomy 22 |
2 (33%) |
1 (7%) |
||
| with del(22q) |
0 (0%) |
2 (14%) |
||
| Complex-karyotype w/o −22/del(22q) | 0 (0%) | 2 (14%) | ||
Figure 1.
NF2 mutations. Detailed description of the 6 NF2 mutations (a 19 bp duplication and 5 deletions) identified among the 20 meningiomas analyzed, including the position of the alterations identified, the potential effect of each mutation and their sequencing chromatograms (the positions of the deleted base(s) are indicated with an arrow).
Association between NF2 mutations and other chromosomal changes
From the 20 meningiomas analyzed, 7 showed a diploid karyotype (35%), another 6 (30%) had an isolated loss of chromosome 22, two or more chromosomal changes including loss of chromosome 22q were found in another 5 cases –monosomy 22 in 3 (15%) and del(22q) in the other 2 patients (10%)– and two meningiomas displayed multiple chromosomal losses/gains in the absence of monosomy 22/del(22q) (n = 2; 10%) (Table 1).
Interestingly, all 6 NF2-mutated tumors carried monosomy 22, which was the only chromosomal alteration in 4/6 cases. Consequently, NF2-mutated meningiomas accounted for most cases associated with monosomy 22 (6/9; 67%), including cases with isolated monosomy 22 (4/6; 67%) or with monosomy 22 combined with other chromosomal alterations (2/6, 33%); conversely, among all other cases except three, which were either diploid for chromosome 22, carried del(22q) or had multiple chromosomal losses/gains in the absence of monosomy 22/del(22q), showed no NF2 mutations (0/11; p = 0.03)(Table 1).
Chromosome 22 copy number alterations and LOH by SNP-arrays
As described above, iFISH showed losses of chromosome 22 in 11 cases; such losses consisted of monosomy 22 (9 cases; 45%) and del(22q) (2 cases; 10%) extending from the 18.207.392 bp position to the telomere of chromosome 22q (Figure 2 and Table 2). Noteworthy, LOH was investigated by SNP-arrays in 15/20 meningiomas, and it was found to involve chromosome 22 in 7/8 cases that had monosomy 22 and in 1/2 cases with del(22q). The number of chromosome 22 SNPs showing LOH varied between 3 and 920 SNPs. Among these patients, four SNPs (e.g., rs2857648, rs2857652, rs2530678 and rs1009147) were found to be associated with LOH for the NF2 gene (Figure 2).
Figure 2.
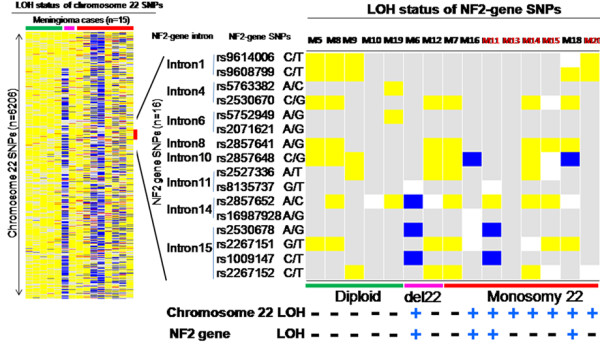
LOH profiles of chromosome 22 and the NF2 gene in sporadic meningiomas (n = 15). Rows (n = 6206) correspond to individual SNPs along the entire chromosome 22 and columns identify different meningiomas. Blue indicates presence of LOH, allele retention is shown in yellow, while red indicates conflict between PB DNA and tumor DNA SNP calls (AA or BB in PB and AB or BB and AB or AA in the tumor sample, respectively), and absence of call for non-informative SNPs (AA or BB) are depicted in white and grey, respectively. Two tumors (M7 and M12) showed a pattern compatible with biclonal allele deletions with copy number value of 1 in the absence of LOH. The NF2 gene locus containing 16 SNPs in the array is amplified in the right side of the figure to better show the status of those SNPs heterozygously distributed in introns 1, 4, 6, 8, 10, 11, 14 and 15. LOH for three NF2-associated SNPs was found in one tumor (M6), for two SNPs in another case (M11) and for one SNP in another two meningiomas (M16 and M18).
Table 2.
Chromosome 22 status by iFISH vs SNP-arrays performed in 15/20 sporadic meningiomas
|
Tumor |
iFISH Karyotype |
Chromosome 22 status by SNP arrays* (n = 6206) |
|||
|---|---|---|---|---|---|
| ID | Chromosome 22 status | Copy number values | N. of SNPs with LOH/N. of informative SNPs (%) | ||
|
M20 |
−22q |
−22 |
1.35 (1.01-1.93) |
108 |
/ 1326 (8%) |
|
M18 |
−22q |
−22 |
1.19 (0.76-1.59) |
365 |
/ 1131 (32%) |
|
M15 |
Complex & -22q |
−22 |
1.30 (0,85-2,03) |
454 |
/ 1158 (39%) |
|
M14 |
−22q |
−22 |
1.34 (0.95-1.77) |
80 |
/ 1472 (5%) |
|
M13 |
−22q |
−22 |
1.24 (0.92-1.8) |
920 |
/ 1188 (77%) |
|
M11 |
Complex & -22q |
−22 |
1.20 (0.91-1.91) |
694 |
/ 1043 (67%) |
|
M16 |
−22q |
−22 |
1.27 (0.81-1.75) |
209 |
/ 1340 (16%) |
|
M7 |
−22q,-Y |
−22 |
1.52 (1.08-2.14) |
18 |
/ 1505 (1%) |
|
M12 |
Complex & del(22q) |
del(22q11.22-qter) |
1.50 (0.98-2) |
19 |
/ 1683 (2%) |
|
M6 |
Complex & del(22q) |
del(22q11.22-qter) |
1.34 (0.8-2.55) |
763 |
/ 1195 (64%) |
|
M19 |
Complex |
Diploid |
1.58 (1.12-2.06) |
7 |
/ 1630 (0.4%) |
|
M10 |
Complex |
Diploid |
1.71 (1.09-2.31) |
9 |
/ 1770 (0.5%) |
|
M9 |
Diploid |
Diploid |
1.75 (1.27-2.32) |
3 |
/ 1712 (0.2%) |
|
M8 |
Diploid |
Diploid |
1.92 (1.6-2.39) |
5 |
/ 1681 (0.3%) |
| M5 | Diploid | Diploid | 1.79 (1.28-2.3) | 3 | / 1696 (0.2%) |
*LOH and copy number analyses involved 6206 SNP localized along the entire chromosome 22.
iFISH interphase fluorescence in situ hybridization, SNP single nucleotide polymorphism, LOH loss of heterozigosity.
Association between NF2 mutations and other features of the disease
Interestingly, all NF2-mutated meningiomas corresponded to female patients (6/6 vs 11/14, p > 0.05) with a higher median age vs all other cases (73 vs 53 years; p = 0.03)(Table 1). By contrast, a similar localization pattern was observed for the 6 NF2-mutated tumors and the other 14 non-mutated meningiomas (Table 1).
From the histopathological point of view, the 6 NF2 mutated benign/grade I meningiomas showed a variable histology including 3 transitional meningiomas, two meningothelial tumors and one fibroblastic tumor, the frequency of transitional tumors being slightly higher than among non-mutated cases (3/6 vs 4/14 cases; p > 0.05) (Table 1).
Discussion
Many studies have confirmed that loss of genetic material from chromosome 22 is by far the most frequent chromosomal alteration in meningiomas, being detected in around half of the cases. Such losses of chromosome 22 are heterogeneous and while they most frequently involve the whole chromosome (monosomy 22), in another substantial proportion of cases it consists of del(22q) with variable breakpoints, both alterations –monosomy 22 and del(22q) − being found either as the only chromosomal alteration or in combination with distinct alterations involving other chromosomes [30,31]. In parallel, multiple studies have also shown a variable frequency (between 14% and 78% of the cases) of NF2 gene mutations in heterogeneous series of meningiomas consisting of between 12 to 170 patients [18,27], some of these reports specifically focusing on the investigation of particular subsets of patients such as recurrent meningiomas [6], rare histopathological subtypes [9] or patients affected by multiple meningiomas [12]. Recurrent chromosomal losses deviating from normal gene dosage present in tumor cells of various types, frequently point out the existence of one or more underlying tumor suppressor genes coded in the deleted DNA sequences. Because of this and the development of meningiomas in patients with neurofibromatosis carrying mutations of the NF2 gene coded at chromosome 22q12.2, NF2 has long been considered as the most relevant gene targeted by chromosome 22 losses in meningiomas [7,32]. However, careful analysis of the studies reported so far reveals that the association between chromosome 22 status and the presence of NF2 mutations has not been investigated in detail in sporadic meningiomas.
In the present study, we demonstrate the existence of a close association between NF2 mutation and monosomy 22 but not del(22q), in sporadic meningiomas. Whereas most cases with monosomy 22 showed NF2 mutations, these were absent in all three patients who showed del(22q). Altogether, these findings suggest that the NF2 gene would only be involved in the pathogenesis of a well-defined subset of all meningiomas, typically in association with monosomy 22. Of note, a few cases showed monosomy 22 in the absence of NF2 mutations; these results support previous observations indicating that in meningiomas, monosomy 22 is typically found at higher frequencies than NF2 mutations, although the frequency of both alterations also depends on the detection method. This, together with the observation that NF2 mutations were always present in heterozygosis, could suggest that mutations of the NF2 gene could be a secondary event in the development of meningiomas which would occur after the loss of chromosome 22 and that therefore, inactivation of other genes coded in chromosome 22 (e.g. SMARCB1[33]) could potentially play a major role in tumor development, even among NF2 mutated cases. In line with this hypothesis, NF2- mutated meningiomas also showed a rather low incidence of LOH of the NF2 gene by SNP-arrays. However, other mechanisms e.g. rearrangements identifiable by MPLA and other techniques, together with mutations in promoter and other non coding regions, and epigenetic mechanisms leading to inactivation of NF2 gene expression, may also be involved and should be investigated to confirm or rule out this hypothesis. Of note, among cases with monosomy 22/del(22q) in the absence of NF2 mutations, two patients showed lack of LOH, which could be related to the greater percentage of non-tumoral diploid cells present in both patients (data not shown) [34].
Despite all the above, detailed analysis of the clinical and histopathological features of the meningiomas patients studied at diagnosis, showed unique features for those sporadic tumors carrying NF2-mutations in association with monosomy 22. Accordingly, these cases systematically corresponded to older menopausal women, they more frequently showed a meningothelial histopathological appearance, and none of them had relapsed so far. These results suggest that NF2-mutated sporadic meningiomas may correspond to a unique cytogenetic and clinical subtype of meningiomas, which emerge at relatively advanced ages. In line with this hypothesis, previous studies also suggested the existence of a relationship between NF2 gene mutations and the histology of meningiomas (e.g. transitional tumors [24]); however, this association remains controversial and in our series, the association between NF2 mutated and transitional meningiomas did not reach statistical significance, in line with the findings of several groups [7,8,21].
Despite different mutations involving distinct exons of the NF2 gene were found in each NF2-mutated meningioma case, they led to a truncated protein or a small in frame deletion and therefore, potentially also to a loss of (normal) function of the nf2 protein. Overall these findings are in line with the great heterogeneity of NF2 mutations reported so far in the literature (Table 3). In fact, previous studies indicate that multiple distinct substitutions or deletions of one or more nucleotides may occur and that all NF2 gene exons, except exon 16, may be involved (Table 3), exon 2 being the most frequently mutated (≈45% of all mutated tumors). Among our cases, deletions of one and three nucleotides together with duplication of a sequence of 20 nucleotides, were observed. Of note, with the exception of one mutation which had been previously described, none of the other five NF2 gene mutations identified in our cases had been reported before in sporadic meningiomas (Table 3).
Table 3.
Frequency of NF2-gene mutations in our patients and other series from the literature reporting >10 sporadic meningiomas
| Reference | No of mutated cases/total cases (%) | Exons involved |
|---|---|---|
| Ruttledge, et al., 1994 [27]** |
24/170 (14%) |
2-7-8-9-10-11-12 |
| LeKanne Deprez, et al., 1994 [26]** |
19/48 (40%) |
1-2-3-4-5-6-11-12 |
| Merel, et al., 1995 [25]** |
15/57 (26%) |
1-2-3-4-5-6-7-8-9-12-13 |
| Wellenreuther, et al., 1995 [24] |
41/70 (59%) |
1-2-3-4-5-6-7-8-10-11-12-13 |
| Ng, et al., 1995 [23] |
7/26 (27%) |
4-5-6-7-10 |
| Papi, et al., 1995 [22]** |
9/61 (15%) |
2-5-7-8-11 |
| Harada, et al., 1996 [21] |
8/23 (35%) |
1-2-5-8-11-12 |
| Ruttledge, et al., 1996 [20] |
67/111 (60%) |
1-2-3-4-6-7-8-10-11-12-13-14-15-17 |
| De Vitis, et al., 1996 [29] |
37/125 (30%) |
2-3-4-5-6-7-8-11-12-13 |
| Stangl, et al., 1997 [18] |
10/12 (83%) |
3-5-6-7-8-11-12 |
| Leone, et al., 1999 [17]** |
11/81 (14%) |
2-7-11 |
| Ueki, et al., 1999 [16] |
10/50 (20%) |
4-5-7-10-12-13 |
| Evans, et al., 2001 [14] |
4/27 (15%) |
3-4-8-13 |
| Joachim, et al., 2001 [13]* |
26/61 (43%) |
1-2-3-4-5-6-7-8-10-11-12-13 |
| Szijan, et al., 2003 [11] |
5/14 (35%) |
2-3-8-12-13 |
| Lomas, et al., 2005 [10]** |
21/88 (24%) |
1-2-3-4-7-11-12-13 |
| Hartmann, et al., 2006 [9]# |
21/80 (20%) |
1-2-3-4-5-6-7-8-9-11-12-13-14-15 |
| Kim, et al., 2006 [8]** |
20/42 (48%) |
1-2-4-5-7-10-11-12 |
| Hansson, et al., 2007 [7]** |
39/100 (39%) |
1-2-3-4-6-7-8-9-10-12-13-14 |
| Goutagny, et al., 2010 [6]& |
14/18 (78%) |
2-5-6-7-8-10-11-12-13 |
| Tabernero et al. (2013) |
6/20 (30%) |
2-3-5-9-12 |
| Total | 414/1284 (32%) | 1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-17 |
*several radiation-induced meningiomas were included in this series; #infrequent subtypes of meningiomas were analyzed in this series; &meningioma cases showing progression were analyzed in this series.
**not all exones of the NF2 gene were investigated in this series.
Conclusions
In the present study we confirm the relatively high frequency of distinct NF2 mutations leading to a truncated protein in sporadic meningiomas, most of the identified genetic changes corresponding to mutations which had not been previously described. Interestingly, a clear association was found among our cases between mutation of the NF2 gene and monosomy 22, but not del(22q); in addition, all NF2-mutated cases corresponded to older menopausal women, supporting the notion that NF2-mutated patients could represent a well-defined and unique cytogenetic and clinical subtype of meningiomas in which acquisition of monosomy 22 could be sequentially followed by NF2 mutation during the development of the disease. Further studies in large series of meningioma patients are required to confirm these observations.
Competing interests
We declare that we do not have received reimbursements, fees, funding, or salary from an organization that may in any way gain or lose financially from the publication of this manuscript, either now or in the future. In the past five years we only have received funding from an governamental research organization to finance this manuscript (the article processing charge) title “Association between mutation of the NF2 gene and monosomy 22 in menopausal women with sporadic meningiomas.”
We do not hold any stocks or shares in an organization that may in any way gain or lose financially from the publication of this manuscript, either now or in the future.
We do not hold or applying for any patents relating to the content of the manuscript and we do not have received reimbursements, fees, funding, or salary from an organization that holds or has applied for patents relating to the content of the manuscript.
We do not have any other financial competing interests. There are not non-financial competing interests (political, personal, religious, ideological, academic, intellectual, commercial or any other) to declare in relation to this manuscript.
Authors’ contributions
MDT conceived and design the study, analyzed/interpreted results and wrote the manuscript, MJA and ARC performed the mutation studies and analyzed/interpreted results, ABN performed the statistical and SNP-arrays analysis, AO, PS and JG provided the samples and clinical follow-up of the patients, PHD collected and performed the initial treatment of the samples and the iFISH studies, AOR participated in the design of the study, supervision, writing and revision of the final version of the manuscript. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
MariaDolores Tabernero, Email: taberner@usal.es.
María Jara-Acevedo, Email: mariajara@usal.es.
Ana B Nieto, Email: ananieto@usal.es.
Arancha Rodríguez Caballero, Email: arocab@usal.es.
Álvaro Otero, Email: aoteror@saludcastillayleon.es.
Pablo Sousa, Email: pasoucas@yahoo.es.
Jesús Gonçalves, Email: goncalves@usal.es.
Patricia H Domingues, Email: patidomingues_6@hotmail.com.
Alberto Orfao, Email: orfao@usal.es.
Acknowledgements
This work has been partially supported by grants from Consejeria Sanidad Junta de Castilla y León, Gerencia Regional de Salud: GRS689/A/11. Fondo de Investigaciones Sanitarias, Instituto de Salud Carlos III, Ministerio de Sanidad y Consumo, Madrid, Spain: RTICC RD06/0020/0035, RD06/0020/0059 and RD12/0036/0048.
MD Tabernero is supported by IECSCYL (Fundación Instituto de Estudios Ciencias de la Salud de Castilla y León).
References
- Zang KD, Singer H. Chromosomal consitution of meningiomas. Nature. 1967;216(5110):84–85. doi: 10.1038/216084a0. [DOI] [PubMed] [Google Scholar]
- Dumanski JP, Carlbom E, Collins VP, Nordenskjold M. Deletion mapping of a locus on human chromosome 22 involved in the oncogenesis of meningioma. Proc Natl Acad Sci USA. 1987;84(24):9275–9279. doi: 10.1073/pnas.84.24.9275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouleau GA, Merel P, Lutchman M, Sanson M, Zucman J, Marineau C, Hoang-Xuan K, Demczuk S, Desmaze C, Plougastel B. et al. Alteration in a new gene encoding a putative membrane-organizing protein causes neuro-fibromatosis type 2. Nature. 1993;363(6429):515–521. doi: 10.1038/363515a0. [DOI] [PubMed] [Google Scholar]
- Perry A, Gutmann DH, Reifenberger G. Molecular pathogenesis of meningiomas. J Neurooncol. 2004;70(2):183–202. doi: 10.1007/s11060-004-2749-0. [DOI] [PubMed] [Google Scholar]
- van Tilborg AA, Al Allak B, Velthuizen SC, de Vries A, Kros JM, Avezaat CJ, de Klein A, Beverloo HB, Zwarthoff EC. Chromosomal instability in meningiomas. J Neuropathol Exp Neurol. 2005;64(4):312–322. doi: 10.1093/jnen/64.4.312. [DOI] [PubMed] [Google Scholar]
- Goutagny S, Yang HW, Zucman-Rossi J, Chan J, Dreyfuss JM, Park PJ, Black PM, Giovannini M, Carroll RS, Kalamarides M. Genomic profiling reveals alternative genetic pathways of meningioma malignant progression dependent on the underlying NF2 status. Clin Cancer Res. 2010;16(16):4155–4164. doi: 10.1158/1078-0432.CCR-10-0891. [DOI] [PubMed] [Google Scholar]
- Hansson CM, Buckley PG, Grigelioniene G, Piotrowski A, Hellstrom AR, Mantripragada K, Jarbo C, Mathiesen T, Dumanski JP. Comprehensive genetic and epigenetic analysis of sporadic meningioma for macro-mutations on 22q and micro-mutations within the NF2 locus. BMC Genomics. 2007;8:16. doi: 10.1186/1471-2164-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Kim IS, Kwon SY, Jang BC, Suh SI, Shin DH, Jeon CH, Son EI, Kim SP. Mutational analysis of the NF2 gene in sporadic meningiomas by denaturing high-performance liquid chromatography. Int J Mol Med. 2006;18(1):27–32. [PubMed] [Google Scholar]
- Hartmann C, Sieberns J, Gehlhaar C, Simon M, Paulus W, von Deimling A. NF2 mutations in secretory and other rare variants of meningiomas. Brain Pathol. 2006;16(1):15–19. doi: 10.1111/j.1750-3639.2006.tb00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomas J, Bello MJ, Arjona D, Alonso ME, Martinez-Glez V, Lopez-Marin I, Aminoso C, de Campos JM, Isla A, Vaquero J, Rey JA. Genetic and epigenetic alteration of the NF2 gene in sporadic meningiomas. Genes Chromosomes Cancer. 2005;42(3):314–319. doi: 10.1002/gcc.20141. [DOI] [PubMed] [Google Scholar]
- Szijan I, Rochefort D, Bruder C, Surace E, Machiavelli G, Dalamon V, Cotignola J, Ferreiro V, Campero A, Basso A, Dumanski JP, Rouleau GA. NF2 Tumor suppressor gene: a comprehensive and efficient detection of somatic mutations by denaturing HPLC and microarray-CGH. Neuromolecular Med. 2003;3(1):41–52. doi: 10.1385/NMM:3:1:41. [DOI] [PubMed] [Google Scholar]
- Heinrich B, Hartmann C, Stemmer-Rachamimov AO, Louis DN, MacCollin M. Multiple meningiomas: investigating the molecular basis of sporadic and familial forms. Int J Cancer. 2003;103(4):483–488. doi: 10.1002/ijc.10840. [DOI] [PubMed] [Google Scholar]
- Joachim T, Ram Z, Rappaport ZH, Simon M, Schramm J, Wiestler OD, von Deimling A. Comparative analysis of the NF2, TP53, PTEN, KRAS, NRAS and HRAS genes in sporadic and radiation-induced human meningiomas. Int J Cancer. 2001;94(2):218–221. doi: 10.1002/ijc.1467. [DOI] [PubMed] [Google Scholar]
- Evans JJ, Jeun SS, Lee JH, Harwalkar JA, Shoshan Y, Cowell JK, Golubic M. Molecular alterations in the neurofibromatosis type 2 gene and its protein rarely occurring in meningothelial meningiomas. J Neurosurg. 2001;94(1):111–117. doi: 10.3171/jns.2001.94.1.0111. [DOI] [PubMed] [Google Scholar]
- Shoshan Y, Chernova O, Juen SS, Somerville RP, Israel Z, Barnett GH, Cowell JK. Radiation-induced meningioma: a distinct molecular genetic pattern? J Neuropathol Exp Neurol. 2000;59(7):614–620. doi: 10.1093/jnen/59.7.614. [DOI] [PubMed] [Google Scholar]
- Ueki K, Wen-Bin C, Narita Y, Asai A, Kirino T. Tight association of loss of merlin expression with loss of heterozygosity at chromosome 22q in sporadic meningiomas. Cancer Res. 1999;59(23):5995–5998. [PubMed] [Google Scholar]
- Leone PE, Bello MJ, de Campos JM, Vaquero J, Sarasa JL, Pestana A, Rey JA. NF2 gene mutations and allelic status of 1p, 14q and 22q in sporadic meningiomas. Oncogene. 1999;18(13):2231–2239. doi: 10.1038/sj.onc.1202531. [DOI] [PubMed] [Google Scholar]
- Stangl AP, Wellenreuther R, Lenartz D, Kraus JA, Menon AG, Schramm J, Wiestler OD, von Deimling A. Clonality of multiple meningiomas. J Neurosurg. 1997;86(5):853–858. doi: 10.3171/jns.1997.86.5.0853. [DOI] [PubMed] [Google Scholar]
- De Vitis LR, Tedde A, Vitelli F, Ammannati F, Mennonna P, Bono P, Grammatico B, Grammatico P, Radice P, Bigozzi U, Montali E, Papi L. Analysis of the neurofibromatosis type 2 gene in different human tumors of neuroectodermal origin. Hum Genet. 1996;97(5):638–641. doi: 10.1007/BF02281875. [DOI] [PubMed] [Google Scholar]
- Ruttledge MH, Andermann AA, Phelan CM, Claudio JO, Han FY, Chretien N, Rangaratnam S, MacCollin M, Short P, Parry D, Michels V, Riccardi VM, Weksberg R, Kitamura K, Bradburn JM, Hall BD, Propping P, Rouleau GA. Type of mutation in the neurofibromatosis type 2 gene (NF2) frequently determines severity of disease. Am J Hum Genet. 1996;59(2):331–342. [PMC free article] [PubMed] [Google Scholar]
- Harada T, Irving RM, Xuereb JH, Barton DE, Hardy DG, Moffat DA, Maher ER. Molecular genetic investigation of the neurofibromatosis type 2 tumor suppressor gene in sporadic meningioma. J Neurosurg. 1996;84(5):847–851. doi: 10.3171/jns.1996.84.5.0847. [DOI] [PubMed] [Google Scholar]
- Papi L, De Vitis LR, Vitelli F, Ammannati F, Mennonna P, Montali E, Bigozzi U. Somatic mutations in the neurofibromatosis type 2 gene in sporadic meningiomas. Hum Genet. 1995;95(3):347–351. doi: 10.1007/BF00225206. [DOI] [PubMed] [Google Scholar]
- Ng HK, Lau KM, Tse JY, Lo KW, Wong JH, Poon WS, Huang DP. Combined molecular genetic studies of chromosome 22q and the neurofibromatosis type 2 gene in central nervous system tumors. Neurosurgery. 1995;37(4):764–773. doi: 10.1227/00006123-199510000-00022. [DOI] [PubMed] [Google Scholar]
- Wellenreuther R, Kraus JA, Lenartz D, Menon AG, Schramm J, Louis DN, Ramesh V, Gusella JF, Wiestler OD, von Deimling A. Analysis of the neurofibromatosis 2 gene reveals molecular variants of meningioma. Am J Pathol. 1995;146(4):827–832. [PMC free article] [PubMed] [Google Scholar]
- Merel P, Hoang-Xuan K, Sanson M, Moreau-Aubry A, Bijlsma EK, Lazaro C, Moisan JP, Resche F, Nishisho I, Estivill X. et al. Predominant occurrence of somatic mutations of the NF2 gene in meningiomas and schwannomas. Genes Chromosomes Cancer. 1995;13(3):211–216. doi: 10.1002/gcc.2870130311. [DOI] [PubMed] [Google Scholar]
- Lekanne Deprez RH, Bianchi AB, Groen NA, Seizinger BR, Hagemeijer A, van Drunen E, Bootsma D, Koper JW, Avezaat CJ, Kley N. et al. Frequent NF2 gene transcript mutations in sporadic meningiomas and vestibular schwannomas. Am J Hum Genet. 1994;54(6):1022–1029. [PMC free article] [PubMed] [Google Scholar]
- Ruttledge MH, Xie YG, Han FY, Peyrard M, Collins VP, Nordenskjold M, Dumanski JP. Deletions on chromosome 22 in sporadic meningioma. Genes Chromosomes Cancer. 1994;10(2):122–130. doi: 10.1002/gcc.2870100207. [DOI] [PubMed] [Google Scholar]
- Lomas J, Bello MJ, Alonso ME, Gonzalez-Gomez P, Arjona D, Kusak ME, de Campos JM, Sarasa JL, Rey JA. Loss of chromosome 22 and absence of NF2 gene mutation in a case of multiple meningiomas. Hum Pathol. 2002;33(3):375–378. doi: 10.1053/hupa.2002.32229. [DOI] [PubMed] [Google Scholar]
- De Vitis LR, Tedde A, Vitelli F, Ammannati F, Mennonna P, Bigozzi U, Montali E, Papi L. Screening for mutations in the neurofibromatosis type 2 (NF2) gene in sporadic meningiomas. Hum Genet. 1996;97(5):632–637. doi: 10.1007/BF02281874. [DOI] [PubMed] [Google Scholar]
- Tabernero MD, Maíllo A, Nieto AB, Diez-Tascón C, Lara M, Sousa P, Otero A, Castrillo A, Patino-Alonso MC, Espinosa A, Mackintosh C, de Alava E, Orfao A. Delineation of commonly deleted chromosomal regions in meningiomas by high-density single nucleotide polymorphism genotyping arrays. Genes Chromosomes Cancer. 2012;51(6):606–617. doi: 10.1002/gcc.21948. [DOI] [PubMed] [Google Scholar]
- Lee Y, Liu J, Patel S, Cloughesy T, Lai A, Farooqi H, Seligson D, Dong J, Liau L, Becker D, Mischel P, Shams S, Nelson S. Genomic landscape of meningiomas. Brain Pathol. 2009;20(4):751–762. doi: 10.1111/j.1750-3639.2009.00356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahronowitz I, Xin W, Kiely R, Sims K, MacCollin M, Nunes FP. Mutational spectrum of the NF2 gene: a meta-analysis of 12 years of research and diagnostic laboratory findings. Hum Mutat. 2007;28(1):1–12. doi: 10.1002/humu.20393. [DOI] [PubMed] [Google Scholar]
- Schmitz U, Mueller W, Weber M, Sevenet N, Delattre O, von Deimling A. INI1 mutations in meningiomas at a potential hotspot in exon 9. Br J Cancer. 2001;84(2):199–201. doi: 10.1054/bjoc.2000.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Wei W, Zhang J, Liu G, Bignell GR, Stratton MR, Futreal PA, Wooster R, Jones KW, Shapero MH. Whole genome DNA copy number changes identified by high density oligonucleotide arrays. Hum Genomic. 2004;1(4):287–299. doi: 10.1186/1479-7364-1-4-287. [DOI] [PMC free article] [PubMed] [Google Scholar]