Summary
For the identification and quantification of methanogenic archaea (methanogens) in environmental samples, various oligonucleotide probes/primers targeting phylogenetic markers of methanogens, such as 16S rRNA, 16S rRNA gene and the gene for the α‐subunit of methyl coenzyme M reductase (mcrA), have been extensively developed and characterized experimentally. These oligonucleotides were designed to resolve different groups of methanogens at different taxonomic levels, and have been widely used as hybridization probes or polymerase chain reaction primers for membrane hybridization, fluorescence in situ hybridization, rRNA cleavage method, gene cloning, DNA microarray and quantitative polymerase chain reaction for studies in environmental and determinative microbiology. In this review, we present a comprehensive list of such oligonucleotide probes/primers, which enable us to determine methanogen populations in an environment quantitatively and hierarchically, with examples of the practical applications of the probes and primers.
Introduction
Methanogenic archaea (methanogens) are strictly anaerobic microorganisms producing methane as a result of their anaerobic respiration (Schink, 1997; Thauer, 1998). For methanogenesis, they can utilize a limited number of substrates such as carbon dioxide, acetate and methyl‐group‐containing compounds under anoxic conditions (Liu and Whitman, 2008). Most of the known methanogens are hydrogenotrophs reducing carbon dioxide to form methane; among them, formate is also often utilized as the electron donor instead of hydrogen. Some of the hydrogenotrophic methanogens can also utilize secondary alcohols such as 2‐propanol as the electron donor. Acetate is an important intermediate substance in the anaerobic decomposition of organic matter, and is generally exclusively utilized by limited groups of methanogens to form methane under anoxic conditions, where external electron acceptors other than carbon dioxide are unavailable. Methyl‐group‐containing compounds, such as methanol and methylamines, are also utilized by some methanogens through disproportionation of methyl groups.
Methanogens are frequently found in anoxic environments, such as rice paddy fields (Iino et al., 2010; Sakai et al., 2010), wetlands (Cadillo‐Quiroz et al., 2009; Bräuer et al., 2010), permafrost (Krivushin et al., 2010; Shcherbakova et al., 2010), landfills (Laloui‐Carpentier et al., 2006), subsurfaces (Doerfert et al., 2009; Mochimaru et al., 2009) and ruminants (Frey et al., 2009), which are known to be the major sources of atmospheric methane. It has been estimated that the annual global emission of methane is 500–600 Tg, and atmospheric methane concentration has risen threefold over the past 200 years (Liu and Whitman, 2008). With the increased interests in global climate change and environmental issues, studies on the diversity and ecophysiological functions of methanogens in such environments have been extensively conducted using cultivation‐dependent and cultivation‐independent approaches (Liu and Whitman, 2008). In addition to such environments, methanogens play key roles in fields of anaerobic digestion technology, which is widely used as a means for treating municipal and industrial waste/wastewater containing high levels of organic compounds (Sekiguchi, 2006; Narihiro and Sekiguchi, 2007; Talbot et al., 2008; Tabatabaei et al., 2010). Methanogens are often critical components of such bioconversion systems, resulting in the recovery of gaseous methane from those wastes as reusable energy resource. To better manage the bioconversion systems and achieve a higher efficiency in removing organic compounds in wastes, methanogens in these systems have been extensively studied and the quantitative monitoring of such methanogenic populations in these systems has been conducted (Narihiro and Sekiguchi, 2007).
To explore the ecological significance of methanogens in these natural and engineered ecosystems, identification and quantification techniques for different methanogen groups are indispensable. For the purpose, analyses of membrane lipid (Weijers et al., 2004; Strapoc et al., 2008), autofluorescence (Neu et al., 2002; Tung et al., 2005; Mochimaru et al., 2007), activity measurement (Lehmann‐Richter et al., 1999; Weijers et al., 2004) and immunoenzymatic profiling (Visser et al., 1991; Sorensen and Ahring, 1997) have been used. In addition to these methods, cultivation‐independent, nucleic acid‐based analysis by using oligonucleotide probe/primers, such as membrane hybridization, fluorescence in situ hybridization (FISH), gene cloning, quantitative polymerase chain reaction (qPCR), and cleavage method with ribonuclease H (RNase H) were most widely and frequently used as means to detect and quantify methanogens more specifically and accurately. In this review, we present a catalogue of previously developed oligonucleotide probes/primers targeting genes of methanogens. Particular emphasis is placed on the probes/primers for 16S rRNA, 16S rRNA gene and the gene for the α‐subunit of methyl coenzyme M reductase (mcrA), which are generally used for the taxonomic classification of methanogens (Friedrich, 2005; Liu and Whitman, 2008).
Phylogeny of methanogens
All the methanogens isolated and characterized to date have been classified into the phylum Euryarchaeota of the domain Archaea (Garrity et al., 2007). They are assigned into 33 genera of the classes ‘Methanomicrobia’, Methanobacteria, Methanococci and Methanopyri (Fig. 1, Table 1). The class ‘Methanomicrobia’ is the most phylogenetically and physiologically diverse group of methanogens consisting of three orders (Methanosarcinales, Methanomicrobiales and Methanocellales); 23 genera belonging to seven families (Fig. 1, Table 1). Within the order Methanosarcinales, the genera Methanosarcina and Methanosaeta are known to play a key role in the conversion of acetate into methane in various anaerobic environments, and the rest are known to metabolize relatively broad ranges of substrates, such as hydrogen, methanol and methylamines (Garrity and Holt, 2001). Known members of the order Methanomicrobiales are all hydrogenotrophs, and some of them are often observed in anaerobic environments as important hydrogen scavengers (Liu and Whitman, 2008). Members of the class Methanobacteria, consisting of the families Methanobacteriaceae and Methanothermaceae, are recognized as important hydrogenotrophs that have also been widely found in anaerobic ecosystems (Garrity and Holt, 2001). Methanobacteriaceae comprises four genera, Methanobacterium, Methanosphaera, Methanobrevibacter and Methanothermobacter. The class Methanococci includes the families Methanococcaceae and Methanocaldococcaceae, which are widely distributed in natural ecosystems such as marine sediments and deep sea geothermal sediments (Liu and Whitman, 2008). The class Methanopyri consists of solely the genus Methanopyrus, a hyperthermophilic, hydrogenotrophic methanogen isolated from the deep‐sea hydrothermal field (Takai et al., 2008).
Figure 1.
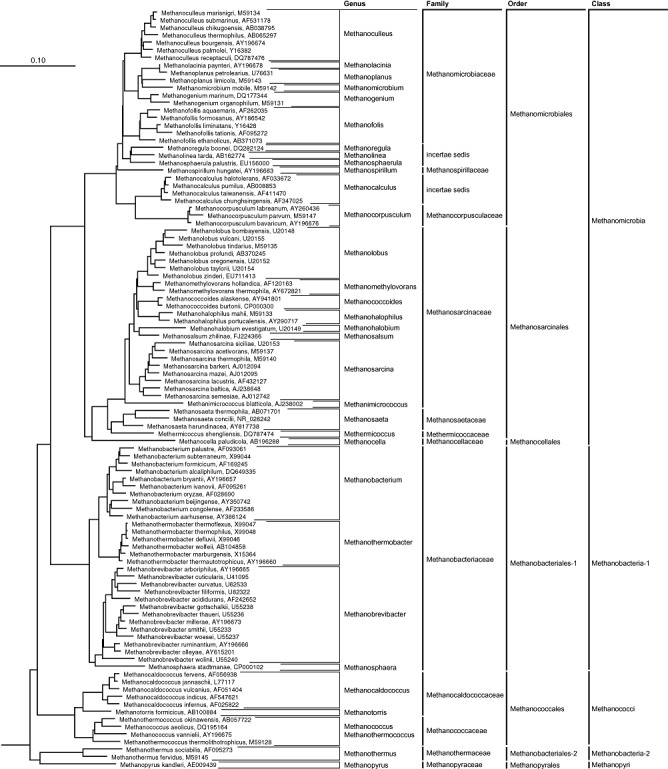
Phylogeny and taxonomy of methanogens. The neighbour‐joining tree was constructed on the basis of 16S rRNA gene sequences using the ARB package (Ludwig et al., 2004) with the data set (Yarza et al., 2008) provided from silva databases (http://silva.mpi‐bremen.de/), showing representative species of methanogens that have been described to date.
Table 1.
Oligonucleotide probes and primers targeting the 16S rRNA gene of methanogens.
| Target group | Probe name | Probe sequence (5′–3′)a | Application | Probe length (mer) | Reference |
|---|---|---|---|---|---|
| Most methanogens | Arch f2b | TTCYGGTTGATCCYGCCRGA | PCR (forward) | 20 | Skillman et al. (2004) |
| Arch r1386 | GCGGTGTGTGCAAGGAGC | PCR (reverse) | 18 | Skillman et al. (2004) | |
| A1f | TCYGKTTGATCCYGSCRGAG | PCR (forward), DGGE | 20 | Embley et al. (1992) | |
| A1100r | TGGGTCTCGCTCGTTG | PCR (forward), DGGE | 16 | Embley et al. (1992) | |
| Met83F | ACKGCTCAGTAACAC | PCR (forward) | 15 | Wright and Pimm (2003) | |
| Met86F | GCTCAGTAACACGTGG | PCR (forward) | 16 | Wright and Pimm (2003) | |
| Met448F | GGTGCCAGCCGCCGC | sequencing | 15 | Wright and Pimm (2003) | |
| Met1027F | GTCAGGCAACGAGCGAGACC | sequencing | 20 | Wright and Pimm (2003) | |
| Met1340R | CGGTGTGTGCAAGGAG | PCR (reverse) | 16 | Wright and Pimm (2003) | |
| 109f | ACKGCTCAGTAACACGT | PCR (forward) | 17 | Grosskopf et al. (1998) | |
| 146f | GGSATAACCYCGGGAAAC | PCR (forward) | 18 | Marchesi et al. (2001) | |
| 1324r | GCGAGTTACAGCCCWCRA | PCR (reverse) | 18 | Marchesi et al. (2001) | |
| ARC344f | ACGGGGYGCAGCAGGCGCGA | PCR (forward), DGGE | 20 | Casamayor et al. (2001) | |
| 25f | CYGGTYGATYCTGCCRG | PCR (forward) | 17 | Dojka et al. (1998) | |
| 1391r | GACGGGCGGTGTGTRCA | PCR (reverse) | 17 | Barns et al. (1994) | |
| A24f | TCYGKTTGATCCYGSCRGA | PCR (forward), DGGE | 19 | Yu et al. (2008) | |
| A357f | CCCTACGGGGCGCAGCAG | PCR (forward), DGGE | 18 | Yu et al. (2008) | |
| A329r | TGTCTCAGGTTCCATCTCCG | PCR (reverse), DGGE | 20 | Yu et al. (2008) | |
| A348r | CCCCRTAGGGCCYGG | PCR (reverse), DGGE | 15 | Yu et al. (2008) | |
| A693r | GGATTACARGATTTC | PCR (reverse), DGGE | 15 | Yu et al. (2008) | |
| Met630F | GGATTAGATACCCSGGTAGT | qPCR (forward), DGGE | 20 | Hook et al. (2009) | |
| Met803R | GTTGARTCCAATTAAACCGCA | qPCR (reverse), DGGE | 21 | Hook et al. (2009) | |
| A1040f | GAGAGGWGGTGCATGGCC | PCR (forward), DGGE | 18 | Reysenbach and Pace (1995) | |
| ARC344 | TCGCGCCTGCTGCICCCCGT | MH | 20 | Raskin et al. (1994b) | |
| ARC915 | GTGCTCCCCCGCCAATTCCT | PCR (reverse), DGGE, MH, FISH | 20 | Raskin et al. (1994b) | |
| MER1 | GGGCACGGGTCTCGCT | PCR (reverse) | 16 | Hales et al. (1996) | |
| Class Methanomicrobia | 1068R | ATGCTTCACAGTACGAAC | PCR (reverse) | 18 | Banning et al. (2005) |
| CMSMM1068m | GGATGCTTCACAGTACGAAC | RNase H | 20 | Narihiro et al. (2009b) | |
| Order Methanocellales | |||||
| Family Methanocellaceae | |||||
| Genus Methanocella | SANAE1136 | GTGTACTCGCCCTCCTCG | FISH | 18 | Sakai et al. (2007) |
| Order Methanomicrobiales | MG1200 | CGGATAATTCGGGGCATGCTG | MH, FISH | 21 | Raskin et al. (1994b) |
| MG1200m | CCGGATAATTCGGGGCATGCTG | RNase H | 22 | Narihiro et al. (2009b) | |
| M(SA/MI)355 | GTAAAGTTTTCGCGCCTG | MH | 18 | Ovreås et al. (1997) | |
| MMB282F | ATCGRTACGGGTTGTGGG | qPCR (forward) | 18 | Yu et al. (2005) | |
| MMB749F | TYCGACAGTGAGGRACGAAAGCTG | qPCR (probe) | 24 | Yu et al. (2005) | |
| MMB832R | CACCTAACGCRCATHGTTTAC | qPCR (reverse) | 21 | Yu et al. (2005) | |
| Family Methanomicrobiaceae | |||||
| Genus Methanoculleus | 298F | GGAGCAAGAGCCCGGAGT | qPCR (forward) | 18 | Franke‐Whittle et al. (2009a) |
| 586R | CCAAGAGACTTAACAACCCA | qPCR (reverse) | 20 | Franke‐Whittle et al. (2009a) | |
| F2SC668 | TCCTACCCCCGAAGTACCCCTC | RNase H | 22 | Narihiro et al. (2009b) | |
| F2SC732 | TCGAAGCCGTTCTGGTGAGGCG | RNase H | 22 | Narihiro et al. (2009b) | |
| AR934F | AGGAATTGGCGGGGGAGCAC | qPCR (forward) | 20 | Shigematsu et al. (2003) | |
| MCU1023TAQ | GAATGATTGCCGGGCTGAAGACTC | qPCR (probe) | 24 | Shigematsu et al. (2003) | |
| MG1200b | CCGGATAATTCGGGGCATGCTG | qPCR (reverse) | 22 | Shigematsu et al. (2003) | |
| Species M. thermophilus | Mc412f | CTGGGTGTCTAAAACACACCCAA | qPCR (forward) | 23 | Hori et al. (2006) |
| Mc578r | ATTGCCAGTATCTCTTAG | qPCR (reverse) | 18 | Hori et al. (2006) | |
| SMCUT1253 | GCCTTTCGGCGTCGATACCC | RNase H | 20 | Narihiro et al. (2009b) | |
| Genus Methanofollis | F3SC984 | CATATCGCTGTCCTACCCGG | RNase H | 20 | Narihiro et al. (2009b) |
| Genus Methanogenium | GMG1128 | CGTTCCGGAGAACAAGCTAG | RNase H | 20 | Narihiro et al. (2009b) |
| Genus Methanomicrobium | GMM829 | CTCGTAGTTACAGGCACACC | FISH, RNase H | 20 | Yanagita et al. (2000) |
| Genus Methanoplanus | |||||
| Species M. limicola | SMPL623c | TTCTCTTAAACGCCTGCAGG | RNase H | 20 | Narihiro et al. (2009b) |
| Species M. endosynbiosus | SMPL623c | TTCTCTTAAACGCCTGCAGG | RNase H | 20 | Narihiro et al. (2009b) |
| Species M. petrolearius | SMPP1252d | CTTCTCAGTGTCGTTGCTCA | RNase H | 20 | Narihiro et al. (2009b) |
| Genus Methanolacinia | SMPP1252d | CTTCTCAGTGTCGTTGCTCA | RNase H | 20 | Narihiro et al. (2009b) |
| Family Methanospirillaceae | |||||
| Genus Methanospirillum | F7SC1260 | TATCCTCACCTCTCGGTGTC | RNase H | 20 | Narihiro et al. (2009b) |
| MSP1025TAQ | GAATGATAGTCGGGATGAAGACTCTA | qPCR (probe) | 26 | Tang et al. (2005) | |
| Genus Methanosphaerula | |||||
| Genus Methanolinea | NOBI109f | ACTGCTCAGTAACACGT | qPCR (forward) | 17 | Imachi et al. (2008) |
| NOBI633 | GATTGCCAGTTTCTCCTG | qPCR (reverse), FISH | 18 | Imachi et al. (2008) | |
| Family Methanocorpusculaceae | |||||
| Genus Methanocorpusculum | F6SC393e | GACAGGCACTCAGGGTTTCC | RNase H | 20 | Narihiro et al. (2009b) |
| GMCP489 | GCCCTGCCCTTTCTTCACAT | RNase H | 20 | Narihiro et al. (2009b) | |
| Family incertae sedis | |||||
| Genus Methanocalculus | F6SC393e | GACAGGCACTCAGGGTTTCC | RNase H | 20 | Narihiro et al. (2009b) |
| GMCL488 | CCCCGCCCTTTCTCCTGGTG | RNase H | 20 | Narihiro et al. (2009b) | |
| Genus Methanoregula | |||||
| Species M. boonei | 6A8 644 | TCTTCCGGTCCCTAGCCTGCCA | FISH | 22 | Bräuer et al. (2006) |
| Species M. formicica | SMSP129 | TATCCCCTTCCATAGGGTAGATT | FISH | 23 | Yashiro et al. (2009) |
| Order Methanosarcinales | MSMX860 | GGCTCGCTTCACGGCTTCCCT | MH | 21 | Raskin et al. (1994b) |
| MSSH859 | TCGCTTCACGGCTTCCCT | FISH | 18 | Boetius et al. (2000) | |
| MSr r859 | TCGCTTCACGGCTTCCCTG | PCR (reverse) | 19 | Skillman et al. (2004) | |
| MSMX860m | GCTCGCTTCACGGCTTCCCT | RNase H | 20 | Narihiro et al. (2009b) | |
| MSL812F | GTAAACGATRYTCGCTAGGT | qPCR (forward) | 20 | Yu et al. (2005) | |
| MSL860F | AGGGAAGCCGTGAAGCGARCC | qPCR (probe) | 21 | Yu et al. (2005) | |
| MSL1159R | GGTCCCCACAGWGTACC | qPCR (reverse) | 17 | Yu et al. (2005) | |
| Family Methanosaetaceae | |||||
| Genus Methanosaeta | MX825 | TCGCACCGTGGCCGACACCTAGC | MH, FISH | 23 | Raskin et al. (1994b) |
| MX825mix | TCGCACCGTGGCYGACACCTAGC | RNase H | 23 | Narihiro et al. (2009b) | |
| MX1361 | ACGTATTCACCGCGTTCTGT | FISH | 20 | Crocetti et al. (2006) | |
| S‐G‐Msae‐0332‐a‐A‐22 | TTAGGTCCGGGATGCXCCACGT | MH, FISH | 22 | Zheng and Raskin (2000) | |
| Mst702F | TAATC CTYGA RGGAC CACCA | qPCR (forward) | 20 | Yu et al. (2005) | |
| Mst753F | ACGGC AAGGG ACGAA AGCTA GG | qPCR (probe) | 22 | Yu et al. (2005) | |
| Mst862R | CCTAC GGCAC CRACM AC | qPCR (reverse) | 17 | Yu et al. (2005) | |
| MS1b | CCGGCCGGATAAGTCTCTTGA | qPCR (forward) | 21 | Shigematsu et al. (2003) | |
| SAE761TAQ | ACCAGAACGGACCTGACGGCAAGG | qPCR (probe) | 24 | Shigematsu et al. (2003) | |
| SAE835R | GACAACGGTCGCACCGTGGCC | qPCR (reverse) | 21 | Shigematsu et al. (2003) | |
| S‐F‐Msaet‐0387‐S‐a‐21 | GATAAGGGRAYCTCGAGTGCY | qPCR (forward) | 21 | Sawayama et al. (2004) | |
| S‐F‐Msaet‐0540‐A‐a‐31 | AGACCCAATAAHARCGGTTACCACTCGRGCC | qPCR (probe) | 31 | Sawayama et al. (2004) | |
| S‐F‐Msaet‐0573‐A‐a‐17 | GGCCGRCTACAGACCCT | qPCR (reverse) | 17 | Sawayama et al. (2004) | |
| Species M. concilii | Rotcl1 | CTCCCGGCCTCGAGCCAGAC | FISH | 20 | Zepp Falz et al. (1999) |
| MS1 | CCGGATAAGTCTCTTGA | MH | 17 | Rocheleau et al. (1999) | |
| MS2 | CTGAATGAGAGCGCTTTCTTT | MH | 21 | Rocheleau et al. (1999) | |
| MS5 | GGCCACGGTGCGACCGTTGTCG | MH, FISH | 22 | Rocheleau et al. (1999) | |
| MMX1273 | GGTTTTAGGAGATTCCCGTC | RNase H | 20 | Narihiro et al. (2009b) | |
| GTMS393m | ACCCAGCACTCGAGGTCCCC | RNase H | 20 | Narihiro et al. (2009b) | |
| Species M. thermophila | Ms413f | CAGATGTGTAAAATACATCTGTT | qPCR (forward) | 23 | Hori et al. (2006) |
| Ms578r | TCTGGCAGTATCCACCGA | qPCR (reverse) | 18 | Hori et al. (2006) | |
| TMX745 | CCCTTGCCGTCGGATCCGTT | RNase H | 20 | Narihiro et al. (2009b) | |
| Family Methanosarcinaceae | MS1414 | CTCACCCATACCTCACTCGGG | MH, FISH | 21 | Raskin et al. (1994b) |
| EelMS240f | CTATCAGGTTGTAGTGGG | FISH | 18 | Boetius et al. (2000) | |
| Msc380F | GAAACCGYGATAAGGGGA | qPCR (forward) | 18 | Yu et al. (2005) | |
| Msc492F | TTAGCAAGGGCCGGGCAA | qPCR (probe) | 18 | Yu et al. (2005) | |
| Msc828R | TAGCGARCATCGTTTACG | qPCR (reverse) | 18 | Yu et al. (2005) | |
| R15Fg | GCTACACGCGGGCTACAATGA | qPCR (forward) | 21 | Zhang et al. (2008a) | |
| FMSC394 | ATGCTGGCACTCGGTGTCCC | RNase H | 20 | Narihiro et al. (2009b) | |
| MS821mh | GCCATGCCTGACACCTAGCG | RNase H | 20 | Narihiro et al. (2009b) | |
| Genus Methanimicrococcus | GMIB1254 | CACCTTTCGGTGTAGTTGCC | RNase H | 20 | Narihiro et al. (2009b) |
| Genus Methanosarcina | MS821 | CGCCATGCCTGACACCTAGCGAGC | MH, FISH | 24 | Raskin et al. (1994b) |
| SARCI551 | GACCCAATAATCACGATCAC | FISH | 20 | Sorensen and Ahring (1997) | |
| SARCI645 | TCCCGGTTCCAAGTCTGGC | FISH | 19 | Sorensen and Ahring (1997) | |
| MB1 | TTTGGTCAGTCCTCCGG | MH | 17 | Rocheleau et al. (1999) | |
| MB3 | CCAGACTTGGAACCG | MH | 15 | Rocheleau et al. (1999) | |
| MB4 | TTTATGCGTAAAATGGATT | MH, FISH | 19 | Rocheleau et al. (1999) | |
| 240F | CCTATCAGGTAGTAGTGGGTGTAAT | qPCR (forward) | 25 | Franke‐Whittle et al. (2009a) | |
| 589R | CCCGGAGGACTGACCAAA | qPCR (reverse) | 18 | Franke‐Whittle et al. (2009a) | |
| MB1b | CGGTTTGGTCAGTCCTCCGG | qPCR (forward) | 20 | Shigematsu et al. (2003) | |
| SAR761TAQ | ACCAGAACGGGTTCGACGGTGAGG | qPCR (probe) | 24 | Shigematsu et al. (2003) | |
| SAR835R | AGACACGGTCGCGCCATGCCT | qPCR (reverse) | 21 | Shigematsu et al. (2003) | |
| S‐G‐Msar‐0450‐S‐a‐19 | TAGCAAGGGCCGGGCAAGA | qPCR (forward) | 19 | Sawayama et al. (2006) | |
| S‐P‐Msar‐0540‐A‐a‐31 | AGACCCAATAATCACGATCACCACTCGGGCC | qPCR (probe) | 31 | Sawayama et al. (2006) | |
| S‐G‐Msar‐0589‐S‐a‐20 | ATCCCGGAGGACTGACCAAA | qPCR (reverse) | 20 | Sawayama et al. (2006) | |
| Genus Methanococcoides | GMCO441 | ACATGCCGTTTACACATGTG | RNase H | 20 | Narihiro et al. (2009b) |
| Genus Methanohalobium | GMHB842 | TCGGCACTAGGAACGGCCGT | RNase H | 20 | Narihiro et al. (2009b) |
| Genus Methanohalophilus | GMHP1258 | CCGTCACTTTTCAGTGTAGG | RNase H | 20 | Narihiro et al. (2009b) |
| Genus Methanolobus | GMLB834 | TGAAACGGTCGCACCGTCCCAG | RNase H | 22 | Narihiro et al. (2009b) |
| Species M. psychrophilus | R15F | GCTACACGCGGGCTACAATGA | qPCR (forward) | 21 | Zhang et al. (2008a) |
| R15R | AATTTAGGTTCGAACACGGCATGAA | qPCR (reverse) | 25 | Zhang et al. (2008a) | |
| Genus Methanomethylovorans | |||||
| Genus Methanosalsum | GMSS261 | GTCGGCTAGCAGGTACCTTG | RNase H | 20 | Narihiro et al. (2009b) |
| Family Methermicoccaceae | |||||
| Genus Methermicoccus | |||||
| Class Methanobacteria | |||||
| Order Methanobacteriales | MB310 | CTTGTCTCAGGTTCCATCTCCG | MH | 22 | Raskin et al. (1994b) |
| MB311 | ACCTTGTCTCAGGTTCCATCTCC | FISH | 23 | Crocetti et al. (2006) | |
| Mbac f331 | CTTGTCTCAGGTTCCATCTC | PCR | 20 | Skillman et al. (2004) | |
| MB1174 | TACCGTCGTCCACTCCTTCCTC | MH, FISH | 22 | Raskin et al. (1994b) | |
| MBT857F | CGWAGGGAAGCTGTTAAGT | qPCR (forward) | 19 | Yu et al. (2005) | |
| MBT929F | AGCACCACAACGCGTGGA | qPCR (probe) | 18 | Yu et al. (2005) | |
| MBT1196R | TACCGTCGTCCACTCCTT | qPCR (reverse) | 18 | Yu et al. (2005) | |
| 1401R | KTTTGGGTGGYGTGACGGGC | PCR (reverse) | 20 | Banning et al. (2005) | |
| Family Methanobacteriaceae | MB1175m | CCGTCGTCCACTCCTTCCTC | RNase H | 20 | Narihiro et al. (2009b) |
| MEB859 | AGGGAAGCTGTTAAGTCC | FISH | 18 | Boetius et al. (2000) | |
| Genus Methanobrevibacter | fMbb1 | CTCCGCAATGTGAGAAATCG | PCR | 20 | Skillman et al. (2004) |
| GMB406 | GCCATCCCGTTAAGAATGGC | RNase H | 20 | Narihiro et al. (2009b) | |
| Species M. ruminantium | MBR1001 | TCAGCCTGGTAATCATACA | FISH | 19 | Yanagita et al. (2000) |
| Species M. smithii | Forward | CCGGGTATCTAATCCGGTTC | qPCR (forward) | 20 | Armougom et al. (2009) |
| Reverse | CTCCCAGGGTAGAGGTGAAA | qPCR (reverse) | 20 | Armougom et al. (2009) | |
| Probe | CCGTCAGAATCGTTCCAGTCAG | qPCR (probe) | 22 | Armougom et al. (2009) | |
| Genus Methanobacterium | fMbium | CGTTCGTAGCCGGCYTGA | PCR | 18 | Skillman et al. (2004) |
| GMBA755 | TGGCTTTCGTTACTCACC | RNase H | 18 | Narihiro et al. (2009b) | |
| S‐F‐Mbac‐0398‐S‐a‐20 | CCCAAGTGCCACTCTTAACG | qPCR (forward) | 20 | Sawayama et al. (2006) | |
| S‐G‐Mbac‐0526‐A‐a‐33 | AAYGGCCACCACTTGAGCTGCCGGTGTTACCGC | qPCR (reverse) | 33 | Sawayama et al. (2006) | |
| S‐G‐Mbac‐0578‐A‐a‐22 | AGACTTATCAARCCGGCTACGA | qPCR (probe) | 22 | Sawayama et al. (2006) | |
| Genus Methanosphaera | GMSP838 | CCGGAACAACTCGAGGCCAT | RNase H | 20 | Narihiro et al. (2009b) |
| Genus Methanothermobacter | Mt392f | ACTCTTAACGGGGTGGCTTTT | qPCR (forward) | 21 | Hori et al. (2006) |
| Mt578r | TCATGATAGTATCTCCAGC | qPCR (reverse) | 19 | Hori et al. (2006) | |
| 410F | CTCTTAACGGGGTGGCTTTT | qPCR (forward) | 20 | Franke‐Whittle et al. (2009a) | |
| 667R | CCCTGGGAGTACCTCCAGC | qPCR (reverse) | 19 | Franke‐Whittle et al. (2009a) | |
| GMTB541 | AAAAGCGGCTACCACTTGAGCT | RNase H | 22 | Narihiro et al. (2009b) | |
| S‐F‐Mbac‐0398‐S‐a‐20 | CCCAAGTGCCACTCTTAACG | qPCR (forward) | 20 | Sawayama et al. (2006) | |
| S‐G‐Mthb‐0549‐S‐a‐32 | CGGACGCTTTAGGCCCAATAAAAGCGGCTACC | qPCR (probe) | 32 | Sawayama et al. (2006) | |
| S‐G‐Mthb‐0589‐A‐a‐25 | GGGATTTCACCAGAGACTTATCAG | qPCR (reverse) | 25 | Sawayama et al. (2006) | |
| Family Methanothermaceae | |||||
| Genus Methanothermus | FMTH1183 | TACGGACCTACCGTCGCCCGCA | RNase H | 22 | Narihiro et al. (2009b) |
| Class Methanococci | |||||
| Order Methanococcales | M(CO/BA)377 | CCCCCGTCGCACTTKCGTG | MH | 19 | Ovreås et al. (1997) |
| Mcc r | WASTVGCAACATAGGGCACGG | PCR (reverse) | 21 | Skillman et al. (2004) | |
| MCC495F | TAAGGGCTGGGCAAGT | qPCR (forward) | 16 | Yu et al. (2005) | |
| MCC686F | TAGCGGTGRAATGYGTTGATCC | qPCR (probe) | 22 | Yu et al. (2005) | |
| MCC832R | CACCTAGTYCGCARAGTTTA | qPCR (reverse) | 20 | Yu et al. (2005) | |
| 1202R | CCAGGRGATTCGGGGCATGC | PCR (reverse) | 20 | Banning et al. (2005) | |
| Family Methanocaldococcaceae | S‐F‐Mcc‐1109‐b‐A‐20 | GCAACATGGGGCRCGGGTCT | MH | 20 | Nercessian et al. (2004) |
| MC504 | GGCTGCTGGCACCGGACTTGCCCA | FISH | 24 | Crocetti et al. (2006) | |
| FMCMT1044i | GTCAACCTGGCCTTCATCCTGC | RNase H | 22 | Narihiro et al. (2009b) | |
| Genus Methanocaldococcus | |||||
| Genus Methanotorris | |||||
| Family Methanococcaceae | MC1109 | GCAACATAGGGCACGGGTCT | MH | 20 | Raskin et al. (1994b) |
| Genus Methanococcus | GMC728 | ACCCGTTCCAGACAAGTGCCTT | RNase H | 22 | Narihiro et al. (2009b) |
| GMC231 | ACTACCTAATCGAGCGCAGTCC | RNase H | 22 | Narihiro et al. (2009b) | |
| GMC416 | TTGATAAAAGCCCATGCTGTGC | RNase H | 22 | Narihiro et al. (2009b) | |
| Genus Methanothermococcus | GMTL416 | TAGAAAAGCCTACGCAGTGC | RNase H | 20 | Narihiro et al. (2009b) |
| Class Methanopyri | |||||
| Order Methanopyrales | |||||
| Family Methanopyraceae | |||||
| Genus Methanopyrus | FMCMT1044i | GTCAACCTGGCCTTCATCCTGC | RNase H | 22 | Narihiro et al. (2009b) |
| S‐G‐Mp‐0431‐a‐A‐20 | TTACACCCCGGTACAGCCGC | MH | 20 | Nercessian et al. (2004) | |
| GMPK1331 | GGTTACTACCGATTCCACCTTC | RNase H | 22 | Narihiro et al. (2009b) |
IUPAC Ambiguity Codes: Y = C or T, R = A or G, K = G or T, S = C or G, W = A or T, M = A or C, H = A or C or T, V = A or C or G
Arch f2 probe covers members of the orders Methanomicrobiales, Methanosarcinales and Methanococcales.
SMPL623 probe covers members of the Methanoplanus limicola and M. endosynbiosus.
SMPP1252 probe covers members of the Methanoplanus petrolearius and Methanolacinia.
F6SC393 probe covers members of the genera Methanocorpusculum and Methanocalculus.
EelMS240 probe targets for members of the genera Methanolobus, Methanohalophilus, Methanococcoides and Methanomethylovorans.
R15F probe covers members of the genera Methanomethylovorans and Methanosarcina and Methanolobus psychrophilus.
MS821m probe covers members of the genera Methanimicrococcus and Methanosarcina.
FMCMT1044 probe covers members of the family Methanocaldococcaceae and genus Methanopyrus.
MH, membrane hybridization.
The isolation and characterization of novel methanogens from various ecosystems are ongoing, and the descriptions of such methanogens have been carried out at an encouraging rate. Recently, hydrogenotrophic methanogens, which are novel at high taxonomic levels (Methanocella paludicola and Methanocella arvoryzae), have been isolated, and the novel order Methanocellales was proposed (Sakai et al., 2008; 2010). These methanogens have long been considered as the uncultivable methanogen group (Rice cluster I), and responsible for the major part of methanogenesis in rice paddy soil (Conrad et al., 2006). In addition, novel hydrogenotrophic methanogens associated with previously uncultivated phylogenetic groups of the order Methanomicrobiales (formerly known as E1/E2 or Fen cluster) were isolated from anaerobic bioreactors (Imachi et al., 2008; Yashiro et al., 2009) and wetlands (Cadillo‐Quiroz et al., 2009; Bräuer et al., 2010). Novel strains of the genera Methanofollis (Imachi et al., 2009), Methanolobus (Doerfert et al., 2009; Mochimaru et al., 2009), Methanospirillum (Iino et al., 2010) and Methanobacterium (Krivushin et al., 2010; Shcherbakova et al., 2010) have also been reported recently.
Despite these efforts in cultivating as yet uncultivable methanogens present in environments, there are still a vast number of uncultivable archaeal taxa that may have similar metabolic functions as those of known methanogens. For example, 16S rRNA gene types assigned into the WSA2 (or ArcI) group were frequently retrieved from methanogenic waste/wastewater treatment systems (Sekiguchi and Kamagata, 2004; Chouari et al., 2005). The WSA2 group is considered to be an archaeal taxon at the class level with no cultured representatives (Hugenholtz, 2002). However, Chouari and colleagues have found that WSA2‐related cells can be enriched using formate‐ or hydrogen‐containing culture media, suggesting that they harbour methanogenic activity (Chouari et al., 2005). Another example similar to the Rice Cluster I group is Rice Cluster II (RC‐II). Members of the RC‐II group were also considered to be methanogens, because the 16S rRNA gene clones affiliated with this group were frequently observed in methanogenic enrichment cultures containing ethanol as an electron donor, and because the RC‐II group is a lineage within the phylogenetic radiation of the orders Methanosarcinales and Methanomicrobiales (Lehmann‐Richter et al., 1999). As can be noted from these examples, there is no doubt that the actual biodiversity of methanogens will be much expanded in the future as the number of isolated and described methanogens continues to increase. However, in this review, we mainly focus on the quantitative monitoring tools for previously cultured methanogens.
Oligonucleotide probes/primers for 16S rRNA and its gene
16S rRNA and its gene are the most frequently used biomarkers for the determination of methanogenic populations in environments. 16S rRNA gene‐targeted probes/primers frequently used for identifying methanogens are listed in Table 1. To entirely describe methanogenic populations in ecosystems of interest, 16S rRNA gene‐targeted primer sets for a wide range of methanogen taxa, such as 146f/1324r (Marchesi et al., 2001) and Met83F (Met86F)/Met1340R (Wright and Pimm, 2003), were developed. In addition, a number of oligonucleotide probes/primers for specifically and hierarchically detecting methanogens at different taxonomic levels were designed to resolve different methanogen populations in waste/wastewater treatment anaerobic sludges (Rocheleau et al., 1999; Zheng and Raskin, 2000; Hori et al., 2006; Ariesyady et al., 2007; Franke‐Whittle et al., 2009a; Narihiro et al., 2009a,b), the rumen (Yanagita et al., 2000; Skillman et al., 2004), subseafloor sediments (Boetius et al., 2000; Nercessian et al., 2004), sediments (Falz et al., 1999), the human gut (Armougom et al., 2009) and wetlands (Bräuer et al., 2006; Zhang et al., 2008a,b) (Table 1). Nowadays, almost all of the known culturable methanogens can be detected using these probes/primers at the class, order, family genus and even species levels; at the genus level, it should be noted that the probes/primers targeting for the genera Methermicoccus, Methanomethylovorans, Methanocaldococcus and Methanotorris are lacking.
Oligonucleotide probes/primers for mcrA gene
The 16S rRNA gene has been best used for the identification of methanogens in environments. However, because archaeal 16S rRNA genes other than those of methanogens can also often be detected using PCR primer sets for a wide range of methanogen taxa, it has limitation in exclusively describing the population structure of methanogens. Therefore, there is a need to detect methanogens on the basis of functional genes that are found to be unique in methanogenesis. Such a functional gene frequently used is mcrA. Methyl coenzyme M reductase (mcr) is the terminal enzyme involved in methanogenesis, which reduces the methyl group bond of methyl coenzyme M with the release of methane (Friedrich, 2005). Because the α‐subunit of mcr (mcrA) and its isoenzyme gene (mrtA) are highly conserved among methanogens, and that these genes are almost exclusively found in methanogens, mcrA/mrtA‐based detection of methanogens has been used. The phylogeny of methanogens determined using mcrA/mrtA (or translated amino acid) sequences is in good accordance with those determined using 16S rRNA gene sequences (Friedrich, 2005). Previously reported, frequently used probes/primers for mcrA/mrtA are categorized into three primer sets, namely, MCR (Springer et al., 1995), ME (Hales et al., 1996) and ML (Luton et al., 2002) (Table 2). The targeted regions of the forward primers of these sets are considerably different, whereas those of the reverse primers are almost the same. The MCR primer set was originally designed to determine the phylogeny of the family Methanosarcinaceae (Springer et al., 1995). The ME primer set was designed to describe methanogenic populations in wetlands (Hales et al., 1996), for which the difficulty in amplifying mcrA/mrtA relevant to Methanosarcinaceae and Methanobacteriaceae was pointed out later (Lueders et al., 2001; Juottonen et al., 2006). The ML primer set was developed on the basis of the mcrA sequences obtained from five orders, comprising Methanosarcinales, Methanomicrobiales, Methanobacteriales, Methanococcales and Methanopyrales (Luton et al., 2002). Four other primer sets and probes for specific taxonomic groups have also been developed recently (Table 2).
Table 2.
Oligonucleotide PCR primers and probes targeting the mcrA gene.
| Probe/primer name | Name | Direction/Application | Probe sequence (5′–3′) | Probe length (mer) | Reference | Specificity |
|---|---|---|---|---|---|---|
| PCR primer | ||||||
| Set 1 | MCRf | Forward | TAYGAYCARATHTGGYT | 17 | Springer et al. (1995) | Most methanogens |
| MCRr | Reverse | ACRTTCATNGCRTARTT | 17 | |||
| Set 2 | ME1 | Forward | GCMATGCARATHGGWATGTC | 20 | Hales et al. (1996) | Most methanogens |
| ME2 | Reverse | TCATKGCRTAGTTDGGRTAGT | 21 | |||
| Set 3 | MLf | Forward | GGTGGTGTMGGATTCACACARTAYGCWACAGC | 32 | Luton et al. (2002) | Most methanogens |
| MLr | Reverse | TTCATTGCRTAGTTWGGRTAGTT | 23 | |||
| Set 4 | ME1 | Forward | GCMATGCARATHGGWATGTC | 20 | Hales et al. (1996) | Most methanogens |
| ME2b | Reverse | TCCTGSAGGTCGWARCCGAAGAA | 23 | Shigematsu et al. (2004) | ||
| Set 5 | MrtA_for | Forward | AAACAATCAACCACGCACTC | 20 | Scanlan et al. (2008) | Methanosphaera stadtmanae |
| MrtA_rev | Reverse | GTGAGCCCAATCGAAGGA | 18 | |||
| Set 6 | METH‐f | Forward | RTRYTMTWYGACCARATMTG | 20 | Colwell et al. (2008) | Most methanogens |
| METH‐r | Reverse | YTGDGAWCCWCCRAAGTG | 18 | |||
| Set 7 | mlas | Forward | GGTGGTGTMGGDTTCACMCARTA | 24 | Steinberg and Regan (2008) | Most methanogens |
| mcrA‐rev | Reverse | CGTTCATBGCGTAGTTVGGRTAGT | 24 | |||
| Set 8 | ME3MF | Forward | ATGTCNGGTGGHGTMGGSTTYAC | 23 | Nunoura et al. (2008) | Most methanogens |
| ME3MF‐e | Forward | ATGAGCGGTGGTGTCGGTTTCAC | 23 | |||
| ME2r' | Reverse | TCATBGCRTAGTTDGGRTAGT | 21 | |||
| Probe | ||||||
| ME3 | Clone screening | GGTGGHGTMGGWTTCACACA | 20 | Hales et al. (1996) | Most of methanogens | |
| SAE716TAQ | TaqMan probe | AGGCCTTCCCCACTCTGCTTGAGGAT | 26 | Shigematsu et al. (2004) | Genus Methanosaeta | |
| SAR716TAQ | TaqMan probe | AGAAATTCCCAACAGCCCTTGAAGAC | 26 | Shigematsu et al. (2004) | Genus Methanosarcina | |
| MCU716TAQ | TaqMan probe | AGCAGTACCCGACCATGATGGAGGAC | 26 | Shigematsu et al. (2004) | Genus Methanoculleus | |
| mbac‐mcrA | TaqMan probe | ARGCACCKAACAMCATGGACACWGT | 25 | Steinberg and Regan (2009) | Family Methanobacteriaceae | |
| mrtA | TaqMan probe | CCAACTCYCTCTCMATCAGRAGCG | 24 | Steinberg and Regan (2009) | Family Methanobacteriaceae | |
| mcp | TaqMan probe | AGCCGAAGAAACCAAGTCTGGACC | 24 | Steinberg and Regan (2009) | Family Methanocorpusculaceae | |
| msp | TaqMan probe | TGGTWCMACCAACTCACTCTCTGTC | 25 | Steinberg and Regan (2009) | Family Methanospirillaceae | |
| Fen | TaqMan probe | AAVCACGGYGGYMTCGGMAAG | 21 | Steinberg and Regan (2009) | Genus Methanoregula | |
| msa | TaqMan probe | CCTTGGCRAATCCKCCGWACTTG | 23 | Steinberg and Regan (2009) | Family Methanosaetaceae | |
| msar | TaqMan probe | TCTCTCWGGCTGGTAYCTCTCCATGTAC | 28 | Steinberg and Regan (2009) | Genus Methanosarcina | |
| McvME0 | FISH | GGAAAAATTCGAAGAAGATC | 20 | Kubota et al. (2006) | Methanococcus vannielii | |
| McvME3r | FISH | TGTGTGAAACCTACGCCACC | 20 | Kubota et al. (2006) | Methanococcus vannielii | |
| McvME1r | FISH | GACATTCCAATCTGCATTGC | 20 | Kubota et al. (2006) | Methanococcus vannielii |
The probes/primers listed here.
Assessing the biodiversity of methanogens in complex communities by PCR detection and cloning of methanogen genes
Some of the noted primers for 16S rRNA and methyl coenzyme M reductase genes have often been used for the detection and identification by PCR to explore the diversity of methanogens in environmental samples (Table 3). For example, the 146f/1324r primer set for most of all the known methanogens was designed for the 16S rRNA gene clone analysis of deep sediment gas hydrate deposit, and the results showed that gene clones (phylotypes) affiliated with Methanosarcina and Methanobrevibacter predominated in the sediments (Marchesi et al., 2001). Similarly, some of these primers shown in Table 1 have been used for PCR to profile methanogen populations by denaturing gradient gel electrophoresis (DGGE) (e.g. (Casamayor et al., 2001; 2002; Yu et al., 2005; 2006; 2008). As examples, Wright and Pimm (2003) developed PCR and sequencing primers for the 16S rRNA gene of methanogens, and used them for the ribotyping of members of the classes ‘Methanomicrobia’ and Methanobacteria. The detection of methanogens by PCR in lamb rumen samples was performed using methanogen‐specific primers targeting different taxonomic levels (Skillman et al., 2004). Banning and colleagues (2005) designed novel reverse primers to provide specific amplification of the 16S rRNA genes of ‘Methanomicrobia’ (Methanomicrobiales and Methanosarcinales), Methanobacteriales and Methanococcales, and successfully used them for the identification of methanogenic population structures in lake sediments.
Table 3.
Examples of oligonucleotide primer sets for PCR‐based analyses for methanogens.
| Type of sample | Application | Target gene | Target group | Probe set (forward/reverse/probe)a | Reference |
|---|---|---|---|---|---|
| Anaerobic process | qPCR | 16S rRNA | Methanomicrobiales | MMB282F/MMB832R/MMB749F | Yu et al. (2005) |
| Methanosarcinales | MSL812F/MSL1159R/MSL860F | ||||
| Methanobacteriales | MBT857F/MBT1196R/MBT929F | ||||
| Methanococcales | MCC495F/MCC832R/MCC686F | ||||
| Methanosarcinaceae | Msc380F/Msc828R/Msc492F | ||||
| Methanosaeta | Mst702F/Mst862R/Mst753F | ||||
| qPCR | mcrA | Methanocorpusculaceae | mlas/mcrA‐rev/mcp | Steinberg and Regan (2009) | |
| Methanospirillaceae | mlas/mcrA‐rev/msp | ||||
| Methanosaetaceae | mlas/mcrA‐rev/msa | ||||
| Methanobacteriaceae | mlas/mcrA‐rev/mbac‐mcrA | ||||
| Methanobacteriaceae | mlas/mcrA‐rev/mrtA | ||||
| Methanoregula | mlas/mcrA‐rev/Fen | ||||
| Methanosarcina | mlas/mcrA‐rev/msar | ||||
| qPCR | 16S rRNA | Methanoculleus | 298F/586R | Franke‐Whittle et al. (2009a) | |
| Methanosarcina | 240F/589R | ||||
| Methanothermobacter | 410F/667R | ||||
| qPCR | 16S rRNA | Methanoculleus thermophilus | Mc412f/Mc578r | Hori et al. (2006) | |
| Methanosaeta thermophila | Ms413f/Ms578r | ||||
| Methanothermobacter | Mt392f/Mt578r | ||||
| qPCR | 16S rRNA | Methanosaeta | MS1b/SAE835R/SAE761TAQ | Shigematsu et al. (2003) | |
| Methanosarcina | MB1b/SAR835R/SAR761TAQ | ||||
| Methanoculleus | AR934F/MG1200b/MCU1023TAQ | ||||
| qPCR | mcrA | Methanosaeta | ME1/ME2b/SAE716TAQ | Shigematsu et al. (2004) | |
| Methanosarcina | ME1/ME2b/SAR716TAQ | ||||
| Methanoculleus | ME1/ME2b/MCU716TAQ | ||||
| qPCR | 16S rRNA | Methanosaeta | S‐F‐Msaet‐0387‐S‐a‐21/S‐F‐Msaet‐0540‐A‐a‐31/S‐F‐Msaet‐0573‐A‐a‐17 | Sawayama et al. (2004) | |
| qPCR | 16S rRNA | Methanosarcina | S‐G‐Msar‐0450‐S‐a‐19/S‐P‐Msar‐0540‐A‐a‐31/S‐G‐Msar‐0589‐S‐a‐20 | Sawayama et al. (2006) | |
| Methanobacterium | S‐F‐Mbac‐0398‐S‐a‐20/S‐G‐Mbac‐0526‐A‐a‐33/S‐G‐Mbac‐0578‐A‐a‐22 | ||||
| Methanothermobacter | S‐F‐Mbac‐0398‐S‐a‐20/S‐G‐Mthb‐0549‐S‐a‐32/S‐G‐Mthb‐0589‐A‐a‐25 | ||||
| qPCR | 16S rRNA | Methanospirillum | AR934F/MG1200b/MSP1025TAQ | Tang et al. (2005) | |
| qPCR | 16S rRNA | Methanolinea | NOBI109f/NOBI633 | Imachi et al. (2008) | |
| PCR‐cloning | 16S rRNA | Most methanogens | 109f/UNIV1492rb | Narihiro et al. (2009a) | |
| PCR‐cloning | 16S rRNA | Most methanogens | 25f/1391r | Ariesyady et al. (2007) | |
| 25f/UNIV1492rb | |||||
| 109f/UNIV1492rb | |||||
| Anaerobic process, wetland | PCR‐cloning | mcrA | Most methanogens | mlas/mcrA‐rev | Steinberg and Regan (2008) |
| Wetland | PCR‐cloning | mcrA | Most methanogens | ME1/ME2 | Hales et al. (1996) |
| qPCR | 16S rRNA | Methanolobus psychrophilus | R15F/R15R | Zhang et al. (2008a) | |
| Rumen | qPCR, DGGE | 16S rRNA | Most methanogens | Met630F/Met803R | Hook et al. (2009) |
| PCR, DGGE | 16S rRNA | Most methanogens | A357f/A693r | Yu et al. (2008) | |
| A24f/A329r | |||||
| A24f/A348r | |||||
| PCR‐typing | 16S rRNA | Most methanogens | Arch f2/Arch r1386 | Skillman et al. (2004) | |
| Methanosarcinales | Arch f2/MSr r859 | ||||
| Methanobacteriales | Mbac f331/Arch r1386 | ||||
| Methanobacterium | fMbium/Arch r1386 | ||||
| Methanococcales | Arch f2/Mcc r | ||||
| Methanobrevibacter | fMbb1/Arch r1386 | ||||
| qPCR | 16S rRNA | Methanobrevibacter smithii | forward/reverse/probe | Armougom et al. (2009) | |
| Gastrointestinal tract | PCR‐cloning | mcrA | Most methanogens | MrtA_for/MrtA_rev | Scanlan et al. (2008) |
| Deep sea sediments | PCR‐cloning | 16S rRNA | Most methanogens | 146f/1324r | Marchesi et al. (2001) |
| qPCR | mcrA | Most methanogens | METH‐f/METH‐r | Colwell et al. (2008) | |
| qPCR | mcrA | Most methanogens | ME3MF and ME3MF‐e/ME2r' | Nunoura et al. (2008) | |
| Lake sediment | PCR‐cloning | 16S rRNA | Methanomicrobia | 355Fc/1068R | Banning et al. (2005) |
| Methanobacteriales | 109f/1401R | ||||
| Methanococcales | 344Fd/1202R | ||||
| Sulfurous lake | PCR, DGGE | 16S rRNA | Most methanogens | ARC344f/ARC915 | Casamayor et al. (2001) |
| Landfill | PCR‐cloning | mcrA | Most methanogens | MLf/MLr | Luton et al. (2002) |
| Ciliate endosymbiont | PCR, DGGE | 16S rRNA | Most methanogens | A1f/A1100r | Embley et al. (1992) |
| Rice paddy soil | PCR, DGGE | 16S rRNA | Most methanogens | 109f/ARC915 | Grosskopf et al. (1998) |
| Pure cultures | PCR‐ribotyping | 16S rRNA | Most methanogens | Met83F (or Met86F)/Met1340R | Wright and Pimm (2003) |
| PCR‐cloning | mcrA | Most methanogens | MCRf/MCRr | Springer et al. (1995) |
UNIV1492r reverse primer was originally referred from Lane (1991) as an universal primer.
355F forward primer was originally referred as M(SA/MI)355 probe developed by Ovreås et al. (1997) as shown in Table 1.
344F forward primer was originally referred as ARC344 probe developed by Raskin and colleagues (1994b) as shown in Table 1.
Massive parallel sequencing of PCR‐amplified 16S rRNA genes using next generation sequencers (such as the FLX pyrosequencers) allows us to obtain a huge number of community sequence tags (for example c. 10 000–100 000 16S pyrotags for each sample), which is more than any Sanger‐based cloning study to date, and have been used for characterizing archaeal populations (including methanogens) in hydrothermal chimneys (Brazelton et al., 2010a,b). The methodological advancements of 16S rRNA gene pyrosequencing include higher resolution (more sequences) for gene‐based community structure analysis, analysis of multiple related samples and use of metadata (Tringe and Hugenholtz, 2008). Because of these advancements, as well as recent development of analytical tools for massive sequence data such as QIIME (Caporaso et al., 2010), the method may be further used for characterizing diversity of methanogens in ecosystems.
Similarly, the primers for methyl coenzyme M reductase genes have often been used for PCR detection and identification to exclusively explore the diversity of methanogens in samples. For example, the MCR set was used to elucidate the diversity of methanogens in various environments with PCR‐based cloning (Kemnitz et al., 2004; Dhillon et al., 2005; Alain et al., 2006) and T‐RFLP analyses (Ramakrishnan et al., 2001; Kemnitz et al., 2004). Such cloning analyses were also conducted using the ME (Hales et al., 1996; Nercessian et al., 1999; Galand et al., 2002; 2005; Tatsuoka et al., 2004) and ML primer sets (Luton et al., 2002; Castro et al., 2004; Juottonen et al., 2005; Nercessian et al., 2005; Ufnar et al., 2007; Smith et al., 2008). Comparative studies using these three primer sets have indicated that the ML primer set is more efficient for retrieving phylogenetically diverse methanogens in the wetland than others (Juottonen et al., 2006; Jerman et al., 2009). Owing to this advantage, the ML set has been used extensively to determine the diversity of methanogens in various anaerobic ecosystems. In addition, it has been noted that these mcrA‐targeted primer sets (especially ME‐related primer set) were also used for the quantitative detection of anaerobic methanotrophic archaea (ANME) in methane seep sediments (Inagaki et al., 2004; Nunoura et al., 2006; 2008). This is due to the fact that anaerobic methane oxidation represented by the ANME group is considered to proceed with mcr‐type enzymes (Thauer and Shima, 2008). Detailed information about the mcrA‐based qPCR for ANMEs is described below.
Polymerase chain reaction‐based molecular techniques, such as PCR‐cloning, pyrosequencing, DGGE and T‐RFLP are adequate to gain entire community composition and diversity of methanogens in ecosystems. Based on the frequency of retrieval of phylotypes in gene library (or relative intensity of DGGE or T‐RF bands in electropherogram), relative abundance of phylotypes of interest can be inferred. However, it should be noted that entire microbial community structure analysis based on bulk cell lysis, DNA extraction, PCR and cloning are often suspect because of several biases involved in each of the steps (Dahllof, 2002). Therefore, one should be careful to discuss on the abundance of phylotypes in samples based solely on the data obtained by these methods. More reliable methods to carry out quantitative detection of different groups of methanogens in samples would be to use the following quantitative molecular techniques.
Identification and quantification of methanogens in complex communities by membrane hybridization method
Quantitative membrane hybridization of labelled DNA probes to community rRNAs has been applied to various environmental rRNAs for the quantitative detection of specific groups of microbes present in complex communities (Stahl et al., 1988; Raskin et al., 1994a). RNA‐dependent community analysis is known to indicate the in situ activity of individual members in ecosystems, because of the reasons that RNA synthesis is known to reflect the in situ growth rates of organisms (Poulsen et al., 1993; Amann et al., 1995), and that the turnover of RNA is thought to be much higher than that of DNA. Therefore, rRNA‐dependent molecular techniques like the present one provide precise information about the dynamic nature of individual microbes in systems. In 1994, Raskin and colleagues carried out the first leading studies on the development of eight oligonucleotide probes for the quantitative detection of methanogens in anaerobic wastewater treatment sludges (Stahl and Amann, 1991; Raskin et al., 1994a,b). In these studies, they established the group‐specific oligonucleotide probes targeting Methanomicrobiales (probes MG1200 and MSMX860), Methanobacteriaceae (probes MB310 and MB1174) and Methanococcales (probe MC1109). Because of the importance of methane production from acetate in anaerobic bioreactors, specific probes for aceticlastic methanogens, such as the members of Methanosarcinaceae (probes MS1414 and MS821) and Methanosaeta (probe MX825), were also developed.
These probes have been successfully applied to the quantification of methanogens in laboratory‐ and full‐scale anaerobic bioreactors based on rRNA (Raskin et al., 1995; Griffin et al., 1998; Liu et al., 2002; McMahon et al., 2004; Zheng et al., 2006). Although membrane hybridization enables the sensitive quantification of individual species of rRNA molecules, this method requires several laborious experimental steps, often radioactively labelled DNA probes, and reference rRNA samples as external standards for each experiment. Thus, the method itself may be replaced by similar but much rapid and simpler methods, such as real‐time RT‐PCR and RNase H methods. However, the probes used for membrane hybridization experiments may be also used as probes/primers in other experiments shown below.
FISH for methanogens
Whole‐cell FISH based on 16S rRNA is now commonly used to detect specific groups of microbes and to quantify populations of interest in environments by direct counting under a microscope (Amann et al., 1995). In addition, FISH is used for visualizing the spatial distribution of the population of interest in biofilms, such as those of methanogens in sludge granules in methanogenic wastewater treatment systems (Sekiguchi et al., 1999). Basically, the probes developed for membrane hybridization of methanogen 16S rRNAs or reverse primers for PCR amplification of methanogen 16S rRNA genes can directly be used as oligonucleotide probes for in situ hybridization studies, the probes previously designed by Raskin and colleagues (Raskin, et al., 1994b) have frequently be used for the purpose of FISH studies as well. These probes have been used for the quantitative detection of methanogens using the FISH technique in various anaerobic ecosystems, such as peat bog (e.g. Horn et al., 2003), aquifer (e.g. Kleikemper et al., 2005), landfills (e.g. Laloui‐Carpentier et al., 2006) and anaerobic wastewater treatment processes (e.g. Sekiguchi et al., 1999; Plumb et al., 2001; Boonapatcharoen et al., 2007; Chen et al., 2009). Recently, the improvement of the specificity and sensitivity of the probes designed by Raskin and colleagues (1994b) has been reported. Crocetti and colleagues (2006) refined the experimental conditions of such probes for FISH analysis to accurately and sensitively detect methanogens.
In addition to the quantification, the probes (Table 1) have also been used for investigating the localization of methanogens in biofilms (sludge granules) [e.g. (Rocheleau et al., 1999; Sekiguchi et al., 1999; Plumb et al., 2001; Zheng et al., 2006; Vavilin et al., 2008; Chen et al., 2009)]. In anaerobic sludge granules, hydrogenotrophic methanogens are often juxtaposed with syntrophic substrate‐degrading bacteria, such as syntrophic propionate‐oxidizing bacteria such as members of the genera Syntrophobacter and Pelotomaculum; such close proximity between syntrophic bacteria and methanogens has been observed by FISH with confocal laser scanning microscopy (Harmsen et al., 1995; 1996; Sekiguchi et al., 1999; Imachi et al., 2000). Anaerobic ciliates often posses endosymbiotic methanogens within their cells, and the distribution of such methanogens in eukaryotic cells has been observed by the FISH method [e.g. (Embley et al., 1992; Shinzato et al., 2007)].
Although FISH is a powerful method for visualizing the cells of interest, there are some drawbacks in detecting cells; one of such problems is concerned with the penetration of oligonucleotide probes into the cells (Amann et al., 1995). For methanogens, FISH staining is often difficult for some Methanobacterium and Methanobrevibacter cells, for which oligonucleotide probes do not penetrate into their cells (Sekiguchi et al., 1999; Yanagita et al., 2000; Nakamura et al., 2006). To solve this problem, fixed cells were subjected to freeze‐thaw cycles before hybridization, resulting in the improvement of probe penetration (Sekiguchi et al., 1999). Another way to solve this problem is the use of recombinant pseudomurein endoisopeptidase, which increases the permeability of oligonucleotide probes into cells, and allows a better visualization of methanogens in anaerobic granular sludge and the endosymbiotic methanogens in the anaerobic ciliate Trimyema compressum (Nakamura et al., 2006). An improved protocol of catalysed reporter deposition‐FISH for methanogens with recombinant pseudomurein endoisopeptidase has also been reported, which can increase fluorescence signal intensity in FISH for detecting cells with a low rRNA content (Kubota et al., 2008).
Recently, mcrA‐based in situ detection of methanogens has been performed using the two‐pass tyramide signal amplification‐FISH approach combined with locked nucleic acids (Kubota et al., 2006; Kawakami et al., 2010). These attempts were, at this point, only partially successful in detecting methanogen cells, because mcrA is generally present as a single copy gene on their chromosome, which results in a low sensitivity of detection.
qPCR
Quantitative PCR of 16S rRNA gene and mcrA has also been used to quantify the abundance of methanogens in recent years. Examples of qPCR primer and probe sets for different taxa of methanogens are listed in Table 3. For example, the primers Met630F/Met803R were developed for the SYBR green‐based real‐time qPCR for almost all the known methanogens in the rumen of the dairy cow (Hook et al., 2009). Yu and colleagues (2005) designed TaqMan‐based qPCR probes/primer sets (six sets in total) for each of the orders Methanomicrobiales, Methanosarcinales, Methanobacteriales and Methanococcales, as well as the families Methanosaetaceae and Methanosarcinaceae. They applied a part of these sets to quantifying aceticlastic methanogens in methanogenic sludges for treating sewage sludges, cheese whey wastewater and synthetic wastewater, and revealed that the population of aceticlastic methanogens is affected by the acetate concentration in the wastewaters (Yu et al., 2006). qPCR detection using specific primers for particular groups of methanogens of interest, such as Methanoculleus (Shigematsu et al., 2003; Hori et al., 2006; Franke‐Whittle et al., 2009a), Methanolinea (Imachi et al., 2008), Methanospirillum (Tang et al., 2005), Methanosaeta (Shigematsu et al., 2003; Sawayama et al., 2004; Hori et al., 2006), Methanosarcina (Shigematsu et al., 2003; Sawayama et al., 2006; Franke‐Whittle et al., 2009a), Methanolobus (Zhang et al., 2008a,b), Methanobrevibacter (Armougom et al., 2009), Methanobacterium (Sawayama et al., 2006) and Methanothermobacter (Hori et al., 2006; Sawayama et al., 2006; Franke‐Whittle et al., 2009a) have also been reported to date (Table 3).
For the qPCR detection of mcrA, the ME primer set was used for the quantification of methanogenic and methanotrophic populations in methane seep sediments (Inagaki et al., 2004; Nunoura et al., 2006). Afterwards, Nunoura and colleagues (2008) slightly modified the ME primer series, and showed that the mixture of the ME3MF and ME3MF‐e forward primers and the ME2' reverse primer is most suitable for the qPCR detection of the methanogens and ANMEs in the environments. The results showed that a significant amount of methanogens and ANMEs was found in anaerobically digested sludge and methane seep sediments. The ML primer set was also used for the quantitation of methanogenic archaeal populations in the rumen (Denman et al., 2007) and human subgingival plaque (Vianna et al., 2008). Moreover, Steinberg and Regan (2008; 2009) developed the mlas/mcrA‐rev primer set, which is a derivative of the ML primer set, for the clone library construction and qPCR analyses of methanogens in oligotrophic fen and anaerobic digester sludge. In addition, the genus‐specific TaqMan probes for the mcrA‐based quantitative detection of the Methanosaeta, Methanosarcina and Methanoculleus resident in acetate‐fed chemostats, and the results showed that dilution rate is a key factor in the acetate bioconversion pathway (Shigematsu et al., 2004).
Quantitative PCR method provides sensitive, quantitative data of gene of interest with a sufficiently high dynamic range of quantification (Zhang and Fang, 2006). Therefore, in addition to the use of digital PCR (Ottesen et al., 2006), qPCR may be further used for quantitative monitoring of methanogen taxa of interests in complex microbial communities. However, it should be noted that the method is PCR‐based and hence their data can be suspect because of biases involved in DNA extraction and primer/probe mismatches.
Assessing methanogen population by RNase H method
Although the above‐mentioned quantitative methods such as membrane hybridization and qPCR are becoming general means to determine the abundance of the population of interest in a complex microbial community, there is a need to develop more simple and rapid techniques that meet the needs for real‐time monitoring of the population of interest in a complex community. Recently, a simple and rapid quantification method, namely, the RNase H method, has been developed (Uyeno et al., 2004). This method is based on the sequence‐specific cleavage of 16S rRNA with ribonuclease H (RNase H) and oligonucleotide (scissor) probes. RNAs from a complex community were first mixed with an oligonucleotide and subsequently digested with RNase H. Because RNase H specifically degrades the RNA strand of RNA : DNA hybrid heteroduplexes, the targeted rRNAs are cleaved at the hybridization site in a sequence‐dependent manner and are consequently cut into two fragments. In contrast, non‐targeted rRNAs remain intact under the same conditions. For the detection of cleaved rRNAs, the resulting RNA fragment patterns can be resolved by gel electrophoresis using RNA‐staining dyes. The relative abundance of the targeted species of 16S rRNA fragments in total 16S rRNA can also be quantified by determining the signal intensity of individual 16S rRNA bands in an electropherogram (without the use of external standards). Because this method does not require an external RNA standard for each experiment, as is required in membrane hybridization, and because the present method is relatively easy to perform within a short time (i.e. within 2–3 h), this technique may provide direct, rapid and easy means of the quantitative detection of particular groups of anaerobes based on their rRNA, such as those of methanogens as well.
This method has been successfully applied to the quantification of active methanogens in anaerobic biological treatment processes (Uyeno et al., 2004; Sekiguchi et al., 2005; Narihiro et al., 2009b). In general, oligonucleotide probes used in FISH and membrane hybridization methods can directly be used as scissor probes in the RNase H method. Recently, a total of 40 probes, including newly designed and previously reported probes listed in Table 1, have been optimized for the specific quantification of methanogens at different taxonomic levels for use in the RNase H method and have been applied to quantitative and comprehensive detection of methanogens in various types of anaerobic biosystems (Narihiro et al., 2009b). As a result, methanogen populations were identified at different taxonomic levels and were influenced by the process temperature and wastewater compositions. Because of the reasons that this method is based on rRNA and that the RNA (rRNA) level is often dependent on the in situ activity of individual cells as described above, this method may be used for real‐time monitoring of active methanogens and other important bacteria in engineered ecosystems such as waste/wastewater treatment systems to better control such bioreactors.
Stable isotope probing (SIP)‐based detection of active methanogen populations in environments
To identify metabolically active populations in environments, SIP of DNA (Radajewski et al., 2000) and RNA (Manefield et al., 2002) has been used in recent years. In principle, SIP technology is based on the incorporation of 13C‐labelled substrates into the nucleic acids. The separation of isotopically labelled (active) fractions from unlabeled (inactive) fractions is generally performed with density gradient centrifugation. The substrate‐assimilated microorganisms in the labelled fractions are identified by a set of PCR‐based molecular techniques such as gene cloning, DGGE and other methods. Therefore, for the purpose of identifying active methanogens that are responsible for particular metabolisms in environments, the probes/primers listed in Tables 1 and 2 can be used.
As examples, active methanogen populations involved in the syntrophic propionate oxidation in anoxic soil were analysed on the basis of rRNA‐SIP, and it was found that the members of the genera Methanobacterium, Methanosarcina and Methanocella play a key role in scavenging hydrogen/formate/acetate in syntrophic association with propionate‐oxidizing bacteria (Lueders et al., 2004). Conrad and coworkers have studied the detection of active methanogen populations using DNA‐SIP combined with 13C‐labelled CO2, and the results of T‐RFLP profiling and phylogenetic analysis for clonal 16S rRNA gene fragments suggest that members of the RC‐I group (Methanocellales) serve as important methanogens in rice paddy fields (Lu and Conrad, 2005; Lu et al., 2005). The active methanogenic populations in enrichment culture of municipal solid waste digester residues spiked with 13C‐labelled substrates (such as cellulose, glucose and sodium acetate) were determined by DNA‐SIP followed by cloning analysis (Li et al., 2009).
Other methods and future perspectives
DNA microarray platform, like PhyloChip, is becoming an important tool for parallel detection of different community members of microbes in ecosystems. For high throughput and comprehensive detection of methanogens in parallel, ANAEROCHIP (Franke‐Whittle et al., 2009b) and GeoChip (Wang et al., 2009) have been developed recently. The primers/probes summarized in this review may be integrated into such a platform for parallel and hierarchical detection of methanogens. These primer/probes for methanogens can also be used in novel PCR‐based techniques, such as the hierarchical oligonucleotide primer extension method (Wu and Liu, 2007), which has recently been developed for quantitative, multiplex detection of targeted microbial genes among PCR‐amplified genes. SIP technology has been noted as an important pretreatment step for functional microbial community analyses, such as Raman microscopy‐FISH (Huang et al., 2007; 2009) and metagenomic approaches (Kalyuzhnaya et al., 2008; Sul et al., 2009). Moreover, recent advances in analytical chemistry, such as isotope ratio mass spectrometry (Penning et al., 2006; Vavilin et al., 2008) and secondary ion mass spectrometry (Orphan et al., 2001), hold great promise for the highly sensitive determination of targeted microbes. Thus, in addition to describing the diversity of methanogens in particular environments of interest on the basis of DNA and RNA, such function‐related analyses of methanogens may become important in the fields of environmental, determinative and applied microbiology.
As described in this minireview, a vast number of probe/primers have been developed for describing and quantifying methanogen populations, covering most parts of the known culturable methanogens described so far. A variety of molecular methods have also been developed that are used in combination with the probe/primers. Because these molecular methods have their own advancements and drawbacks, researchers need to select appropriate combinations of methods and probe/primers depending on what the researchers need to know. For details, recent reviews may be helpful for the selection of molecular techniques to be used (Talbot et al., 2008; Tabatabaei et al., 2010). In molecular ecology, multiple approaches are best to gain a complete picture of methanogen populations in environments. Therefore, the use of appropriate (multiple) molecular techniques in combinations with other non‐molecular based methods like membrane lipid, autofluorescence, activity measurement and immunoenzymatic profiling should be considered. It should also be noted that there are still a number of uncultivated methanogens in various environments, and that they should be further isolated and characterized in detail. Monitoring tools for such uncultured methanogens remain to be developed to further increase in the coverage of methanogens present in environments.
Acknowledgments
This work was supported by the Environment Research and Technology Development Fund (S2‐03) and the Global Environment Research Fund (RF‐076) of the Ministry of the Environment, Japan.
References
- Alain K., Holler T., Musat F., Elvert M., Treude T., Kruger M. Microbiological investigation of methane‐ and hydrocarbon‐discharging mud volcanoes in the Carpathian Mountains, Romania. Environ Microbiol. 2006;8:574–590. doi: 10.1111/j.1462-2920.2005.00922.x. [DOI] [PubMed] [Google Scholar]
- Amann R.I., Ludwig W., Schleifer K.H. Phylogenetic identification and in‐situ detection of individual microbial‐cells without cultivation. Microbiol Rev. 1995;59:143–169. doi: 10.1128/mr.59.1.143-169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariesyady H.D., Ito T., Okabe S. Functional bacterial and archaeal community structures of major trophic groups in a full‐scale anaerobic sludge digester. Water Res. 2007;41:1554–1568. doi: 10.1016/j.watres.2006.12.036. [DOI] [PubMed] [Google Scholar]
- Armougom F., Henry M., Vialettes B., Raccah D., Raoult D. Monitoring bacterial community of human gut microbiota reveals an increase in Lactobacillus in obese patients and methanogens in anorexic patients. PLoS ONE. 2009;4:e7125. doi: 10.1371/journal.pone.0007125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banning N., Brock F., Fry J.C., Parkes R.J., Hornibrook E.R.C., Weightman A.J. Investigation of the methanogen population structure and activity in a brackish lake sediment. Environ Microbiol. 2005;7:947–960. doi: 10.1111/j.1462-2920.2004.00766.x. [DOI] [PubMed] [Google Scholar]
- Barns S.M., Fundyga R.E., Jeffries M.W., Pace N.R. Remarkable archaeal diversity detected in a Yellowstone National Park hot‐spring environment. Proc Natl Acad Sci USA. 1994;91:1609–1613. doi: 10.1073/pnas.91.5.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boetius A., Ravenschlag K., Schubert C.J., Rickert D., Widdel F., Gieseke A. A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature. 2000;407:623–626. doi: 10.1038/35036572. et al. [DOI] [PubMed] [Google Scholar]
- Boonapatcharoen N., Meepian K., Chaiprasert P., Techkarnjanaruk S. Molecular monitoring of microbial population dynamics during operational periods of anaerobic hybrid reactor treating cassava starch wastewater. Microb Ecol. 2007;54:21–30. doi: 10.1007/s00248-006-9161-6. [DOI] [PubMed] [Google Scholar]
- Bräuer S.L., Cadillo‐Quiroz H., Yashiro E., Yavitt J.B., Zinder S.H. Isolation of a novel acidiphilic methanogen from an acidic peat bog. Nature. 2006;442:192–194. doi: 10.1038/nature04810. [DOI] [PubMed] [Google Scholar]
- Bräuer S.L., Cadillo‐Quiroz H., Ward R.J., Yavitt J., Zinder S. Methanoregula boonei gen. nov., sp. nov., an acidiphilic methanogen isolated from an acidic peat bog. Int J Syst Evol Microbiol. 2010 doi: 10.1099/ijs.0.021782-0. [DOI] [PubMed] [Google Scholar]
- Brazelton W.J., Ludwig K.A., Sogin M.L., Andreishcheva E.N., Kelley D.S., Shen C.C. Archaea and bacteria with surprising microdiversity show shifts in dominance over 1,000‐year time scales in hydrothermal chimneys. Proc Natl Acad Sci USA. 2010a;107:1612–1617. doi: 10.1073/pnas.0905369107. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazelton W.J., Sogin M.L., Baross J.A. Multiple scales of diversification within natural populations of archaea in hydrothermal chimney biofilms. Environ Microbiol Rep. 2010b;2:236–242. doi: 10.1111/j.1758-2229.2009.00097.x. [DOI] [PubMed] [Google Scholar]
- Cadillo‐Quiroz H., Yavitt J.B., Zinder S.H. Methanosphaerula palustris gen. nov., sp nov., a hydrogenotrophic methanogen isolated from a minerotrophic fen peatland. Int J Syst Evol Microbiol. 2009;59:928–935. doi: 10.1099/ijs.0.006890-0. [DOI] [PubMed] [Google Scholar]
- Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K. QIIME allows analysis of high‐throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casamayor E.O., Muyzer G., Pedros‐Alio C. Composition and temporal dynamics of planktonic archaeal assemblages from anaerobic sulfurous environments studied by 16S rDNA denaturing gradient gel electrophoresis and sequencing. Aquat Microb Ecol. 2001;25:237–246. [Google Scholar]
- Casamayor E.O., Massana R., Benlloch S., Ovreas L., Diez B., Goddard V.J. Changes in archaeal, bacterial and eukaryal assemblages along a salinity gradient by comparison of genetic fingerprinting methods in a multipond solar saltern. Environ Microbiol. 2002;4:338–348. doi: 10.1046/j.1462-2920.2002.00297.x. et al. [DOI] [PubMed] [Google Scholar]
- Castro H., Ogram A., Reddy K.R. Phylogenetic characterization of methanogenic assemblages in eutrophic and oligotrophic areas of the Florida Everglades. Appl Environ Microbiol. 2004;70:6559–6568. doi: 10.1128/AEM.70.11.6559-6568.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.L., Wu J.H., Tseng I.C., Liang T.M., Liu W.T. Characterization of active microbes in a full‐scale anaerobic fluidized bed reactor treating phenolic wastewater. Microbes Environ. 2009;24:144–153. doi: 10.1264/jsme2.me09109. [DOI] [PubMed] [Google Scholar]
- Chouari R., Le Paslier D., Daegelen P., Ginestet P., Weissenbach J., Sghir A. Novel predominant archaeal and bacterial groups revealed by molecular analysis of an anaerobic sludge digester. Environ Microbiol. 2005;7:1104–1115. doi: 10.1111/j.1462-2920.2005.00795.x. [DOI] [PubMed] [Google Scholar]
- Colwell F.S., Boyd S., Delwiche M.E., Reed D.W., Phelps T.J., Newby D.T. Estimates of biogenic methane production rates in deep marine sediments at Hydrate Ridge, Cascadia margin. Appl Environ Microbiol. 2008;74:3444–3452. doi: 10.1128/AEM.02114-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad R., Erkel C., Liesack W. Rice Cluster I methanogens, an important group of Archaea producing greenhouse gas in soil. Curr Opin Biotechnol. 2006;17:262–267. doi: 10.1016/j.copbio.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Crocetti G., Murto M., Bjornsson L. An update and optimisation of oligonucleotide probes targeting methanogenic Archaea for use in fluorescence in situ hybridisation (FISH) J Microbiol Methods. 2006;65:194–201. doi: 10.1016/j.mimet.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Dahllof I. Molecular community analysis of microbial diversity. Curr Opin Biotechnol. 2002;13:213–217. doi: 10.1016/s0958-1669(02)00314-2. [DOI] [PubMed] [Google Scholar]
- Denman S.E., Tomkins N., McSweeney C.S. Quantitation and diversity analysis of ruminal methanogenic populations in response to the antimethanogenic compound bromochloromethane. FEMS Microbiol Ecol. 2007;62:313–322. doi: 10.1111/j.1574-6941.2007.00394.x. [DOI] [PubMed] [Google Scholar]
- Dhillon A., Lever M., Lloyd K.G., Albert D.B., Sogin M.L., Teske A. Methanogen diversity evidenced by molecular characterization of methyl coenzyme M reductase A (mcrA) genes in hydrothermal sediments of the Guaymas Basin. Appl Environ Microbiol. 2005;71:4592–4601. doi: 10.1128/AEM.71.8.4592-4601.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfert S.N., Reichlen M., Iyer P., Wang M.Y., Ferry J.G. Methanolobus zinderi sp nov., a methylotrophic methanogen isolated from a deep subsurface coal seam. Int J Syst Evol Microbiol. 2009;59:1064–1069. doi: 10.1099/ijs.0.003772-0. [DOI] [PubMed] [Google Scholar]
- Dojka M.A., Hugenholtz P., Haack S.K., Pace N.R. Microbial diversity in a hydrocarbon‐ and chlorinated‐solvent‐contaminated aquifer undergoing intrinsic bioremediation. Appl Environ Microbiol. 1998;64:3869–3877. doi: 10.1128/aem.64.10.3869-3877.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embley T.M., Finlay B.J., Thomas R.H., Dyal P.L. The use of rRNA sequences and fluorescent probes to investigate the phylogenetic positions of the anaerobic ciliate Metopus palaeformis and its archaeobacterial endosymbiont. J Gen Microbiol. 1992;138:1479–1487. doi: 10.1099/00221287-138-7-1479. [DOI] [PubMed] [Google Scholar]
- Falz K.Z., Holliger C., Grosskopf R., Liesack W., Nozhevnikova A.N., Muller B. Vertical distribution of methanogens in the anoxic sediment of Rotsee (Switzerland) Appl Environ Microbiol. 1999;65:2402–2408. doi: 10.1128/aem.65.6.2402-2408.1999. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke‐Whittle I.H., Goberna M., Insam H. Design and testing of real‐time PCR primers for the quantification of Methanoculleus, Methanosarcina, Methanothermobacter, and a group of uncultured methanogens. Can J Microbiol. 2009a;55:611–616. doi: 10.1139/w08-157. [DOI] [PubMed] [Google Scholar]
- Franke‐Whittle I.H., Goberna M., Pfister V., Insam H. Design and development of the ANAEROCHIP microarray for investigation of methanogenic communities. J Microbiol Methods. 2009b;79:279–288. doi: 10.1016/j.mimet.2009.09.017. [DOI] [PubMed] [Google Scholar]
- Frey J.C., Pell A.N., Berthiaume R., Lapierre H., Lee S., Ha J.K. Comparative studies of microbial populations in the rumen, duodenum, ileum and faeces of lactating dairy cows. J Appl Microbiol. 2009;108:1982–1993. doi: 10.1111/j.1365-2672.2009.04602.x. et al. [DOI] [PubMed] [Google Scholar]
- Friedrich M.W. Methyl‐coenzyme M reductase genes: unique functional markers for methanogenic and anaerobic methane‐oxidizing Archaea. Methods Enzymol. 2005;397:428–442. doi: 10.1016/S0076-6879(05)97026-2. [DOI] [PubMed] [Google Scholar]
- Galand P.E., Saarnio S., Fritze H., Yrjala K. Depth related diversity of methanogen Archaea in Finnish oligotrophic fen. FEMS Microbiol Ecol. 2002;42:441–449. doi: 10.1111/j.1574-6941.2002.tb01033.x. [DOI] [PubMed] [Google Scholar]
- Galand P.E., Juottonen H., Fritze H., Yrjala K. Methanogen communities in a drained bog: effect of ash fertilization. Microbial Ecol. 2005;49:209–217. doi: 10.1007/s00248-003-0229-2. [DOI] [PubMed] [Google Scholar]
- Garrity G.M., Holt J.G. Phylum AII. Euryarchaeota phy. nov. In: Boone D.R., Castenholz R.W., Garrity G.M., editors. 2nd. Springer‐Verlag; 2001. pp. 211–355. [Google Scholar]
- Garrity G.M., Lilburn T.G., Cole J.R., Harrison S.H., Euzeby J., Tindall B.J. 2007. , and Part 1 The‘Archaea’, Phyla‘Crenarchaeota’ and‘Euryarchaeota’[WWW document]. URL http://www.taxonomicoutline.org/index.php/toba/article/view/178/210.
- Griffin M.E., McMahon K.D., Mackie R.I., Raskin L. Methanogenic population dynamics during start‐up of anaerobic digesters treating municipal solid waste and biosolids. Biotechnol Bioeng. 1998;57:342–355. doi: 10.1002/(sici)1097-0290(19980205)57:3<342::aid-bit11>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Grosskopf R., Janssen P.H., Liesack W. Diversity and structure of the methanogenic community in anoxic rice paddy soil microcosms as examined by cultivation and direct 16S rRNA gene sequence retrieval. Appl Environ Microbiol. 1998;64:960–969. doi: 10.1128/aem.64.3.960-969.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales B.A., Edwards C., Ritchie D.A., Hall G., Pickup R.W., Saunders J.R. Isolation and identification of methanogen‐specific DNA from blanket bog feat by PCR amplification and sequence analysis. Appl Environ Microbiol. 1996;62:668–675. doi: 10.1128/aem.62.2.668-675.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmsen H.J.M., Kengen K.M.P., Akkermans A.D.L., Stams A.J.M. Phylogenetic analysis of two syntrophic propionate‐oxidizing bacteria in enrichments cultures. Syst Appl Microbiol. 1995;18:67–73. [Google Scholar]
- Harmsen H.J.M., Kengen H.M.P., Akkermans A.D.L., Stams A.J.M., deVos W.M. Detection and localization of syntrophic propionate‐oxidizing bacteria in granular sludge by in situ hybridization using 16S rRNA‐based oligonucleotide probes. Appl Environ Microbiol. 1996;62:1656–1663. doi: 10.1128/aem.62.5.1656-1663.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook S.E., Northwood K.S., Wright A.D.G., McBride B.W. Long‐term monensin supplementation does not significantly affect the quantity or diversity of methanogens in the rumen of the lactating dairy cow. Appl Environ Microbiol. 2009;75:374–380. doi: 10.1128/AEM.01672-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori T., Haruta S., Ueno Y., Ishii M., Igarashi Y. Dynamic transition of a methanogenic population in response to the concentration of volatile fatty acids in a thermophilic anaerobic digester. Appl Environ Microbiol. 2006;72:1623–1630. doi: 10.1128/AEM.72.2.1623-1630.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn M.A., Matthies C., Kusel K., Schramm A., Drake H.L. Hydrogenotrophic methanogenesis by moderately acid‐tolerant methanogens of a methane‐emitting acidic peat. Appl Environ Microbiol. 2003;69:74–83. doi: 10.1128/AEM.69.1.74-83.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W.E., Stoecker K., Griffiths R., Newbold L., Daims H., Whiteley A.S., Wagner M. Raman‐FISH: combining stable‐isotope raman spectroscopy and fluorescence in situ hybridization for the single cell analysis of identity and function. Environ Microbiol. 2007;9:1878–1889. doi: 10.1111/j.1462-2920.2007.01352.x. [DOI] [PubMed] [Google Scholar]
- Huang W.E., Ferguson A., Singer A.C., Lawson K., Thompson I.P., Kalin R.M. Resolving genetic functions within microbial populations: in situ analyses using rRNA and mRNA stable isotope probing coupled with single‐cell raman‐fluorescence in situ hybridization. Appl Environ Microbiol. 2009;75:234–241. doi: 10.1128/AEM.01861-08. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugenholtz P. Exploring prokaryotic diversity in the genomic era. Genome Biol. 2002;3:REVIEWS0003. doi: 10.1186/gb-2002-3-2-reviews0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino T., Mori K., Suzuki K. Methanospirillum lacunae sp. nov., a methane‐producing archaeon isolated from a puddly soil, and the emendation of the genus Methanospirillum and Methanospirillum hungatei. Int J Syst Evol Microbiol. 2010;60:2563–2566. doi: 10.1099/ijs.0.020131-0. [DOI] [PubMed] [Google Scholar]
- Imachi H., Sekiguchi Y., Kamagata Y., Ohashi A., Harada H. Cultivation and in situ detection of a thermophilic bacterium capable of oxidizing propionate in syntrophic association with hydrogenotrophic methanogens in a thermophilic methanogenic granular sludge. Appl Environ Microbiol. 2000;66:3608–3615. doi: 10.1128/aem.66.8.3608-3615.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imachi H., Sakai S., Sekiguchi Y., Hanada S., Kamagata Y., Ohashi A., Harada H. Methanolinea tarda gen. nov., sp nov., a methane‐producing archaeon isolated from a methanogenic digester sludge. Int J Syst Evol Microbiol. 2008;58:294–301. doi: 10.1099/ijs.0.65394-0. [DOI] [PubMed] [Google Scholar]
- Imachi H., Sakai S., Nagai H., Yamaguchi T., Takai K. Methanofollis ethanolicus sp nov., an ethanol‐utilizing methanogen isolated from a lotus field. Int J Syst Evol Microbiol. 2009;59:800–805. doi: 10.1099/ijs.0.003731-0. [DOI] [PubMed] [Google Scholar]
- Inagaki F., Tsunogai U., Suzuki M., Kosaka A., Machiyama H., Takai K. Characterization of C‐1‐metabolizing prokaryotic communities in methane seep habitats at the Kuroshima Knoll, southern Ryukyu arc, by analyzing pmoA, mmoX, mxaF, mcrA, and 16S rRNA genes. Appl Environ Microbiol. 2004;70:7445–7455. doi: 10.1128/AEM.70.12.7445-7455.2004. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerman V., Metje M., Mandic‐Mulec I., Frenzel P. Wetland restoration and methanogenesis: the activity of microbial populations and competition for substrates at different temperatures. Biogeosciences. 2009;6:1127–1138. [Google Scholar]
- Juottonen H., Galand P.E., Tuittila E.S., Laine J., Fritze H., Yrjala K. Methanogen communities and Bacteria along an ecohydrological gradient in a northern raised bog complex. Environ Microbiol. 2005;7:1547–1557. doi: 10.1111/j.1462-2920.2005.00838.x. [DOI] [PubMed] [Google Scholar]
- Juottonen H., Galand P.E., Yrjala K. Detection of methanogenic Archaea in peat: comparison of PCR primers targeting the mcrA gene. Res Microbiol. 2006;157:914–921. doi: 10.1016/j.resmic.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Kalyuzhnaya M.G., Lapidus A., Ivanova N., Copeland A.C., McHardy A.C., Szeto E. High‐resolution metagenomics targets specific functional types in complex microbial communities. Nat Biotechnol. 2008;26:1029–1034. doi: 10.1038/nbt.1488. et al. [DOI] [PubMed] [Google Scholar]
- Kawakami S., Kubota K., Imachi H., Yamaguchi T., Harada H., Ohashi A. Detection of single copy genes by two‐pass tyramide signal amplification fluorescence in situ hybridization (two‐pass TSA‐FISH) with single oligonucleotide probes. Microbes Environ. 2010;25:15–21. doi: 10.1264/jsme2.me09180. [DOI] [PubMed] [Google Scholar]
- Kemnitz D., Chin K.J., Bodelier P., Conrad R. Community analysis of methanogenic archaea within a riparian flooding gradient. Environ Microbiol. 2004;6:449–461. doi: 10.1111/j.1462-2920.2004.00573.x. [DOI] [PubMed] [Google Scholar]
- Kleikemper J., Pombo S.A., Schroth M.H., Sigler W.V., Pesaro M., Zeyer J. Activity and diversity of methanogens in a petroleum hydrocarbon‐contaminated aquifer. Appl Environ Microbiol. 2005;71:149–158. doi: 10.1128/AEM.71.1.149-158.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivushin K.V., Shcherbakova V.A., Petrovskaya L.E., Rivkina E.M. Methanobacterium veterum sp nov., from ancient Siberian permafrost. Int J Syst Evol Microbiol. 2010;60:455–459. doi: 10.1099/ijs.0.011205-0. [DOI] [PubMed] [Google Scholar]
- Kubota K., Ohashi A., Imachi H., Harada H. Visualization of mcr mRNA in a methanogen by fluorescence in situ hybridization with an oligonucleotide probe and two‐pass tyramide signal amplification (two‐pass TSA‐FISH) J Microbiol Methods. 2006;66:521–528. doi: 10.1016/j.mimet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Kubota K., Imachi H., Kawakami S., Nakamura K., Harada H., Ohashi A. Evaluation of enzymatic cell treatments for application of CARD‐FISH to methanogens. J Microbiol Methods. 2008;72:54–59. doi: 10.1016/j.mimet.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Laloui‐Carpentier W., Li T., Vigneron V., Mazeas L., Bouchez T. Methanogenic diversity and activity in municipal solid waste landfill leachates. Antonie Van Leeuwenhoek. 2006;89:423–434. doi: 10.1007/s10482-005-9051-9. [DOI] [PubMed] [Google Scholar]
- Lane D.J. 16S/23S rRNA sequencing. In: Stackebrandt E., Goodfellow M., editors. John Wiley & Sons; 1991. pp. 115–175. [Google Scholar]
- Lehmann‐Richter S., Grosskopf R., Liesack W., Frenzel P., Conrad R. Methanogenic archaea and CO2‐dependent methanogenesis on washed rice roots. Environ Microbiol. 1999;1:159–166. doi: 10.1046/j.1462-2920.1999.00019.x. [DOI] [PubMed] [Google Scholar]
- Li T.L., Mazeas L., Sghir A., Leblon G., Bouchez T. Insights into networks of functional microbes catalysing methanization of cellulose under mesophilic conditions. Environ Microbiol. 2009;11:889–904. doi: 10.1111/j.1462-2920.2008.01810.x. [DOI] [PubMed] [Google Scholar]
- Liu W.T., Chan O.C., Fang H.H.P. Microbial community dynamics during start‐up of acidogenic anaerobic reactors. Water Res. 2002;36:3203–3210. doi: 10.1016/s0043-1354(02)00022-2. [DOI] [PubMed] [Google Scholar]
- Liu Y.C., Whitman W.B. Metabolic, phylogenetic, and ecological diversity of the methanogenic archaea. Ann N Y Acad Sci. 2008;1125:171–189. doi: 10.1196/annals.1419.019. [DOI] [PubMed] [Google Scholar]
- Lu Y.H., Conrad R. In situ stable isotope probing of methanogenic archaea in the rice rhizosphere. Science. 2005;309:1088–1090. doi: 10.1126/science.1113435. [DOI] [PubMed] [Google Scholar]
- Lu Y.H., Lueders T., Friedrich M.W., Conrad R. Detecting active methanogenic populations on rice roots using stable isotope probing. Environ Microbiol. 2005;7:326–336. doi: 10.1111/j.1462-2920.2005.00697.x. [DOI] [PubMed] [Google Scholar]
- Ludwig W., Strunk O., Westram R., Richter L., Meier H., Yadhukumar ARB: a software environment for sequence data. Nucleic Acids Res. 2004;32:1363–1371. doi: 10.1093/nar/gkh293. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueders T., Chin K.J., Conrad R., Friedrich M. Molecular analyses of methyl‐coenzyme M reductase alpha‐subunit (mcrA) genes in rice field soil and enrichment cultures reveal the methanogenic phenotype of a novel archaeal lineage. Environ Microbiol. 2001;3:194–204. doi: 10.1046/j.1462-2920.2001.00179.x. [DOI] [PubMed] [Google Scholar]
- Lueders T., Pommerenke B., Friedrich M.W. Stable‐isotope probing of microorganisms thriving at thermodynamic limits: syntrophic propionate oxidation in flooded soil. Appl Environ Microbiol. 2004;70:5778–5786. doi: 10.1128/AEM.70.10.5778-5786.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luton P.E., Wayne J.M., Sharp R.J., Riley P.W. The mcrA gene as an alternative to 16S rRNA in the phylogenetic analysis of methanogen populations in landfill. Microbiology. 2002;148:3521–3530. doi: 10.1099/00221287-148-11-3521. [DOI] [PubMed] [Google Scholar]
- McMahon K.D., Zheng D.D., Stams A.J.M., Mackie R.I., Raskin L. Microbial population dynamics during start‐up and overload conditions of anaerobic digesters treating municipal solid waste and sewage sludge. Biotechnol Bioeng. 2004;87:823–834. doi: 10.1002/bit.20192. [DOI] [PubMed] [Google Scholar]
- Manefield M., Whiteley A.S., Griffiths R.I., Bailey M.J. RNA stable isotope probing, a novel means of linking microbial community function to Phylogeny. Appl Environ Microbiol. 2002;68:5367–5373. doi: 10.1128/AEM.68.11.5367-5373.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi J.R., Weightman A.J., Cragg B.A., Parkes R.J., Fry J.C. Methanogen and bacterial diversity and distribution in deep gas hydrate sediments from the Cascadia Margin as revealed by 16S rRNA molecular analysis. FEMS Microbiol Ecol. 2001;34:221–228. doi: 10.1111/j.1574-6941.2001.tb00773.x. [DOI] [PubMed] [Google Scholar]
- Mochimaru H., Yoshioka H., Tamaki H., Nakamura K., Kaneko N., Sakata S. Microbial diversity and methanogenic potential in a high temperature natural gas field in Japan. Extremophiles. 2007;11:453–461. doi: 10.1007/s00792-006-0056-8. et al. [DOI] [PubMed] [Google Scholar]
- Mochimaru H., Tamaki H., Hanada S., Imachi H., Nakamura K., Sakata S., Kamagata Y. Methanolobus profundi sp nov., a methylotrophic methanogen isolated from deep subsurface sediments in a natural gas field. Int J Syst Evol Microbiol. 2009;59:714–718. doi: 10.1099/ijs.0.001677-0. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Terada T., Sekiguchi Y., Shinzato N., Meng X.Y., Enoki M., Kamagata Y. Application of pseudomurein endoisopeptidase to fluorescence in situ hybridization of methanogens within the family Methanobacteliaceae. Appl Environ Microbiol. 2006;72:6907–6913. doi: 10.1128/AEM.01499-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narihiro T., Sekiguchi Y. Microbial communities in anaerobic digestion processes for waste and wastewater treatment: a microbiological update. Curr Opin Biotechnol. 2007;18:273–278. doi: 10.1016/j.copbio.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Narihiro T., Terada T., Kikuchi K., Iguchi A., Ikeda M., Yamauchi T. Comparative analysis of bacterial and archaeal communities in methanogenic sludge granules from upflow anaerobic sludge blanket reactors treating various food‐processing, high‐strength organic wastewaters. Microbes Environ. 2009a;24:88–96. doi: 10.1264/jsme2.me08561. et al. [DOI] [PubMed] [Google Scholar]
- Narihiro T., Terada T., Ohashi A., Wu J.H., Liu W.T., Araki N. Quantitative detection of culturable methanogenic archaea abundance in anaerobic treatment systems using the sequence‐specific rRNA cleavage method. ISME J. 2009b;3:522–535. doi: 10.1038/ismej.2009.4. et al. [DOI] [PubMed] [Google Scholar]
- Nercessian D., Upton M., Lloyd D., Edwards C. Phylogenetic analysis of peat bog methanogen populations. FEMS Microbiol Lett. 1999;173:425–429. doi: 10.1111/j.1574-6968.2000.tb09436.x. [DOI] [PubMed] [Google Scholar]
- Nercessian O., Prokofeva M., Lebedinski A., L'Haridon S., Cary C., Prieur D., Jeanthon C. Design of 16S rRNA‐targeted oligonucleotide probes for detecting cultured and uncultured archaeal lineages in high‐temperature environments. Environ Microbiol. 2004;6:170–182. doi: 10.1046/j.1462-2920.2003.00560.x. [DOI] [PubMed] [Google Scholar]
- Nercessian O., Bienvenu N., Moreira D., Prieur D., Jeanthon C. Diversity of functional genes of methanogens, methanotrophs and sulfate reducers in deep‐sea hydrothermal environments. Environ Microbiol. 2005;7:118–132. doi: 10.1111/j.1462-2920.2004.00672.x. [DOI] [PubMed] [Google Scholar]
- Neu T.R., Kuhlicke U., Lawrence J.R. Assessment of fluorochromes for two‐photon laser scanning microscopy of biofilms. Appl Environ Microbiol. 2002;68:901–909. doi: 10.1128/AEM.68.2.901-909.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunoura T., Oida H., Toki T., Ashi J., Takai K., Horikoshi K. Quantification of mcrA by quantitative fluorescent PCR in sediments from methane seep of the Nankai Trough. FEMS Microbiol Ecol. 2006;57:149–157. doi: 10.1111/j.1574-6941.2006.00101.x. [DOI] [PubMed] [Google Scholar]
- Nunoura T., Oida H., Miyazaki J., Miyashita A., Imachi H., Takai K. Quantification of mcrA by fluorescent PCR in methanogenic and methanotrophic microbial communities. FEMS Microbiol Ecol. 2008;64:240–247. doi: 10.1111/j.1574-6941.2008.00451.x. [DOI] [PubMed] [Google Scholar]
- Orphan V.J., House C.H., Hinrichs K.U., McKeegan K.D., DeLong E.F. Methane‐consuming archaea revealed by directly coupled isotopic and phylogenetic analysis. Science. 2001;293:484–487. doi: 10.1126/science.1061338. [DOI] [PubMed] [Google Scholar]
- Ottesen E.A., Hong J.W., Quake S.R., Leadbetter J.R. Microfluidic digital PCR enables multigene analysis of individual environmental bacteria. Science. 2006;314:1464–1467. doi: 10.1126/science.1131370. [DOI] [PubMed] [Google Scholar]
- Ovreas L., Forney L., Daae F.L., Torsvik V. Distribution of bacterioplankton in meromictic lake Saelenvanner, as determined by denaturing gradient gel electrophoresis of PCR‐amplified gene fragments coding for 16S rRNA. Appl Environ Microbiol. 1997;63:3367–3373. doi: 10.1128/aem.63.9.3367-3373.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penning H., Claus P., Casper P., Conrad R. Carbon isotope fractionation during acetoclastic methanogenesis by Methanosaeta concilii in culture and a lake sediment. Appl Environ Microbiol. 2006;72:5648–5652. doi: 10.1128/AEM.00727-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumb J.J., Bell J., Stuckey D.C. Microbial populations associated with treatment of an industrial dye effluent in an anaerobic baffled reactor. Appl Environ Microbiol. 2001;67:3226–3235. doi: 10.1128/AEM.67.7.3226-3235.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen L.K., Ballard G., Stahl D.A. Use of ribosomal‐RNA fluorescence in situ hybridization for measuring the activity of single cells in young and established biofilms. Appl Environ Microbiol. 1993;59:1354–1360. doi: 10.1128/aem.59.5.1354-1360.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radajewski S., Ineson P., Parekh N.R., Murrell J.C. Stable‐isotope probing as a tool in microbial ecology. Nature. 2000;403:646–649. doi: 10.1038/35001054. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan B., Lueders T., Dunfield P.F., Conrad R., Friedrich M.W. Archaeal community structures in rice soils from different geographical regions before and after initiation of methane production. FEMS Microbiol Ecol. 2001;37:175–186. [Google Scholar]
- Raskin L., Poulsen L.K., Noguera D.R., Rittmann B.E., Stahl D.A. Quantification of methanogenic groups in anaerobic biological reactors by oligonucleotide probe hybridization. Appl Environ Microbiol. 1994a;60:1241–1248. doi: 10.1128/aem.60.4.1241-1248.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin L., Stromley J.M., Rittmann B.E., Stahl D.A. Group‐specific 16S ribosomal‐RNA hybridization probes to describe natural communities of methanogens. Appl Environ Microbiol. 1994b;60:1232–1240. doi: 10.1128/aem.60.4.1232-1240.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin L., Zheng D.D., Griffin M.E., Stroot P.G., Misra P. Characterization of microbial communities in anaerobic bioreactors using molecular probes. Antonie Van Leeuwenhoek. 1995;68:297–308. doi: 10.1007/BF00874140. [DOI] [PubMed] [Google Scholar]
- Reysenbach A.‐L., Pace N.R. Thermophiles. In: Robb F.T., Place A.R., editors. Cold Spring Harbour Press; 1995. pp. 101–107. [Google Scholar]
- Rocheleau S., Greer C.W., Lawrence J.R., Cantin C., Laramee L., Guiot S.R. Differentiation of Methanosaeta concilii and Methanosarcina barkeri in anaerobic mesophilic granular sludge by fluorescent in situ hybridization and confocal scanning laser microscopy. Appl Environ Microbiol. 1999;65:2222–2229. doi: 10.1128/aem.65.5.2222-2229.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai S., Imachi H., Hanada S., Ohashi A., Harada H., Kamagata Y. Methanocella paludicola gen. nov., sp nov., a methane‐producing archaeon, the first isolate of the lineage ‘Rice Cluster I’, and proposal of the new archaeal order Methanocellales ord. nov. Int J Syst Evol Microbiol. 2008;58:929–936. doi: 10.1099/ijs.0.65571-0. [DOI] [PubMed] [Google Scholar]
- Sakai S., Imachi H., Sekiguchi Y., Ohashi A., Harada H., Kamagata Y. Isolation of key methanogens for global methane emission from rice paddy fields: a novel isolate affiliated with the clone cluster rice cluster I. Appl Environ Microbiol. 2007;73:4326–4331. doi: 10.1128/AEM.03008-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai S., Conrad R., Liesack W., Imachi H. Methanocella arvoryzae sp. nov., a hydrogenotrophic methanogen, isolated from Italian rice field soil. Int J Syst Evol Microbiol. 2010;60:2918–2923. doi: 10.1099/ijs.0.020883-0. [DOI] [PubMed] [Google Scholar]
- Sawayama S., Tada C., Tsukahara K., Yagishita T. Effect of ammonium addition on methanogenic community in a fluidized bed anaerobic digestion. J Biosci Bioeng. 2004;97:65–70. doi: 10.1016/S1389-1723(04)70167-X. [DOI] [PubMed] [Google Scholar]
- Sawayama S., Tsukahara K., Yagishita T. Phylogenetic description of immobilized methanogenic community using real‐time PCR in a fixed‐bed anaerobic digester. Bioresour Technol. 2006;97:69–76. doi: 10.1016/j.biortech.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Scanlan P.D., Shanahan F., Marchesi J.R. Human methanogen diversity and incidence in healthy and diseased colonic group using mcrA gene analysis. BMC Microbiol. 2008;8:79. doi: 10.1186/1471-2180-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schink B. Energetics of syntrophic cooperation in methanogenic degradation. Microbiol Mol Biol Rev. 1997;61:262–280. doi: 10.1128/mmbr.61.2.262-280.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi Y. Yet‐to‐be cultured microorganisms relevant to methane fermentation processes. Microbes Environ. 2006;21:1–15. [Google Scholar]
- Sekiguchi Y., Kamagata Y. Microbial community structure and functions in methane fermentation technology for wastewater treatment. In: Nakano M.M., Zuber P., editors. Horizon Bioscience; 2004. pp. 361–384. [Google Scholar]
- Sekiguchi Y., Kamagata Y., Nakamura K., Ohashi A., Harada H. Fluorescence in situ hybridization using 16S rRNA‐targeted oligonucleotides reveals localization of methanogens and selected uncultured bacteria in mesophilic and thermophilic sludge granules. Appl Environ Microbiol. 1999;65:1280–1288. doi: 10.1128/aem.65.3.1280-1288.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi Y., Uyeno Y., Sunaga A., Yoshida H., Kamagata Y. Sequence‐specific cleavage of 16S rRNA for rapid and quantitative detection of particular groups of anaerobes in bioreactors. Water Sci Technol. 2005;52:107–113. [PubMed] [Google Scholar]
- Shcherbakova V.A., Rivkina E.M., Pecheritsyna S., Laurinavichuis K., Suzina N.E., Gilichinsky D. Methanobacterium arcticum sp.nov., methanogenic archaeon from Holocene Arctic permafrost. Int J Syst Evol Microbiol. 2010 doi: 10.1099/ijs.0.021311-0. [DOI] [PubMed] [Google Scholar]
- Shigematsu T., Tang Y.Q., Kawaguchi H., Ninomiya K., Kijima J., Kobayashi T. Effect of dilution rate on structure of a mesophilic acetate‐degrading methanogenic community during continuous cultivation. J Biosci Bioeng. 2003;96:547–558. doi: 10.1016/S1389-1723(04)70148-6. et al. [DOI] [PubMed] [Google Scholar]
- Shigematsu T., Tang Y.Q., Kobayashi T., Kawaguchi H., Morimura S., Kida K. Effect of dilution rate on metabolic pathway shift between aceticlastic and nonaceticlastic methanogenesis in chemostat cultivation. Appl Environ Microbiol. 2004;70:4048–4052. doi: 10.1128/AEM.70.7.4048-4052.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinzato N., Watanabe I., Meng X.Y., Sekiguchi Y., Tamaki H., Matsui T., Kamagata Y. Phylogenetic analysis and fluorescence in situ hybridization detection of archaeal and bacterial endosymbionts in the anaerobic ciliate Trimyema compressum. Microb Ecol. 2007;54:627–636. doi: 10.1007/s00248-007-9218-1. [DOI] [PubMed] [Google Scholar]
- Skillman L.C., Evans P.N., Naylor G.E., Morvan B., Jarvis G.N., Joblin K.N. 16S ribosomal DNA‐directed PCR primers for ruminal methanogens and identification of methanogens colonising young lambs. Anaerobe. 2004;10:277–285. doi: 10.1016/j.anaerobe.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Smith J.M., Green S.J., Kelley C.A., Prufert‐Bebout L., Bebout B.M. Shifts in methanogen community structure and function associated with long‐term manipulation of sulfate and salinity in a hypersaline microbial mat. Environ Microbiol. 2008;10:386–394. doi: 10.1111/j.1462-2920.2007.01459.x. [DOI] [PubMed] [Google Scholar]
- Sorensen A.H., Ahring B.K. An improved enzyme‐linked immunosorbent assay for whole‐cell determination of methanogens in samples from anaerobic reactors. Appl Environ Microbiol. 1997;63:2001–2006. doi: 10.1128/aem.63.5.2001-2006.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer E., Sachs M.S., Woese C.R., Boone D.R. Partial gene sequences for the A subunit of methyl‐coenzyme M reductase (mcrI) as a phylogenetic tool for the family Methanosarcinaceae. Int J Syst Bacteriol. 1995;45:554–559. doi: 10.1099/00207713-45-3-554. [DOI] [PubMed] [Google Scholar]
- Stahl D.A., Amann R. Development and application of nucleic acid probes. In: Stackebrandt E., Goodfellow M., editors. Wiley; 1991. pp. 205–248. [Google Scholar]
- Stahl D.A., Flesher B., Mansfield H.R., Montgomery L. Use of phylogenetically based hybridization probes for studies of ruminal microbial ecology. Appl Environ Microbiol. 1988;54:1079–1084. doi: 10.1128/aem.54.5.1079-1084.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L.M., Regan J.M. Phylogenetic comparison of the methanogenic communities from an acidic, oligotrophic fen and an anaerobic digester treating municipal wastewater sludge. Appl Environ Microbiol. 2008;74:6663–6671. doi: 10.1128/AEM.00553-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L.M., Regan J.M. mcrA‐targeted real‐time quantitative PCR method to examine methanogen communities. Appl Environ Microbiol. 2009;75:4435–4442. doi: 10.1128/AEM.02858-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strapoc D., Picardal F.W., Turich C., Schaperdoth I., Macalady J.L., Lipp J.S. Methane‐producing microbial community in a coal bed of the Illinois Basin. Appl Environ Microbiol. 2008;74:2424–2432. doi: 10.1128/AEM.02341-07. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sul W.J., Park J., Quensen J.F., Rodrigues J.L.M., Seliger L., Tsoi T.V. DNA‐stable isotope probing integrated with metagenomics for retrieval of biphenyl dioxygenase genes from polychlorinated biphenyl‐contaminated river sediment. Appl Environ Microbiol. 2009;75:5501–5506. doi: 10.1128/AEM.00121-09. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabatabaei M., Rahim R.A., Abdullah N., Wright A.‐D.G., Shirai Y., Sakai K. Importance of the methanogenic archaea populations in anaerobic wastewater treatments. Process Biochem. 2010;45:1214–1225. et al. [Google Scholar]
- Takai K., Nakamura K., Toki T., Tsunogai U., Miyazaki M., Miyazaki J. Cell proliferation at 122 degrees C and isotopically heavy CH4 production by a hyperthermophilic methanogen under high‐pressure cultivation. Proc Natl Acad Sci USA. 2008;105:10949–10954. doi: 10.1073/pnas.0712334105. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot G., Topp E., Palin M.F., Masse D.I. Evaluation of molecular methods used for establishing the interactions and functions of microorganisms in anaerobic bioreactors. Water Res. 2008;42:513–537. doi: 10.1016/j.watres.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Tang Y.Q., Shigematsu T., Morimura S., Kida K. Microbial community analysis of mesophilic anaerobic protein degradation process using bovine serum albumin (BSA)‐fed continuous cultivation. J Biosci Bioeng. 2005;99:150–164. doi: 10.1263/jbb.99.150. [DOI] [PubMed] [Google Scholar]
- Tatsuoka N., Mohammed N., Mitsumori M., Hara K., Kurihara M., Itabashi H. Phylogenetic analysis of methyl coenzyme‐M reductase detected from the bovine rumen. Lett Appl Microbiol. 2004;39:257–260. doi: 10.1111/j.1472-765X.2004.01566.x. [DOI] [PubMed] [Google Scholar]
- Thauer R.K. Biochemistry of methanogenesis: a tribute to Marjory Stephenson. Microbiology. 1998;144:2377–2406. doi: 10.1099/00221287-144-9-2377. [DOI] [PubMed] [Google Scholar]
- Thauer R.K., Shima S. Methane as fuel for anaerobic microorganisms. Ann N Y Acad Sci. 2008;1125:158–170. doi: 10.1196/annals.1419.000. [DOI] [PubMed] [Google Scholar]
- Tringe S.G., Hugenholtz P. A renaissance for the pioneering 16S rRNA gene. Curr Opin Microbiol. 2008;11:442–446. doi: 10.1016/j.mib.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Tung H.C., Bramall N.E., Price P.B. Microbial origin of excess methane in glacial ice and implications for life on Mars. Proc Natl Acad Sci USA. 2005;102:18292–18296. doi: 10.1073/pnas.0507601102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ufnar J.A., Ufnar D.F., Wang S.Y., Ellender R.D. Development of a swine‐specific fecal pollution marker based on host differences in methanogen mcrA genes. Appl Environ Microbiol. 2007;73:5209–5217. doi: 10.1128/AEM.00319-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyeno Y., Sekiguchi Y., Sunaga A., Yoshida H., Kamagata Y. Sequence‐specific cleavage of small‐subunit (SSU) rRNA with oligonucleotides and RNase H: a rapid and simple approach to SSU rRNA‐based quantitative detection of microorganisms. Appl Environ Microbiol. 2004;70:3650–3663. doi: 10.1128/AEM.70.6.3650-3663.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavilin V.A., Qu X., Mazeas L., Lemunier M., Duquennoi C., He P.J., Bouchez T. Methanosarcina as the dominant aceticlastic methanogens during mesophilic anaerobic digestion of putrescible waste. Antonie Van Leeuwenhoek. 2008;94:593–605. doi: 10.1007/s10482-008-9279-2. [DOI] [PubMed] [Google Scholar]
- Vianna M.E., Holtgraewe S., Seyfarth I., Conrads G., Horz H.P. Quantitative analysis of three hydrogenotrophic microbial groups, methanogenic archaea, sulfate‐reducing bacteria, and acetogenic bacteria, within plaque biofilms associated with human periodontal disease. J Bacteriol. 2008;190:3779–3785. doi: 10.1128/JB.01861-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser F.A., van Lier J.B., Macario A.J.L., de Macario E.C. Diversity and population dynamics of methanogenic bacteria in a granular consortium. Appl Environ Microbiol. 1991;57:1728–1734. doi: 10.1128/aem.57.6.1728-1734.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F.P., Zhou H.Y., Meng J., Peng X.T., Jiang L.J., Sun P. GeoChip‐based analysis of metabolic diversity of microbial communities at the Juan de Fuca Ridge hydrothermal vent. Proc Natl Acad Sci USA. 2009;106:4840–4845. doi: 10.1073/pnas.0810418106. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weijers J.W.H., Schouten S., van der Linden M., van Geel B., Damste J.S.S. Water table related variations in the abundance of intact archaeal membrane lipids in a Swedish peat bog. FEMS Microbiol Lett. 2004;239:51–56. doi: 10.1016/j.femsle.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Wright A.D.G., Pimm C. Improved strategy for presumptive identification of methanogens using 16S riboprinting. J Microbiol Methods. 2003;55:337–349. doi: 10.1016/s0167-7012(03)00169-6. [DOI] [PubMed] [Google Scholar]
- Wu J.H., Liu W.T. Quantitative multiplexing analysis of PCR‐amplified ribosomal RNA genes by hierarchical oligonucleotide primer extension reaction. Nucleic Acids Res. 2007;35:e82. doi: 10.1093/nar/gkm413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagita K., Kamagata Y., Kawaharasaki M., Suzuki T., Nakamura Y., Minato H. Phylogenetic analysis of methanogens in sheep rumen ecosystem and detection of Methanomicrobium mobile by fluorescence in situ hybridization. Biosci Biotechnol Biochem. 2000;64:1737–1742. doi: 10.1271/bbb.64.1737. [DOI] [PubMed] [Google Scholar]
- Yarza P., Richter M., Peplies J., Euzeby J., Amann R., Schleifer K.H. The All‐Species Living Tree project: a 16S rRNA‐based phylogenetic tree of all sequenced type strains. Syst Appl Microbiol. 2008;31:241–250. doi: 10.1016/j.syapm.2008.07.001. et al. [DOI] [PubMed] [Google Scholar]
- Yashiro Y., Sakai S., Ehara M., Miyazaki M., Yamaguchi T., Imachi H. Methanoregula formicica sp. nov., a novel methane‐producing archaeon isolated from methanogenic sludge. Int J Syst Evol Microbiol. 2009 doi: 10.1099/ijs.0.014811-0. [DOI] [PubMed] [Google Scholar]
- Yu Y., Lee C., Kim J., Hwang S. Group‐specific primer and probe sets to detect methanogenic communities using quantitative real‐time polymerase chain reaction. Biotechnol Bioeng. 2005;89:670–679. doi: 10.1002/bit.20347. [DOI] [PubMed] [Google Scholar]
- Yu Y., Kim J., Hwang S. Use of real‐time PCR for group‐specific quantification of aceticlastic methanogens in anaerobic processes: population dynamics and community structures. Biotechnol Bioeng. 2006;93:424–433. doi: 10.1002/bit.20724. [DOI] [PubMed] [Google Scholar]
- Yu Z.T., Garcia‐Gonzalez R., Schanbacher F.L., Morrison M. Evaluations of different hypervariable regions of archaeal 16S rRNA genes in profiling of methanogens denaturing by Archaea‐specific PCR and gradient gel electrophoresis. Appl Environ Microbiol. 2008;74:889–893. doi: 10.1128/AEM.00684-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G.S., Jiang N., Liu X.L., Dong X.Z. Methanogenesis from methanol at low temperatures by a novel psychrophilic methanogen, ‘Methanolobus psychrophilus’ sp nov., prevalent in Zoige wetland of the Tibetan plateau. Appl Environ Microbiol. 2008a;74:6114–6120. doi: 10.1128/AEM.01146-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G.S., Tian J.Q., Jiang N., Guo X.P., Wang Y.F., Dong X.Z. Methanogen community in Zoige wetland of Tibetan plateau and phenotypic characterization of a dominant uncultured methanogen cluster ZC‐I. Environ Microbiol. 2008b;10:1850–1860. doi: 10.1111/j.1462-2920.2008.01606.x. [DOI] [PubMed] [Google Scholar]
- Zhang T., Fang H.H.P. Applications of real‐time polymerase chain reaction for quantification of microorganisms in environmental samples. Appl Microbiol Biotechnol. 2006;70:281–289. doi: 10.1007/s00253-006-0333-6. [DOI] [PubMed] [Google Scholar]
- Zheng D., Raskin L. Quantification of Methanosaeta species in anaerobic bioreactors using genus‐ and species‐specific hybridization probes. Microbial Ecol. 2000;39:246–262. doi: 10.1007/s002480000003. [DOI] [PubMed] [Google Scholar]
- Zheng D., Angenent L.T., Raskin L. Monitoring granule formation in anaerobic upflow bioreactors using oligonucleotide hybridization probes. Biotechnol Bioeng. 2006;94:458–472. doi: 10.1002/bit.20870. [DOI] [PubMed] [Google Scholar]


