Summary
Insects form an extremely large group of animals and bear a consequently large variety of associated microbes. This microbiota includes very specific and obligate symbionts that provide essential functions to the host, and facultative partners that are not necessarily required for survival. The Tephritidae is a large family that includes many fruit pests such as the Mediterranean fruit fly (the medfly, Ceratitis capitata) and the Olive fly (Bactrocera oleae). Community and functional analyses showed that the microbiota of both flies contribute to their diet, and affect host fitness parameters. The analysis of the microbiota's community structure of mass‐reared, sterilized medfly males used in the sterile insect technique revealed a strong reduction in Klebsiella spp. compared with non‐sterile and wild flies. Inoculation of sterile males with this gut population affected female mating behaviour as they preferentially mated with inoculated versus non‐inoculated males. These studies suggest that control can be significantly improved by manipulating symbionts in pest animals.
The biological world, as we know it today, comprises 1.5–1.8 millions of species of organisms that have been described and another 5–100 million that await discovery and/or description (Wilson, 2003). Only a tiny fraction of these species (around 10 000; Euzeby, 2010) are prokaryotes. The reason for this paucity is the requirement to obtain a culture of the organism, an unattainable task in most cases today, in order to formally define a prokaryotic species (Gevers et al., 2005). Nevertheless, a tremendous prokaryotic genetic diversity is now well documented (Torsvik et al., 2002). The profound influence of microbial genomes on the biology, including the evolution, of eukaryotic hosts with which they can associate is being recognized (Rosenberg et al., 2009). Foremost among these hosts are the insects with this clade comprising about half of the total described species (Mayhew, 2007). Insects are major contributors to natural as well as to man‐managed ecosystems and largely bear on human affairs, both positively (e.g. plant pollinators and natural enemies) and negatively (e.g. pests and disease vectors).
The microbiota insects support is usually less complex than those of higher animals such as mammals, including humans. In the latter case, at least 5 million genes can be found in the microbiota, a figure well above the 20 000 or so human genes (Rosenberg and Rosenberg, 2008). However, community complexity is only one part of the whole picture: ecological and evolutionary differences between the lifestyles of different animals shape different relationships between hosts and symbionts (a term used thereafter in its widest meaning). These differences are reflected at the physiological, ecological and anatomical levels. For example, P symbionts (see below) are not known in mammals and no dedicated organ or cellular structure has been found to specifically enclose bacteria, this in contrast to insects. Yet, rules governing the interactions between host and microbial symbionts may emerge from the study of insect models (Chaston and Goodrich‐Blair, 2010); insects can be relatively easily grown under controlled conditions, the host and the symbionts can be manipulated, the interactions precisely determined and effects measured. In addition, the added benefit of studying insect–microbe interactions is that their understanding may quite readily be applied to contribute to increasing human well‐being by reducing insect‐caused damages.
Different types of symbiotic associations are found in insects
Insect symbionts have been broadly categorized into two main types: primary (P) and secondary (S) symbionts. P symbionts are mostly found within specialized structures called bacteriomes that contain bacteriocytes (or mycetocytes) cells within which the symbiotic bacteria are enclosed. Bacteriomes are located within cavities of the insect body, or associated with a gut compartment, and the intracellular bacteria are generally unculturable. P symbionts are usually deemed essential for their host with which they share long evolutionary histories, and are generally transmitted vertically (Baumann et al., 2006). S symbionts are heritable, mostly facultative symbionts, with variable contributions to their host. They originate in multiple independent infections, horizontal transmission or both (Baumann et al., 2006).
Wolbachia symbionts may be considered P symbionts but they are not essential for their host. Instead, by and large, they have detrimental effects on their host's fitness, although beneficial effects have also been described (Dean, 2006; Koukou et al., 2006; Brownlie et al., 2009). Wolbachia manipulate host reproduction to increase their spread in host populations (Turelli and Hoffmann, 1991). In cytoplasmic incompatibility, maybe the most common mechanism used by Wolbachia to alter the sex ratio in the favour of females, the mating of an uninfected female – or of female and male carrying incompatible strains – with an infected male results in early embryonic development arrest (O'Neill and Karr, 1990).
Genome erosion is a feature of P symbionts, leading to a Muller's ratchet (Moran, 1996). An accelerated rate of molecular evolution in P symbionts leads to size‐reduced genomes with an AT‐biased nucleotide composition, the loss of open reading frames, including of DNA repair functions and of regulatory sequences (Tamas et al., 2002; McCutcheon and Moran, 2007). These characteristics are detectable in Sodalis glossinidius, the S symbiont in the Tsetse fly (Glossina morsitans ssp. morsitans). In fact, this symbiont may be undergoing a transition between an S type to a P type: it is inherited by the intrauterine route just like the fly's P symbiont Wigglesworthia (Pais et al., 2008), its genome has reduced coding capacity and contains numerous pseudogenes (Toh et al., 2006), but the bacterium can be cultured and appears to have only recently diverged from a free‐living ancestor (Beard et al., 1992). Thus, genome erosion, and the recruitment of S symbionts may lead to symbiont displacement. In Curculionoidea (weevils), congruence between host and symbiont phylogenies, 16S rDNA gene evolutionary rates and AT content suggest that the older Candidatus Nardonella symbiont was replaced as the insects shifted to different diets or habitats (Lefevre et al., 2004; Conord et al., 2008).
The P/S classification is very useful but does not cover the case in which the insect‐associated microbiota is facultative, consisting of culturable bacteria that significantly contribute to the host's fitness: in a word, S symbionts in the absence of P symbionts. This appears to be the case in Ceratitis capitata (Wiedemann), the Mediterranean fruit fly (Diptera:Tephritidae), one of the most polyphagous, widespread and destructive pest species (Liquido et al., 1991). Thus, there are various degrees of tightness in the symbiotic associations linking insects and microbes, and they overlap, are dynamic and may evolve rapidly. Research of this reservoir of interactions may yield principles governing the symbioses; once understood, principles may be put to use.
The Tephritidae is a large family that includes many fruit pests
The family Tephritidae includes almost 5000 species in about 500 genera (Evenhuis et al., 2008). It is part of the order Diptera that contains the flies, the midges, gnats, bots and other two‐winged forms and may encompass 20% of insect diversity, with more than 150 000 described species (Yeates and Wiegmann, 2005). Within the Tephritidae, approximately 70 species are considered as important agricultural pests while many others may cause minor damage or are potentially harmful (White and Elson‐Harris, 1992). Fruits are the main hosts of the most deleterious genera: Anastrepha, Ceratitis, Bactrocera, Dacus and Rhagoletis. Many of these flies are highly polyphagous, utilizing a large variety of fruits or other food sources. In addition, in the subfamily Tephritinae, the larvae feed on Asteraceae flower heads and often induce formation of galls (Headrick and Goeden, 1994).Therefore there are substantial differences in the feeding behaviours of flies and in their developmental stages: the females of the medfly oviposit in many types of fruits, and larvae may experience quite varied growth conditions; in contrast, the larvae of the olive fly (Bactrocera oleae) only grow in olive fruits. In both flies, the adults feed on sugar rich diets such as fruit juices, honeydew, nectar, fruit and plant exudates as well as on microorganisms (Drew and Yuval, 2000), and occasionally on bird droppings and pollen (Christenson and Foote, 1960; Drew and Yuval, 2000). If symbionts are needed at both the adult and the larval stages, their contributions should be consequently adapted to the needs of each stage: populations may consequently shift, or a dominant symbiont may be metabolically versatile or plurivalent.
Tephritidae are therefore diverse, as are their associations with bacteria. The digestive tract of many of these flies has evolved to contain specialized cavities or organs within which bacterial symbionts are hosted (Stammer 1929; Mazzon et al., 2008). In fruit flies such as the medfly and the olive fly, a bacteria‐filled esophageal bulb is present, and the gut symbionts are restricted to the intestinal lumen (Capuzzo et al., 2005). The main colonizers in medfly are culturable (Behar et al., 2008a). In the olive fly, Capuzzo and colleagues (2005) characterized Candidatus Erwinia dacicola as the dominant, uncultured symbiont, while the cultured Acetobacter tropicalis was proposed by Kounatidis and colleagues (2009).
In the Tephritinae, the esophageal bulb appears to be devoid of microorganisms, bacteria are found in the gut lumen but also outside the peritrophic membrane (a thin chitinous‐proteinaceous membrane that separates food from midgut tissue) in contact with midgut epithelial cells; these bacteria could not be cultured (Mazzon et al., 2008). The causes and effects of these anatomical differences are not known. Yet, Enterobacteriaceae appear to constitute the largely dominant symbiotic clade in these associations (Table 1) with their populations and functions exhibiting large variations between the different life stages of the hosts (see below), with certain symbionts being readily culturable while others still remain uncultured.
Table 1.
Bacterial genera found in association with Tephritids.
| Tephritid species | Source organ | Bacterial genus | Reference |
|---|---|---|---|
| Anastrepha ludens | Gut | C, E, K, Pr, Ps | Kuzina et al. (2001) |
| Anastrepha ludens | Crop, gut | C, E, K, P | Martinez et al. (1994) |
| Bactrocera cacuminata | Gut | C, K, Pa, Ps, | Fitt and Obrien (1985); Raghu et al. (2002) |
| Bactrocera jarvisi | Gut | E, K, Pr, Ps, | Fitt and Obrien (1985) |
| Bactrocera neolumeralis | Gut | E, Ps | Fitt and Obrien (1985) |
| Bactrocera oleae | Esophageal bulb, gut, ovipositor | A, Er, Ps | Petri (1909); Capuzzo et al. (2005); Kounatidis et al. (2009) |
| Bactrocera tryoni | Crop, gut, host plant, mouthparts | E, K, Pa, Pr, Ps | Fitt and Obrien (1985); Drew and Lloyd (1987) |
| Ceratitis capitata | Gut, esophageal bulb | C, E, Er, K, Pa, Pc, Pr, Ps | Marchini et al. (2002); Lauzon (2003); Behar et al. (2008a) |
| Rhagoletis alternata | Gut | E, Er | Daser and Brandl (1992) |
| Rhagoletis completa | Esophageal bulb | K | Howard et al. (1985) |
| Rhagoletis cornivora | Esophageal bulb | E, K | Howard et al. (1985) |
| Rhagoletis electromorpha | Esophageal bulb | E, K | Howard et al. (1985) |
| Rhagoletis mendax | Esophageal bulb | E, K | Howard et al. (1985) |
| Rhagoletis pomonella | Esophageal bulb, crop, gut | E, K, Ps | Rossiter et al. (1983); Howard et al. (1985); Lauzon (1998; 2003) |
| Rhagoletis suavis | Esophageal bulb | E, K | Howard et al. (1985) |
| Rhagoletis tabellaria | Esophageal bulb | E, K | Howard et al. (1985) |
| Tephritis conura | Gut | Er | Daser and Brandl (1992) |
| Tephritis dilacerata | Gut | E | Daser and Brandl (1992) |
| Urophora cuspidata | Gut | Er | Daser and Brandl (1992) |
| Urophora solstitialis | Gut | Er | Daser and Brandl (1992) |
Key to genus name: A, Acetobacter; C, Citrobacter; E, Enterobacter; Er, Erwinia; K, Klebsiella; Pa, Pantoea; Pc, Pectobacterium; Pr, Providencia; Ps, Pseudomonas. Adapted from Behar and colleagues (2008c), references within.
Insect symbionts contribute to the nutrition of their hosts
Symbiotic bacteria associated with insects affect their hosts in many ways. Some of these effects are major and arise when the microbe's contribution is essential for survival or has extreme consequences on the fitness of the host. For example, P symbionts providing nutritional complementation of monotonous diets largely deficient in one or more essential nutrients upgrade the food source. This enables the host insect to obtain the necessary compounds when just ingesting more food does not help. This is the situation in wood‐eating termites, blood‐feeding insects and plant sap‐feeding insects. Aphids, psyllids, whiteflies, mealybugs and stinkbugs feed on phloem sap, and sharpshooters feed on xylem sap. These saps differ in sugar content but both are lacking essential amino acids (Kato, 1981; Sandström and Pettersson, 1994). A large body of research has demonstrated the role of the Buchnera symbiont in nutritional complementation in aphids; in sharpshooters, the two P symbionts Candidatus Sulcia muelleri and of Candidatus Baumannia cicadellinicola complement their host's requirements for essential amino acids, and vitamins and cofactors respectively. For a recent review on symbiont‐based nutritional complementation in insects, the reader is invited to consult Douglas (2009).
Insects feeding on complex food sources may still require symbionts to overcome deficiencies when the full required dietary complement is not available. A now well‐cited example is that of the opportunistic feeders Carpenter ants (Camponotus) and their obligate Candidatus Blochmania symbiont, which provides the colony with essential amino acids and recycled nitrogen when nutrition alone cannot provide these compounds (Feldhaar et al., 2007).
As described above, adult fruit flies such as the olive fly and the medfly are also opportunistic feeders; yet, they can experience unbalanced diets containing high levels of carbohydrates and low, deficient levels of amino acids, including essential ones (Wackers, 2005). Provisioning of insufficient quantities of metabolizable nitrogen or of qualitatively inadequate nitrogenous compounds – e.g. a lack in essential amino acids – reduces the reproductive potential of the fly (Tsitsipis, 1989; Drew and Yuval, 2000). Ben‐Yosef and colleagues (2008a; 2010) investigated the effects of the gut microbiota on fitness parameters, in both species. Antibiotic compounds were added to diets of various qualities and their effects on female oviposition were observed. Control treatments were included to measure the efficiency of the antibiotic treatment on the one hand – reducing the size of the gut microbiota by order of magnitudes – and the extent of its effects on the metabolism of the fly – food intake, weight and else, on the other hand; these were minor.
Female medflies fed a full diet (sugar and yeast hydrolysate) were not affected by the antibiotic treatment, as the quantity of eggs they deposited and the dynamics of oviposition were unaltered. Strongly nutritionally stressed females fed a sugar diet laid significantly less eggs than flies fed the rich diet, confirming the effect of diet quality on egg production; in addition, the flies exposed to antibiotics significantly accelerated their oviposition rate (Ben‐Yosef et al., 2008a). Female olive flies were tested in a similar but more extensive series of treatments and were fed a poor (sugar only), a rich (yeast hydrolysate) or a deficient (non‐essential amino acids as the only nitrogen source) diet, with or without antibiotic in the diet (Ben‐Yosef et al., 2010). No significant differences between flies fed or not fed antibiotics were observed in the poor and in the rich diets. In the deficient diet lacking essential amino acids, the flies bearing their natural complement of bacteria produced more eggs than the antibiotic‐treated ones. Further, female olive flies fed a honeydew diet with or without antibiotics, behaved similarly to flies fed on a deficient diet (M. Ben‐Yosef, unpubl. results).
A conclusion of these experiments is that bacteria do not contribute to egg production in strongly nutritionally deprived, sugar fed females and do not provide supplementary capacities to females feeding on a complete diet. Overall, these tests suggest that bacteria in these fruit flies can fill ‘deficiency gaps’ by complementing deficient nitrogenous nutrients under suboptimal conditions. However, a minimal level of metabolizable nitrogen seems to be required to achieve complementation.
Interestingly, nitrogen fixation was shown to occur in the gut of the medfly (Behar et al., 2005). The bacterial community inhabiting the medfly's gut is essentially composed of Enterobacteriaceae and includes Citrobacter, Enterobacter, Klebsiella, Pantoea and Pectobacterium as dominant genera (Behar et al., 2005; 2008a; Ben Ami et al., 2010) and nitrogen fixation is known to be performed by members of these clades. In their work, Behar and colleagues (2005) demonstrated nitrogen fixation in the gut of the adult medfly, using RT‐PCR and an acetylene reduction assay. It was inferred from the reaction rate that a significant portion of the fly's nitrogen requirements could thus be provided by fixing atmospheric nitrogen. Larvae also express the nitrogenase gene in fruits, and may therefore also enjoy an increased source of nitrogen (Behar et al., 2008a). In addition to fixing nitrogen, the gut microbiota may help recycle nitrogenous waste products into usable compounds such as uric acid and ammonia. Bacterial species like Enterobacter are present in the medfly's gut and may produce uricase, an enzyme that degrades uric acid into allantoin (Lauzon et al., 2000), which is then further processed to urea. In turn, urea is transformed into ammonia by various Enterobacteriaceae like Klebsiella oxytoca or Enterobacter gergoviae (Zinder and Dworkin, 2000); as mentioned, these bacterial taxa are commonly associated with the medfly gut.
The bacterial populations provisioning nitrogen to the medfly may also be involved in providing additional carbon to their hosts during larval growth. While the lytic enzymes produced by medfly larvae can digest proteins and simple sugars, they do not degrade polysaccharide efficiently (Silva et al., 2006). Klebsiella oxytoca and Pectobacterium are dominant, rot‐causing pectinolytic bacterial populations. They can generate an ample pool of sugars metabolizable by the growing larva in the fruit (Behar et al., 2008a), helping it to graduate to the next ontogenetic stage (Kaspi et al., 2002). This flow of sugar may also provide energy for the energy‐demanding nitrogen fixation process; more than half of the pectin‐degrading colonies isolated from medfly larvae and fruit rot were also able to fix nitrogen (Behar et al., 2008a). In contrast, the diets rich in available sugars experienced by the adult fly may explain the reduced levels of pectin‐degrading bacteria found at this stage in the gut of the insect (Behar et al., 2008a).
The importance of keeping a controlled microbiota may be observed in both the medfly and the olive fly, as bacterial populations are transmitted from parents to offspring, suggesting a tight association between the host and its symbiotic organisms. Behar and colleagues (2008a) working with flies caught in the field identified a number of genetically identical bacterial populations in deposited eggs, in the rot developing after oviposition, in next‐generation larvae and in the guts of these F1 adults; Lauzon and colleagues (2009) demonstrated the establishment and transgenerational transmission of marker strains inoculated in adult medflies, for at least two generations. It can be proposed that in the medfly, a consortium of closely related Enterobacteriaceae populations is transmitted from parents to offspring, and that their relative abundances during the different stages of the life cycle fluctuate according to the fly's needs. While our knowledge of symbiotic relationships in the olive fly is still less advanced, current data suggest that one bacterial species dominates both at the larval and at the adult stage (Capuzzo et al., 2005; Estes et al., 2009; Kounatidis et al., 2009). As described above, the adult gut symbionts significantly improved the insects' fitness.
In the Tsetse fly two Wigglesworthia populations are harboured: one that is enclosed in the bacteriome, and is active in vitamin (and possible other nutrients) provisioning, and free‐living cells populating the milk gland organ that are involved in maternal transmission to progeny. Wigglesworthia are required for female but not for male fertility. Other effects of this mutualist include alterations in longevity, digestion and vectorial competence (Pais et al., 2008). Noticeably, the Ca. E. dacicola symbiont of the olive fly also fractionates into an intracellular state in the larval midgut and an extracellular population in the adult foregut. Although this is still to be demonstrated, and in contrast to the medfly, these results suggest functional plurivalence of the same symbiont in the olive fly.
Insect symbionts can alter mating behaviour in fruit flies
As exemplified by the Tsetse case, insect symbionts can exert a variety of effects on their hosts. Here, we shall only focus on tephritid flies. Little is known on the role bacteria may have at the larval stage of the olive fly but Hagen (1966) and Hagen and Tassan (1972) showed that larvae growing from eggs deprived on their bacteria were unable to develop in the olive fruit. It can be postulated that the bacteria may be active in the detoxification of the antimicrobial compounds produced at high concentrations in the green olive (Amiot et al., 1989; Ryan et al., 1999). In the medfly, the microbiota affects longevity in a diet‐dependent, and complex way: flies with gut microbes carried a cost when they were nutritionally stressed (only fed sugar) but not when they were fed a full diet (Ben‐Yosef et al., 2008b). However, feeding a high concentration mixture of Enterobacteriaceae to sugar‐fed flies increased longevity; a similar treatment in which the enterobacteria were substituted with a low concentration of Pseudomonas aeruginosa isolated from the fly's gut strongly decreased their lifespan (Behar et al., 2008b). Pseudomonads form a quantitatively tiny but stable cryptic community in the fly's gut (Behar et al., 2008b). Ryu and colleagues (2008) demonstrated that a dominant bacterial population is sufficient to prevent the establishment of a minor, pathogenic bacterial strain in the Drosophila gut. This may also be the situation in the medfly, with the dominant enterobacteria controlling the size of the pseudomonad community. Yet, it should be noted that pseudomonads are not inherently pathogenic to the medfly. Very high levels of this genus are found in healthy flies produced for the sterile insect technique (SIT) (Ben Ami et al., 2010).
SIT is an environmentally friendly approach in which mass‐produced sterile males are released in the field where they compete for mating with females against wild males thus bringing about a reduction of the pest population (Hendrichs et al., 1995). A main drawback of SIT has been the rather low performance of the sterile males; they compete poorly for matings with females against wild males and therefore require frequent introductions in large numbers into the field, resulting in high operational costs. Male sterility is brought about by irradiation, and this procedure was shown to significantly alter the composition of the insect gut's microbiota (Ben Ami et al., 2010): Klebsiella spp. formed about 20% of the total gut population when males were not irradiated but only 4% when they were. In a first study, Ben‐Yosef and colleagues (2008a) showed that wild male flies not treated with antibiotics and fed a full diet had a better mating performance than antibiotic‐treated males, measured as mating latency (the amount of time taken for a female to accept copulation with a male, with treated and untreated males separated). This suggested that bacteria alter reproductive behaviour. Following this study, Ben Ami and colleagues (2010) introduced a marked Klebsiella strain isolated from a wild medfly into the irradiated male diet. Consequently, the performance in a mating latency test in 100 l tents of the males receiving this probiotic treatment was significantly improved. This experiment showed that the constitution of the microbiota affects sexual performance of males by altering female behaviour. The cues used by females to gauge males and upon which bacteria may have an effect are not known. They may be chemical (pheromones), behavioural (courtship intensity and complexity), visual, tactile or a combination thereof.
Although encouraging, a reduction in mating latency is only one parameter for measuring the competitiveness of SIT males. An effective probiotic should improve the treated males in direct competition with wild males, reduce female remating (the more a female mates, the higher the probability to encounter a wild male and lay fertilized eggs), increase longevity and be effective in the field, not only under laboratory conditions. Gavriel and colleagues (2011) set a series of competitive mating tests of increasing scales, starting with probiotic‐treated SIT males competing against untreated SIT males for females in 100 l tents, and up to probiotic‐treated or untreated SIT males competing with wild males for matings in field cages (2 × 2 × 2 m cages including a citrus tree; these are semi‐field conditions). In another test, females mated with probiotic‐treated or untreated SIT males were presented with wild males and remating frequency was measured. In all three tests, the inoculated SIT males performed significantly better than the untreated, or even the wild males.
Conclusion
Biocontrol approaches have to be upscaled from the lab to the field scale if they are to become applicative. In the medfly case, SIT is already well implemented; there are production facilities around the world and large‐scale applications (Klassen and Curtis, 2005). Yet, improving its efficiency is important for increasing its use; the medfly may also serve as an example for developing control methods for other insect pests. The results detailed here show that probiotics hold a great potential and can be introduced into SIT facilities to improve the performance of irradiated males. Other benefits may also be reaped by controlling the microbiota during all the productions stages, from the egg to the released fly. Probiotics can be applied as prophylactics that may prevent pathogens from establishing in the production and reproduction lines; they may improve larval growth, and as seen, male performance (Fig. 1). Although applications may not need waiting for all answers, central questions are still open: we do not understand the mechanisms by which the bacterial inoculum provided to the SIT male improves its performance; how ‘relaxed’ is the host–bacteria relationship in the medfly, i.e. do different species or different bacterial strains have different effects at different growth stages; what checks the growth of pathogens present in the insect? With the adequate answers, this can lead to novel applications such as stage‐specific inoculation; the use of different adapted strains or mixtures, and the manipulation of the microbiota in wild flies to promote pathogen takeover.
Figure 1.
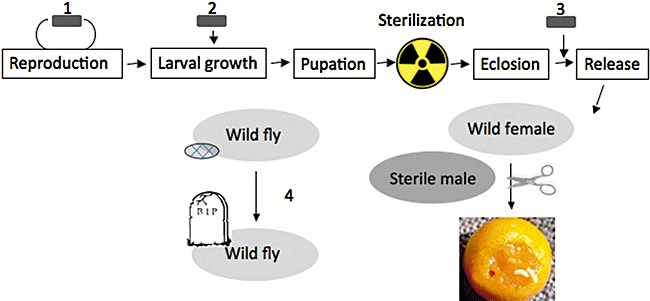
Microbiota manipulation during SIT male production can take place: (1) during egg production, with a probiotic bacterium, or a mixture of bacteria introduced in the fertile fly stock; (2) during larval growth; (3) after eclosion, before release of the sterilized adult males. The sterile males mate with wild females, which in turn do not produce larvae. (4) Wild fly microbiota may be altered, leading to increased mortality, e.g. by favouring the growth of indigenous detrimental bacteria present in the fly, or by the direct introduction of a pathogen ( ). To date, stage (3) has been investigated (see text).
). To date, stage (3) has been investigated (see text).
The contribution of its microbiota to the olive fly's life cycle and fitness demonstrates a potential for exploitation in biocontrol schemes like SIT. Alternatively, specifically targeting the main symbiont upon which the fly appears to rely at both at the larval and at the adult stage could deliver a one–two punch to this pest.
Acknowledgments
I would like to sincerely thank Professor Boaz Yuval for his helpful comments on the manuscript. My research is supported by a grant from the Binational Agricultural Research and Development Fund.
References
- Amiot M.‐J., Fleuriet A., Macheix J.‐J. Accumulation of oleuropein derivatives during olive maturation. Phytochemistry. 1989;28:67–69. [Google Scholar]
- Baumann P., Moran N.A., Baumann L. Bacteriocyte‐associated endosymbionts of insects. In: Dworkin M., Rosenberg E., Schleifer K.‐H., Stackebrandt E., editors. Springer; 2006. pp. 403–438. [Google Scholar]
- Beard C.B., O'Neill S.L., Mason P., Mandelco L., Woese C.R., Tesh R.B. Genetic transformation and phylogeny of bacterial symbionts from tsetse. Insect Mol Biol. 1992;1:123–131. doi: 10.1111/j.1365-2583.1993.tb00113.x. et al. [DOI] [PubMed] [Google Scholar]
- Behar A., Yuval B., Jurkevitch E. Enterobacteria‐mediated nitrogen fixation in natural populations of the fruit fly, Ceratitis capitata. Mol Ecol. 2005;14:2637–2643. doi: 10.1111/j.1365-294X.2005.02615.x. [DOI] [PubMed] [Google Scholar]
- Behar A., Jurkevitch E., Yuval B. Bringing back the fruit into fruit fly–bacteria interactions. Mol Ecol. 2008a;17:1375–1386. doi: 10.1111/j.1365-294X.2008.03674.x. [DOI] [PubMed] [Google Scholar]
- Behar A., Yuval B., Jurkevitch E. Gut bacterial communities in the Mediterranean fruit fly (Ceratitis capitata) and their impact on host longevity. J Insect Physiol. 2008b;54:1377–1383. doi: 10.1016/j.jinsphys.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Behar A., Ben‐Yosef M., Lauzon C.R., Yuval B., Jurkevitch E. Structure and function of the bacterial community associated with the Mediterranean fruit fly. In: Bourtzis K., Miller T., editors. CRC; 2008c. pp. 251–271. [Google Scholar]
- Ben Ami E., Yuval B., Jurkevitch E. Manipulation of the microbiota of mass‐reared Mediterranean fruit flies Ceratitis capitata (Diptera: Tephritidae) improves sterile male sexual performance. ISME J. 2010;4:28–37. doi: 10.1038/ismej.2009.82. [DOI] [PubMed] [Google Scholar]
- Ben‐Yosef M., Jurkevitch E., Yuval B. Effect of bacteria on nutritional status and reproductive success of the Mediterranean fruit fly Ceratitis capitata. Physiol Entomol. 2008a;33:145–154. [Google Scholar]
- Ben‐Yosef M., Behar A., Jurkevitch E., Yuval B. Bacteria–diet interactions affect longevity in the medfly –Ceratitis capitata. J Appl Entomol. 2008b;132:690–694. [Google Scholar]
- Ben‐Yosef M., Aharon Y., Jurkevitch E., Yuval B. Give us the tools and we will do the job: symbiotic bacteria affect olive fly fitness in a diet dependent fashion. Proc R Soc Lond B Biol Sci. 2010;277:1545–1552. doi: 10.1098/rspb.2009.2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlie J.C., Cass B.N., Riegler M., Witsenburg J.J., Iturbe‐Ormaetxe I., McGraw E.A., O'Neill S.L. Evidence for metabolic provisioning by a common invertebrate endosymbiont, Wolbachia pipientis, during periods of nutritional stress. PLoS Pathog. 2009;5:e1000368. doi: 10.1371/journal.ppat.1000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuzzo C., Firrao G., Mazzon L., Squartini A., Girolami V. ‘Candidatus Erwinia dacicola’, a coevolved symbiotic bacterium of the olive fly Bactrocera oleae (Gmelin) Int J Syst Evol Microbiol. 2005;55:1641–1647. doi: 10.1099/ijs.0.63653-0. [DOI] [PubMed] [Google Scholar]
- Chaston J., Goodrich‐Blair H. Common trends in mutualism revealed by model associations between invertebrates and bacteria. FEMS Microbiol Rev. 2010;34:41–58. doi: 10.1111/j.1574-6976.2009.00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christenson L.D., Foote R.H. Biology of fruit flies. Annu Rev Entomol. 1960;5:171–192. [Google Scholar]
- Conord C., Despres L., Vallier A., Balmand S., Miquel C. Long‐term evolutionary stability of bacterial endosymbiosis in Curculionoidea: additional evidence of symbiont replacement in the Dryophthoridae family. Mol Biol Evol. 2008;25:859–868. doi: 10.1093/molbev/msn027. et al. [DOI] [PubMed] [Google Scholar]
- Daser U., Brandl R. Microbial gut floras of 8 species of Tephritids. Biol J Linn Soc. 1992;45:155–165. [Google Scholar]
- Dean M.D. A Wolbachia‐associated fitness benefit depends on genetic background in Drosophila simulans. Proc R Soc B. 2006;273:1415–1420. doi: 10.1098/rspb.2005.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas A. The microbial dimension in insect nutritional ecology. Funct Ecol. 2009;23:38–47. [Google Scholar]
- Drew R.A.I., Lloyd A.C. Relationship of fruit‐flies (Diptera, Tephritidae) and their bacteria to host plants. Ann Entomol Soc Am. 1987;80:629–636. [Google Scholar]
- Drew R.A.I., Yuval B. The evolution of fruit fly feeding behavior. In: Aluja M., Norrbom A., editors. CRC; 2000. pp. 731–749. [Google Scholar]
- Estes A.M., Hearn D.J., Bronstein J.L., Pierson E.A. The olive fly endosymbiont, ‘Candidatus Erwinia dacicola’, switches from an intracellular existence to an extracellular existence during host insect development. Appl Environ Microbiol. 2009;75:7097–7106. doi: 10.1128/AEM.00778-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euzeby J. 2010. ) List of prokaryotic names with standing in nomenclature [WWW document]. URL http://www.bacterio.cict.fr/number.html#total.
- Evenhuis N.L., Pape T., Pont A.C., Thompson F.C. 2008. , and ) The Diptera Site [WWW document]. URL http://www.sel.barc.usda.gov/Diptera/tephriti/tephriti.htm.
- Feldhaar H., Straka J., Krischke M., Berthold K., Stoll S., Mueller M.J., Gross R. Nutritional upgrading for omnivorous carpenter ants by the endosymbiont Blochmannia. BMC Biol. 2007;5:48. doi: 10.1186/1741-7007-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitt G.P., Obrien R.W. Bacteria associated with 4 species of Dacus (Diptera, Tephritidae) and their role in the nutrition of the larvae. Oecologia. 1985;67:447–454. doi: 10.1007/BF00384954. [DOI] [PubMed] [Google Scholar]
- Gavriel S., Jurkevitch E., Gazit Y., Yuval B. Bacterially enriched diet improves sexual performance of sterile male Mediterranean fruit flies. J Appl Entomol. 2011 [Google Scholar]
- Gevers D., Cohan F.M., Lawrence J.G., Spratt B.G., Coenye T., Feil E.J. Re‐evaluating prokaryotic species. Nat Rev Microbiol. 2005;3:733–739. doi: 10.1038/nrmicro1236. et al. [DOI] [PubMed] [Google Scholar]
- Hagen K.S. Dependence of the olive fly, Dacus oleae, larvae on symbiosis with Pseudomonas savastanoi for the utilization of olive. Nature. 1966;209:423–424. [Google Scholar]
- Hagen K.S., Tassan R.L. Exploring nutritional roles of extracellular symbiotes on the reproduction of honeydews feeding adult Chrysopids and Tephritids. In: Rodriguez J.G., editor. North‐Holland; 1972. pp. 323–351. [Google Scholar]
- Headrick D.H., Goeden R.D. Reproductive behavior of California fruit flies and the classification and evolution of Tephritidae (Diptera) mating systems. Stud Dipterol. 1994;1:195–252. [Google Scholar]
- Hendrichs J., Franz G., Rendon P. Increased effectiveness and applicability of the sterile insect technique through male‐only releases for control of Mediterranean fruit flies during fruiting seasons. J Appl Entomol. 1995;119:371–377. [Google Scholar]
- Howard D.J., Bush G.L., Breznak J.A. The evolutionary significance of bacteria associated with Rhagoletis. Evolution. 1985;39:405–417. doi: 10.1111/j.1558-5646.1985.tb05677.x. [DOI] [PubMed] [Google Scholar]
- Kato T. Major nitrogen compounds transported in xylem vessels from roots to top in Citrus trees. Physiol Plant. 1981;52:275–279. [Google Scholar]
- Kaspi R., Mossinson S., Drezner T., Kamensky B., Yuval B. Effects of larval diet on development rates and reproductive maturation of male and female Mediterranean fruit flies. Physiol Entomol. 2002;27:29–38. [Google Scholar]
- Klassen W., Curtis C.F. History of the sterile insect technique. In: Dyck V.A., Hendrichs J., Robinson A.S., editors. Springer; 2005. pp. 3–38. [Google Scholar]
- Koukou K., Pavlikaki H., Kilias G., Werren J.H., Bourtzis K., Alahiotis S.N. Influence of antibiotic treatment and Wolbachia curing on sexual isolation among Drosophila melanogaster cage populations. Evolution. 2006;60:87–96. [PubMed] [Google Scholar]
- Kounatidis I., Crotti E., Sapountzis P., Sacchi L., Rizzi A., Chouaia B. Acetobacter tropicalis is a major symbiont of the olive fruit fly (Bactrocera oleae. Appl Environ Microbiol. 2009;75:3281–3288. doi: 10.1128/AEM.02933-08. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzina L.V., Peloquin J.J., Vacek D.C, Miller T.A. Isolation and identification of bacteria associated with adult laboratory Mexican fruit flies, Anastrepha ludens (Diptera: Tephritidae) Cur Microbiol. 2001;42:290–294. doi: 10.1007/s002840110219. [DOI] [PubMed] [Google Scholar]
- Lauzon C.R. Symbiotic relationships of Tephritids. In: Bourtzis K., Miller T.A., editors. CRC; 2003. pp. 115–129. [Google Scholar]
- Lauzon C.R., McCombs S.D., Potter S.E., Peabody N.C. Establishment and vertical passage of EnterobacterPantoeaagglomerans and Klebsiella pneumoniae through all life stages of the Mediterranean fruit fly (Diptera: Tephritidae) Ann Entomol Soc Am. 2009;102:85–95. [Google Scholar]
- Lauzon C.R., Sjogren R.E., Wright S.E., Prokopy R.J. Attraction of Rhagoletis pomonella (Diptera : Tephritidae) flies to odor of bacteria: Apparent confinement to specialized members of Enterobacteriaceae. Environ Entomol. 1998;27:853–857. [Google Scholar]
- Lauzon C.R., Sjogren R.E., Prokopy R.J. Enzymatic capabilities of bacteria associated with apple maggot flies: a postulated role in attraction. J Chem Ecol. 2000;26:953–967. [Google Scholar]
- Lefevre C., Charles H., Vallier A., Delobel B., Farrell B., Heddi A. Endosymbiont phylogenesis in the Dryophthoridae weevils: evidence for bacterial replacement. Mol Biol Evol. 2004;21:965–973. doi: 10.1093/molbev/msh063. [DOI] [PubMed] [Google Scholar]
- Liquido N., Shinoda L.A., Cunningham R.T. 1991.
- Marchini D., Rosetto M., Dallai R., Marry L. Bacteria associated with the oesophageal bulb of the medfly Ceratitis capitata (Diptera : Tephritidae) Curr Microbiol. 2002;44:120–124. doi: 10.1007/s00284-001-0061-1. [DOI] [PubMed] [Google Scholar]
- Martinez A.J., Robacker D.C., Garcia J.A., Esau K.L. Laboratory and field olfactory attraction of the Mexican fruit‐fly (Diptera: Tephritidae) to metabolites of bacterial species. Flo Entomol. 1994;77:117–126. [Google Scholar]
- Mayhew P.J. Why are there so many insect species? Biol Rev. 2007;82:425–454. doi: 10.1111/j.1469-185X.2007.00018.x. [DOI] [PubMed] [Google Scholar]
- Mazzon L., Piscedda A., Simonato M., Martinez‐Sanudo I., Squartini A., Girolami V. Presence of specific symbiotic bacteria in flies of the subfamily Tephritinae (Diptera Tephritidae) and their phylogenetic relationships: proposal of ‘Candidatus Stammerula tephritidis’. Int J Syst Evol Microbiol. 2008;58:1277–1287. doi: 10.1099/ijs.0.65287-0. [DOI] [PubMed] [Google Scholar]
- McCutcheon J., Moran N.A. Parallel genomic evolution and metabolic interdependence in an ancient symbiosis. Proc Natl Acad Sci USA. 2007;104:19392–19397. doi: 10.1073/pnas.0708855104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran N.A. Accelerated evolution and Muller's rachet in endosymbiotic bacteria. Proc Natl Acad Sci USA. 1996;93:2873–2878. doi: 10.1073/pnas.93.7.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill S.L., Karr T.L. Bidirectional incompatibility between conspecific populations of Drosophila simulans. Nature. 1990;348:178–180. doi: 10.1038/348178a0. [DOI] [PubMed] [Google Scholar]
- Pais R., Lohs C., Wu Y., Wang J., Aksoy S. The obligate mutualist Wigglesworthia glossinidia influences reproduction, digestion, and immunity processes of its host, the Tsetse fly. Appl Environ Microbiol. 2008;74:5965–5974. doi: 10.1128/AEM.00741-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri L. Memorie della Regia Stazione di Patologia Vegetale di Roma; 1909. [Google Scholar]
- Raghu S., Clarke A.R., Bradley J. Microbial mediation of fruit fly‐host plant interactions: Is the host plant the “centre of activity”? Oikos. 2002;97:319–328. [Google Scholar]
- Rosenberg E., Sharon G., Zilber‐Rosenberg I. The hologenome theory of evolution contains Lamarckian aspects within a Darwinian framework. Environ Microbiol. 2009;11:2959–2962. doi: 10.1111/j.1462-2920.2009.01995.x. [DOI] [PubMed] [Google Scholar]
- Rossiter M.C., Howard D.J., Bush G.L. Symbiotic bacteria of Rhagoletis pomonella. In: Cavalloro R., editor. CRC Press; 1983. pp. 77–84. [Google Scholar]
- Ryan D., Robards K., Lavee S. Accumulation of oleuropein derivatives during olive maturation. Int J Food Sci Technol. 1999;34:265–274. [Google Scholar]
- Ryu J.‐H., Kim S.‐H., Lee H.‐Y., Bai J.Y., Nam Y.‐D., Bae J.‐W. Innate immune homeostasis by the homeobox gene Caudal and commensal‐gut mutualism in Drosophila. Science. 2008;319:777–782. doi: 10.1126/science.1149357. et al. [DOI] [PubMed] [Google Scholar]
- Sandström J., Pettersson J. Amino acid composition of phloem sap and the relation to intraspecific variation in pea aphid (Acyrthosiphon pisum) performance. J Insect Physiol. 1994;40:947–955. [Google Scholar]
- Silva F., Alcazar A., Macedo L.L.P., Oliveira A.S., Macedo F.P., Abreu L.R. Digestive enzymes during development of Ceratitis capitata (Diptera : Tephritidae) and effects of SBTI on its digestive serine proteinase targets. Insect Biochem Mol Biol. 2006;36:561–569. doi: 10.1016/j.ibmb.2006.04.004. et al. [DOI] [PubMed] [Google Scholar]
- Stammer H.J. Die bakteriensymbiose der trypetiden (Diptera) Zoomorphology. 1929;15:481–523. [Google Scholar]
- Tamas I.K.L., Canback B., Naslund K.A., Eriksson A.‐S., Wernegreen J.J., Sandstrom J.P. 50 Million years of genomic stasis in endosymbiotic bacteria. Science. 2002;296:2376–2379. doi: 10.1126/science.1071278. et al. [DOI] [PubMed] [Google Scholar]
- Toh H., Weiss B.L., Perkin S.A.H., Yamashita A., Oshima K., Hattori M., Aksoy S. Massive genome erosion and functional adaptations provide insights into the symbiotic lifestyle of Sodalis glossinidius in the tsetse host. Gen Res. 2006;16:149–156. doi: 10.1101/gr.4106106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torsvik V., Øvreås L., Thingstad T.F. Prokaryotic diversity – magnitude, dynamics, and controlling factors. Science. 2002;296:1064–1066. doi: 10.1126/science.1071698. [DOI] [PubMed] [Google Scholar]
- Tsitsipis J.A. Nutrition: requirements. In: Robinson A.S., Hooper G., editors. Elsevier; 1989. pp. 103–119. [Google Scholar]
- Turelli M., Hoffmann A.A. Rapid spread of an inherited incompatibility factor in California Drosophila. Nature. 1991;353:440–442. doi: 10.1038/353440a0. [DOI] [PubMed] [Google Scholar]
- Wackers F.L. Suitability of (extra‐) floral nectar, pollen, and honeydew as insect food sources. In: Wackers F.L., van Rijn P.C.J., Bruin J., editors. Cambridge University Press; 2005. pp. 17–74. [Google Scholar]
- White I.M., Elson‐Harris M.M. CAB International; 1992. [Google Scholar]
- Wilson E.O. The encyclopedia of life. Trends Ecol Evol. 2003;18:77–80. [Google Scholar]
- Yeates D.K., Wiegmann B.M. Phylogeny and evolution of Diptera: recent insight and new perspective. In: Yeates D.K., Wiegmann B.M., editors. Colombia University Press; 2005. pp. 14–44. [Google Scholar]
- Zinder D.E., Dworkin M. Morphological and physiological diversity. In: Dworkin M., Falkow S., Rosenberg E., Schleifer K.‐H., Stackebrandt E., editors. Springer Verlag; 2000. pp. 185–220. [Google Scholar]
- Zilber‐Rosenberg E., Rosenberg E. Role of microorganisms in the evolution of animals and plants: the hologenome theory of evolution. FEMS Microbiol Rev. 2008;32:723–735. doi: 10.1111/j.1574-6976.2008.00123.x. [DOI] [PubMed] [Google Scholar]


