Summary
A fungal strain isolated from a microbial consortium growing in a natural asphalt lake is able to grow in purified asphaltenes as the only source of carbon and energy. The asphaltenes were rigorously purified in order to avoid contamination from other petroleum fractions. In addition, most of petroporphyrins were removed. The 18S rRNA and β‐tubulin genomic sequences, as well as some morphologic characteristics, indicate that the isolate is Neosartorya fischeri. After 11 weeks of growth, the fungus is able to metabolize 15.5% of the asphaltenic carbon, including 13.2% transformed to CO2. In a medium containing asphaltenes as the sole source of carbon and energy, the fungal isolate produces extracellular laccase activity, which is not detected when the fungus grow in a rich medium. The results obtained in this work clearly demonstrate that there are microorganisms able to metabolize and mineralize asphaltenes, which is considered the most recalcitrant petroleum fraction.
Introduction
The world is running out of conventional crude oils; however, there are large deposits of heavy and extra‐heavy crude oils worldwide. In the USA the heavy oil reserves are estimated at more than seven times the known reserves of conventional crude oils (Meyer and Schenk, 1988; Meyer and deWitt Jr, 1990). The largest heavy and extra‐heavy crude oil reserves in the world are found in the Orinoco oil belt of Venezuela, the Athabasca oil sands in Alberta, Canada, and the Olenik oil sands in Siberia, Russia. Asphaltenic and viscous heavy oils represent a huge energy reserve to be exploited in the next decades. In Canada only, the heavy oil reserve considered to be potentially recoverable is estimated to be 280–315 BBL (billion barrels of petroleum liquids), larger than the Saudi Arabia oil reserves estimated at 264 BBL (OPEC, 2009; Government of Alberta, Canada, 2011).
Heavy crude oils are known as unconventional crude oils because they cannot be produced, transported or refined by conventional methods. They have a specific gravity approaching or even exceeding that of water. Asphaltene and wax contents define the high viscosity and many problems associated with recovery, separation and processing of heavy oils and bitumens. Oil is considered to be a colloidal system where the asphaltenes are the disperse phase. In crude oil, the interaction between resins and asphaltenes render the latter stabilized. As in the case of waxes, asphaltenes deposition is the consequence of oil instability. In reservoirs, paraffins and asphaltenes remain in equilibrium, but when crude oil is extracted, this equilibrium is lost due to temperature and pressure changes, and as a consequence, asphaltenes and waxes tend to precipitate. This precipitate forms deposits during extraction in oil wells, blending of oils from different origins, storage, transportation and refining of heavy fractions (Mullins and Sheu, 1998; Mullins et al., 2007).
Asphaltenes are the heaviest and most polar fraction of crude oil. Asphaltenes are defined as the fraction of crude oil that is insoluble in n‐heptane or n‐pentane, but is soluble in benzene or toluene. Despite that the asphaltenes structure has not been fully elucidated, it is widely accepted that they are constituted by interacting systems of polyaromatic sheets bearing alkyl side‐chains. Asphaltene molecules have a high content of O, N and S heteroatoms as well as metals (V, Ni and Fe) (Strausz et al., 1992; Mullins et al., 2007). The problems associated with asphaltenes have increased due to the need to extract heavier crude oils, as well as the trend to extract larger amounts of light fractions out of crude oil by cracking and visbreaking.
The asphaltenic fraction is recognized as the most recalcitrant oil fraction. There is no clear evidence that asphaltenes can be degraded or transformed by microbial activity. Microorganisms have been found associated with bitumens (Wyndham and Costerton, 1981) and natural asphalt lake (Naranjo et al., 2007), which contain high amounts of asphaltenes. A molecular study by Kim and Crowley (2007) revealed a wide range of phylogenetic groups within the Archaea and Bacteria domains in natural asphalt‐rich tar pits; interestingly, genes encoding novel oxygenases were also detected in such samples. On the other hand, an extensive screening involving more than 750 strains of filamentous fungi was carried out to select strains able to modify untreated hard coal (Bublitz et al., 1994; Hofrichter et al., 1997). Only six of the 750 strains tested exhibited some activity, from which the most active fungi, Panus tigrinus, growing on wood shavings coated with coal asphaltenes led to a decrease of the average molecular weight (Hofrichter et al., 1997). Most of studies on asphaltenes biodegradation should be considered cautiously as the asphaltene content was usually determined gravimetrically after n‐alkane precipitation, and thus the reported changes may be attributed to the disruption of the asphaltenic matrix by the production of surfactants during bacterial growth, liberating trapped hydrocarbons. Other studies have reported that the asphaltenic fraction does not support bacterial growth, and no changes in asphaltene content are found after bioconversion of heavy oils and asphaltenes (Lacotte et al., 1996; Thouand et al., 1999). A few reports on oil biodegradation have claimed the degradation of asphaltenic fraction by mixed bacteria (Bertrand et al., 1983; Rontani et al., 1985). However, none of these reports described the analytical results of extractable materials recovered from appropriate sterile controls. Therefore, most of the asphaltene losses during microbial activity could be considered abiotic losses (Lacotte et al., 1996). A study conducted by Pineda and colleagues (2004) reported a bacterial consortium able to grow in asphaltenes as the sole carbon source. Asphaltenes mineralization was estimated by measuring CO2 production. The authors found in two control experiments (inoculum without asphaltenes and non‐inoculated asphaltenes), a CO2 production equivalent to 39% and 26%, respectively, of that found in the consortium growing in asphaltenes. The microbial inoculum for consortium stabilization contained 1% of crude oil, which could serve as carbon source. Thus, it is not possible to distinguish the origin of the CO2 production.
The first clear experimental evidence that enzymes are able to modify asphaltene molecules has been reported by Fedorak and colleagues (1993). Chloroperoxidase from the fungus Caldariomyces fumago and a chemically modified cytochrome c were able to transform petroporphyrins and asphaltenes in reaction mixtures containing organic solvents (Fedorak et al., 1993; Mogollon et al., 1998; Garcia‐Arellano et al., 2004). Notable spectral changes in the petroporphyrin‐rich fraction of asphaltenes were observed and the enzymatic oxidation of petroporphyrins led to the removal up to 74% of Ni and 95% of V. According to Fourier Transform Infrared Spectroscopy (FTIR) spectra, the chemically modified cytochrome c catalysed the oxidation of sulfur and carbon atoms in asphaltene molecules (Garcia‐Arellano et al., 2004). The enzymatic treatment of asphaltenes is an interesting alternative for the removal of heavy metals. It would result in reduced catalyst poisoning during hydrotreatment and cracking processes. On the other hand, the introduction of polar groups in asphaltene molecules could positively affect their sedimentation properties and improve their behaviour.
In this report we describe a fungus isolated from a natural asphalt lake that is able to grow using asphaltenes as the sole source of carbon and energy. To our knowledge, this is the first report of a microorganism able to grow in asphaltenes with rigorous control experiments.
Results
In order to determine the ability of the fungal strain to mineralize asphaltenes, mycelium was inoculated in a medium containing petroporphyrin‐free asphaltenes as the only source of carbon and energy. The fungal inocula for experiments were obtained from two previous cultures with asphaltenes as the sole source of carbon to avoid any contamination from an alternative carbon source. The kinetic of biomass production during 7‐week cultures is shown in Fig. 1. The maximal biomass was reached at the fourth week, and afterward the dry biomass started declining. For these experiments the asphaltene preparation was reprecipitated three times, and the precipitate was exhaustively washed. In addition, a procedure to reduce the content of petroporphyrins was performed. It is then possible to conclude that the fungus is clearly growing on asphaltenes as the sole source of carbon and energy.
Figure 1.
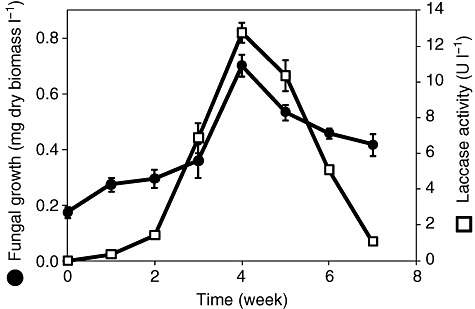
Kinetics of biomass production (filled circles) and extracellular laccase activity (open squares) of Neosartorya fischeri cultures with oil asphaltenes as sole source of carbon. The fungus mycelia was inoculated in conical flasks containing 50 ml of modified Czapek minimal medium supplemented with 20 mg of asphaltenes as the sole carbon source. Laccase activity was estimated as the ABTS oxidation. The cultures were carried out in three independent experiments.
A preliminary methylene blue stain and optical microscopic observation showed coenocytic mycelia characteristic of sexual Aspergillus, such as Neosartorya fischeri. In order to identify the fungal strain, the genomic sequence of the 18S rRNA was determined and compared with the NCBI database. Five matches with high identity, shown in Table 1, were found: Neosartorya fischeri (GenBank AY373894.1), Aspergillus lentulus (GenBank AB250399.1), Aspergillus novofumigatus (GenBank AB299411.1), Aspergillus fumisynnematus (GenBank AB250779.1) and Aspergillus fumigatus (GenBank FR733870.1). Due to the high scores of the five strains in the18S rRNA gene analysis and in order to conclusively identify the strain, the genomic sequence of the β‐tubulin was determined and compared with the NCBI database. The values obtained of sequence analysis of the β‐tubulin gene for the five higher matches of 18S rRNA are shown in Table 1: Neosartorya fischeri (GenBank EF669844), Aspergillus lentulus (GenBank FR775351), Aspergillus novofumigatus (GenBank HQ127280), Aspergillus fumisynnematus (GenBank AB248076) and Aspergillus fumigatus (GenBank NC007194). Neosartorya fischeri showed the higher score of the database. Thus, from the analysis of the β‐tubulin gene sequence it is clear that the fungal isolate from the natural asphalt Lake of Guanoco located in Sucre, Venezuela is Neosartorya fischeri.
Table 1.
Comparison of the 18S rRNA and β‐tubulin gene sequences from fungal isolate.
| Strain | 18S rRNA Identity (%) | β‐Tubulin Identity (%) |
|---|---|---|
| Neosartorya fischeria | 99 | 98 |
| Aspergillus lentulus | 99 | 94 |
| Aspergillus novofumigatus | 99 | 96 |
| Aspergillus fumisynnematus | 99 | 94 |
| Aspergillus fumigatus | 98 | 93 |
Also known as Neosartorya fischeri var. spinosa.
During fungal cultivation in asphaltenes, the extracellular medium was tested for peroxidase, monooxygenase and laccase activities. Only very low laccase activity was detected (Fig. 1). A maximum extracellular activity of 12.7 mU ml−1 (±0.6) was found at the fourth week using 2,2′‐azino‐bis(3‐ethylbenzthiazoline‐6‐sulfonic acid) (ABTS) as substrate. The concentration of extracellular protein was especially low during fungal growth. After 7 weeks culture, the extracellular media showed 1.73 µg ml−1 (±0.11) of protein; thus, a specific laccase activity of 7.3 U mg−1 of protein was obtained. In order to verify the identity of the enzyme, the catalytic activity was confirmed using the laccase‐specific substrate syringaldazine (Harkin et al., 1974). Laccase activity was only detected in medium with asphaltenes as the sole source of carbon and energy; no activity was detected when the fungus was grown in Sabouraud media. A 1300 bp DNA fragment was amplified by PCR using degenerated oligonucleotides, which were designed according to the DNA sequences of different laccases and oxidoreductases encoding genes from various Aspergillus species. The partial DNA sequence, which represents 72% of the complete protein sequence, shows remarkable identity with a putative laccase TilA from Neosartorya fischeri (Accession number GenBank: XM 001264182.1; 92% identity) and a laccase from Aspergillus fumigatus (Accession number GenBank: XM 747840.1; 90% identity).
The asphaltene mineralization was quantified by measuring CO2 production during 11 weeks cultures (Fig. 2). The CO2 evolution steadily increased until the ninth week. None of the control experiments, medium with asphaltenes without inoculum and medium with inoculum without asphaltenes, showed significant CO2 production (Fig. 2). According to elemental analysis, carbon represents 83.5% of the total weight in asphaltenes from Maya crude oil (Durand et al., 2010). A carbon balance of cultures carried out in flasks with serological stoppers showed that 13.2% (±2.6) of carbon mass contained in the asphaltenes was transformed to CO2. On the other hand, the fungal dry biomass after 11 weeks of culture was 1.5 (±0.10) mg l−1. Considering the elemental formula reported for Aspergillus niger biomass (Nielsen et al., 1994) and subtracting the inoculum weight, 2.3% (±0.04) of asphaltenic carbon was incorporated into the fungal biomass. Based on these calculations, it could be estimated that at least 15.5% of asphaltene was metabolized.
Figure 2.
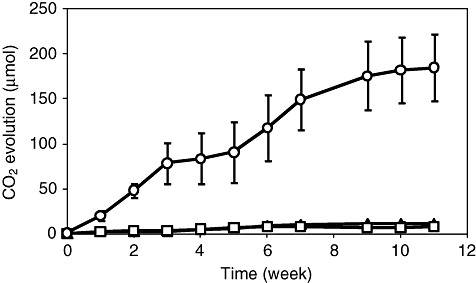
Kinetics of CO2 evolution in cultures of Neosartorya fischeri growing in asphaltenes as the sole source of carbon. Cultures were carried out in flasks with serological stoppers, containing 20 mg of asphaltenes in 30 ml of Czapek medium incubated at 37°C (circles). Control cultures, inoculated Czapek medium without asphaltenes (squares), and Czapek medium with asphaltenes and without inoculum (triangles).
After 4 weeks' culture, the cell‐free extracellular medium was solvent extracted. The extracted metabolites were then silylated and analysed by GC‐MS. Low amounts of oxidized metabolites, such as hydroxyphenylacetic acid, 9‐nitroso carbazole, thianaphthene‐2‐carboxylic acid, and hydroxypyrenedione, were detected, among traces of unidentified organic compounds. These compounds were not detected in the extracted control cultures, indicating that they are originated from the fungal metabolization of asphaltenes.
The physical interaction between the fungus strain and the solid asphaltene was analysed through scanning electron microscopy. As shown in the images in Fig. 3, the fungus surrounds the solid asphaltene and no adhesion of the hyphae to the substrate is observed. Polysaccharide‐like structures that enclose the hyphae and the solid asphaltene can be observed (Fig. 3C and D). This extracellular material could be glycoproteins that facilitate the interaction between the hydrophobic surface of the solid substrate and the fungi, as in the case of hydrophobins (Muñoz et al., 1997).
Figure 3.
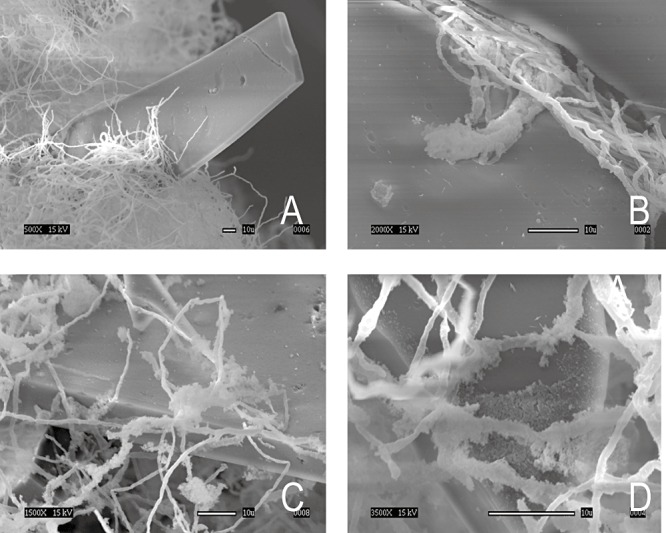
Scanning electron microscopy (SEM) of the interaction between hyphae and solid oil asphaltenes in cultures of Neosartorya fischeri. The white bar indicates 10 µm. A. Fungal mycelium embedding solid asphaltenes particle. B. Fungal hyphae showing no clear adhesion on the asphaltenic material. C and D. Fungal hyphae with extracellular material.
In order to explore the fungal preference for certain asphaltene constituents, the asphaltenes were fractionated according to their polarity. For fungal cultivation, 20 mg of each fraction was used. The fraction abundance and fungal growth are shown in Table 2. The less polar fraction (80/20) was not tested because not enough material could be obtained for cultures. No significant differences were found for fungal growth on the different asphaltene fractions.
Table 2.
Fungal growth on asphaltene fractions with different polarity.
| Fraction (acetone/toluene) | Weight percentage (%) | Fungi growth (mg of dry biomass l−1) |
|---|---|---|
| 80/20 | 0.39 | NA |
| 60/40 | 61.09 | 1.33 |
| 40/60 | 16.49 | 1.40 |
| 20/80 | 5.42 | 1.32 |
| Residue | 9.04 | 1.58 |
| Total | 92.43 | – |
NA, not assayed.
On the other hand, model compounds such as aliphatic and polycyclic aromatic hydrocarbons, and sulfur, nitrogen and oxygen heterocycles were assayed for fungal growth. After 4 weeks' culture, the produced dry biomass in mg l−1 were for each substrate as follows: heptadecane, 18.4; nonadecane, 16.2; naphthalene, 3.8;, phenanthrene, 8.5; pyrene, 2.6; fluoranthene, 3.3; 1,2:5,6 dibenzanthracene, 9.2; 4,4 thiodiphenol, 14.6; dibenzothiophene, 6.2; imidazole, 4.8; carbazole, 6.5 and benzofuran, 0.2. As expected the aliphatic compounds supported high fungal growth, while polycyclic aromatic hydrocarbons and aromatic heterocyclic compounds supported moderate fungal growth. Interestingly, benzofuran supported very low growth, which may be due to a toxicity effect.
Discussion
For a long time there has been controversy on the capacity of microorganisms, bacteria and fungi, to grow on asphaltenes as sole source of carbon and energy. There are reports on oil biodegradation that claim the degradation of the asphaltenic fraction by mixed bacteria (Bertrand et al., 1983; Rontani et al., 1985); however, there are also reports concluding that the asphaltenic fraction does not support bacterial growth (Lacotte et al., 1996; Thouand et al., 1999). This controversy was unsolved because most of the studies used gravimetric methods to determine the amount of residual asphaltenes and the degradation was evaluated in the presence of other oil constituents. It is well known that asphaltenes are aggregated structures that could be disrupted by both changes in its structure or by the presence of surfactant compounds produced by the microbial degradation of other petroleum constituents. In this work, rigorous asphaltene purification was carried out to avoid the presence of pentane‐soluble material. Monitoring fungal biomass and CO2 production appears to be a good technique for evaluating the asphaltene metabolization and mineralization. Figures 1 and 2 clearly show that the Neosartorya fischeri strain isolated from natural asphalt Lake of Guanoco is able to grow using asphaltenes as sole source of carbon and energy. The control experiments demonstrate that CO2 evolution occurs only in the presence of both asphaltenes and fungal biomass (Fig. 2).
On the other hand, asphaltene metabolization is also supported by biomass production. After 11 weeks' fungal growth, 1.5 mg l−1 of dry biomass was produced. The fungal growth on asphaltenes is significantly lower than these obtained in a rich medium. Nevertheless, the carbon balance shows that around 15% of the asphaltene carbon is metabolized. In addition, oxidized aromatic compounds could be detected in the culture supernatant after fungal growth on asphaltenes, while in the control experiments no aromatic metabolites could be detected.
No significant differences in fungal growth were detected when different polarity fractions from petroporphyrin‐free asphaltenes were used as substrates (Table 2). Asphaltenes are constituted by interacting systems of polyaromatic sheets bearing alkyl side‐chains, and they have a high content of O, N and S heteroatoms. The use of model compounds may give information on the specificity of the fungal enzymatic system. The model compounds used were chosen as representative moieties of the asphaltene molecules (Strausz et al., 1992; Mullins et al., 2007), and most of them are solid and water‐insoluble as asphaltenes. Aspergillus sp. are not especially known as good hydrocarbon degraders (Sutherland, 1992), nevertheless there are reports on their capacity to degrade aliphatic hydrocarbons (Markovetz et al., 1968; Voigt et al., 1981; Amin and Modi, 1987; Volke‐Sepúlveda et al., 2006), polycyclic aromatic hydrocarbons (Ghosh et al., 1983; Yogambal and Karegoudar, 1997; Salicis et al., 1999; Garon et al., 2000; Capotorti et al., 2004; Naranjo et al., 2007), carbazole (Lobastova et al., 2004) and petroleum derived complex mixtures (Elshafie et al., 2007; Itah et al., 2009; Okoro and Amund, 2010). In addition, there is experimental evidence that the monooxygenase system cytochrome P450 is involved in the metabolism of these substrates (Dutta et al., 1983; Baillie et al., 1996; Prenafeta‐Boldúet al., 2001; Vatsyayan et al., 2008). On the other hand, the low growth obtained with benzofuran could be due to its toxicity. It is well known that benzofuran derivatives show antifungal activity (Pfefferle et al., 1990; Masubuchi et al., 2003).
Regarding the strain identification, our data suggest that the Venezuelan fungus strain could be Neosartorya fischeri. The analysis of the 18S rRNA gene sequence generated five matches with high identity to our isolate: Neosartorya fischeri, Aspergillus lentulus, Aspergillus novofumigatus, Aspergillus fumisynnematus and Aspergillus fumigatus (Table 1). On the other hand, the analysis of the β‐tubulin gene sequence showed as the best match Neosartorya fischeri (Table 1). The molecular probes, specially the 18S rRNA, do not have enough phylogenetic strength to resolve the evolutionary relationship among the Asperguillus species from Fumigati section. The sequence analysis of the β‐tubulin gene revealed to be useful to differentiate the species related to Aspergillus fumigatus (Alcazar‐Fuoli et al., 2008). The Aspergillus gender is classified as Ascomycota and is believed to reproduce only by non‐sexual sporulation, but it has been recently found that some species that produce both sexual and asexual spores (O'Gorman et al., 2009; Machida and Gomi, 2010). The currently accepted name for sexual genera with the closest phylogenetic relationship with A. fumigatus is Neosartorya fischeri (Machida and Gomi, 2010). Rigid interpreters of nomenclature rules believe it is wrong to describe a fungus with known sexual stage as Aspergillus. Fungi with sexual sporulation are called teleomorphs, referring to the sexual meiotic spore bearing morphological phase. Thus, Neosartorya fischeri is a telomorphic species that is closely related to A. fumigatus. A methylene blue stain of the Venezuelan isolate allowed visual analysis of the hyphae morphology, which included coenocytic mycelia as well as the presence of cleistothecia, characteristic of sexual Aspergillus such as Neosartorya fischeri (McClenny, 2005; Machida and Gomi, 2010). Both genomes were compared by Wortman and colleagues (2006), showing that N. fischeri has a slightly bigger genome with more genes in orthologue clusters, the same content of GC and introns and less proteome than A. fumigatus strains. Nevertheless, whereas the nucleotide sequence of core genes may be 99.8% identical between the two fungi, there are at least 2% unique genes in each genome (Fedorova et al., 2008). Thus, the morphological examination and both genetic sequence analyses support that the strain isolated from the natural asphalt Lake of Guanoco is Neosartorya fischeri.
As mentioned above, it has been reported that the Aspergillus sp. multi‐enzymatic system cytochrome P450 is involved in the aromatic hydrocarbon metabolization. However, cytochromes P450 are not extracellular enzymes and the SEM images (Fig. 3) show no adhesion of the fungal hyphae on the asphaltene material. Thus, it seems possible that extracellular enzymes are involved in the initial stages of asphaltene transformation. An extracellular laccase activity was detected during fungi growth (Fig. 1). The 1300 bp fragment amplified from cDNA with degenerate oligonucleotides displayed the highest identity with a putative laccase TilA from Neosartorya fischeri (92%) and a laccase from Aspergillus fumigatus (90%). These results are consistent with the identification of the Venezuelan fungus strain discussed above. Laccases are known to catalyse the oxidation of aromatic moieties of simple and complex polycyclic aromatic compounds, albeit more efficiently in the presence of small molecules which serve as redox mediators (Collins et al., 1996; Majcherczyk et al., 1998; Pickard et al., 1999; Johannes and Majcherczyk, 2000). The products are usually more polar compounds, such as quinones or diones. The capacity of laccases to catalyse the formation of free radicals that may delocalize and lead to covalent bond disruption (Torres et al., 2003) could be involved in the asphaltene metabolization. This free radical formation and further oxidation could improve the bioavailability and/or biodegradability of asphaltene molecules. Further studies on the enzymatic activities involved in asphaltenes transformation are currently being performed.
The Neosartorya fischeri isolate from Guanoco asphalt lake is able to mineralize 13.2% of asphaltenes. To our knowledge, this is the first report of microbial growth on asphaltenes as the sole carbon source, with rigorous asphaltenes preparation and control experiments. An extracellular laccase activity is induced during the asphaltene metabolization and the partial DNA sequence shows remarkable identity with a putative laccase TilA. Neosartorya fischeri is also able to grow on diverse solid and water‐insoluble model compounds, suggesting that only some asphaltene molecule moieties are metabolized.
Experimental procedures
Fungal strain, asphaltenes and chemicals
Fungus strain was isolated from natural asphalt Lake of Guanoco located in Sucre, Venezuela. It is one of the largest deposits of asphalt in the world with more than 445 hectares and contains an estimated of six million tons of asphalt. It was used as a commercial source of asphalt from 1891 to 1935, and currently not being exploited. The isolate was maintained in mineral liquid media with asphaltenes. Petroporphyrin‐free asphaltenes were obtained according to Buenrostro‐Gonzalez and colleagues (2002) by precipitation with n‐pentane from Mexican Maya crude oil as follows: 100 g of crude oil was suspended in 5 l of n‐pentane and stirred for 1 h. The mixture was left in repose for 16 h at 20°C in a stopped flask, then the suspension was centrifuged at 10 000 g and the pellet was re‐dissolved in 100 ml of dichloromethane. The asphaltenes solution was reprecipitated with 5 l of n‐pentane, maintained 16 h in repose and centrifuged. This procedure was repeated one more time and the precipitate was washed with n‐pentane to improve the separation of the asphaltenes fraction. The petroporphyrin‐free asphaltenes were prepared as previously reported (Garcia‐Arellano et al., 2004): 6 g of asphaltenes was diluted in 300 ml of toluene and stirred during 2 h at 20°C. Then, 3 l of acetone was added and the mixture stirred during 3 h at 20°C and maintained in repose for 48 h. The mixture was centrifuged at 12 000 g for 30 min and the pellet was washed three times with acetone. The red‐brownish supernatant and subsequent washes contained the petroporphyrins (showing an A407/A390 absorbance ratio of 1.2), whereas the pellet contained the petroporphyrin‐free asphaltenes.
Asphaltenes fractionation by polarity
Porphyrin‐free asphaltenes were fractionated by differential precipitation according to Buenrostro‐Gonzalez and colleagues (2002). To a 2% w/v porphyrin‐free asphaltenes solution in toluene, a 20% v/v of acetone as precipitant was added; the mixture was sonicated to ensure the correct dispersion of asphaltenes, and the suspension was left stand for 16 h. The mixture was then centrifuged at 12 000 g for 30 min. The precipitated asphaltenes were dried in an oven at 70°C overnight and represented the less polar fraction named 80/20. The supernatant was recovered and acetone was added to reach 40% v/v, then sonicated, maintained 16 h, and centrifuged. The procedure was repeated for 20/80 toluene‐acetone solution. The residual fraction, representing the most polar fraction, was soluble in a mixture of 20% toluene and 80% acetone.
Strain identification
The identification of the fungus strain was performed by using selective media, macroscopic and microscopic examination of morphological characteristics and also by molecular techniques through 18S rRNA and β‐tubulin (Glass and Donaldson, 1995) gene sequencing. Mycelium was grown in Sabouraud media (glucose 40 g ml−1 and peptone 10 g ml−1, pH 5.5) for 3 days at 37°C and 150 r.p.m. Total RNA was extracted with TRIZOL Reagent (Invitrogen) following the protocol of the manufacturer, after cell lysis with liquid nitrogen. cDNA was amplified using the following oligonucleotides, which were generated from the multiple alignment of various 18S rRNA DNA sequences from Aspergillus strains from the NCBI database: Fwd: (5′‐ACCTGCGGAAGGATCATTACC‐3′), Rev: (5′‐ACAGAGCAGGTGACAAAGCCC‐3′). PCR reactions were performed in a Bio‐Rad MyCycler thermocycler using a final concentration of 10 pM of each deoxyribonucleotide triphosphate, and 3 µl of fungal cDNA as template. PCR conditions consisted of an initial denaturation at 94°C for 2 min, 30 cycles of amplification at 94°C for 30 s, annealing at 60°C for 40 s, extension at 72°C for 30 s, and a final extension at 68°C for 10 min. The PCR product (300 bp) was purified using the DNA Clean and Concentrator kit (Zymo Research). The DNA fragment was cloned in the Topo TA PCR Cloning Vector (Invitrogen) and sequenced in the Institute of Biotechnology DNA Sequencing Facilities.
To amplify the β‐tubulin gene, 2 µl of genomic DNA as template and 25 µl of oligonucleotides Bt2a: (5′‐GGTAACCAAATCGGTGCTGCTTTC‐3′) and Bt2b: (5′‐ACCCTCAGTGTAGTGACCCTTGGC‐3′) were used. PCR conditions consisted of an initial denaturation at 95°C for 2 min, 32 cycles of amplification at 94°C for 1 min, annealing at 68°C for 1 min, extension at 72°C for 1 min, and a final extension at 72°C for 7 min. The PCR product (500 bp) was purified using the DNA Clean and Concentrator kit (Zymo Research) and sequenced in the Institute of Biotechnology DNA Sequencing Facilities. All other nucleic acid manipulations were carried out by standard methods (Sambrook et al., 1989).
Fungal growth on asphaltenes
The fungus mycelia was inoculated in flasks containing 50 ml of modified Czapek minimal medium without the original carbon source (4 g NaNO3, 2 g K2HPO4, 1 g MgSO4•7H2O, 1 g KCl and 0.02 g, FeSO4•7H2O) supplemented with 20 mg of asphaltenes as sole carbon source. The cultures were incubated at 37°C and stirred at 100 r.p.m. Every week, three flasks were centrifuged at 12 000 g and the fungal pellet was grinded in a mortar with liquid nitrogen in order to break the cells. The grinded biomass was centrifuged at 12 000 g and the soluble protein content was then measured in the supernatant using the Bio‐Rad protein reagent with a standard curve of BSA. An equivalence curve was obtained to transform between soluble protein and dry biomass. Biomass from cultures grown in Sabouraud medium was used and the following equation was obtained: 1 mg of dry biomass = 2.234 µg of soluble protein (linear correlation coefficient of 0.95). Control experiments with inoculum in mineral media without asphaltenes and also with asphaltenes without inoculum were performed. All experiments were carried out in triplicate.
Cultures with asphaltenes, fractionated by polarity, were performed under the same growth conditions described above, in which 20 mg of asphaltenes was substituted by 20 mg of each fraction. Fungal growth on different model compounds was also estimated. The cultures were performed in 50 ml of Czapek medium containing 10 mg of diverse compounds as sole carbon source and energy: phenanthrene, carbazole, benzo(a)pyrene, 4,4‐thiodiphenol, benzofurane, nonadecane, heptadecane, 1,2:5,6‐dibenzoanthracene, dibenzothiophene, naphthalene, fluoranthene, pyrene or imidazole. Flasks were incubated for 4 weeks at 37°C and 100 r.p.m., and the biomass production was estimated as described above.
Asphaltenes mineralization
CO2 evolution was determined in cultures carried out in flasks with serological stoppers. Twenty milligrams of asphaltenes in 30 ml of Czapek medium was incubated at 37°C and 150 r.p.m. Controls were made with inoculum in mineral media without asphaltenes and also with asphaltenes without inoculum. A headspace analysis was performed every week taking 100 µl and analysing in an Altech gas chromatographer equipped with a CTR‐1 column and a TCD detector. The oven temperature was 100°C, the injector temperature was 150°C and the detector temperature was 250°C. A standard curve was previously generated in order to relate CO2 concentrations to the peak area. Data are reported as total expelled CO2 in mg. After 11 weeks the cultures were stopped and the dry biomass was estimated as described above.
Metabolite analysis
The extracellular medium after 4 weeks' growth was acidified and extracted with dichloromethane. The organic extract was reduced under vacuum and the residue was silylated with N,O‐bis(trimethylsilyl)acetamide (BSA). Then, the silylated products were analysed in an Agilent Gas Chromatographer equipped with a Mass detector (GC‐MS).
Electron microscopy. Fungi from cultures in 50 ml of Czapek media containing 20 mg of asphaltenes were harvested after 3 weeks of incubation at 37°C and 100 r.p.m. Samples were fixed in ethanol and observed using a JEOL JSM S410‐LV scanning electron microscope with a 4 nm resolution at Microscopy Unit of the Cell Physiology Institute (UNAM).
Extracellular enzyme assays
The extracellular medium was filtered through a 0.22 µm pore membrane and then concentrated in an ultrafiltration cell (Amicon) with a 10 kDa cut‐off membrane. The concentrated culture supernatant was assayed for both peroxidase and laccase activities following the protocols previously described by Chance and Maehly (1995) and Niku‐Paavola and colleagues (1988) respectively.
Detection of a putative laccase encoding gene
Total RNA was extracted as mentioned above. cDNA was synthesized by PCR using total RNA as template and the following degenerated oligonucleotides obtained from the multiple alignment of the DNA sequences of laccases and oxidoreductases from various Aspergillus strains: Fwdlac (5′‐CARGGVGAYGATGTYGARTTYTTRGT‐3′); Revlac (5′‐CGWAGAAYTTGTTVGMGTGYTTGTG‐3′).
Sequence analysis
Computer analysis of nucleotide and amino acid sequences was performed with ExPASy proteomics server. Multiple alignments were performed with CLUSTALW (http://www.ebi.ac.uk/tools/msa/clustalw2/), and the partial DNA sequence was compared with the NCBI database using BLASTN software package (http://www.ncbi.nlm.nih.gov/BLAST/) (Altschul et al., 1997). PCR reactions were performed in a Bio‐Rad MyCycler thermocycler using a final concentration of 10 pM of each deoxyribonucleotide triphosphate, and 3 µl of fungal cDNA as template. PCR conditions were as follows: initial denaturation at 95°C for 2 min; 30 cycles of amplification at 94°C, annealing at 50°C for 40 s, extension at 72°C for 1.5 min and a final extension at 68°C for 10 min. The PCR fragment of 1300 bp was purified as mentioned above and cloned in the PCR Topo TA vector, according to manufacturer's protocol.
Acknowledgments
We thank Rosa Roman for her technical assistance. This work has been funded by the National Council of Science and Technology (CONACYT) of Mexico.
References
- Alcazar‐Fuoli L., Mellado E., Alastruey‐Izquierdo A., Cuenca‐Estrella M., Rodriguez‐Tudela J.L. Aspergillus section Fumigati: antifungal susceptibility patterns and sequence‐based identification. Antimicrob Agents Chemother. 2008;52:1244–1251. doi: 10.1128/AAC.00942-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul S.F., Madden T.L., Schaffer A.A., Zhang J., Zhang Z., Miller W., Lipman D.J. Gapped BLASTand PSI‐BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin A.R., Modi V.V. Metabolism of n‐alkanes by Aspergillus japonicus. Folia Microbiol. 1987;32:24–28. doi: 10.1007/BF02877254. [DOI] [PubMed] [Google Scholar]
- Baillie G.S., Hitchcock C.A., Burnet F.R. Increased cytochrome P‐450 activity in Aspergillus fumigatus after xenobiotic exposure. J Med Vet Mycol. 1996;34:341–347. [PubMed] [Google Scholar]
- Bertrand J.C., Rambeloarisoa E., Rontani J.F., Giusti G., Mattei G. Microbial degradation of crude oil in sea water in continuous culture. Biotechnol Lett. 1983;5:567–572. [Google Scholar]
- Bublitz F., Guenther T., Fritsche W. Screening of fungi for the biological modification of hard coal and coal derivatives. Fuel Proc Technol. 1994;40:347–354. [Google Scholar]
- Buenrostro‐Gonzalez E., Andersen S.I., Garcia‐Martinez J.A., Lira‐Galeana C. Solubility/molecular structure relationships of asphaltenes in polar and nonpolar media. Energy Fuels. 2002;16:732–741. [Google Scholar]
- Capotorti G., Digianvincenzo P., Cesti P., Bernardi A., Guglielmetti G. Pyrene and benzo(a)pyrene metabolism by an Aspergillus terreus strain isolated from a polycylic aromatic hydrocarbons polluted soil. Biodegradation. 2004;15:79–85. doi: 10.1023/b:biod.0000015612.10481.e6. [DOI] [PubMed] [Google Scholar]
- Chance B., Maehly A.C. Assay of catalases and peroxidases. Methods Enzymol. 1995;2:764–775. doi: 10.1002/9780470110171.ch14. [DOI] [PubMed] [Google Scholar]
- Collins P.J., Kotterman M.J.J., Field J.A., Dobson A.D.W. Oxidation of anthracene and benzo[a]pyrene by laccases from Trametes versicolor. Appl Environ Microbiol. 1996;62:4563–4567. doi: 10.1128/aem.62.12.4563-4567.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand E., Clemancey M., Lancelin J.J., Verstraete J., Espinat D., Quoineaud A.A. Effect of chemical composition on asphaltenes aggregation. Energy Fuels. 2010;24:1051–1062. [Google Scholar]
- Dutta D., Ghosh D.K., Mishra A.K., Samanta T.B. Induction of benzo(a)pyrene hydroxylase in Aspergillus ochraceus TS: evidences of multiple forms of cytochrome P‐450. Biochem Biophys Res Commun. 1983;115:692–699. doi: 10.1016/s0006-291x(83)80200-9. [DOI] [PubMed] [Google Scholar]
- Elshafie A., AlKindi A.Y., Al‐Busaidi S., Bakheit C., Albahry S.N. Biodegradation of crude oil and n‐alkanes by fungi isolated from Oman. Mar Pollut Bull. 2007;54:1692–1696. doi: 10.1016/j.marpolbul.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Fedorak P.M., Semple K.M., Vazquez‐Duhalt R., Westlake D.W.S. Chloroperoxidase mediated modifications of petroporphyrins and asphaltenes. Enzyme Microb Technol. 1993;15:429–437. doi: 10.1016/0141-0229(93)90169-3. [DOI] [PubMed] [Google Scholar]
- Fedorova N.D., Khaldi N., Joardar V.S., Maiti R., Amedeo P., Anderson M.J. Genomic islands in the pathogenic filamentous fungus Aspergillus fumigatus. PLoS Genet. 2008;4:e1000046. doi: 10.1371/journal.pgen.1000046. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Arellano H., Buenrostro‐Gonzalez E., Vazquez‐Duhalt R. Biocatalytic transformation of petroporphyrins by chemical modified cytochrome c. Biotechnol Bioeng. 2004;85:790–798. doi: 10.1002/bit.20023. [DOI] [PubMed] [Google Scholar]
- Garon D., Krivobok S., Seigle‐Murandi F. Fungal degradation of fluorene. Chemosphere. 2000;40:91–97. doi: 10.1016/s0045-6535(99)00250-7. [DOI] [PubMed] [Google Scholar]
- Ghosh D.K., Dutta D., Samanta T.B., Mishra A.K. Microsomal benzo(a)pyrene hydroxylase in Aspergillus ochraceus TS: assay and characterization of the enzyme system. Biochem Biophys Res Commun. 1983;113:497–505. doi: 10.1016/0006-291x(83)91753-9. [DOI] [PubMed] [Google Scholar]
- Glass N.L., Donaldson G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous Ascomycetes. Appl Environ Microbiol. 1995;61:1323–1330. doi: 10.1128/aem.61.4.1323-1330.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Government of Alberta, Canada. 2011. Oil sands. Resource & Assessment. Alberta Energy [WWW document]. URL http://www.energy.alberta.ca/OilSands/1715.asp.
- Harkin J.M., Larsen M.J., Obst J.R. Use of syringaldazine for detection of laccase in sporophores of wood rotting fungi. Mycologia. 1974;66:469–476. [PubMed] [Google Scholar]
- Hofrichter M., Bublitz F., Fritsche W. Fungal attack on coal: I. Modification of hard coal by fungi. Fuel Proc Technol. 1997;52:43–53. [Google Scholar]
- Itah A.Y., Brooks A.A., Ogar B.O., Okure A.B. Biodegradation of international jet A‐1 aviation fuel by microorganisms isolated from aircraft tank and joint hydrant storage systems. Bull Environ Contam Toxicol. 2009;83:318–327. doi: 10.1007/s00128-009-9770-0. [DOI] [PubMed] [Google Scholar]
- Johannes C., Majcherczyk A. Natural mediators in the oxidation of polycyclic aromatic hydrocarbons by laccase mediator systems. Appl Environ Microbiol. 2000;66:524–528. doi: 10.1128/aem.66.2.524-528.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.‐S., Crowley D.E. Microbial diversity in natural asphalts of the Rancho La Brea tar pits. Appl Environ Microbiol. 2007;73:4579–4591. doi: 10.1128/AEM.01372-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacotte D.J., Mille G., Acquaviva M., Bertrand J.C. Arabian light 150 asphaltene biotransformation with n‐alkanes as co‐substrate. Chemosphere. 1996;32:1755–1761. [Google Scholar]
- Lobastova T.G., Sukhodolskaya G.V., Nikolayeva V.M., Baskunov B.P., Turchin K.F., Donova M.V. Hydroxylation of carbazoles by Aspergillus flavus VKM F‐1024. FEMS Microbiol Lett. 2004;235:51–56. doi: 10.1016/j.femsle.2004.04.008. [DOI] [PubMed] [Google Scholar]
- McClenny N. Laboratory detection and identification of Aspergillus species by microscopic observation and culture: the traditional approach. Med Mycol. 2005;43:S125–S128. doi: 10.1080/13693780500052222. [DOI] [PubMed] [Google Scholar]
- Machida M., Gomi K. Caister Academic Press; 2010. [Google Scholar]
- Majcherczyk A., Johannes C., Huttermann A. Oxidation of polycyclic aromatic hydrocarbons (PAH) by laccase of Trametes versicolor. Enzyme Microb Technol. 1998;22:335–341. [Google Scholar]
- Markovetz A.J., Jr, Cazin J., Allen J.E. Assimilation of alkanes and alkenes by fungi. Appl Microbiol. 1968;16:487–489. doi: 10.1128/am.16.3.487-489.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masubuchi M., Ebiike H., Kawasaki K., Sogabe S., Morikami K., Shiratori Y. Synthesis and biological activities of benzofuran antifungal agents targeting fungal N‐myristoyltransferase. Bioorg Med Chem. 2003;11:4463–4478. doi: 10.1016/s0968-0896(03)00429-2. et al. [DOI] [PubMed] [Google Scholar]
- Meyer R.F., Schenk C.J. 1988.
- Meyer R.F., deWitt W., Jr 1990.
- Mogollon L., Rodriguez R., Larrota W., Ortiz C., Torres R. Biocatalytic removal of nickel and vanadium from petroporphyrins and asphaltenes. Appl Biochem Biotechnol. 1998;70–72:765–777. doi: 10.1007/BF02920187. [DOI] [PubMed] [Google Scholar]
- Mullins O.C., Sheu E.Y. Plenum Press; 1998. [Google Scholar]
- Mullins O.C., Sheu E.Y., Hammami A., Marshall A.G. Spinger Science + Business Media LCC; 2007. [Google Scholar]
- Muñoz G., Nakari‐Setälä T., Merja‐Penttilä E.A. Hydrophobin gene srh 1, expressed during sporulation of the biocontrol agent Trichoderma harzianum. Curr Genet. 1997;32:225–230. doi: 10.1007/s002940050270. [DOI] [PubMed] [Google Scholar]
- Naranjo L., Urbina H., De Sisto A., León V. Isolation of autochthonous non‐white rot fungi with potential for enzymatic upgrading of Venezuelan extra‐heavy crude oil. Biocatal Biotransformation. 2007;25:1–9. doi: 10.1080/10242420701379908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J., Villadsen J., Liden G. Kluwer Academic/Plenum Publishers; 1994. [Google Scholar]
- Niku‐Paavola M.L., Karhunen E., Salola P., Raunio V. Ligninolytic enzymes of the white‐rot fungus Phlebia radiata. Biochem J. 1988;254:877–884. doi: 10.1042/bj2540877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Gorman C.M., Fuller H.T., Dyer P.S. Discovery of a sexual cycle in the opportunistic fungal pathogen Aspergillus fumigatus. Nature. 2009;457:471–475. doi: 10.1038/nature07528. [DOI] [PubMed] [Google Scholar]
- Okoro C.C., Amund O.O. Biodegradation of produced water hydrocarbons by Aspergillus fumigatus. J Am Sc. 2010;6:143–149. [Google Scholar]
- OPEC. 2009. OPEC annual statistical bulletin 2009[WWW document]. URL http://www.opec.org/opec_web/en/
- Pfefferle W., Anke H., Bross M., Steffan B., Vianden R., Steglich W. Asperfuran, a novel antifungal metabolite from Aspergillus oryzae. J Antibiot. 1990;43:648–654. doi: 10.7164/antibiotics.43.648. [DOI] [PubMed] [Google Scholar]
- Pickard M.A., Roman R., Tinoco R., Vazquez‐Duhalt R. Polycyclic aromatic hydrocarbon metabolism by white rot fungi and oxidation by Coriolopsis gallica UAMH 8260 laccase. Appl Environ Microbiol. 1999;65:3805–3809. doi: 10.1128/aem.65.9.3805-3809.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda F.G., Mesta‐HoWard A.M., Boll‐Argúello G., Lira‐Galeana C. A microbial consortium isolated from a crude oil sample that uses asphaltenes as a carbon and energy source. Biodegradation. 2004;15:145–151. doi: 10.1023/b:biod.0000026476.03744.bb. [DOI] [PubMed] [Google Scholar]
- Prenafeta‐Boldú F.X., Luykx D.M., Vervoort J., de Bont J.A. Fungal metabolism of toluene: monitoring of fluorinated analogs by (19)F nuclear magnetic resonance spectroscopy. Appl Environ Microbiol. 2001;67:1030–1034. doi: 10.1128/AEM.67.3.1030-1034.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rontani J.F., Bosser‐Joulak F., Rambeloarisoa E., Bertrand J.C., Giusti G., Faure R. Analytical study of Asyhart crude oil biodegradation. Chemosphere. 1985;14:1413–1422. [Google Scholar]
- Salicis F., Krivobok S., Jack M., Benoit‐Guyod J.L. Biodegradation of fluoranthene by soil fungi. Chemosphere. 1999;38:3031–3039. doi: 10.1016/s0045-6535(98)00504-9. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Fritsch E.F., Maniatis T. 2nd. Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Strausz O.P., Mojelsky T.W., Lown E.M. The molecular structure of asphaltenes: an unfolding story. Fuel. 1992;71:1355–1363. [Google Scholar]
- Sutherland J.B. Detoxification of polycyclic aromatic hydrocarbons by fungi. J Ind Microbiol. 1992;9:53–61. doi: 10.1007/BF01576368. [DOI] [PubMed] [Google Scholar]
- Thouand G., Bauda P., Oudot J., Kirsh G., Sutton C., Vidalie J.F. Laboratory evaluation of crude oil biodegradation with commercial or natural microbial inocula. Can J Microbiol. 1999;45:106–115. [PubMed] [Google Scholar]
- Torres E., Bustos‐Jaimes I., Le Borgne S. Potential use of oxidative enzymes for the detoxification of organic pollutants. Appl Catal B: Environ. 2003;46:1–15. [Google Scholar]
- Vatsyayan P., Kumar A.K., Goswami P., Goswami P. Broad substrate cytochrome P450 monooxygenase activity in the cells of Aspergillus terreus MTCC 6324. Bioresour Technol. 2008;99:68–75. doi: 10.1016/j.biortech.2006.11.055. [DOI] [PubMed] [Google Scholar]
- Voigt A., Bemmann W., Tröger R. The growth of thermophilic fungi strains Aspergillus fumigatus and Mucor lusitanicus in n‐alkane medium. Zentralbl Bakteriol Naturwiss. 1981;136:590–602. [PubMed] [Google Scholar]
- Volke‐Sepúlveda T., Gutiérrez‐Rojas M., Favela‐Torres E. Biodegradation of high concentrations of hexadecane by Aspergillus niger in a solid‐state system: kinetic analysis. Bioresour Technol. 2006;97:1583–1591. doi: 10.1016/j.biortech.2005.07.026. [DOI] [PubMed] [Google Scholar]
- Wortman J.R., Fedorova N., Crabtree J., Joardar V., Maiti R., Haas J. Whole genome comparison of the A. fumigatus family. Med Mycol. 2006;44:S3–S7. doi: 10.1080/13693780600835799. et al. [DOI] [PubMed] [Google Scholar]
- Wyndham R.C., Costerton J.W. In vitro microbial degradation of bituminous hydrocarbons and in situ colonization of bitumen surfaces within the Athabasca oil sands deposit. Appl Environ Microbiol. 1981;41:791–800. doi: 10.1128/aem.41.3.791-800.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogambal R.K., Karegoudar T.B. Metabolism of polycyclic aromatic hydrocarbons by Aspergillus niger. Indian J Exp Biol. 1997;35:1021–1023. [PubMed] [Google Scholar]


