Abstract
Objectives
To describe the prevalence of co-existing conditions that affect clinical decision-making among adults with coronary heart disease (CHD).
Design
Cross-sectional
Setting
NHANES, 1999–2004
Participants
8654 people aged ≥ 45; 1259 with CHD.
Measurements
Co-existing conditions relevant to clinical decision-making and implementing therapy for CHD across 3 domains: 1)chronic diseases; 2) self-reported and laboratory-based clinical measures; 3) health status factors of self-reported and observed function. We estimated prevalence by gender and age, examined mutually exclusive patterns, and modeled the odds ratios (OR) of having incurred repeated hospitalization in the last year among participants with CHD and each complexity pattern, versus CHD alone.
Results
The prevalence of comorbid chronic diseases among subjects with CHD was: arthritis (56.7%), chronic lower respiratory tract disease (25.5%), diabetes (24.8%), stroke (13.8%), and congestive heart failure (29.0%); clinical factors adding to complexity of clinical decision-making for CHD were: use of > 4 meds (54.5%), UI (48.6%), dizziness or falls (34.8%), low GFR (24.4%), anemia (10.1%), high ALT (5.9%), use of warfarin (10.2%), and health status factors were: cognitive impairment (29.9%), mobility difficulty (40.4%), frequent mental distress (14.3%), visual impairment (16.7%), and hearing impairment (17.9%). Several comorbidity patterns were associated with elevated odds of hospitalization.
Conclusion
Co-existing conditions that may modify the effectiveness of or interact with CHD therapies, influence the feasibility of CHD therapies, or alter patients' priorities concerning their healthcare should be considered in the development of trials and guidelines in order to better inform real-world clinical decision-making.
Keywords: Comorbidity, heart disease, guideline, prevalence
INTRODUCTION
Coronary heart disease (CHD) is a common disease among older adults, with a prevalence of 37% among men, and 26% among women, aged 65 and older in the United States.1 It is negatively associated with quality of life and is the leading cause of death in this country.2, 3 Among older adults with CHD, 79% of women and 69% of men have at least one additional major chronic disease.4 There is increasing recognition that people with CHD and additional chronic conditions experience high levels of health care utilization and poor outcomes.5, 6
Prior work in the Medicare population has found that select non-cardiac comorbidities increase the risk of preventable hospitalizations and death in people with CHD.7 These adverse outcomes may be caused by conditions that worsen the specific pathophysiology of CHD, reduce the person's physiologic ability to compensate for CHD and thereby worsen its effects, interact with CHD therapies to alter their actions, both beneficial and unintended, affect patients' or physicians' priorities for treatment, or function as competing demands. In this respect, CHD is typical, rather than an exception, among major chronic diseases. However, which comorbidities are important to consider in patients with CHD is unknown, and the highest priorities are likely to be determined by prevalence and the potential for the condition to affect real-world clinical decision-making. Feinstein defined comorbidity as “any distinct additional clinical entity that has existed or may occur during the clinical course of a patient who has the index disease under study.”8 Comorbidity has been studied for the purposes of prognostication, risk adjustment, and, more recently, understanding heterogeneity of treatment effect.9, 10 To date, however, there has been little work to frame how comorbidities which may confer health status complexity at the patient level affect clinical decision-making.11, 12 Thus, which comorbid conditions should be considered in the development of more relevant clinical practice guidelines (CPGs) and their implementation is unknown. Such a framework would help establish priorities for their development.
Therefore, we sought to describe the prevalence of co-existing factors in nationally-representative data with the specific purpose of articulating influential factors that affect clinical decision-making and that should potentially inform the development and implementation of clinical guidelines for CHD. In this paper we draw from a nationally-representative dataset, the National Health and Nutrition Survey (NHANES), to estimate the prevalence and impact of patterns of chronic diseases, clinical factors and health status factors that increase the complexity of management of CHD as a basis for deciding which clinically important factors should be studied and incorporated into management for people with CHD. We then model the cross-sectional relationship between these factors and health care utilization, specifically repeated hospitalization in the past year, comparing the most common mutually exclusive patterns of these factors with CHD to CHD alone.
METHODS
Study Population
NHANES is a nationally representative study of non-institutionalized civilians that employs a complex multi-stage probability design to over-sample persons aged 60 or older. Collection of information occurs through home interviews or exams in mobile centers. Study details including operations manuals are publicly available.13 To ensure adequate sample size in age and gender strata, we joined three survey waves (1999–2000, 2001–2002 and 2003–2004) to create an analytic sample of 8654 observations of people aged 45 and older including 1259 with CHD. NHANES interview response rates ranged from 76% to 80% for the 3 waves.
Choice of conditions
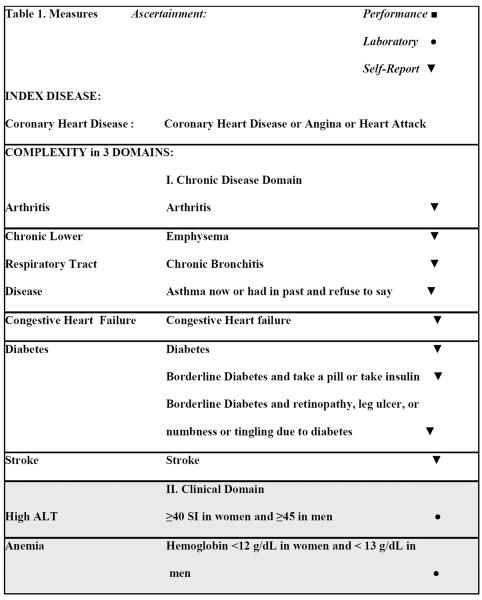
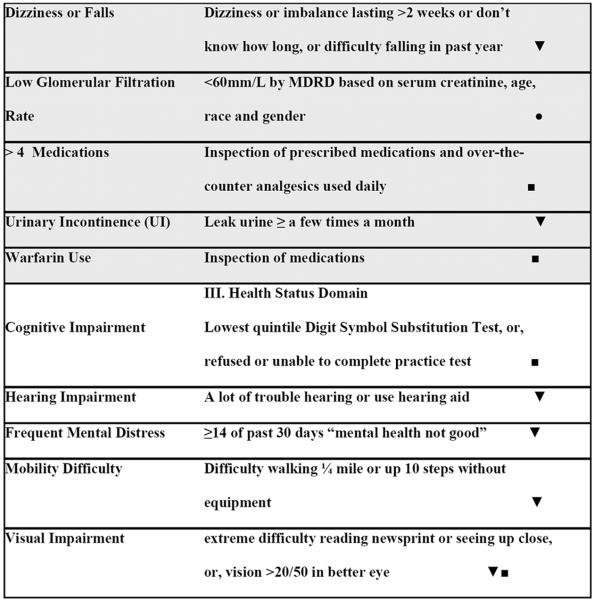
We considered conditions that are likely to alter the clinical course or ability to treat CHD, focusing on intrinsic conditions that are relevant when making decisions related to prescribing medications or other treatments or achieving adherence. Though we acknowledge that health system and societal factors are also significant, these factors were beyond the scope of this study. Specifically, the clinician authors created a consensus list of conditions that physiologically interact with recommended therapies or that alter a patient's ability to achieve treatment benefit. We grouped these factors into “disease”, “clinical”, and “health status” domains to reflect a progressively widening scope from classic disease definitions, to conditions, and finally whole-person relevance (Table 1). The disease domain encompassed other traditional chronic diseases that are considered of major importance because they are established as leading causes of death or morbidity, and there are known interactions between each disease and CHD, CHD treatments and each disease, one of their treatments and CHD, or between treatments for both conditions. The clinical domain consisted of physiologic conditions and factors that should be weighed when prescribing therapies because they may be a contra-indication or relative contra-indication (e.g. the presence of elevated liver enzymes when considering the use of statin drugs, or complaints of dizziness when considering the use and dose of therapy lowering blood pressure). The health status domain was reserved for conditions that affect function and quality of life, are likely to affect a person's ability to adhere to therapy, and are often caused by several processes in older adults.
Table 1.
Definitions of Factors Adding to Complexity
|
Diseases
Comorbid disease status was ascertained through NHANES questions asking “has a doctor or other health professional ever told you had [disease]?” Arthritis (ART), congestive heart failure (CHF) and stroke (CVA) were identified in this manner through single questions. CHD included individuals who responded affirmatively to any of 3 questions about coronary heart disease, angina or a heart attack. Likewise, chronic lower respiratory tract disease (CLRT) included emphysema, chronic bronchitis, or, those who currently had asthma or had a history of asthma and refused to answer if they still had it. Apart from this exception, individuals were counted as disease-free only if they responded “No” to relevant questions. Regarding diabetes (DM), participants were able to answer that they had borderline diabetes. Among those reporting borderline diabetes, individuals were counted as having DM if they took insulin or a pill for diabetes, or, suffered from retinopathy, a lower extremity ulcer that took more than 4 weeks to heal, or numbness or tingling in the hands or feet due to diabetes.
Clinical factors
Low glomerular filtration rate (GFR) was calculated using the Modification of Diet in Renal Disease (MDRD) equation based on serum creatinine, age, race and gender as <60 mm/L.14 Low hemoglobin was defined as <12 g/dL in women and < 13 g/dL in men.15 A high alanine aminotransferase (ALT) was defined as serum level >40 SI in women and >45 in men. Urinary incontinence was ascertained by self-report of leaking urine at least a few times a month. Individuals who reported dizziness or imbalance lasting at least 2 weeks or for an unknown duration, or, difficulty with falls in the last year were counted as having problems with dizziness or falls. Using >4 medications was defined following a previously established cutpoint16 and from inspection of prescribed medications and over-the-counter analgesics used daily. Supplements, vitamins and minerals were not counted as medications. Use of warfarin was defined following inspection of prescribed medications.
Health status factors
Cognitive impairment was ascertained through performance on the Digit Symbol Substitution test, which was conducted for those 60 years of age and older.17 People who refused or were unable to perform the practice test for cognitive reasons, or, performed in the lowest quintile (less than 30 correct answers in 120 seconds) were counted as cognitively impaired. Mobility difficulty was defined as present if the individual reported difficulty walking ¼ mile or up 10 steps without equipment.18 Frequent Mental Distress was present if participants reported that for at least 14 of the past 30 days mental health was not good.19, 20 Visual impairment was ascertained through self-reported extreme difficulty reading newsprint or seeing up close, or, examined visual acuity score >20/50 in the better eye. Hearing impairment was defined according to self-reporting a lot of trouble hearing or use of a hearing aid.
Additional Variables
We examined the effects of age, gender, education, income, having health insurance, and race using self-reported responses to questions. Access to care was defined as self-report of two or more physician visits in the last year.
Repeated Hospitalization Outcome
Study participants were asked whether they experienced an overnight hospital stay in the last year, not including spending a night in an emergency room. Those who replied affirmatively were also asked how many times this had occurred in the last year. The outcome was categorized as at least two hospitalizations in the last year. We performed sensitivity analyses with alternative outcomes of more than 1, more than 3 hospitalizations in the last year, and a continuous outcome analyzed through negative binomial regression.
Analytic Plan
The National Center for Health Statistics (NCHS) provides sampling weights that account for sampling strategy and survey non-response. Using methods provided by NCHS, we modified the original weights in our combined sample to maintain national representation. Protocols for each wave were compared to detect changes. No changes in protocol were observed for the variables used in these analyses, though revisions were made for inconsistencies in naming and coding. We performed analyses with statistical software designed to conduct subpopulation analyses using masked variance units to estimate appropriate standard errors. We summarize baseline characteristics using means and standard deviations. Differences in these variables between subjects with and without CHD were compared using either a two-sided t-test for continuous variables or a χ2 test for categorical data.
The decision to group age into categories of 45–64, 65+ was made following convention and after confirming that the relatively small number of people above age 85 limited analyses in that group. Prevalence was estimated with binomial Wald 95% confidence intervals for the complexity factors in disease, clinical and health status domains in older adults with CHD for different gender and age groups. We then estimated and tested the impact of these factors on the outcome of repeated hospitalization using a logistic regression model. In the model we adjusted for age and access to care (at least 2 physician visits in the last year). In each domain, i.e. disease, clinical factors, health status factors, we identified the 5 most common mutually exclusive complexity patterns, taking into account the presence or absence of conditions in a domain. For the clinical domain, 6 patterns were examined because there was not a substantial drop in prevalence between the 5th and 6th most common patterns. We employed interactive terms as appropriate to model odds ratios (OR) for at least 2 hospitalizations in adults aged 45 and over with CHD plus a complexity pattern versus CHD alone. We examined the effects of age, access to care, gender, education, income and race through multivariate models accounting for complexity factors. All analyses were carried out in SAS version 9.1 and Stata version 9.2 (Stata Corp, College Station, Texas). The study protocol was approved by The Johns Hopkins University School of Medicine Institutional Review Board.
RESULTS
Table 2 describes the baseline demographic and complexity factors by CHD status. People with CHD were more likely be older, male, white, to be less educated, to possess health insurance and to have lower household income. Compared to their counterparts without CHD, complexity factors across all three domains were all statistically significant and more prevalent for participants with CHD, except for elevated alanine aminotransferase.
Table 2.
Baseline Characteristics (Demographics and Complexity Factors) in Adults Aged ≥ 45years, Overall and By Coronary Heart Disease Status: NHANES 1999–2004.
| Characteristics | Overall sample (n=8654) | Subjects without any CHD (n=7306) | Subjects with CHD (n=1259) | P Value |
|---|---|---|---|---|
|
| ||||
| Demographic Variables | ||||
|
| ||||
| Age, mean (SD) y | 60.3 (0.2) | 59.3 (0.2) | 67.6 (0.6) | <.0001 |
| Age categories: | ||||
| 45–64, N (%) | 3925 (69.4) | 355 (39.4) | ||
| 65–74, N (%) | 1688 (17.4) | 1693 (28.7) | ||
| 75+, N (%) | 366 (13.2) | 538 (31.8) | ||
|
| ||||
| Gender | ||||
|
| ||||
| Male, N. (%) | 4212 (46.1) | 3444 (44.8) | 731 (56.1) | <.0001 |
|
| ||||
| Female, N. (%) | 4442 (53.9) | 3862 (55.2) | 528 (43.9) | |
|
| ||||
| Race | ||||
|
| ||||
| White, N. (%) | 4846 (80.6) | 3981 (80.0) | 829 (85.6) | <.0001 |
|
| ||||
| Black, N. (%) | 1544 (10.0) | 1352 (10.2) | 172 (8.2) | |
|
| ||||
| Hispanic, N. (%) | 2031 (9.4) | 1781 (9.8) | 224 (6.2) | |
|
| ||||
| Education (at least High School), N. (%) | 5350 (76.7) | 4583 (78.1) | 727 (66.9) | <.0001 |
|
| ||||
| Has Health Insurance, N. (%) | 7578 (90.6) | 6328 (90.1) | 1175 (94.7) | .0001 |
|
| ||||
| Household Income less than 20,000, N. (%) | 2368 (21.6) | 1888 (19.8) | 449 (33.3) | <.0001 |
|
| ||||
| Disease Variables (in descending overall prevalence) | ||||
|
| ||||
| Arthritis, N. (%) | 3517 (37.3) | 2767 (34.6) | 703 (56.7) | <.001 |
|
| ||||
| CLRT, N. (%) | 1254 (15.4) | 950 (14.0) | 287 (25.5) | <.001 |
|
| ||||
| Diabetes, N. (%) | 1443 (12.5) | 1063 (10.6) | 347 (24.8) | <.0001 |
|
| ||||
| Stroke, N. (%) | 568 (4.5) | 357 (3.2) | 199 (13.8) | <.0001 |
|
| ||||
| CHF, N. (%) | 530 (4.5) | 173 (1.7) | 343 (29.0) | <.0001 |
|
| ||||
| Clinical Factors (in descending overall prevalence) | ||||
|
| ||||
| Urinary Incontinence, N. (%) | 2127 (42.8) | 1698 (41.8) | 394 (48.5) | .0030 |
|
| ||||
| Dizziness or Falls, N. (%) | 2034 (20.9) | 1552 (18.8) | 447 (34.8) | <.0001 |
|
| ||||
| Use of > 4 meds, N. (%) | 1961 (20.8) | 1258 (16.0) | 671 (54.5) | <.0001 |
|
| ||||
| Low GFR, N. (%) | 915 (10.0) | 626 (8.0) | 278 (24.4) | <.0001 |
|
| ||||
| Anemia, N. (%) | 636 (5.9) | 487 (5.2) | 137 (10.1) | <.0001 |
|
| ||||
| High ALT, N. (%) | 503 (7.3) | 451 (7.5) | 49 (5.9) | 0.1585 |
|
| ||||
| Warfarin Use, N. (%) | 300 (2.7) | 152 (1.6) | 138 (10.2) | <.0001 |
|
| ||||
| Health Status Factors (in descending overall prevalence) | ||||
|
| ||||
| Cognitive Impairment, N. (%) | 813 (23.9) | 619 (22.2) | 178 (29.9) | 0.0022 |
|
| ||||
| Mobility Difficulty, N. (%) | 2035 (20.0) | 1561 (17.5) | 447 (40.4) | <.0001 |
|
| ||||
| Frequent Mental Distress, N. (%) | 504 (10.4) | 401 (10.0) | 98 (14.3) | 0.0022 |
|
| ||||
| Visual Impairment, N. (%) | 989 (10.3) | 751 (9.0) | 215 (16.7) | <.0001 |
|
| ||||
| Hearing Impairment, N. (%) | 1134 (9.8) | 848 (8.6) | 265 (17.9) | <.0001 |
Footnote:
CHD: coronary heart disease
CLRT: chronic lower respiratory tract disease
CHF: congestive heart failure
GFR: glomerular filtration rate
The prevalence of comorbid chronic diseases among subjects with CHD was: arthritis (56.7%), chronic lower respiratory tract disease (25.5%), diabetes (24.8%), stroke (13.8%), and congestive heart failure (29.0%); clinical factors adding to complexity of clinical decision-making for CHD were: use of > 4 meds (54.5%), UI (48.6%), dizziness or falls (34.8%), low GFR (24.4%), anemia (10.1%), high ALT (5.9%), use of warfarin (10.2%), and health status factors were: cognitive impairment (29.9%), mobility difficulty (40.4%), frequent mental distress (14.3%), visual impairment (16.7%), and hearing impairment (17.9%).
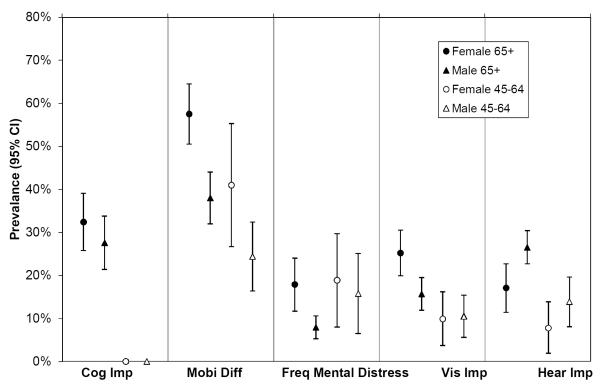
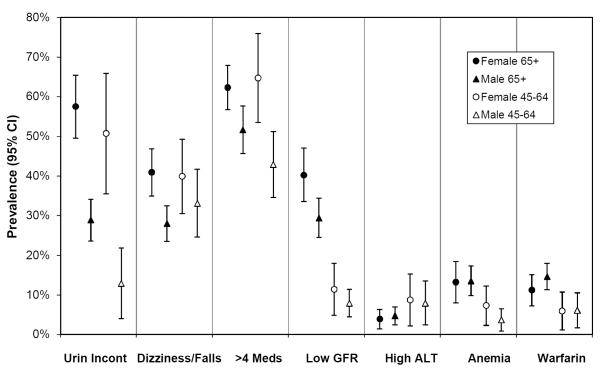
Figure 1 (a–c) depicts prevalence rates of chronic disease, clinical, and health status complexity among people with CHD. While some conditions were significantly more common in the 65+ population (UI, low GFR, anemia, mobility difficulty, visual and hearing impairment), many were equally common among those 45–64 (> 4 meds, dizziness or falls, arthritis).
Figure 1a.
Prevalence of diseases among people with Coronary Heart Disease, Stratified by Age and Gender, 95% CI
Footnote:
CHD: coronary heart disease
CLRT: chronic lower respiratory tract disease
CHF: congestive heart failure
GFR: glomerular filtration rate
CVA: stroke
Figure 1c.
Prevalence of health status factors among people with Coronary Heart Disease, Stratified by Age and Gender, 95% CI
Footnote:
Cog Imp: Cognitive impairment
Mobi Diff: Mobility Difficulty
Freq Mental Distress: Frequent Mental Distress
Vis Imp: Visual Impairment
Hear Imp: Hearing Impairment
Table 3 depicts the prevalence of mutually exclusive patterns of the presence of CHD alone versus CHD with other specific complexity patterns of disease, clinical factors, or health status factors and the OR and 95% CI of experiencing at least 2 hospitalizations in the prior year. Seventy per cent (70.4%) of people with CHD were captured by the most prevalent 6 complexity disease patterns, 58.1% of people were captured by the 7 most prevalent clinical complexity patterns, and 44.2% of people with CHD were included in the 6 most prevalent health status patterns.(Table 3)
Table 3.
Odds Ratios for Reporting at least 2 Hospitalizations in Last Year in CHD plus Multimorbidity Pattern versus CHD alone among Adults Aged 45+, NHANES 1999–2004
| Pattern | ||||
|---|---|---|---|---|
| Coronary Heart Disease+… diseases | Prevalence (%) | Odds Ratio * | 95% CI | |
| none, i.e. Coronary Heart Disease | 22.7 | ref. | ||
| Arthritis | 22.2 | 1.36 | 0.77 | 2.38 |
| Arthritis + Diabetes Mellitus | 7.7 | 2.33 | 1.18 | 4.62 |
| Arthritis + Chronic Lower Respiratory Tract Disease | 7.1 | 1.48 | 0.84 | 2.59 |
| Arthritis + Congestive Heart Failure | 5.5 | 2.20 | 1.04 | 4.63 |
| Diabetes Mellitus | 5.2 | 1.72 | 1.16 | 2.55 |
| Coronary Heart DIsease+…clinical factors | ||||
| none of clinical factors | 24.1 | ref. | ||
| >4 medications | 13.1 | 3.15 | 1.75 | 5.66 |
| >4 medications+Dizziness or Falls+Urinary Incontinence | 7.0 | 4.65 | 1.37 | 15.79 |
| >4 medications+Urinary Incontinence | 6.0 | 3.51 | 1.32 | 9.32 |
| Urinary Incontinence | 4.5 | 1.11 | 0.53 | 2.33 |
| >4 medications+Low GFR+Urinary Incontinence | 3.4 | 4.44 | 1.28 | 15.44 |
| Dizziness or Falls+Urinary Incontinence | 2.9 | 1.48 | 0.59 | 3.70 |
| Coronary Heart Disease+…health status factors | ||||
| none of functional factors | 22.4 | ref. | ||
| Mobility Difficulty | 11.4 | 2.05 | 1.15 | 3.67 |
| Mobility Difficulty +Hearing Impairment | 3.3 | 2.11 | 0.87 | 5.16 |
| Hearing Impairment | 2.8 | 1.03 | 0.52 | 2.05 |
| Mobility Difficulty +Visual Impairment | 2.4 | 3.24 | 1.65 | 6.38 |
| Visual Impairment | 1.9 | 1.58 | 0.84 | 2.99 |
Odds Ratio using adults with Coronary Heart Disease as referent, i.e. odds if have Coronary Heart Disease+multimorbidity pattern / odds if have Coronary Heart Disease. Bold identifies patterns with statistically significant odds ratios. Models adjusted for age (continuous) and access to care (at least 2 physician visits in last year). Gender, education, income and race were not significant predictors after accounting for complexity factors, age and access to care.
GFR: glomerular filtration rate
A subset of complexity factors across domains were associated with repeated hospitalizations. For example, compared to people with CHD alone, the 22.2% of participants with CHD who also had arthritis were not at significantly greater risk for repeated hospitalization (OR: 1.36, 95%CI: 0.77–2.38). In contrast, the 7.7% of participants with CHD who also had arthritis and diabetes were more than twice as likely to have been hospitalized at least twice (2.33, 1.18–4.62). In the clinical domain, the 13.1% with CHD also taking > 4 meds (3.15, 1.75–5.66) and the 7.0% with CHD taking > 4 meds and experiencing dizziness and urinary incontinence (4.65, 1.37–15.79) were at greater risk for repeated hospitalization than those with CHD alone; those with urinary incontinence were not. In the health status domain, mobility difficulty was consistently associated with repeated hospitalization: 11.4% with CHD and mobility difficulty (2.05, 1.15–3.67), 3.3% with CHD and mobility difficulty and hearing impairment (2.11, 0.87–5.16), and 2.4% with CHD mobility difficulty and visual impairment (3.24, 1.65–6.38) were at elevated risk.
CONCLUSION
In this paper, we describe a novel and replicable approach to considering clinical complexity that may assist in choosing conditions to be considered in clinical trials, guideline development and implementation and, therefore, eventually, in clinical decision-making with multimorbid patients. We found that three quarters of adults with CHD have at least one condition contributing to complexity, and many people have several. The high prevalence of complexity of health status in people with CHD, which has not been previously identified or quantified, underscores the importance of recognizing these co-existing conditions in the conduct of research and clinical practice and the development and application of clinical practice guidelines for adults with CHD. We found that clinical and health factors that are not disease-specific were as prevalent, and sometimes more strongly associated with repeated hospitalization as major chronic diseases. These findings suggest that efforts to reduce hospitalizations among people with CHD should potentially target interventions at conditions that are not captured by classic disease labels and focus on other clinical and health status factors.
The mechanism by which coexisting diseases, clinical conditions, or health status conditions influence an outcome such as repeated hospitalization is likely complex, and dependent on specific diseases and conditions; as well as influenced by social and health system factors that we have not examined here. We found that not all clinical patterns of complexity were associated with risk of repeated hospitalization. Specific combinations of co-existing conditions influenced the outcome of repeated hospitalization underscoring that health status complexity is influenced not only by the number of conditions that a patient may have, but by the specific conditions a person has. Many of the co-existing conditions may function as competing demands for physicians, patients, and family or friends.21, 22 Some studies suggest that having more chronic conditions is not associated with worse performance on disease-specific process measures, perhaps in part due to more frequent contact with the health system.23–25 While disease-specific process measures are commonly used in patients with multimorbidity, there is no consensus on what are the best quality measures for the population with multimorbidity.26 Considering whether co-existing conditions are concordant, that is sharing pathophysiology and likely to have an overlapping management plan, or discordant, that is not directly related in either their pathogenesis or management and not sharing an underlying predisposing factor, illustrates that the relationship may be more complex.27,28 For example, conditions that are “discordant” with diabetes decrease the likelihood of intensification of hypertension management.27, 29–31 The acuity, severity or dominance of comorbid conditions may be most important at a particular point in time.29, 32, 33
Our study is particularly important in the context of the use of clinical practice guidelines (CPGs). CPGs are used to guide management of chronic medical conditions. Translation of CPGs into practice can be difficult. However, they are often adopted by payors and others to define quality standards and provide focus for quality improvement efforts.34, 35 Historically, CPGs have been developed with a focus on specific chronic conditions.36 However, little has been known about how or whether CPGs can be applied to the management of multimorbid people.28, 37–39 Our data suggest that strict provider adherence to CPGs among complex adults with CHD may be associated with harm. For example, following the recommendations in a CPG for CHD may lead to taking more medications and could result in medication side effects such as dizziness or falls, all factors we have demonstrated to be associated with repeated hospitalization. In addition, taking more medications can lead to decreased adherence, and influence patient safety and clinical outcomes.40 CPG use in the clinical setting requires a substantial effort by the clinician to prioritize and make choices about all possible recommendations for CHD, and other conditions.39, 41–44 Currently, clinicians are without explicit guidance or evidence as to how to approach care decisions for such patients. A first step towards developing such guidance is to understand the common patterns of co-existing conditions that are relevant to clinical decision-making for adults with CHD.
These results also have implications for those adults with CHD who do not exhibit co-existing factors in any domain. Of this subpopulation, those who do not receive proven therapies may represent a prime opportunity to increase effectiveness given that they have fewer likely treatment interactions and contraindications to therapies, and may have fewer barriers to adherence.
Neglect of these co-existing factors which may add to complexity of clinical decision-making in the design, conduct and reporting of clinical and health services trials raises serious concerns about external validity of most CPGs. The patients studied in clinical trials which form the basis of CPGs do not adequately reflect the true population in terms of burden of comorbidity, due in part to emphasis on efficacy by funding and regulatory agencies and perceived barriers to the participation of older adults in clinical trials.45–47 Many older patients and patients with major comorbidities are still excluded from many clinical trials.45, 48 While the number of trials with age exclusions has decreased, the number of heart failure trials excluding participants with specific comorbidities increased from 1985 to 1999, with more than half of such trials excluding people with major hepatic, renal or hematologic comorbidities.49 By providing a framework for evaluating complexity along with empiric data for CHD, it may be possible to find a balance between trial safety and the need to include an adequate representation of the targeted population. As a first step to improving the ability to assess external validity, we have provided nationally-representative estimates for CHD and factors which may add to the complexity of clinical decision-making. If multimorbid patients are to be enrolled in more pragmatic trials, new analytic techniques which account for heterogeneity of treatment effects, as opposed to emphasizing a single overall “average”, should be explored.50
Currently, studies of CHD report on the prevalence of a small number of conditions. In order to develop CPGs more relevant to people with CHD (or any index condition) and comorbidities, first it must be decided what are the common and/or clinically relevant conditions to consider. We have considered these on 2 levels, conditions and/or treatments which interact with CHD or its treatments, and conditions which may make the implementation of guidelines more challenging. We described how common these conditions are in people with CHD in order to have this inform the processes that CHD guideline developers will undertake during guideline development of new or revised CHD guidelines. This paper does not mandate a specific set of conditions that should be considered in the development of CPGs. This choice will need to be part of priority setting at the outset of CPG development; the results reported here are necessary for a thoughtful deliberation.
LIMITATIONS
While this work is generalizable to the community-dwelling population of the United States, we were limited to factors included in the NHANES dataset and were not able to fully assess all factors that would add to complexity of management of CHD, including depression or other diagnosed mental illnesses.51 Frequent mental distress is likely associated with true mental illness, but not identical. We are unable to address the perceived treatment burden experienced by patients and their family and friends from therapies for CHD and co-existing conditions. We were only able to assess cognitive impairment in those over 65, and visual impairment was fully measured only in participants age 50 or older. We were not able to assess health literacy, language barriers, or neighborhood characteristics. The institutional population is not represented in NHANES. Similarly, there are many conditions that would greatly add to complexity of management of a person with CHD which were not assessed by NHANES, including active cancer, aspirin sensitivity and many others. We are unable to ascertain the length of stay or cause of hospitalization. The hospitalizations occurred in the year prior to the NHANES assessment. We relied on self-report of conditions for many of the assessed conditions, and while agreement between self-report of chronic conditions and a thorough adjudication process has been demonstrated to be reasonably good among community-dwelling disabled women, there is still some misclassification.52 Despite these limitations, the empiric data on the burden of complexity experienced by adults with CHD that we provide is useful, and more than has been previously recognized in clinical practice, clinical practice guidelines, and both clinical and health services research.
CONCLUSION
Complexity of clinical management for people with CHD is the rule, not the exception. While half of older adults have ≥ 3 chronic conditions, the current paradigm of evidence-based medicine and health care quality focuses largely on single-diseases.35, 39, 53–55 Currently, there are limited data, and thus a limited knowledge base, to guide clinicians on how to deliver the best quality care to these patients.39, 41–44 Clinical practice guidelines focused on single diseases do not apply well to those with multimorbidity.39 Describing the complexity of patients with CHD is a necessary first step to develop evidence and strategies to guide the care of these patients. The findings presented here support the idea that a persons' health status complexity can be better understood using a framework that incorporates disease, clinical and health status domains. Understanding how to best care for patients with CHD in terms of all of their health needs may lead to improvements in quality of life, utilization of health care, safety, morbidity and mortality.
Figure 1b.
Prevalence of clinical factors among people with Coronary Heart Disease, Stratified by Age and Gender, 95% CI
Footnote:
Urin Incont: urinary incontinence
GFR: glomerular filtration rate
ACKNOWLEDGMENT
We appreciate the assistance in manuscript preparation provided by Dr. Travonia Hughes, PhD.
Financial Disclosures: None of the authors received corporate financial support, consultantships, speaker arrangements, company holdings, or patents related to this research, and the materials described in this article. For this work, Dr. Boyd was supported by the Johns Hopkins Bayview Center for Innovative Medicine, The Robert Wood Johnson Foundation Physician Faculty Scholars Program, and the Paul Beeson Career Development Award Program (NIA K23 AG032910, AFAR, The John A. Hartford Foundation, The Atlantic Philanthropies, The Starr Foundation and an anonymous donor). Dr. Weiss was supported by the Robert Wood Johnson Foundation Amos Medical Faculty Development Program. Dr. Wolff was supported by NIMH K01 MH082885-2.
Sponsor's Role: The sponsors had no role in the design, methods, analysis, or preparation of this paper.
Conflict of Interest Drs. Boyd, Leff, Wolff, Rand, Weiss, Yu, and Ms. Zhou have been supported by the federal government through the NIA, AHRQ, NHLBI and NIMH. Drs. Boyd, Leff, Wolff and Weiss have been supported by the non-profit Center for HealthCare Strategies for work on the prevalence of multimorbidity in the Medicaid population. Dr. Boyd is a Robert Wood Johnson Physician Faculty Scholar.
Footnotes
Author Contributions: Dr. Boyd conceptualized this paper, obtained funding, designed and interpreted the analysis with Drs Weiss, Yu, and Ms. Zhou, and wrote the manuscript. Dr. Yu collaborated in the design and supervision of the statistical analysis and interpretation of data, critical revision of this manuscript for important intellectual content. Dr. Leff was responsible for collaborating in the following activities for this paper: study design and implementation, analysis and interpretation of data and critical revision of the manuscript for important intellectual content. Dr. Rand was a co-investigator responsible for collaborating in the following activities: conceptualization of the paper, interpretation of data, and critical revision of the manuscript for important intellectual content. Ms. Zhou was a co-investigator responsible for collaborating in the following activities for this paper: the analysis and interpretation of data and critical revision of the manuscript for important intellectual content. Dr. Wolff was a co-investigator responsible for collaborating in the following activities for this paper: design and implementation, analysis and interpretation of data, and critical revision of the manuscript for important intellectual content. Dr. Weiss was responsible for collaborating in the following activities for this paper: study design and implementation, analysis and interpretation of data and critical revision of the manuscript for important intellectual content.
REFERENCES
- [1].Federal Interagency Forum on Aging-Related Statistics . Older Americans 2008: Key Indicators of Well-Being. US Govt. Printing Office; Washington, DC: Mar, 2008. [Google Scholar]
- [2].Gijsen R, Hoeymans N, Schellevis FG, et al. Causes and consequences of comorbidity: A review. J Clin Epidemiol. 2001;54:661–674. doi: 10.1016/s0895-4356(00)00363-2. [DOI] [PubMed] [Google Scholar]
- [3].Jemal A, Ward E, Hao Y, et al. Trends in the leading causes of death in the United States, 1970–2002. JAMA. 2005;294:1255–1259. doi: 10.1001/jama.294.10.1255. [DOI] [PubMed] [Google Scholar]
- [4].Weiss CO, Boyd CM, Yu Q, et al. Patterns of prevalent major chronic disease among older adults in the United States. JAMA. 2007;298:1160–1162. doi: 10.1001/jama.298.10.1160-b. [DOI] [PubMed] [Google Scholar]
- [5].Glynn LG, Buckley B, Reddan D, et al. Multimorbidity and risk among patients with established cardiovascular disease: A cohort study. Br J Gen Pract. 2008;58:488–494. doi: 10.3399/bjgp08X319459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ritchie C, Ekundayo OJ, Muchimba M, et al. Effects of diabetes mellitus in patients with heart failure and chronic kidney disease: A propensity-matched study of multimorbidity in chronic heart failure. Int J Cardiol. 2009;134:330–335. doi: 10.1016/j.ijcard.2008.12.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Braunstein JB, Anderson GF, Gerstenblith G, et al. Noncardiac comorbidity increases preventable hospitalizations and mortality among Medicare beneficiaries with chronic heart failure. J Am Coll Cardiol. 2003;42:1226–1233. doi: 10.1016/s0735-1097(03)00947-1. [DOI] [PubMed] [Google Scholar]
- [8].Feinstein AR. The Pre-therapeutic classification of co-morbidity in chronic disease. J Chronic Dis. 1970;23:455–468. doi: 10.1016/0021-9681(70)90054-8. [DOI] [PubMed] [Google Scholar]
- [9].Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- [10].Greenfield S, Billimek J, Pellegrini F, et al. Comorbidity affects the relationship between glycemic control and cardiovascular outcomes in diabetes: A cohort study. Ann Intern Med. 2009;151:854–860. doi: 10.7326/0003-4819-151-12-200912150-00005. [DOI] [PubMed] [Google Scholar]
- [11].Yancik R, Ershler W, Satariano W, et al. Report of the national institute on aging task force on comorbidity. J Gerontol A Biol Sci Med Sci. 2007;62:275–280. doi: 10.1093/gerona/62.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Boyd CM, Ritchie CS, Tipton EF, et al. From Bedside to Bench: Summary from the American Geriatrics Society/National Institute on Aging Research Conference on Comorbidity and Multiple Morbidity in Older Adults. Aging Clin Exp Res. 2008;20:181–188. doi: 10.1007/bf03324775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].National Center for Health Statistics National Health and Nutrition Examination Survey. Volume 2008 [Google Scholar]
- [14].Stevens LA, Coresh J, Greene T, et al. Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- [15].Woodman R, Ferrucci L, Guralnik J. Anemia in older adults. Curr Opin Hematol. 2005;12:123–128. doi: 10.1097/01.moh.0000154030.13020.85. [DOI] [PubMed] [Google Scholar]
- [16].Hanlon JT, Weinberger M, Samsa GP, et al. A randomized, controlled trial of a clinical pharmacist intervention to improve inappropriate prescribing in elderly outpatients with polypharmacy. Am J Med. 1996;100:428–437. doi: 10.1016/S0002-9343(97)89519-8. [DOI] [PubMed] [Google Scholar]
- [17].Wechsler D. WAIS-R Manual. The Psychological Corporation; San Antonio, TX: 1981. [Google Scholar]
- [18].Chaves PH, Ashar B, Guralnik JM, et al. Looking at the relationship between hemoglobin concentration and prevalent mobility difficulty in older women. Should the criteria currently used to define anemia in older people be reevaluated? J Am Geriatr Soc. 2002;50:1257–1264. doi: 10.1046/j.1532-5415.2002.50313.x. [DOI] [PubMed] [Google Scholar]
- [19].From the Centers for Disease Control and Prevention Self-reported frequent mental distress among adults--United States, 1993–1996. JAMA. 1998;279:1772–1773. [PubMed] [Google Scholar]
- [20].Strine TW, Greenlund KJ, Brown DW, et al. Characteristics of people aged 45 years or older with heart disease by frequent mental distress status, 2001. Prev Med. 2004;39:191–196. doi: 10.1016/j.ypmed.2004.01.022. [DOI] [PubMed] [Google Scholar]
- [21].Yarnall KS, Pollak KI, Ostbye T, et al. Primary care: is there enough time for prevention? Am J Public Health. 2003;93:635–641. doi: 10.2105/ajph.93.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ostbye T, Yarnall KS, Krause KM, et al. Is there time for management of patients with chronic diseases in primary care? Ann Fam Med. 2005;3:209–214. doi: 10.1370/afm.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Min LC, Reuben DB, MacLean CH, et al. Predictors of overall quality of care provided to vulnerable older people. J Am Geriatr Soc. 2005;53:1705–1711. doi: 10.1111/j.1532-5415.2005.53520.x. [DOI] [PubMed] [Google Scholar]
- [24].Min LC, Wenger NS, Fung C, et al. Multimorbidity is associated with better quality of care among vulnerable elders. Med Care. 2007;45:480–488. doi: 10.1097/MLR.0b013e318030fff9. [DOI] [PubMed] [Google Scholar]
- [25].Higashi T, Wenger NS, Adams JL, et al. Relationship between number of medical conditions and quality of care. N Engl J Med. 2007;356:2496–2504. doi: 10.1056/NEJMsa066253. [DOI] [PubMed] [Google Scholar]
- [26].Forum NQ. 2010;Volume 2010 [Google Scholar]
- [27].Piette JD, Kerr EA. The impact of comorbid chronic conditions on diabetes care. Diabetes Care. 2006;29:725–731. doi: 10.2337/diacare.29.03.06.dc05-2078. [DOI] [PubMed] [Google Scholar]
- [28].Sales AE, Tipton EF, Levine DA, et al. Are co-morbidities associated with guideline adherence? The MI-Plus study of Medicare patients. J Gen Intern Med. 2009;24:1205–1210. doi: 10.1007/s11606-009-1096-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kerr EA, Zikmund-Fisher BJ, Klamerus ML, et al. The role of clinical uncertainty in treatment decisions for diabetic patients with uncontrolled blood pressure. Ann Intern Med. 2008;148:717–727. doi: 10.7326/0003-4819-148-10-200805200-00004. [DOI] [PubMed] [Google Scholar]
- [30].Turner BJ, Hollenbeak CS, Weiner M, et al. Effect of unrelated comorbid conditions on hypertension management. Ann Intern Med. 2008;148:578–586. doi: 10.7326/0003-4819-148-8-200804150-00002. [DOI] [PubMed] [Google Scholar]
- [31].Lagu T, Weiner MG, Hollenbeak CS, et al. The impact of concordant and discordant conditions on the quality of care for hyperlipidemia. J Gen Intern Med. 2008;23:1208–1213. doi: 10.1007/s11606-008-0647-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kerr EA, Heisler M, Krein SL, et al. Beyond comorbidity counts: How do comorbidity type and severity influence diabetes patients' treatment priorities and self-management? J Gen Intern Med. 2007;22:1635–1640. doi: 10.1007/s11606-007-0313-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bolen SD, Samuels TA, Yeh HC, et al. Failure to intensify antihypertensive treatment by primary care providers: A cohort study in adults with diabetes mellitus and hypertension. J Gen Intern Med. 2008;23:543–550. doi: 10.1007/s11606-008-0507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Casalino L, Gillies RR, Shortell SM, et al. External incentives, information technology, and organized processes to improve health care quality for patients with chronic diseases. JAMA. 2003;289:434–441. doi: 10.1001/jama.289.4.434. [DOI] [PubMed] [Google Scholar]
- [35].Garber AM. Evidence-based guidelines as a foundation for performance incentives. Health Aff (Millwood) 2005;24:174–179. doi: 10.1377/hlthaff.24.1.174. [DOI] [PubMed] [Google Scholar]
- [36].The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC VII) Volume 2005 doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- [37].Schellevis FG, van der Velden J, van de Lisdonk E, et al. Comorbidity of chronic diseases in general practice. J Clin Epidemiol. 1993;46:469–473. doi: 10.1016/0895-4356(93)90024-u. [DOI] [PubMed] [Google Scholar]
- [38].Tinetti ME, Bogardus ST, Jr., Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med. 2004;351:2870–2874. doi: 10.1056/NEJMsb042458. [DOI] [PubMed] [Google Scholar]
- [39].Boyd CM, Darer J, Boult C, et al. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: Implications for pay for performance. JAMA. 2005;294:716–724. doi: 10.1001/jama.294.6.716. [DOI] [PubMed] [Google Scholar]
- [40].May C, Montori VM, Mair FS. We need minimally disruptive medicine. BMJ. 2009;339:b2803. doi: 10.1136/bmj.b2803. [DOI] [PubMed] [Google Scholar]
- [41].Kravitz RL, Duan N, Braslow J. Evidence-based medicine, heterogeneity of treatment effects, and the trouble with averages. Milbank Q. 2004;82:661–687. doi: 10.1111/j.0887-378X.2004.00327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Greenfield S, Kravitz R, Duan N, et al. Heterogeneity of treatment effects:Implications for guidelines, payment, and quality assessment. Am J Med. 2007;120:S3–9. doi: 10.1016/j.amjmed.2007.02.002. [DOI] [PubMed] [Google Scholar]
- [43].Fortin M, Soubhi H, Hudon C, et al. Multimorbidity's many challenges. BMJ. 2007;334:1016–1017. doi: 10.1136/bmj.39201.463819.2C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Fortin M, Dionne J, Pinho G, et al. Randomized controlled trials: Do they have external validity for patients with multiple comorbidities? Ann Fam Med. 2006;4:104–108. doi: 10.1370/afm.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Van Spall HG, Toren A, Kiss A, et al. Eligibility criteria of randomized controlled trials published in high-impact general medical journals: A systematic sampling review. JAMA. 2007;297:1233–1240. doi: 10.1001/jama.297.11.1233. [DOI] [PubMed] [Google Scholar]
- [46].Mody L, Miller DK, McGloin JM, et al. Recruitment and retention of older adults in aging research. J Am Geriatr Soc. 2008;56:2340–2348. doi: 10.1111/j.1532-5415.2008.02015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kitzman DW, Rich MW. Age disparities in heart failure research. JAMA. 2010;304:1950–1951. doi: 10.1001/jama.2010.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wedzicha JA, Calverley PM, Seemungal TA, et al. The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med. 2008;177:19–26. doi: 10.1164/rccm.200707-973OC. [DOI] [PubMed] [Google Scholar]
- [49].Heiat A, Gross CP, Krumholz HM. Representation of the elderly, women, and minorities in heart failure clinical trials. Arch Intern Med. 2002;162:1682–1688. doi: 10.1001/archinte.162.15.1682. [DOI] [PubMed] [Google Scholar]
- [50].Kent DM, Kitsios G. Against pragmatism: On efficacy, effectiveness and the real world. Trials. 2009;10:48. doi: 10.1186/1745-6215-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Safford MM, Allison JJ, Kiefe CI. Patient complexity: more than comorbidity. The vector model of complexity. J Gen Intern Med. 2007;22(Suppl 3):382–390. doi: 10.1007/s11606-007-0307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Simpson CF, Boyd CM, Carlson MC, Griswold ME, Guralnik JM, Fried LP. Agreement between self-report of disease diagnoses and medical record validation in disabled older women: factors that modify agreement. J Am Geriatr Soc. 2004;52:123–127. doi: 10.1111/j.1532-5415.2004.52021.x. [DOI] [PubMed] [Google Scholar]
- [53].Anderson G, Horvath J. Chronic Conditions: Making the Case for Ongoing Care: Partnership for Solutions. 2002. [Google Scholar]
- [54].Marengoni A, Rizzuto D, Wang HX, et al. Patterns of chronic multimorbidity in the elderly population. J Am Geriatr Soc. 2009;57:225–230. doi: 10.1111/j.1532-5415.2008.02109.x. [DOI] [PubMed] [Google Scholar]
- [55].Lee PG, Cigolle C, Blaum C. The co-occurrence of chronic diseases and geriatric syndromes: The health and retirement study. J Am Geriatr Soc. 2009;57:511–516. doi: 10.1111/j.1532-5415.2008.02150.x. [DOI] [PubMed] [Google Scholar]