Abstract
The health-promoting effects of seaweeds have been linked to antioxidant activity that may counteract cancer-causing oxidative stress-induced damage and inflammation. While antioxidant activity is commonly associated with direct radical scavenging activity, an alternative way to increase the antioxidant status of a cell is to enhance the endogenous (phase II) defense system consisting of cytoprotective antioxidant enzymes, including NAD(P)H:quinone oxidoreductase 1 (NQO1). These enzymes are transcriptionally regulated by the antioxidant response element (ARE) via the transcription factor Nrf2. Extracts derived from cultivated Ulva sp., a green alga regarded as a marine vegetable (sea lettuce), potently activated the Nrf2-ARE pathway in IMR-32 neuroblastoma and LNCaP prostate cancer cells. RNA interference studies demonstrated that Nrf2 and PI3 kinase are essential for the phase II response in IMR-32 cells. Activity-enriched fractions induced Nrf2 nuclear translocation and target gene transcription, and boosted the cellular glutathione level and therefore antioxidant status. A single-dose gavage feeding of Ulva-derived fractions increased Nqo1 transcript levels in various organs. Nqo1 induction spiked in different tissues, depending on the specific chemical composition of each administered fraction. We purified and characterized four ARE inducers in this extract, including loliolide (1), isololiolide (2), a megastigmen (3), and a novel chlorinated unsaturated aldehyde (4). The ARE-active fractions attenuated lipopolysaccharide-induced iNOS and Cox2 gene expression in macrophagic RAW264.7 cells, decreasing nitric oxide (NO) and prostaglandin E2 (PGE2) production, respectively. Nqo1 activity and NO production were abrogated in nrf2−/− mouse embryonic fibroblasts, providing a direct link between the induction of phase II response and anti-inflammatory activity.
Keywords: natural products, seaweed, Ulva species, antioxidant response element, oxidative stress
Introduction
Marine algae (seaweeds) have been used as a food source and medicine for centuries (1). This includes green algae (Chlorophyta), red algae (Rhodophyta) and brown algae (Ochrophyta). Consumption of seaweed, which predominantly occurs in Japan, was found to be inversely related to various cancers, including colon, rectal and stomach cancer (2,3). Seaweed is a major part of the Okinawan food culture, and Okinawans have the longest life expectancy in the world and low disability rates (4). Numerous beneficial properties of algal extracts and constituents have been reported, however, usually only in a descriptive manner, without pinpointing specific bioactive components or invoking specific molecular pathways. Green algae of the genus Ulva, also known as sea lettuce, are among the most commonly consumed seaweeds. They reportedly have anti-inflammatory and antitumoral properties and are implicated in cancer prevention and detoxification. For example, crude extracts of U. reticulata given to rats attenuated acetaminophen-induced hepatotoxicity by improving the hepatic antioxidant status (5). It has been postulated that Ulva extract protects the membrane from damage by toxic reactive metabolites produced by acetaminophen biotransformation (5). U. conglobata has neuroprotective and anti-inflammatory activity (6), while U. lactuca has antitumor and immunostimulating effects (7). The antioxidant activity of U. pertusa has been attributed to polysaccharides with high sulfate content (8). We recently described that U. lactuca can increase the cellular antioxidant status through an alternative mechanism, and attributed this activity in part to the presence of monounsaturated fatty acid constituents (9).
While antioxidant activity is commonly associated with direct radical scavenging activity, an alternative way to increase the antioxidant status of a cell or body is to enhance the endogenous defense system consisting of antioxidant enzymes and detoxification enzymes, which presumably causes a more sustained, longer-lasting effect. Phase II and other antioxidant enzymes are commonly regulated by the antioxidant response element (ARE) on the transcriptional level (10). Increased expression of these enzymes correlates with a decrease in cellular damage by reactive oxygen species (ROS), which are implicated in inflammation and the pathogenesis of many age-related disorders, including cancer, neurodegeneration, and aging itself (11–13). In humans, the antioxidant response element (ARE) regulates the expression of cytoprotective antioxidant enzymes [e.g., heme oxygenase-1 (HO-1), glutathione-S-transferases (GSTs), NAD(P)H:quinone oxidoreductase 1 (NQO1)], which contribute to the endogenous defense against oxidative stress (10). The major transcription factor involved in the induction of phase II enzymes is nuclear factor erythroid-derived 2-related factor 2 (Nrf2), a Cap ‘n’ Collar (CNC) type basic region-leucine zipper (bZip) transcription factor that, upon activation by ARE inducers, translocates to the nucleus, binds to the ARE sequence as a heterodimer with one of the small bZip proteins, Mafs, and activates ARE-dependent genes. Nrf2 is negatively regulated by the cysteine-rich protein Keap1. Keap1 serves to sequester Nrf2 in the cytoplasm and interacts with Cul3-based E3 ubiquitin ligase to target Nrf2 for proteasomal degradation (14–16).
Nrf2 knockout mice show diminished detoxification capabilities (17), decreased responsiveness to chemoprotective agents (18), and enhanced susceptibility to oxidative stress induced cell death (19). Conversely, Nrf2 overexpression protects from oxidative stress (19). NQO1-deficient individuals are at a considerably higher risk of developing leukemia following occupational exposure to benzene (20). The activation of the Nrf2-ARE pathway is a valid cancer preventive strategy, and sulforaphane, a constituent of broccoli, is an example of a cancer preventive natural product that acts through this mechanism (12). We hypothesized and preliminarily demonstrated that some seaweeds are able to activate this signaling pathway and that some of the beneficial, particularly antioxidant, properties may be mediated through ARE activation as opposed to only direct scavenging properties (9).
ARE activation may be particularly relevant to prostate cancer (21). The most common hallmark in prostate cancer is the silencing of glutathione-S-transferase (GST)-π (GSTP1) due to DNA methylation, which is nearly universal (22–24). Because of the lack of GSTP1 expression in prostate cancer (regardless of grade or stage) (22–24), induction of GSTs and other phase II enzymes through ARE activation is a promising prostate cancer-preventive strategy (25). While prostate cancer is the second leading cause of cancer death in American men (26), prostate cancer is rarely diagnosed and contributes little to cancer mortality in Asia (27,28). However, men migrating from Asia to the USA increase their risk, which remains elevated in their male descendants (29–32), likely attributable to lifestyle and dietary changes. Notably, diet in Asia largely includes seaweed, suggesting a possible connection between algae consumption and decreased prostate cancer risk. Many other diseases, including those with an inflammation component, are caused by excessive oxidative stress and may be prevented or interfered with via enhancing the cellular antioxidant status.
This study follows on previous research that showed that extracts of marine algae can activate the Nrf2-ARE pathway, and that extracts of Ulva spp. were particularly active among a variety of seaweeds tested (9). We extracted large amounts of Ulva sp. available through cultivation to isolate individual compounds that are ARE inducers, tested enriched fractions in prostate cancer cells and other cellular models for their ability to activate the Nrf2-ARE pathway and assessed downstream effects on inflammatory markers. We also correlated the cellular activity with in vivo activity in physiologically relevant settings to ultimately assess the chemopreventive potential of Ulva.
Materials and Methods
All chemicals and solvents used for extraction, fractionation and isolation of compounds were purchased from Fisher Scientific unless specified. For chemicals, reagents, cells, culture media, RNA extraction, quantitative RT-PCR, immunoblot analysis, and glutathione assays, see Supplementary Information for details.
ARE Luciferase Reporter Assay in IMR-32 and LNCaP Cells
ARE luciferase reporter plasmid (50 ng/well) and CMV-GFP (10 ng/well) plasmid for monitoring transfection efficiency were co-transfected into IMR-32 cells (3 × 104 cells/well) and LNCaP (2 × 104 cells/well) using FUGENE HD (Roche). Transfected cells were seeded in 96-well plates and incubated for 24 h. Cells were treated with Ulva fractions, compounds, positive controls (tBHQ and sulforaphane at 10 μM) and solvent control (1% DMSO) and allowed to incubate for an additional 24 h before luciferase activity was measured using BriteLite (PerkinElmer) luminescence detection reagent.
Inhibitor Studies
IMR-32 cells were seeded in 6-well dishes (6 × 105 cells/well) one day before treatment. After pretreatment with the PI3K inhibitor LY294002 (25 μM) or the MEK1 inhibitor PD98059 (50 μM) for 30 min, cells were treated with fractions for 24 h, and then proteins were harvested and subjected to immunoblot analysis.
RNA Interference Experiments
Nontargeting control siRNA and siGENOME SMART pool siRNA reagents targeting NRF2 (mixture of 4 siRNAs) were obtained from Dharmacon. IMR-32 cells were seeded in 6-well dishes (3 × 105 cells/well) 24 h prior to transfection. SiRNAs were transfected with siLentFect at the effective dose of 50 nM. 60 h after siRNA transfection, cells were treated with fractions or vehicle for 24 h before total protein lysates were collected and subjected to immunoblot analysis.
In Vivo Studies
ARE-human placental alkaline phosphatase transgenic mice were as described (see Supplementary Information) (33). Male transgenic mice (~35 g; n = 3) between 12–16 weeks of age were gavaged with a single dose (5 mg; ~140 mg/kg) of prioritized fractions NP3, NP4, NP5 and P4. Male control mice (n=3) from both transgenic and non-transgenic (for hPAP activity) sets were fed with 200 μL of the vehicle (10% DMSO; 10% Cremophor in PBS) and various tissues harvested 12 h post feeding. Total RNA for RT-PCR was isolated using Trizol reagent (Invitrogen) and protein for hPAP enzyme activity using freshly prepared TMNC buffer as described (33).
hPAP Enzyme Activity Assay
For alkaline phosphatase tissue activity, tissues were homogenized in TMNC (0.05 M Tris, 0.005 M MgCl2, 0.1 M NaCl, 1% [CHAPS]) lysis buffer and refrozen at −80 °C. Endogenous phosphatases were heat inactivated at 65 °C for 30 min, and the samples then incubated at room temperature in the presence of a chemiluminescent CSPD substrate (Tropix) for alkaline phosphatase. Activity was assessed by measuring the resulting luminescent signal representing relative hPAP activity according to manufacturer’s instruction for Phospho-Light Reporter Gene Assay System (Applied Biosystems).
Assay for Induction of iNOS and Cox2 in Macrophage Cells
RAW264.7 cells were seeded in 6-wells for RNA experiments and treated with different concentrations of Ulva fractions for 1 h. Cells were treated with 1 μg/mL of LPS or 10 ng/mL of IFN-γ and incubated for 12 h. Total RNA was extracted using RNeasy Mini Kit. PCR analyses were performed on the aliquots of the cDNA preparations to detect iNOS, Cox2, Nqo1 and β-actin (internal standard) expression.
RT-qPCR for NRF2 and NQO1 Expression in IMR-32 Cells (Purified Compounds)
IMR-32 cells (1×106 cells/well) were seeded in 6-well plates one day before treatment. Cells were treated with variable concentrations of compounds for 12 h. Total RNA was extracted and real-time PCR was performed in triplicate using GAPDH expression as internal control for normalization.
PGE2 Assay
RAW264.7 cells were seeded in 96-well plates (4 × 104 cells/well) 24 h prior to treatment. Fractions or vehicle were pretreated for 1 h before adding LPS (1 μg/mL), and incubated for further 24 h. The supernatant was transferred to fresh collection tubes and used for the assay. Amersham Prostaglandin E2 Biotrak Enzyme immunoassay (EIA) system (GE Healthcare) was used for the assay and the manufacturer’s instructions were followed.
NO Assay
RAW 264.7 cells (2 × 104cells/well), wild-type (104cells/well), Nrf2−/− (104cells/well) and Keap1−/− (5 × 103 cells/well) mouse embryonic fibroblasts (MEFs) were seeded in 96-well plates and pre-treated for 1 h with different concentrations of Ulva fractions or solvent control (1% EtOH), prior to adding LPS (1 μg/mL), IFN-γ (10 ng/mL), and/or TNF-α (10 ng/mL). NO production in culture supernatant was assessed after 24 h (RAW cells) or 20 h (MEFs) by measuring nitrite concentration, an oxidative product of NO. Nitrite production was measured by mixing 50 μL of culture supernatant with 50 μL of Griess reagent (Promega), and absorbance was measured at 540 nm against a calibration curve generated for fresh sodium nitrite standard.
Nqo1 Activity Assay
Wild-type (8 × 103 cells/well), Nrf2−/− (8 × 103 cells/well) and Keap1−/− (4 × 103 cells/well) fibroblasts were plated in 96-well plates, and 24 h later the cells were incubated with various concentrations of compound or solvent control for 40 h. The activity was measured as described (34).
Extraction of Cultivated Ulva sp. and Bioassay-guided Isolation
For detailed procedures for extraction, isolation and characterization of compounds, see Supplementary Information. Material from cultured Ulva was freeze-dried (2.23 kg) and extracted with nonpolar (EtOAc, NP), and polar organic (EtOH, P) and polar aqueous (1:1 EtOH/H2O, W) solvents, as shown (Supplementary Scheme S1). Si gel fractions (NP1–NP6) from the nonpolar EtOAc extract and the C18 fractions from both EtOH (P1–P6) and 1:1 EtOH/H2O (W1–W6) extracts were investigated for ARE activity to establish previously identified activity profiles. NP3 (424 mg), NP4 (129 mg), NP5 (354 mg) from the nonpolar extract and P4 (420 mg) from the polar EtOH extract were prioritized based on ARE activity. Each of the four prioritized fractions was chromatographed further employing size exclusion chromatography (Sephadex LH20) and several sequences of reversed-phase HPLC to give the following compounds.
Loliolide (1)
Colorless amorphous solid; [α]20D −52 (c 0.06, CHCl3); 1H NMR (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3) spectra, see Supplementary Information; HRESIMS m/z [M + Na]+ 219.0990 (calcd for C11H16NaO3, 219.0997).
Isololiolide (2)
Colorless amorphous solid; [α]20D +44 (c 0.1, CHCl3); 1H NMR (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3) spectra, see Supplementary Information; HRESIMS m/z [M + Na]+ 219.0988 (calcd for C11H16NaO3, 219.0997).
3,5,6-Trihydroxy-7-megastigmen-9-one (3)
Colorless amorphous solid; [α]20D −68 (c 0.09, MeOH) ; 1H NMR (400 MHz) and 2D NMR (600 MHz, CD3OD) and 13C NMR (100 MHz, CD3OD) spectra, see Supplementary Information; HRESIMS m/z [2M + Na]+ 507.3286 (calcd for C26H44NaO8, 507.2934) and [M − H2O + Na]+ 247.1314 (calcd for C13H20NaO3, 247.1310).
8-Chloro-6,7-dihydroxy-deca-2,4-dienal (4)
Colorless oil; [α]20D −188 (c 0.0008, MeOH); 1H and 2D NMR (500 MHz, CD3OD) data and spectra, see Table 1 and Supplementary Information; HRESIMS m/z [M + Cl]− 253.0404, 255.0376 (ratio 9:6, calcd for C10H1535Cl2O3, 253.0398; C10H1537Cl2O3, 255.0369).
Table 1.
NMR (500 MHz) data for 8-chloro-6,7-dihydroxy-deca-2,4-dienal (4) in CD3OD
| δH (J in Hz) | δCb | COSY | HMBC | |
|---|---|---|---|---|
| 1 | 9.53, d (8.0) | 196.4, CH | H-2 | C-2 |
| 2 | 6.15, dd (15.0, 8.0) | 132.6, CH | H-1, H-3 | C-4 |
| 3 | 7.33, dd (15.0, 11.0) | 154.4, CH | H-2, H-4 | C-1, C-5 |
| 4 | 6.67, ddd (15.2, 11.0, 1.8) | 129.5, CH | H-3, H-5 | C-3, C-6 |
| 5 | 6.47, dd (15.2, 5.2) | 147.6, CH | H-4, H-6 | C-3, C-6 |
| 6 | 4.66, dd (5.2, 2.0, 1.8) | 71.9, CH | H-5, H-7 | |
| 7 | 3.54, dd (8.8, 2.0) | 77.6, CH | H-6, H-8 | C-8 |
| 8 | 4.01, dd (8.8, 2.8,) | 64.6, CH | H-7, H2-9 | |
| 9a | 2.10, ddd (14.3, 7.2, 2.8) | 27.9, CH2 | H-8, Hb-9, H3-10 | C-10 |
| 9b | 1.70, ddd (14.3, 7.2, 1.8) | H-8, Ha-9, H3-10 | C-8 | |
| 10 | 1.06, d (7.2) | 10.6, CH3 | H-8, H2-9, H3-10 | C-9 |
| 6-OHa | 5.21, d (6.2) | H-6 | C-8 | |
| 7-OHa | 5.11, d (7.7) | H-7 |
Observed in DMSO-d6;
assigned from HSQC experiment
Results
Extract from Cultivated Ulva sp. is a Potent ARE Activator in Cellular In Vitro Systems
We previously sampled a variety of field-collected seaweeds from the Florida coastline and, using an ARE-luciferase reporter gene assay, found that Ulva lactuca is a particularly potent activator of the ARE. These U. lactuca field collections yielded monounsaturated fatty acid type compounds as representative ARE-active components (9). Large-scale cultivation of Ulva sp. now enabled us to rigorously evaluate the biological activity of the resulting extracts after carrying out a bioassay-guided fractionation. We used two cell lines for our initial assessment of ARE activity: (i) IMR-32 human neuroblastoma cells, a commonly used cellular model of oxidative stress which we have previously used to identify ARE activators (9,35,36), and (ii) androgen-sensitive (LNCaP) prostate cancer cells, since a common genetic feature of prostate cancer is the silencing of a GST gene (ARE-regulated) (22–24). Prostate cancer may be prevented most suitably by compounds acting through an antioxidant-type mechanism; however, the cancer preventive agent sulforaphane shows diminished responsiveness in androgen-insensitive cell lines (25).
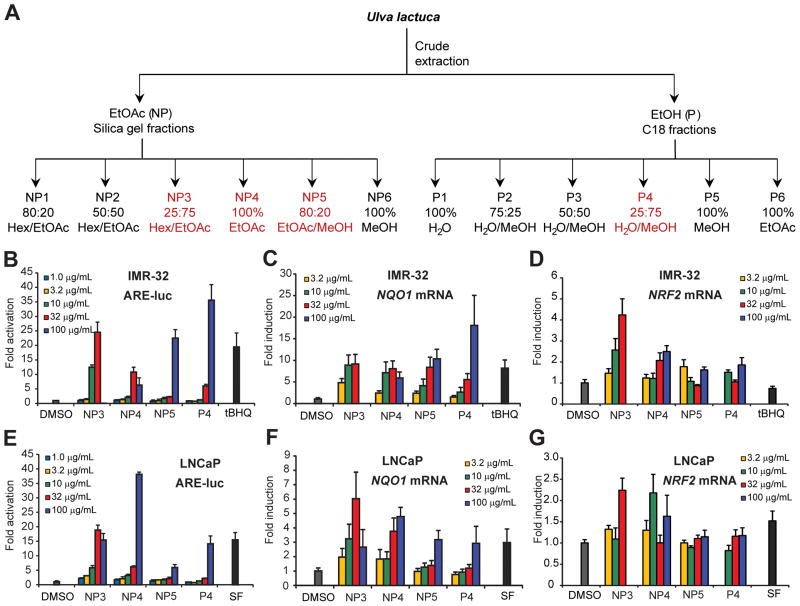
The freeze-dried sample was successively extracted with EtOAc and EtOH, followed by silica gel or C18 reversed-phase chromatography, respectively (Figure 1A), to yield several activity-enriched fractions, suggesting the presence of multiple bioactive components. Further chemical profiling and purification indeed confirmed that the ARE activity is attributed to an array of metabolites, prompting us to initially characterize the biological activities of the chromatography fractions rather than individual components and to also capture potential synergistic effects. The EtOAc (nonpolar, NP) extract yielded the most active fractions, designated NP3, NP4 and NP5 (Figure 1A), in the reporter assay (Figure 1B). NP3 and NP4 activated the reporter 25- and 11-fold, respectively, at 32 μg/mL and showed toxicity at higher concentrations (100 μg/mL) in the IMR-32 cells. NP5 and the EtOH (polar, P) extract-derived P4 fraction (Figure 1A) were highly active at 100 μg/mL, with 23- and 36-fold activity, respectively (Figure 1B). These activities correlated with the effects observed on the transcription of endogenous NQO1, an ARE-regulated target gene, as determined by quantitative PCR (qPCR) after reverse transcription (RT). The four active fractions induced NQO1 expression up to 8- to 18-fold (Figure 1C). Transcript levels of NRF2 were at most marginally increased in NP4, NP5 and P4 (1.8- to 2.5-fold) and to a slightly greater extent in fraction NP3 (4.2-fold) (Figure 1D). Overall these data suggest that Nrf2 transcription factor activation on the protein level rather than gene expression level must have been largely responsible for the potent NQO1 induction. The reporter activation and induction of NQO1 transcription were not cell-type specific, as similar results were obtained in LNCaP cells (Figures 1E–G), although different fractions were more effective in different reporter cell types (Figures 1B vs. 1E). In both cell lines the magnitudes of activation rivaled those of the corresponding positive controls tert-butylhydroquinone (tBHQ) and sulforaphane (SF) (Figures 1B–G).
Figure 1.
Extraction and fractionation of cultivated Ulva sp. and assessment of ARE-related transcriptional effects in neuroblastoma and prostate cancer cells. (A) Cultivated Ulva was extracted successively with EtOAc and EtOH and extracts were subjected to chromatography. Fractions indicated in red showed greatest ARE activity and were used for more detailed studies. (B–D) Dose-dependent ARE activities (normalized to vehicle control) of prioritized fractions in IMR-32 neuroblastoma cells. Tert-butylhydroquinone (tBHQ, 10 μM) served as a positive control. (B) Reporter assay. IMR-32 cells were transfected with ARE-luciferase reporter and treated 24 h later with NP and P fractions for another 24 h. Reporter activation was measured by luminescence (n = 3, ± SD). (C, D) Effects on endogenous NQO1 (C) and NRF2 (D) transcript levels as measured by RT-qPCR. IMR-32 cells were treated with fractions for 12 h, total RNA was isolated and reverse-transcribed to cDNA that was then subjected to TaqMan analysis (n = 3). GAPDH expression was used as internal control for normalization. (E–G) Dose-dependent ARE activities (normalized to vehicle control) of prioritized fractions in LNCaP prostate cancer cells as carried out for IMR-32 cells (panels B–D, respectively). Sulforaphane (SF, 10 μM) served as a positive control.
Ulva-promoted ARE-driven Antioxidant Expression Requires Nrf2 and a Functional PI3K Pathway in IMR-32 Cells and Leads to Enhanced Glutathione Levels
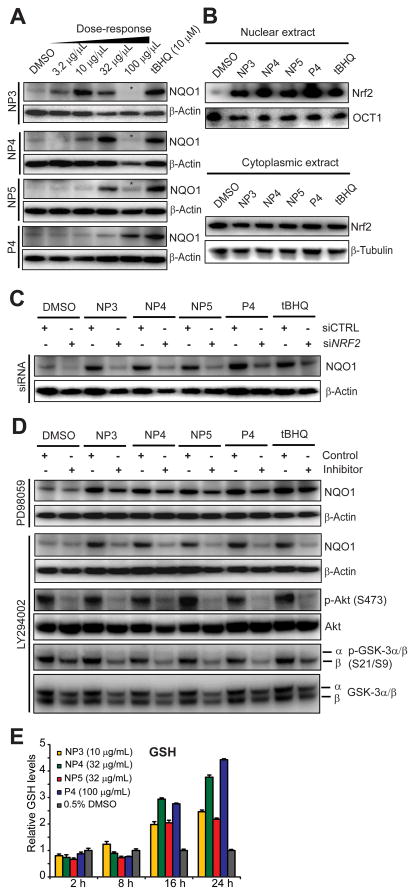
Next, we tested whether NQO1 protein levels paralleled the increased transcript levels. We chose the IMR-32 cell line, which gave a more robust induction of NQO1 transcription. Again, all four fractions strongly elevated NQO1 protein levels as measured by immunoblot analysis, and the optimal concentrations were sample-dependent (Figure 2A). We found that nuclear extracts derived from all treated cells were highly enriched in Nrf2 (Figure 2B), suggesting that the fractions caused Nrf2 nuclear translocation. To determine if the increase in NQO1 protein relied on Nrf2, we depleted cells of Nrf2 via RNA interference using previously validated NRF2-specific siRNAs (9,36) and then treated with fractions at the active concentrations. Expectedly, we found that none of the Ulva fractions was able to promote NQO1 levels in siNRF2-treated IMR-32 cells (Figure 2C), indicating that Nrf2 is the essential transcription factor mediating NQO1 induction by the Ulva fractions. Furthermore, pretreatment with a PI3K inhibitor prevented NQO1 induction, while a MEK1 inhibitor had only marginal effects (Figure 2D), indicating that the activity of the fractions in this cell type relies on a functional PI3K signaling pathway, but only to a much lesser extent on MAPK signaling, as observed for field-collected U. lactuca (9). The fractions did not appear to activate PI3K signaling, since they did not pronouncedly increase the phosphorylation status of Akt (downstream of PI3K) and GSK-3β (downstream of Akt); the latter is a key regulator of Nrf2 stability and downstream effector of Nrf2 inducers (37). PI3K inhibitor LY29002 effectively abrogated Akt and GSK-3β phosphorylation (Figure 2D), potentially decreasing Nrf2 stability. Additionally, PI3K has also recently been implicated in Nrf2 translation (35), augmenting the PI3K contribution to net Nrf2 levels. We then tested the effects of the fractions at their nontoxic active concentration on levels of the major small-molecule antioxidant, glutathione, in a time-dependent manner. We observed a 10–30% drop of glutathione levels after 2 h (Figure 2E), possibly due to transient disruption of the cellular redox status. Upon longer incubation times (16 and 24 h) we measured a strong increase of glutathione levels of up to 220–440%, depending on the fraction, consistent with ARE activation since glutathione biosynthetic genes are regulated by the Nrf2-ARE pathway (9).
Figure 2.
Downstream responses and mechanism of ARE-controlled gene expression of active fractions in IMR-32 cells. (A) Effect on NQO1 protein levels. Cells were treated with various concentrations of NP3, NP4, NP5 and P4 for 24 h, total protein lysates prepared, resolved by SDS/PAGE and subjected to Western blot analysis. β-Actin served as loading control. Asterisks (*) indicate toxic concentrations. The most active nontoxic concentration from this analysis for each fraction was used for subsequent studies (NP3: 10 μg/mL, NP4: 32 μg/mL, NP5: 32 μg/mL, P4: 100 μg/mL). (B) Analysis of Nrf2 nuclear translocation and stabilization by Western blot analysis using previously established active fraction concentrations. Nuclear and cytoplasmic extracts were prepared with the NE-PER reagent kit (Pierce) 24 h after treatment, resolved by SDS/PAGE and the Western blots were probed with Nrf2 antibody. OCT1 and β-tubulin levels served as loading controls for nuclear and cytoplasmic extracts, respectively. (C) Cells were transfected with siRNAs targeting NRF2 or with non-targeting control siRNAs (50 nM). Upon 60 h incubation, cells were treated with the indicated fractions at active concentrations (see panel A) for another 24 h before total protein lysates were collected, separated by SDS/PAGE and subjected to Western blot analysis. (D) Effect of pharmacological kinase inhibitors on natural products-induced NQO1 protein expression. Cells were pretreated with PI3K inhibitor LY294002 (25 μM) or MEK1 inhibitor PD98059 (50 μM) for 30 min and then exposed for 24 h to active concentrations of the indicated Ulva fractions (see panel A). Total protein was collected and subjected to SDS/PAGE followed by Western blot analysis to monitor NQO1 levels and phosphorylation status of Akt and GSK-3α/β. (E) Time-dependent effects on glutathione levels. Cells were treated for the indicated times with the active concentration of Ulva fractions (see panel A), cells were harvested and analyzed for total glutathione (GSH and GSSG), which was measured using the Glutathione Assay Kit (Sigma). Results are represented as means ± SD (n = 3).
Enriched Extracts from Cultivated Ulva sp. Activate ARE-driven Gene Transcription In Vivo
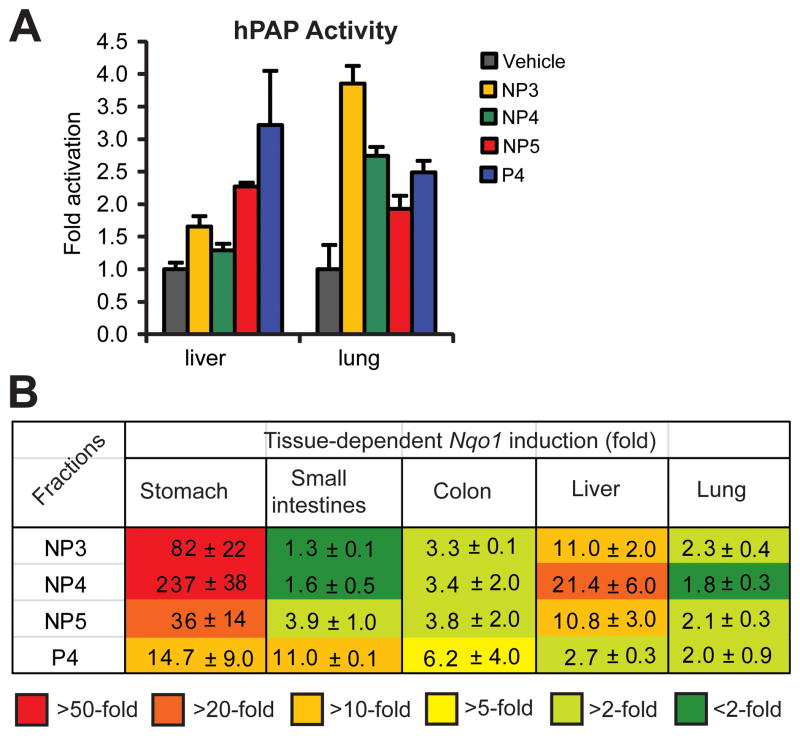
To test if the in vitro ARE activity translates into in vivo activity, and thereby if the mixtures are sufficiently bioavailable and/or have a low enough clearance rate and, by extrapolation, if Ulva sp. consumption can lead to activation of the endogenous ARE-regulated defense system, we treated mice with the bioactive fractions by oral gavage. We used transgenic mice developed by Johnson (33) in which the ARE sequence of the rat Nqo1 enhancer region has been linked to human placental alkaline phosphatase (hPAP) reporter gene, a model that is suitable to evaluate enzymatic and immunohistochemical responses. These mice were previously used to test the effectiveness of triterpenoids as inducers of the Nrf2-ARE pathway in vivo (38). Analogously, we fed prioritized concentrated fractions to male mice (single dose of 140 mg/kg, n = 3), harvested tissues 12 h later and, to assess hPAP activity, focused on two tissues with (i) detoxification responsibilities (liver) and (ii) a potentially high degree of oxidative activity (lung). In both cases we observed increased activity by the treatments, up to 3.2-fold in liver and up to 3.9-fold in lung (Figure 3A), suggesting that the active Ulva components are sufficiently bioavailable based on the measured functional response.
Figure 3.
Activation of ARE reporter and induction of endogenous Nqo1 transcript levels in vivo 12 h after gavage-feeding of Ulva extract mixtures. Male ARE-hPAP reporter mice (n = 3) were gavaged with a single dose (200 μL) of prioritized fractions NP3, NP4, NP5, P4 or vehicle control (10% DMSO, 10% Cremophor in PBS) and various tissues harvested 12 h post feeding. (A) Lung and liver tissues were analyzed for hPAP activity. For each mouse, tissue samples were analyzed in triplicate. (B) Relative Nqo1 transcript changes and heatmap to visualize trends in fraction-dependent Nqo1 induction for various tissues. Total RNA was isolated from each tissue and mRNA was reverse-transcribed to cDNA that was then analyzed by TaqMan-based qPCR. For each mouse, tissue samples were analyzed in triplicate. Results are represented as fold induction ± SEM.
These encouraging results from the reporter assay prompted us to analyze in detail the endogenous Nqo1 expression by RT-qPCR relative to controls, as a measure of the true antioxidant enzyme status potential in the tissues. We also extended the analysis to additional tissues. All of the prioritized fractions derived from cultured Ulva showed in vivo activity, but the upregulation of Nqo1 spiked in different tissues, as roughly represented in a heat map for averages of all mice for active fractions (Figure 3B). These data suggest that different organs may be targeted with distinctive fractions to achieve protective effects. This is likely due to different chemistry and bioavailability of different components, which remains to be investigated.
Isolation, Structure Determination and Activity of Representative ARE Inducers from Cultivated Ulva sp
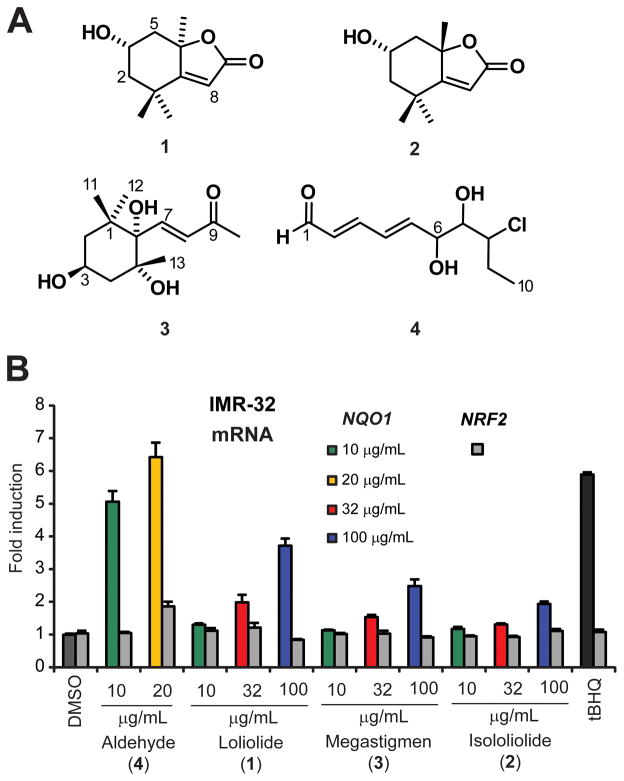
As pointed out above, there appeared to be a wide array of ARE activators in the samples. In an effort to deconvolute the complex mixtures present in NP3, NP4, NP5 and P4, we subjected the fractions to a series of size exclusion chromatography using Sephadex LH20, and subsequently to several sequences of reversed-phase HPLC. We identified that NP3, the most active fraction of the four, comprised of several small molecules of interest (Figure 4A). More specifically, the later eluting fractions 7 and 8 from LH20 chromatography (1:1 DCM/MeOH) were identified as mixtures of small molecules, potentially electrophiles with α,β-unsaturated carbonyl moieties. Additional LH20 chromatography (eluent: 2:5:1 hexanes/DCM/MeOH) and subsequent reversed-phase HPLC provided the known compounds loliolide (1), isololiolide (2), 3,5,6-trihydroxy-7-megastigmen-9-one (3) (39–42), along with a new aldehyde, 8-chloro-6,7-dihydroxy-deca-2,4-dienal (4) (Figure 4A). Based on 1H NMR and HPLC-DAD profiles we concluded that the remaining LH20 fractions mostly consisted of fatty acid glycerides. The structures of all known compounds (1–3) were determined utilizing NMR and MS experiments and by comparison of data to literature (40–42).
Figure 4.
Chemical analysis and biological characterization of purified active components. (A) Chemical structures of ARE activators isolated from fraction NP3. These structures represent only a small subset of active compounds in the extract and may also be present in other fractions. (B) Effects of purified compounds on NQO1 and NRF2 transcript levels in IMR-32 cells as measured by RT-qPCR. IMR-32 cells were treated with the indicated compounds for 12 h, total RNA was isolated and reverse-transcribed to cDNA that was then subjected to TaqMan analysis (n = 3, ± SD). GAPDH expression was used as internal control for normalization.
The HRMS analysis of 4 showed a distinctive isotopic cluster for [M + Cl]− at m/z 253.0404/255.0376 consistent with a molecular formula C10H15ClO3 (calcd. 253.0398/255.0369). The 1H NMR spectrum of 4 indicated the existence of an aldehyde function δ 9.53 (1H, d, J = 8.0 Hz), in addition to four signals characteristic for conjugated olefinic protons at δ 6.15 (1H, dd, J = 15.0 and 8.0 Hz), δ 7.33 (1H, dd, J = 15.0 and 11.0 Hz), δ 6.67 (1H, ddd, J = 15.2, 11.0 and 1.8 Hz) and δ 6.47 (1H, dd, J = 15.2 and 5.2 Hz). The latter were attributed to H-2, H-3, H-4 and H-5 of a linear system. The COSY experiment firmly established a single spin system from H-1 to H-10. In the absence of a direct carbon experiment due to insufficient sample, the carbon shifts were assigned with the aid of edited-HSQC and HMBC experiments (Table 1). The data revealed compound 4 to have an oxylipin structure. In addition to the aldehyde carbonyl (δC 196.4) and olefinic carbons (δC 132.6, 154.4, 129.5 and 147.6), three additional low-field resonances were assigned at δC 71.9 (C-6) and 77.6 (C-7) for two oxymethines, and at δC 64.6 (C-8) indicative of bearing a chlorine atom. The connectivity from C-1 to C-10 was further established by HMBC experiment. Two additional resonances at δ 5.21 (1H, d, J = 6.2 Hz) and δ 5.11 (1H, d, J = 7.7 Hz) in the 1H NMR spectrum acquired in DMSO-d6 supported the presence of exchangeable protons that were assigned to the hydroxy groups at C-6 and C-7, respectively. Therefore the planar structure for 4 was determined as 8-chloro-6,7-dihydroxy-deca-2,4-dienal and reported herein as an unprecedented oxylipin analog (Figure 4A).
Metabolites 1–4 were investigated for ARE activity by evaluating the activation of NQO1 transcription in IMR-32 cells in a dose dependent manner (Figure 4B). The oxylipin showed the most pronounced activity among the purified metabolites (6.4-fold at 20 μg/mL) comparable to tBHQ (5.9-fold) under identical conditions. At the same time, all purified compounds had no or only marginal effects on NRF2 transcript levels. However, the purification from the activity-enriched fractions did not increase the potency, suggesting that the combination with other active and/or matrix components may contribute to the overall activity. 1H NMR and HPLC-DAD profiling of the chromatographic fractions from NP4, NP5 and P4 indicated complex mixtures of fatty acid glycerides as the major constituents in addition to the compounds discussed above and trace amounts of fatty acids. We previously isolated and characterized representative monounsaturated fatty acids and a corresponding amide derivative from field-collected U. lactuca as ARE activators, which appeared to be more dominant in that species (9).
Anti-inflammatory Activity of Fractions Derived from Cultivated Ulva sp
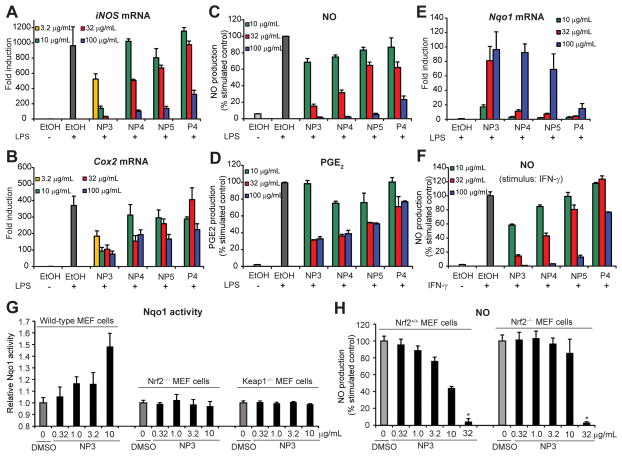
We then decided to measure downstream physiological effects as a result of phase II and antioxidant enzyme upregulation in a disease-relevant context. Since there is evidence that links the induction of phase II enzymes with protection from inflammatory stress (11,43), we used the RAW 264.7 macrophage cell line to correlate ARE activation to LPS- and IFN-γ stimulated iNOS and Cox2 transcription. All fractions were able to abrogate LPS-induced expression of iNOS and Cox2, two pro-inflammatory target genes, almost completely at higher concentrations (iNOS, Figure 5A) or partially (Cox2) (Figure 5B). We then measured the products of iNOS and Cox2 enzyme action, i.e., nitric oxide (NO) and prostaglandin E2 (PGE2), respectively. All fractions strongly reduced NO production in a dose-dependent manner (Figure 5C). Similarly, using ELISA we determined that these fractions also partially decreased PGE2 levels (Figure 5D), paralleling the RT-qPCR results. The knockdown was inversely correlated with Nqo1 transcript levels, which were strongly elevated in RAW264.7 cells in a dose-dependent manner (Figure 5E), suggesting that ARE activation is a relevant mechanism by which the Ulva fractions exert their anti-inflammatory effect. Furthermore, NP3 appeared to be most potent in both the knockdown of iNOS and Cox2 and the induction of Nqo1 mRNA levels, consistent with the data obtained in IMR-32 and LNCaP cells. To determine if the anti-inflammatory activity is stimulus-dependent, we found that three of the fractions (NP3, NP4, NP5) also inhibited IFN-γ induced NO production, while the weakest ARE activator, P4, did not have a substantial effect (Figure 5F).
Figure 5.
Effects of prioritized fractions on pro-inflammatory gene expression and mediators in macrophage RAW 264.7 cells and mouse embryonic fibroblasts (MEFs). (A–E) RAW 264.7 cells were pretreated for 1 h with various doses of the prioritized fractions and then stimulated with LPS (1 μg/mL). RNA samples were collected upon 12 h treatment, reverse transcribed and cDNAs analyzed by RT-qPCR (n = 3, ± SD) for iNOS (A), Cox2 (B) or Nqo1 (E) gene expression. Gapdh expression was used as internal control for normalization. Supernatants to measure NO (C) and PGE2 (D) levels were collected 24 h after treatment and assayed using Griess reagent and ELISA, respectively (n = 3, ± SD). (F) RAW 264.7 cells were pretreated for 1 h with the fractions and then stimulated with IFN-γ (10 ng/mL). NO levels were measured 24 h later using Griess reagent (n = 3, ± SD). (G) Wild-type, Nrf2−/− and Keap1−/− MEFs were incubated with different doses of Ulva fraction NP3 for 40 h and Nqo1 activity measured (n = 5, ± SD) (H) Wild-type and Nrf2−/− MEFs were pretreated for 1 h with various doses of NP3, stimulated with IFN-γ (10 ng/mL) and TNF-α (10 ng/mL) and NO levels measured 20 h later using Griess reagent (n = 5, ± SD). Asterisk (*) denotes toxicity.
These data suggest that the anti-inflammatory effect of Ulva fractions is largely stimulus-independent, yet there are some notable differences that may be attributed to the different chemical composition of the fractions. We then used the most potent and chemically most characterized fraction (NP3) to test for Nqo1 activity and NO synthesis inhibition in isogenic mouse embryonic fibroblast (MEF) cells as previously described (43). Fraction NP3 induced Nqo1 activity only in wild-type MEFs but not in Nrf2 knockout (Nrf2−/−) and Keap1-knockout (Keap1−/−) cells (Figure 5G). When MEFs were stimulated with IFN-γ and TNF-α, fraction NP3 reduced NO levels only in wild-type MEFs but not Nrf2−/− MEFs up to 10 μg/mL. We cannot exclude the possibility of non-Nrf2 mediated anti-inflammatory effects at higher concentration as previously observed for other ARE activators (44), since the apparent reduction of NO in Nrf2−/− MEFs at 32 μg/mL overlapped with the toxicity. In summary, the anti-inflammatory effects of the investigated Ulva fraction appear to be mediated by an Nrf2/ARE-dependent mechanism.
Discussion
ARE activation is linked to cancer prevention and is a novel therapeutic approach for numerous oxidative stress mediated diseases (10–13). In particular, natural products present in terrestrial cruciferous vegetables have been extensively investigated, but were usually found to have low stability and bioavailability. In alternative drug discovery efforts, high-throughput screening campaigns have yielded novel synthetic ARE activators (35). Reata Pharmaceutical’s bardoxolone methyl (RTA 402) is an ARE activator with anti-inflammatory properties which has reached phase 3 clinical trials for patients with chronic kidney disease and type 2 diabetes following promising phase 2 study results, and is most advanced towards translation to the bedside (45). Our interest in exploring marine natural products for drug discovery and the amount of descriptive literature about potential benefits of algae consumption, combined with epidemiological evidence about low cancer rates in the major geographical areas of seaweed consumption, led us to investigate various marine algae for their ability to activate the ARE. Species of Ulva can be easily cultivated and are already consumed by humans. Further investigation is warranted to focus on characterization and development of cultivated Ulva as a protective natural remedy against cancer, inflammation and other oxidative stress mediated chronic diseases.
Induction of the Keap1/Nrf2-ARE pathway is due to a multitude of extract components, some of which we have structurally characterized here. Loliolide (1) and isololiolide (2) are well represented in several species of algae belonging to the genera Undaria, Padina, Cytosphora, Dictyota and Ulva (41,46). The origin of loliolide has been extensively discussed and concluded to be either a photo oxidation or degradation product of algal carotenoids such as fucoxanthin (47); in fact, in certain other preparations of Ulva, we encountered fucoxanthin which was recently shown to exert ARE activity (48). Loliolide is a phytotoxic compound displaying inhibition of algae growth, germination, ant-repellent and immunosuppressive properties (46). The immunosuppressive properties of this monoterpene were attributed to disruption of T-lymphocyte function (40). The norisoprenoid megastigmen (3) was previously reported from U. lactuca (42), although no biological importance had been linked to this compound. A common feature of ARE inducers extends to their ability to modify sulfhydryl groups, by oxidation, reduction or alkylation (49). The structural features of compounds 1–4 are consistent with Michael acceptors, which are prominent among the classes of inducers of cytoprotective enzymes (50). Compounds 1–4 are all electrophiles with α,β-unsaturated carbonyl moieties that can act as Michael acceptors which could alkylate Keap1, thereby modifying the Keap1-Nrf2 complex and leading to Nrf2 nuclear translocation and target gene induction. We previously identified 7(E)-9-keto-octadec-7-enoic acid, 7(E)-9-keto-hexadec-7-enoic acid and 7(E)-9-keto-octadec-7-enamide as ARE-active components in field-collected U. lactuca (9), all three of which contain the same reactive functionality to alkylate Cys residues in a similar fashion. These and/or similar unsaturated fatty acid components may also have contributed to the overall activity of the cultivated Ulva sp.
While we have been able to enrich the extract through bioassay-guided fractionation, further purification of individual components did not lead to activity improvement, suggesting that several agents may have either additive, synergistic and/or potentiating effects; this remains to be rigorously tested. The importance of matrix components was also demonstrated in our single-dose in vivo study, which indicated differential activation in different tissues. The diversity and dissemination of active and other (possibly by themselves) inactive compounds may affect the net ARE activation, presumably due to modulation of bioavailability, clearance rate and absorption profile. Expectedly, the mice did not show any adverse effects, and in ongoing and future work we will investigate the effects of chronic administration on various organs. Interestingly, field-collected U. lactuca extracts enriched in monounsaturated fatty acids showed preferential cardioprotective properties (9). In the future one may envision that specific organs or tissues could be targeted with tailored natural products preparations, depending on the disease condition to be prevented or treated. U. lactuca and other species of Ulva can be easily cultivated on a large scale and thus may be a renewable source for effective cancer chemoprevention and to prevent and/or relieve other oxidative stress mediated disease conditions. Furthermore, secondary metabolite production of this green alga may be manipulated through environmental conditions to ultimately increase the chemopreventive potential of the resulting extracts. The different therapeutic properties reported for different Ulva species may be explained by the differences in chemical composition (5–7), but many activities may also be at least partially attributed to ARE activation.
Supplementary Material
Acknowledgments
Financial support: National Institutes of Health, NCI Grant R21CA133681
We thank Dr. Sarath Gunasekera and Ellen Sewejkis for assistance with extraction and large-scale chromatography, Dr. Dennis Hanisak for providing the cultured Ulva, Professor Jeffrey Johnson for providing ARE reporter mice, Professor David Borchelt and Susan Fromholt for genotyping, and Dr. Albena Dinkova-Kostova for providing isogenic MEF cells. This is contribution #921 of the Smithsonian Marine Station.
Grant Support. National Institutes of Health, NCI Grant R21CA133681
Footnotes
Disclosure: HL, VJP and RR are co-inventors on a patent application related to the content of the manuscript.
References
- 1.Chapman VJ, Chapman DJ. Seaweeds and Their Uses. Chapman and Hall; New York: 1980. pp. 62–67. [Google Scholar]
- 2.Hoshiyama Y, Sekine T, Sasaba T. A case-control study of colorectal-cancer and its relation to diet, cigarettes, and alcohol-consumption in Saitama Prefecture, Japan. Tohoku J Exp Med. 1993;171:153–165. doi: 10.1620/tjem.171.153. [DOI] [PubMed] [Google Scholar]
- 3.Hoshiyama Y, Sasaba T. A case-control study of single and multiple stomach cancers in Saitama Prefecture, Japan. Jpn J Cancer Res. 1992;83:937–943. doi: 10.1111/j.1349-7006.1992.tb02004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sho H. History and characteristics of Okinawan longevity food. Asia Pac J Clin Nutr. 2001;10:159–164. doi: 10.1111/j.1440-6047.2001.00235.x. [DOI] [PubMed] [Google Scholar]
- 5.Balaji Raghavendra Rao H, Sathivel A, Devaki T. Antihepatotoxic nature of Ulva reticulata (Chlorophycaeae) on acetaminophen-induced hepatotoxicity in experimental rats. J Med Food. 2004;7:495–497. doi: 10.1089/jmf.2004.7.495. [DOI] [PubMed] [Google Scholar]
- 6.Jin D-Q, Lim CS, Sung J-Y, Choi HG, Ha I, Han J-S. Ulva conglobata, a marine algae, has neuroprotective and anti-inflammatory effects in murine hippocampal and microglial cells. Neurosci Lett. 2006;402:154–158. doi: 10.1016/j.neulet.2006.03.068. [DOI] [PubMed] [Google Scholar]
- 7.Lee DG, Hyun JW, Kang KA, Lee JO, Lee SH, Ha BJ, et al. Ulva lactuca: a potential seaweed for tumor treatment and immune stimulation. Biotechnol and Bioprocess E. 2004;9:236–238. [Google Scholar]
- 8.Qi H, Zhang Q, Zhao T, Chen R, Zhang H, Niu X, et al. Antioxidant activity of different sulfate content derivatives of polysaccharide extracted from Ulva pertusa (Chlorophyta) in vitro. Int J Biol Macromol. 2005;37:195–199. doi: 10.1016/j.ijbiomac.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 9.Wang R, Paul VJ, Luesch H. Seaweed extracts and unsaturated fatty acid constituents from the green alga Ulva lactuca as activators of the cytoprotective Nrf2-ARE pathway. Free Rad Biol Med. 2013;57:141–153. doi: 10.1016/j.freeradbiomed.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 11.Chen X-L, Kunsch C. Induction of cytoprotective genes through Nrf2/antioxidant response element pathway: a new therapeutic approach for the treatment of inflammatory diseases. Curr Pharm Des. 2004;10:879–891. doi: 10.2174/1381612043452901. [DOI] [PubMed] [Google Scholar]
- 12.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 13.van Muiswinkel FL, Kuiperij HB. The Nrf2-ARE signaling pathway: promising drug target to combat oxidative stress in neurodegenerative disorders. Curr Drug Targets CNS Neurol Disord. 2005;4:267–281. doi: 10.2174/1568007054038238. [DOI] [PubMed] [Google Scholar]
- 14.Dinkova-Kostova AT, Holtzclaw WD, Kensler TW. The role of Keap1 in cellular protective responses. Chem Res Toxicol. 2005;18:1779–1791. doi: 10.1021/tx050217c. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi M, Yamamoto M. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv Enzyme Regul. 2006;46:113–140. doi: 10.1016/j.advenzreg.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Zhang DD. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev. 2006;38:769–789. doi: 10.1080/03602530600971974. [DOI] [PubMed] [Google Scholar]
- 17.Chan K, Han X-D, Kan YW. An important function of Nrf2 in combating oxidative stress: detoxification of acetaminophen. Proc Natl Acad Sci USA. 2001;98:4611–4616. doi: 10.1073/pnas.081082098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramos-Gomez M, Kwak M-K, Dolan PM, Itoh K, Yamamoto M, Talalay P, et al. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc Natl Acad Sci USA. 2001;98:3410–3415. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calkins MJ, Jakel RJ, Johnson DA, Chan K, Kan YW, Johnson JA. Protection from mitochondrial complex II inhibition in vitro and in vivo by Nrf2-mediated transcription. Proc Natl Acad Sci USA. 2005;102:244–249. doi: 10.1073/pnas.0408487101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nebert DW, Roe AL, Vandale SE, Bingham E, Oakley GG. NAD(P)H:quinone oxidoreductase (NQO1) polymorphism, exposure to benzene, and predisposition to disease: a HuGE review. Genet Med. 2002;4:62–70. doi: 10.1097/00125817-200203000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Sikka SC. Role of oxidative stress response elements and antioxidants in prostate cancer pathobiology and chemoprevention -- a mechanistic approach. Curr Med Chem. 2003;10:2679–2692. doi: 10.2174/0929867033456341. [DOI] [PubMed] [Google Scholar]
- 22.Lee WH, Morton RA, Epstein JI, Brooks JD, Campbell PA, Bova GS, et al. Cytidine methylation of regulatory sequences near the π-class glutathione S-transferase gene accompanies human prostatic carcinogenesis. Proc Natl Acad Sci USA. 1994;91:11733–11737. doi: 10.1073/pnas.91.24.11733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee W-H, Isaacs WB, Bova GS, Nelson WG. CG island methylation changes near the GSTP1 gene in prostatic carcinoma cells detected using the polymerase chain reaction: a new prostatic biomarker. Cancer Epidemiol Biomark Prev. 1997;6:443–450. [PubMed] [Google Scholar]
- 24.Lin X, Tascilar M, Lee WH, Vles WJ, Lee BH, Veeraswamy R, et al. GSTP1 cpG island hypermethylation is responsible for the absence of GSTP1 expression in human prostate cancer cells. Am J Pathol. 2001;159:1815–1826. doi: 10.1016/S0002-9440(10)63028-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brooks JD, Paton VG, Vidanes G. Potent induction of phase 2 enzymes in human prostate cells by sulforaphane. Cancer Epidemiol Biomark Prev. 2001;10:949–954. [PubMed] [Google Scholar]
- 26.Greenlee RT, Hill-Harmon MB, Murray T, Thun M. Cancer statistics, 2001. CA Cancer J Clin. 2001;51:15–36. doi: 10.3322/canjclin.51.1.15. [DOI] [PubMed] [Google Scholar]
- 27.Carter BS, Carter HB, Isaacs JT. Epidemiologic evidence regarding predisposing factors to prostate cancer. Prostate. 1990;16:187–197. doi: 10.1002/pros.2990160302. [DOI] [PubMed] [Google Scholar]
- 28.Yu H, Harris RE, Gao YT, Gao R, Wynder EL. Comparative epidemiology of cancers of the colon, rectum, prostate, and breast in Shanghai, China versus the United States. Int J Epidemiol. 1991;20:76–81. doi: 10.1093/ije/20.1.76. [DOI] [PubMed] [Google Scholar]
- 29.Shimizu H, Ross RK, Bernstein L, Yatani R, Henderson BE, Mack TM. Cancers of the prostate and breast among Japanese and white immigrants in Los Angeles County. Br J Cancer. 1991;63:963–966. doi: 10.1038/bjc.1991.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whittemore AS, Kolonel LN, Wu AH, John EM, Gallagher RP, Howe GR, et al. Prostate cancer in relation to diet, physical activity, and body size in blacks, whites, and Asians in the United States and Canada. J Natl Cancer Inst. 1995;87:652–661. doi: 10.1093/jnci/87.9.652. [DOI] [PubMed] [Google Scholar]
- 31.Haenzel W, Kurihara M. Mortality from cancer and other diseases among Japanese men in the United States. J Natl Cancer Inst. 1968;40:43–68. [PubMed] [Google Scholar]
- 32.Danley KL, Richardson JL, Bernstein L, Langholz B, Ross RK. Prostate cancer: trends in mortality and stage-specific incidence rates by racial/ethnic group in Los Angeles County, California (United States) Cancer Cause Control. 1995;6:492–498. doi: 10.1007/BF00054156. [DOI] [PubMed] [Google Scholar]
- 33.Johnson DA, Andrews GK, Xu W, Johnson JA. Activation of the antioxidant response element in primary cortical neuronal cultures derived from transgenic reporter mice. J Neurochem. 2002;81:1233–1241. doi: 10.1046/j.1471-4159.2002.00913.x. [DOI] [PubMed] [Google Scholar]
- 34.Prochaska HJ, Santamaria AB. Direct measurement of NAD(P)H:quinone reductase from cells cultured in microtiter wells: a screening assay for anticarcinogenic enzyme inducers. Anal Biochem. 1988;169:328–336. doi: 10.1016/0003-2697(88)90292-8. [DOI] [PubMed] [Google Scholar]
- 35.Hur W, Sun Z, Jiang T, Mason DE, Peters EC, Zhang DD, et al. A small-molecule inducer of the antioxidant response element. Chem Biol. 2010;17:537–547. doi: 10.1016/j.chembiol.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y, Kern JT, Walker JR, Johnson JA, Schultz PG, Luesch H. A genomic screen for activators of the antioxidant response element. Proc Natl Acad Sci USA. 2007;104:5205–5210. doi: 10.1073/pnas.0700898104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rojo AI, Medina-Campos ON, Rada P, Zuniga-Toala A, Lopez-Gazcon A, Espada S, Pedraza-Chaverri J, et al. Signaling pathways activated by the phytochemical nordihydroguaiaretic acid contribute to a Keap1-independent regulation of Nrf2 stability: Role of glycogen synthase kinase-3. Free Radic Biol Med. 2012;52:473–487. doi: 10.1016/j.freeradbiomed.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 38.Yates MS, Tauchi M, Katsuoka F, Flanders KC, Liby KT, Honda T, et al. Pharmacodynamic characterization of chemopreventive triterpenoids as exceptionally potent inducers of Nrf2-regulated genes. Mol Cancer Ther. 2007;6:154–162. doi: 10.1158/1535-7163.MCT-06-0516. [DOI] [PubMed] [Google Scholar]
- 39.Chen S-Y, Huang K-J, Wang S-K, Wen Z-H, Chen P-W, Duh C-Y, et al. Antiviral and anti-inflammatory metabolites from the soft coral Sinularia capillosa. J Nat Prod. 2010;73:771–775. doi: 10.1021/np9008078. [DOI] [PubMed] [Google Scholar]
- 40.Okada N, Shirata K, Niwano M, Koshino H, Uramoto M. Immunosuppressive activity of a monoterpene from Eucommia ulmoides. Phytochem. 1994;37:281–282. [Google Scholar]
- 41.Kimura J, Maki N. New loliolide derivatives from the brown alga Undaria pinnatifida. J Nat Prod. 2002;65:57–58. doi: 10.1021/np0103057. [DOI] [PubMed] [Google Scholar]
- 42.Sun Y, Zhan Y-C, Sha Y, Pei Y-H. Norisoprenoids from Ulva lactuca. J Asian Nat Prod Res. 2007;9:321–325. doi: 10.1080/10286020600727491. [DOI] [PubMed] [Google Scholar]
- 43.Dinkova-Kostova AT, Liby KT, Stephenson KK, Holtzclaw WD, Gao X, Suh N, et al. Extremely potent triterpenoid inducers of the phase 2 response: Correlations of protection against oxidant and inflammatory stress. Proc Natl Acad Sci USA. 2005;102:4584–4589. doi: 10.1073/pnas.0500815102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu H, Dinkova-Kostova AT, Talalay P. Coordinate regulation of enzyme markers for inflammation and for protection against oxidants and electrophiles. Proc Natl Acad Sci USA. 2008;105:15926–15931. doi: 10.1073/pnas.0808346105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pergola PE, Raskin P, Toto RD, Meyer CJ, Huff JW, Grossman EB, et al. BEAM Study Investigators. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med. 2011;365:327–336. doi: 10.1056/NEJMoa1105351. [DOI] [PubMed] [Google Scholar]
- 46.Percot A, Yalcin A, Aysel V, Erdugan H, Dural B, Guven KC. Loliolide in marine algae. J Nat Prod. 2009;23:460–465. doi: 10.1080/14786410802076069. [DOI] [PubMed] [Google Scholar]
- 47.Repeta DJ. Carotenoid digenesis in recent marine sediments: II. Degradation of fucoxanthin to loliolide. Geochim Cosmochim Ac. 1989;53:699–707. [Google Scholar]
- 48.Liu CL, Chiu YT, Hu ML. Fucoxanthin enhances HO-1 and NQO1 expression in murine hepatic BNL CL. 2 cells through activation of the Nrf2/ARE system partially by its pro-oxidant activity. J Agric Food Chem. 2011;59:11344–11351. doi: 10.1021/jf2029785. [DOI] [PubMed] [Google Scholar]
- 49.Dinkova-Kostova AT, Massiah MA, Bozak RE, Hicks RJ, Talalay P. Potency of Michael reaction acceptors as inducers of enzymes that protect against carcinogenesis depends on their reactivity with sulfhydryl groups. Proc Natl Acad Sci USA. 2001;98:3404–3409. doi: 10.1073/pnas.051632198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dinkova-Kostova AT, Talalay P. Direct and indirect antioxidant properties of inducers of cytoprotective proteins. Mol Nutr Food Res. 2008;52:S128–S138. doi: 10.1002/mnfr.200700195. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.