Abstract
Urothelial carcinoma (UC) accounts for 5% to 10% of malignancies in men in Europe and the United States. For locally advanced or metastatic disease, there are two standard firstline chemotherapy regimens: MVAC (methotrexate, vinblastine, doxorubicin, cisplatin) and gemcitabine/cisplatin. For refractory disease, there is currently no standard treatment. Vinflunine, a second-generation Vinca alkaloid, is the first chemotherapeutic agent to be evaluated in a large UC second-line population. This review discusses the pre-clinical and clinical data published, and compares vinflunine to alternative single agents and combination regimens tested in this setting. Based on the results of the phase II and III clinical trials, there appears to be sufficient evidence to support the use of vinflunine in the second-line setting.
Keywords: vinflunine, Vinca alkaloid, urothelial carcinoma, transitional cell carcinoma, bladder cancer
Introduction
Bladder cancer is the ninth most common cancer worldwide, and accounted for an estimated 14,330 deaths in 2009 in the United States.1 It affects men in the majority (77%) of cases2 with 63% of cases occurring in the developed world. In Europe and the United States bladder cancer accounts for 5% to 10% of malignancies in men.3 Cigarette smoking is the primary risk factor,4–10 but other risk factors include exposure to arsenic,11,12 aromatic amines,13 and aniline dyes.14 Additionally, chronic Schistosoma haematobium infection has been linked to the squamous cell type of bladder carcinoma, explaining the higher rates observed in parts of Africa and the Middle East.15 Urothelial or transitional cell carcinoma (UC) accounts for 95% of bladder tumors, with the remaining cases consisting of squamous cell carcinoma and adenocarcinoma.16
While the majority of UC cases present as superficial disease,17 15% to 25% are at high risk for progression to muscle invasion.18 For locally advanced or metastatic disease, there are two standard first-line chemotherapy regimens: MVAC (methotrexate, vinblastine, doxorubicin, cisplatin) and gemcitabine/cisplatin. Unfortunately, the median overall survival (OS) following MVAC therapy is only 14.8 months and gemcitabine + cisplatin is associated with 7.4 months median time to progressive disease (TTP) and 13.8 months OS.19 Although many patients are not eligible for further treatment due to poor performance status (PS), there is nonetheless an unmet clinical need for effective second-line therapy for patients having progressed on these platinum-based regimens.
Various single agents and combination regimens have been evaluated in clinical trials. Single agents having clinical activity include docetaxel,20 paclitaxel,21–23 pemetrexed,24,25 bortezomib,26,27 and vinblastine.28 Combination regimens have often included taxanes combined with a second drug such as carboplatin29–31 or gemcitabine.32–36 Unfortunately, many of these studies have limited efficacy.
Vinflunine (VFL), a novel microtubule inhibitor, is undergoing clinical evaluation in this setting. Studies reported in the literature show encouraging results with VFL as a single agent in the treatment of refractory UC. This review discusses VFL’s safety and efficacy profile in this setting, and compares it to competing single-agent and combination regimens.
Pharmacology and mechanism of action
Chemistry of vinflunine
The origins of VFL (Javlor®; Pierre Fabre Medicament Laboratories), a third-generation Vinca alkaloid, date back to 1958 when vinblastine was first isolated from the Madagascan periwinkle Catharanthus roseus (also known as Vinca rosea).37 The discovery of vincristine and vinorelbine soon followed. VFL was discovered in 1988 when novel superacidic chemistry was applied to vinorelbine, allowing selective addition of two fluorine atoms at the 20’ position of the catharanthine moiety to create 20’,20’-difluoro-3’,4’-dihydrovinorelbine, or VFL (see Figure 1). Previously, the 20’ site had been inaccessible using classical synthetic chemistry.38
Figure 1.
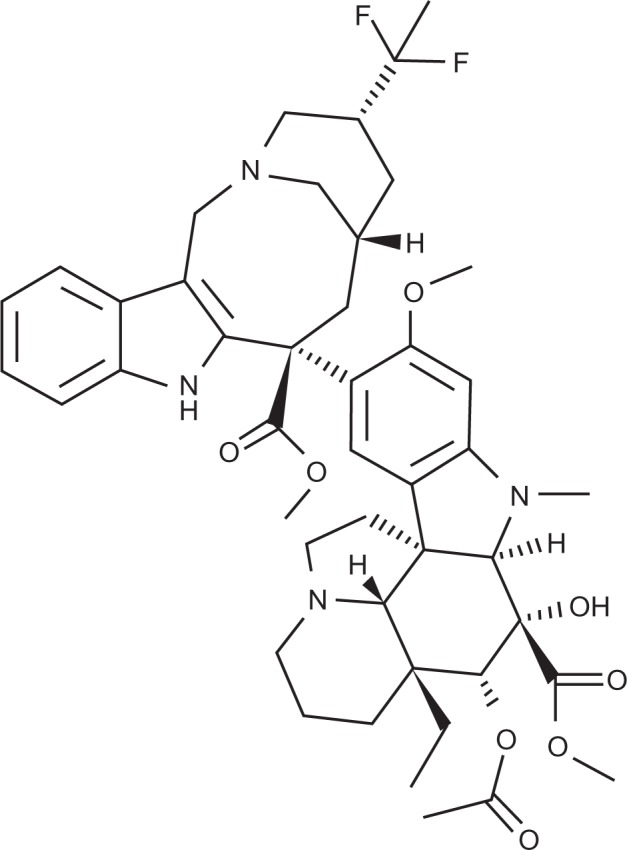
Structure of vinflunine.
In contrast to other microtubule inhibitors, VFL is freely water-soluble. Thus, there is no risk of solvent-related hyper-sensitivity reactions. Phase I trials showed a mean terminal half-life of VFL to be 40 hours and a terminal half-life of 4 to 6 days for 4-O-deacetylvinflunine, VFL’s only active metabolite. The volume of distribution is approximately 1,517 L suggesting significant tissue distribution. Excretion is both fecal (two thirds) and urinary (one third), an important consideration given that many patients with advanced bladder cancer suffer renal impairment.39–41
Mechanism of action
Microtubules make up the mitotic spindle required for chromosome separation and cell division, and are the target of anti-cancer agents such as Vinca alkaloids and taxanes. While taxanes stabilize microtubules resulting in “frozen mitosis”, Vinca alkaloids exert their antiproliferative effect largely by preventing tubulin polymerization. This destabilizes microtubules and prevents mitotic progression.42 At high concentrations, they depolymerize microtubules and destroy mitotic spindles; at lower concentrations, they suppress the rate and extent of microtubule treadmilling, resulting in mitotic block and apoptotic cell death.43,44
Advantages relative to other Vinca alkaloids
VFL interacts with microtubules in a different way to other Vinca alkaloids. It binds to tubulin less readily, requiring 3- to 17-fold higher concentrations for similar biological effects relative to other members of its class.45 Consequently fewer and smaller spiral polymers are induced and its interaction with tubulin is more readily reversible.46 These different binding properties represent an advantage for VFL because they confer greater antitumor efficacy45 and reduced neurotoxicity,43 an important clinical consideration. The precise reason for VFL’s superior antitumor efficacy is unknown, but may involve high intracellular accumulation and interaction with undefined targets.47
A general problem with the Vinca alkaloid class of drugs is the development of multidrug resistance (MDR) due to over-expression of P-glycoprotein (Pgp), a membrane efflux pump. Although VFL does induce Pgp-mediated resistance, cross-resistance to VFL occurred at a lower rate than to other Vinca alkaloids in human cancer cell lines including leukemia, breast carcinoma and bladder carcinoma.48 Additionally, mice implanted with P388 murine leukemia cells developed complete resistance to vinorelbine at 11 weeks, compared to 36 weeks for VFL.49
Vascular-disrupting and anti-angiogenic action
In addition to its anti-proliferative effects, studies have demonstrated that VFL has vascular-disrupting and anti-angiogenic properties in vitro.50 In 2006 Kruczynski et al showed that in vitro VFL disrupts the network of capillary-like structures and inhibits endothelial cell migration and capacity to organize. These experiments were done at concentrations that only mildly affected endothelial cell proliferation.50 In addition, in vivo studies in mice showed reduced numbers of liver metastases after intrasplenic injection of LS174T (tumor) cells. Moreover, at doses 20- to 40-fold lower than its maximal therapeutic dose (MTD), VFL inhibited bFGF-induced angiogenesis in Matrigel™ implants.50
Pre-clinical properties
Efficacy
VFL has shown higher in vivo anticancer activity than other microtubule inhibitors. In mice implanted with P388 murine leukemia cells, VFL has shown the maximum antitumor activity and survival prolongation compared to the other Vinca alkaloids.51 Hill et al52 treated nude mice implanted with a series of human tumor xenografts, and reported a 64% overall response rate with VFL compared to 27% with vinorelbine. In another important study Bonfil et al reported complete tumor eradication and 100% survival at 60 days when VFL (5–20 mg/kg) was administered intraperitoneally into mice that had had murine bladder cancer cells (MB49) implanted transurethrally.53 The success of these studies led to the beginning of clinical trials by Bennouna et al39 and others.
Toxicity profile
Common toxicities of Vinca alkaloids include myelosuppression and neuropathy due to axonal degeneration resulting in peripheral neuropathy typically beginning as paresthesia of the fingers and toes that spreads proximally in a ‘glove and stocking’ distribution.54 Compared to other Vinca alkaloids, VFL has a better safety profile because it binds tubulin weakly and reversibly. These characteristics may explain the smaller degree of neuropathy and noncumulative nature of VFL-related toxicities, respectively. In most cases, neuropathy resolves after drug withdrawal.47
Synergistic effects
In a pre-clinical study evaluating in vitro activity against a human non-small-cell lung cancer (NSCLC) cell line, VFL demonstrated synergistic cytotoxicity with cisplatin, mitomycin C, doxorubicin and 5-fluorouracil (5-FU). Similar results were obtained against human leukemia cells. Importantly, no antagonistic drug interactions occurred,55 suggesting the potential for combination therapies. When these synergies were examined in vivo using P388 murine leukemia cells grafted intravenously, the authors found superior activity for combinations of single intraperitoneal doses of VFL with either doxorubicin, mitomycin C, cisplatin or F 11782.56 VFL’s synergistic effects with cisplatin and 5-FU were further evaluated in a transplantable murine colon adenocarcinoma model, MAC 29. While synergy was demonstrated with cisplatin, this was not shown with 5-FU.57
VFL may also exhibit synergy with radiotherapy. Microtubule inhibitors such as VFL synchronize cells in the mitotic phase, rendering them more sensitive to radiation.58 Indeed, Simoens et al59 found a concentration-dependent G2/M block and consequent radiosensitization of different tumor cell lines.
Clinical trials
Because of its broader spectrum of activity and advantages over other Vinca alkaloids, VFL has been evaluated as monotherapy and as combination chemotherapy in a number of different cancers, including breast cancer,60–66 NSCLC,67–71 small cell lung cancer (SCLC),72,73 prostate cancer,74 gastric cancer,75 malignant pleural mesothelioma76–78 and renal cell carcinoma.79 The recommended dose for clinical studies is 320 mg/m2 administered intravenously every 21 days. For patients with a lower performance status or those having received prior pelvic irradiation, the dose is reduced to 280 mg/m2.69,80 Prior pelvic irradiation is reported to exacerbate hematological toxicities.81 In the literature, there are currently two phase II and one phase III study reported of VFL as second-line therapy in metastatic UC.
Phase I trials
On the basis of strong pre-clinical data, VFL was evaluated in several phase I trials using a variety of schedules in patients with solid tumors. Bennouna et al39 conducted a study in 31 patients with advanced malignancy. Patients were treated with escalating doses from 30 to 400 mg/m2 VFL 3-weekly, and the authors reported a maximum tolerated dose (MTD) of 400 mg/m2. Adverse events reported were mucositis, constipation and neutropenia of short duration. Three partial responses (PR) were observed. The authors recommended a dose of 350 mg/m2 3-weekly.
In 2006, Johnson et al41 reported a similar study in 16 patients with advanced solid tumors. VFL was given 3-weekly on days 1 and 8 as a 10-minute infusion. A maximum tolerated dose (MTD) was established at 190 mg/m2 based on 2 of 4 patients developing constipation and neutropenia which were dose limiting. There were no objective responses. The authors recommended a dose of 170 mg/m2 on days 1 and 8 3-weekly for future studies.
Phase II trials in urothelial carcinoma
Based on the safety data reported in phase I trials and the complete tumor eradication reported in a murine bladder carcinoma model,53 phase II trials of vinflunine in UC were begun. Culine et al82 conducted an open-label, multicenter, noncomparative phase II study in 51 patients from 16 European centers with advanced UC who failed first-line platinum-based regimens. Patients were treated with 320 mg/m2 VFL. The primary endpoint was efficacy as measured by tumor response rate; secondary objectives were duration of response, progression-free survival (PFS), OS, and treatment-related toxicity. Eligible patients were aged ≥18 years with a Karnofsky performance status (KPS) ≥80. Tumor response was assessed after the initial 2 cycles. Nine patients (18%; 95% CI: 8.4%–30.9%) achieved PR and 25 achieved stable disease (SD) for an overall disease control rate of (PR + SD) of 67% (95% CI: 52.1%–79.3%). Additionally, objective response was seen in 8 of 34 patients (24%) previously treated for metastatic disease and in 1 of 17 (6%) patients previously treated in the neoadjuvant or adjuvant setting. The median duration of response was 9.1 months (95% CI: 4.2–15.0) and median PFS was 3.0 months (95% CI: 2.4–3.8). Median OS was 6.6 months (95% CI: 4.8–7.6). Significant toxicities included grade 3–4 leukopenia in 45% patients and grade 3–4 neutropenia in 67% patients. Five patients (10%) experienced febrile neutropenia, 2 of whom died of drug related toxicity. Importantly, grade 3–4 sensory neuropathy was not observed, nor was grade 3–4 renal function impairment.
In 2009, Vaughn et al83 reported results of a similar study done in the United States evaluating the safety and efficacy of VFL in patients having progressed within 12 months after platinum-based chemotherapy. Eligibility criteria were similar to the Culine et al trial,82 and patients were treated with 320 mg/m2 VFL unless they had impaired renal function, KPS ≥ 80, prior pelvic irradiation, or were aged ≥75 years. These patients received an initial dose of 280 mg/m2, which was escalated to 320 mg/m2 in cycle 2 if well tolerated. Of 175 patients enrolled, 151 received at least one dose of VFL. The primary objective was response rate as defined by an independent response review committee (IRRC); secondary endpoints included duration of response, time to response, disease control rate, PFS, OS, and toxicity. Twenty-two of 151 subjects achieved PR (15%; 95% CI: 9%–21%) and 64 achieved SD (42%), for an overall response rate (ORR) of 14.6% (95% CI: 9.4%–21.2%). The median duration of response was 6.0 months (95% CI: 5.4–9.5 months), and the median duration of disease stabilization was 4.0 months (95% CI: 3.5–4.7 months). Median PFS was 2.8 months, and median OS was 8.2 months. The main toxicity of VFL was hematologic: grade 3 or 4 neutropenia was reported in 58% patients, leucopoenia in 49%, and anemia in 16%. Grade 3–4 febrile neutropenia was seen in 10 patients (7%). Grade 3–4 non-hematologic toxicities included constipation (17%), fatigue (13%), ileus (5%), and abdominal pain (5%). Patients with renal impairment had a similar safety profile, and VFL did not damage renal function.
Phase III trials in urothelial carcinoma
On the basis of the two phase II trials, Bellmunt et al81 conducted a randomized, multinational, phase III trial of VFL and best supportive care (BSC) as second-line treatment in patients progressing after platinum-containing chemotherapy. Three hundred and seventy patients were recruited: 253 were randomized to the VFL + BSC arm, and 117 to the BSC only arm. Eligibility criteria were similar to the phase II trials. Patients with Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0 were given 320 mg/m2 VFL, while patients with ECOG PS 0 and prior pelvic irradiation or ECOG PS 1 were given 280 mg/m2 VFL. The primary objective was obtaining a median 2-month survival advantage favoring the VFL + BSC group. This was achieved (6.9 versus 4.6 months) in both the intent-to-treat and eligible population, but the difference in OS was only significant (P = 0.040) in the eligible population. The intent-to-treat population was the randomly assigned population, and the eligible population excluded 13 patients with at least one major protocol violation at baseline. Secondary endpoints were ORR, disease control rate and duration, and median PFS. Sixteen (8.6%) patients achieved PR with a 7.4 months (95% CI: 4.5–17.0) median duration of response, and 86 (46.5%) achieved SD. Significant (grade 3–4) toxicities were primarily hematologic, including neutropenia (50%), febrile neutropenia (6%) and anemia (19%).
The VINCENT (vinflunine in cisplatin-ineligible patients) phase III clinical trial is currently ongoing. It compares gemcitabine plus VFL versus gemcitabine plus placebo in chemotherapy-naïve patients. The target accrual is 450 patients.84
Safety and tolerability
In all phase II and III trials evaluating VFL in UC the drug was reported to have an acceptable safety profile. The main grade 3–4 toxicities were hematologic (see Table 1). Common non-hematologic adverse effects were fatigue and constipation. Importantly, the dose-limiting neurotoxicity seen in other Vinca alkaloids was not observed in VFL, and toxicities were non-cumulative. In general, the safety profile of VFL compared relatively favorably to that of other single agents (see Table 2).
Table 1.
Grade 3–4 adverse events in patients with advanced or metastatic urothelial carcinoma treated with vinflunine
| Trial description | Vinflunine, phase II | Vinflunine, phase II | VFL + BSC vs BSC, phase III |
|---|---|---|---|
| Investigators | Culine82 | Vaughn83 | Bellmunt81 |
| Dose and schedule | 320 mg/m2 IV every 21 days | Dose escalation of 280 to 320 mg/m2 IV every 21 days | Two arms with 280 or 320 mg/m2 IV every 21 days |
| Hematologic | No patients (%) | No patients (%) | No patients (%) |
| Anemia | 7 (14) | 23 (15.5) | 47 (19.1) |
| Leukopenia | 23 (45) | 73 (49.3) | NR |
| Neutropenia | 34 (67) | 86 (58.1) | 123 (50.0) |
| Thrombocytopenia | 3 (6) | 5 (3.4) | 14 (5.7) |
| Febrile neutropenia | 5 (10) | 10 (6.6) | 15 (6.0) |
| Neutropenia with infection | 3 (6) | 3 (2.0) | NR |
| Nonhematologic toxicities | |||
| Nausea | 2 (4) | 5 (3.3) | 6 (2.4) |
| Vomiting | 3 (6) | 3 (2.0) | 7 (2.8) |
| Constipation | 4 (8) | 25 (16.6) | 40 (16.1) |
| Stomatitis/mucositis | 3 (6) | 5 (3.3) | 4 (1.6) |
| Asthenia/fatigue | 5 (10) | 19 (12.6) | 48 (19.3) |
| Abdominal pain | 4 (8) | 7 (4.6) | 10 (4.0) |
| Peripheral sensory neuropathy | 0 | 1 (0.7) | 3 (1.2) |
| Injection site reactions | 0 | 1 (0.7) | 1 (0.4) |
| Myalgia | 2 (4) | 4 (2.6) | 8 (3.2) |
Abbreviation: NR, not reported.
Table 2.
Trials of single agents in second-line treatment of advanced or metastatic urothelial carcinoma
| Agent | No of evaluable patients | ORR: patients responding (%) | Median TTP (months) | Median OS (months) | Main grade 3–4 toxicities: no patients (%) | Reference |
|---|---|---|---|---|---|---|
| Vinflunine | 51 | 9 (18%) | 3.0 | 6.6 | 34 (67%) neutropenia; 23 (45%) leukopenia; 7 (14%) anemia |
Culine82 |
| Vinflunine | 151 | 22 (15%) | 2.8 | 8.2 | 86 (58.1%) neutropenia; 73 (49%) leukopenia; 23 (16%) anemia |
Vaughn83 |
| Vinflunine | 370 | 16 (8.6%) | 3.0 | 6.9 | 123 (50%) neutropenia; 47 (19%) anemia |
Bellmunt81 |
| Vinblastine | 28 | 5 (18%) | N/A | N/A | N/A | Blumenreich28 |
| Paclitaxel | 14 | 1 (7.1%) | N/A | N/A | Hematological toxicities in 23 of 42 courses; 2 (14%) with other grade 3–4 toxicities | Papamichael21 |
| Paclitaxel | 31 | 3 (10%) | 2.2 | 7.2 | 4 (13%) anemia; 2 (7%) asthenia | Vaughn22 |
| Paclitaxel | 45 | 4 (9%) | 3 | 7 | N/A | Joly23 |
| Docetaxel | 30 | 4 (13.3%) | N/A | 9 | 18 (60%) | McCaffrey20 |
| Ifosfamide | 20 | 1 (5%) | 6.0 | 8.0 | 2 (10%) | Pronzato87 |
| Ifosfamide | 56 | 11 (20%) | 2.2 | 5.1 | N/A | Witte88 |
| Topotecan | 44 | 4 (9.1%) | 1.4 | 6.3 | 34 (77%) leukopenia; 27 (61%) anemia; 19 (43%) thrombocytopenia |
Witte89 |
| Pyrazoloacridine | 14 | 0 | N/A | 9 months | 8 (57%) neutropenia; 2 (14%) thrombocytopenia |
Dodd90 |
| Piritrexim | 27 | 2 (7%) | 2.1 | 7.0 | 4 (15%) thrombocytopenia; 3 (11%) anemia; 5 (19%) neuropathy; 2 (7%) hepatotoxicity; 2 (7%) nausea |
Roth91 |
| Piritrexim | 22 | 0 | N/A | N/A | 6 (27%) | Lassiter92 |
| Oxaliplatin | 20 | 1 (5%) | N/A | N/A | 11 (55%) | Winquist93 |
| Pemetrexed | 47 | 13 (27.7%) | 2.9 | 9.6 | 4 (9%) thrombocytopenia; 4 (9%) neutropenia; 2 (4%) anemia; 3 (6%) fatigue; 2 (4%) diarrhea |
Sweeney24 |
| Pemetrexed | 12 | 1 (8%) | N/A | N/A | 3 (23%) anemia; 3 (23%) neutropenia; 3 (23%) thrombocytopenia | Galsky25 |
| Bortezomib | 21 | 0 | 1.9 | 3.5 | 15 (71%) | Gomez-Abuin26 |
| Bortezomib | 25 | 0 | 1.4 | 5.7 | 5 (21%) hematological toxicity | Rosenberg27 |
| Gemcitabine | 44 | 11 (25%) | 3.1 | 12.6 | 21 (48%) neutropenia; 4 (9%) anorexia | Akaza94 |
| Epothilone B analog BMS-247550 (ixabepilone) | 45 | 5 (11.9%) | 2.7 | 8.0 | 16 (36%) granulocytopenia; 5 (11%) thrombocytopenia; 3 (7%) sensory neuropathy; 5 (11%) fatigue; 4 (9%) dehydration |
Dreicer95 |
| Irinotecan | 40 | 2 (5%) | 2.1 | 5.4 | N/A | Beer96 |
| Lapatinib | 59 | 1 (1.7%) | 2.0 | 4.1 | 4 (7%) vomiting; 2 (3%) diarrhea; 2 (3%) dehydration; 2 (3%) hyponatremia |
Wulfing85 |
Abbreviations: N/A, not available or not reported; ORR, overall response rate; OS, overall survival; TTP, time to progression.
The potential role of vinflunine in the management of urothelial carcinoma
The wide variety of single agents (see Table 2) and combination of agents (see Table 3) studied in the second-line setting of metastatic UC begs the question: is there sufficient evidence to recommend a standard second-line treatment in metastatic cisplatin-resistant UC?
Table 3.
Trials of combination chemotherapy in second-line treatment of advanced or metastatic urothelial carcinoma
| Agents | No of evaluable patients | ORR: patients responding (%) | Median TTP (months) | Median OS (months) | Reference |
|---|---|---|---|---|---|
| Paclitaxel, cisplatin, methotrexate | 25 | 10 (40%) | NR | 3.7 | Tu97 |
| 5-Fluorouracil, cisplatin, interferon-alpha | 40 | 5 (12.5%) | 2.3 | 4.8 | De Mulder98 |
| Docetaxel, ifosfamide | 20 | 4 (25%) | NR | 4.0 | Krege99 |
| Gemcitabine, paclitaxel | 40 | 24 (60%) | 6.4 | 14.4 | Sternberg32 |
| Gemcitabine, paclitaxel | 27 | 12 (44%) | 11.0 | 13.0 | Fechner33 |
| Gemcitabine, paclitaxel | 10 | 7 (70%) | 4.1 | 10.3 | Matsumoto34 |
| Gemcitabine, paclitaxel | 20 | 6 (30%) | 4.5 | 11.5 | Kanai35 |
| Gemcitabine, paclitaxel | 30 | 10 (33.3%) | 5.5 | 11.3 | Suyama36 |
| Gemcitabine, ifosfamide | 34 | 7 (21%) | 4.0 | 9.0 | Pectasides100 |
| Cisplatin, gemcitabine, ifosfamide | 49 | 20 (40.8%) | NR | 9.5 | Pagliaro101 |
| Gemcitabine, docetaxel | 29 | 5 (17%) | NR | 7.7 | Dreicer102 |
| Oxaliplatin, fluorouracil, folinic acid (FOLFOX-4) | 16 | 3 (19%) | NR | 4.0 | Di Lorenzo103 |
| Paclitaxel, carboplatin | 44 | 7 (16%) | 4.0 | 6.0 | Vaishampayan29 |
| Paclitaxel, carboplatin | 31 | 10 (32.3%) | 3.7 | 7.9 | Kouno30 |
| Paclitaxel, carboplatin | 18 | 6 (33%) | 4.0 | 11.0 | Soga31 |
| Paclitaxel, ifosfamide, nedaplatin | 32 | 24 (75%) | 8.0 | 22.0 | Shinohara104 |
| Paclitaxel, cisplatin | 28 | 10 (36%) | 6.2 | 10.3 | Uhm105 |
| Gemcitabine, ifosfamide | 23 | 5 (22%) | NR | 4.8 | Lin106 |
| Methotrexate, vinblastine, doxorubicin, cisplatin | 30 | 9 (30%) | 5.3 | 10.9 | Han107 |
Abbreviations: NR, not reported; ORR, overall response rate; OS, overall survival; TTP, time to progression.
Single agents
Second-line single agents, mainly studied in phase II trials, have not demonstrated impressive response rates (0%–27.7%), median TTP, or OS. Molecularly targeted agents in particular have generally fared poorly, as exemplified by the trials of bortezomib26,27 and lapatinib.85 In addition, many studies enrolled relatively few patients, making it difficult to draw conclusions about efficacy. VFL has been the only drug evaluated in a randomized controlled phase III trial that has demonstrated moderate activity and a good safety profile.
Combination regimens
Combination regimens generally offer the compromise of higher response rates and longer OS in exchange for more severe toxicities. In assessing the potential role of VFL it is important to consider combinations for which there is most evidence. Gemcitabine/paclitaxel has been one of the more extensively studied regimens recently, particularly in Japan. Different doses and schedules have been tried, with response rates ranging from 30% to 70% and median OS of 10.3 to 14.4 months. Reported grade 3–4 toxicities have been mainly hematological, but also include peripheral neuropathy and allergic reactions to paclitaxel. Kanai et al35 observed grade 3–4 neutropenia in 6 patients (30%), anemia in 3 patients (15%), and thrombocytopenia in 1 patient (5%). No instances of grade 3–4 peripheral neuropathy were reported. Matsumoto et al34 also observed mainly hematologic toxicities as well as hypersensitivity reactions to paclitaxel. Of the 10 patients in the study, 5 developed grade 3 neutropenia and 1 experienced grade 4 thrombocytopenia. Seven patients experienced minor (lower than grade 3) hypersensitivity reactions to paclitaxel, suggesting that allergic reactions are a major limitation of this regimen. Sternberg et al32 reported grade 3–4 neutropenia in 13 patients (32%) and febrile neutropenia in 3 patients (7%). Granulocyte colony-stimulating factor was given to 10 patients (24%). Suyama et al36 reported 15 patients (45%) experiencing grade 3–4 neutropenia, all of whom responded well to granulocyte colony-stimulating factor. Peripheral neuropathy occurred in 19 patients: 2 cases (6%) were grade 3 and 17 (52%) were grade 2.
The paclitaxel/carboplatin combination has also yielded good response rates, but fared the worst in terms of OS in the largest study.29 Toxicities were also significant: 28 patients (64%) experienced grade 3–4 hematologic toxicities, and 11 patients (25%) experienced neurologic toxicities.
Given the relatively large number of patients enrolled in phase II and III clinical trials in UC and clinical efficacy and safety data available, there appears to be sufficient evidence to support the use of VFL in the second-line setting. Randomized trials comparing VFL with combinations most commonly used, such as Gemcitabine/paclitaxel and paclitaxel/carboplatin, would establish the clinical utility of VFL. However, such trials would necessarily be large in order to prove superiority of one regimen to another.
Conclusion
There is currently no standard second-line treatment for managing cisplatin-resistant metastatic UC. Most single agents have yielded unimpressive results, and combination regimens have shown improved response rates and OS, but also greater toxicity. Previous studies have shown the importance of prognostic factors such as chemosensitivity to first-line therapy86 or the presence of visceral metastasis,32 making comparisons among smaller trials difficult given their necessarily more heterogeneous patient populations.
VFL is the first chemotherapeutic agent to be evaluated in a large UC second-line population: the phase II and III trials included a total of 572 patients. In these studies, VFL has demonstrated relevant clinical activity and, perhaps more importantly, an acceptable and manageable toxicity profile in advanced and refractory disease. Approval of VFL in this setting would provide a safe and moderately effective standard of care against which other single agents or combination regimens could be compared.
Footnotes
Disclosure
The authors report no conflicts of interest.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Kirkali Z, Chan T, Manoharan M, et al. Bladder cancer: epidemiology, staging and grading, and diagnosis. Urology. 2005;66(6 Suppl 1):4–34. doi: 10.1016/j.urology.2005.07.062. [DOI] [PubMed] [Google Scholar]
- 4.Brennan P, Bogillot O, Cordier S, et al. Cigarette smoking and bladder cancer in men: a pooled analysis of 11 case-control studies. Int J Cancer. 2000;86(2):289–294. doi: 10.1002/(sici)1097-0215(20000415)86:2<289::aid-ijc21>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 5.Howe GR, Burch JD, Miller AB, et al. Tobacco use, occupation, coffee, various nutrients, and bladder cancer. J Natl Cancer Inst. 1980;64(4):701–713. [PubMed] [Google Scholar]
- 6.Hartge P, Silverman D, Hoover R, et al. Changing cigarette habits and bladder cancer risk: a case-control study. J Natl Cancer Inst. 1987;78(6):1119–1125. [PubMed] [Google Scholar]
- 7.Augustine A, Hebert JR, Kabat GC, Wynder EL. Bladder cancer in relation to cigarette smoking. Cancer Res. 1988;48(15):4405–4408. [PubMed] [Google Scholar]
- 8.Burch JD, Rohan TE, Howe GR, et al. Risk of bladder cancer by source and type of tobacco exposure: a case-control study. Int J Cancer. 1989;44(4):622–628. doi: 10.1002/ijc.2910440411. [DOI] [PubMed] [Google Scholar]
- 9.Clavel J, Cordier S, Boccon-Gibod L, Hemon D. Tobacco and bladder cancer in males: increased risk for inhalers and smokers of black tobacco. Int J Cancer. 1989;44(4):605–610. doi: 10.1002/ijc.2910440408. [DOI] [PubMed] [Google Scholar]
- 10.Engeland A, Andersen A, Haldorsen T, Tretli S. Smoking habits and risk of cancers other than lung cancer: 28 years’ follow-up of 26,000 Norwegian men and women. Cancer Causes Control. 1996;7(5):497–506. doi: 10.1007/BF00051881. [DOI] [PubMed] [Google Scholar]
- 11.Chiou HY, Chiou ST, Hsu YH, et al. Incidence of transitional cell carcinoma and arsenic in drinking water: a follow-up study of 8,102 residents in an arseniasis-endemic area in northeastern Taiwan. Am J Epidemiol. 2001;153(5):411–418. doi: 10.1093/aje/153.5.411. [DOI] [PubMed] [Google Scholar]
- 12.Rahman MM, Ng JC, Naidu R. Chronic exposure of arsenic via drinking water and its adverse health impacts on humans. Environ Geochem Health. 2009;31(Suppl 1):189–200. doi: 10.1007/s10653-008-9235-0. [DOI] [PubMed] [Google Scholar]
- 13.de Vocht F, Sobala W, Wilczynska U, Kromhout H, Szeszenia-Dabrowska N, Peplonska B. Cancer mortality and occupational exposure to aromatic amines and inhalable aerosols in rubber tire manufacturing in Poland. Cancer Epidemiol. 2009;33(2):94–102. doi: 10.1016/j.canep.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 14.Gan J, Skipper PL, Gago-Dominguez M, et al. Alkylaniline-hemoglobin adducts and risk of non-smoking-related bladder cancer. J Natl Cancer Inst. 2004;96(19):1425–1431. doi: 10.1093/jnci/djh274. [DOI] [PubMed] [Google Scholar]
- 15.Bedwani R, Renganathan E, El Kwhsky F, et al. Schistosomiasis and the risk of bladder cancer in Alexandria, Egypt. Br J Cancer. 1998;77(7):1186–1189. doi: 10.1038/bjc.1998.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kantor AF, Hartge P, Hoover RN, Fraumeni JF., Jr Epidemiological characteristics of squamous cell carcinoma and adenocarcinoma of the bladder. Cancer Res. 1988;48(13):3853–3855. [PubMed] [Google Scholar]
- 17.Amling CL. Diagnosis and management of superficial bladder cancer. Curr Probl Cancer. 2001;25(4):219–278. doi: 10.1067/mcn.2001.117539. [DOI] [PubMed] [Google Scholar]
- 18.Heney NM. Natural history of superficial bladder cancer. Prognostic features and long-term disease course. Urol Clin North Am. 1992;19(3):429–433. [PubMed] [Google Scholar]
- 19.von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18(17):3068–3077. doi: 10.1200/JCO.2000.18.17.3068. [DOI] [PubMed] [Google Scholar]
- 20.McCaffrey JA, Hilton S, Mazumdar M, et al. Phase II trial of docetaxel in patients with advanced or metastatic transitional-cell carcinoma. J Clin Oncol. 1997;15(5):1853–1857. doi: 10.1200/JCO.1997.15.5.1853. [DOI] [PubMed] [Google Scholar]
- 21.Papamichael D, Gallagher CJ, Oliver RT, Johnson PW, Waxman J. Phase II study of paclitaxel in pretreated patients with locally advanced/metastatic cancer of the bladder and ureter. Br J Cancer. 1997;75(4):606–607. doi: 10.1038/bjc.1997.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaughn DJ, Broome CM, Hussain M, Gutheil JC, Markowitz AB. Phase II trial of weekly paclitaxel in patients with previously treated advanced urothelial cancer. J Clin Oncol. 2002;20(4):937–940. doi: 10.1200/JCO.2002.20.4.937. [DOI] [PubMed] [Google Scholar]
- 23.Joly F, Houede N, Noal S, et al. Do patients with advanced urothelial carcinoma benefit from weekly paclitaxel chemotherapy? A GETUG Phase II Study. Clin Genitourin Cancer. 2009;7(2):E28–E33. doi: 10.3816/CGC.2009.n.018. [DOI] [PubMed] [Google Scholar]
- 24.Sweeney CJ, Roth BJ, Kabbinavar FF, et al. Phase II study of pemetrexed for second-line treatment of transitional cell cancer of the urothelium. J Clin Oncol. 2006;24(21):3451–3457. doi: 10.1200/JCO.2005.03.6699. [DOI] [PubMed] [Google Scholar]
- 25.Galsky MD, Mironov S, Iasonos A, Scattergood J, Boyle MG, Bajorin DF. Phase II trial of pemetrexed as second-line therapy in patients with metastatic urothelial carcinoma. Invest New Drugs. 2007;25(3):265–270. doi: 10.1007/s10637-006-9020-9. [DOI] [PubMed] [Google Scholar]
- 26.Gomez-Abuin G, Winquist E, Stadler WM, et al. A phase II study of PS-341 (Bortezomib) in advanced or metastatic urothelial cancer. A trial of the Princess Margaret Hospital and University of Chicago phase II consortia. Invest New Drugs. 2007;25(2):181–185. doi: 10.1007/s10637-006-9009-4. [DOI] [PubMed] [Google Scholar]
- 27.Rosenberg JE, Halabi S, Sanford BL, et al. Phase II study of bortezomib in patients with previously treated advanced urothelial tract transitional cell carcinoma: CALGB 90207. Ann Oncol. 2008;19(5):946–950. doi: 10.1093/annonc/mdm600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blumenreich MS, Yagoda A, Natale RB, Watson RC. Phase II trial of vinblastine sulfate for metastatic urothelial tract tumors. Cancer. 1982;50(3):435–438. doi: 10.1002/1097-0142(19820801)50:3<435::aid-cncr2820500309>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 29.Vaishampayan UN, Faulkner JR, Small EJ, et al. Phase II trial of carboplatin and paclitaxel in cisplatin-pretreated advanced transitional cell carcinoma: a Southwest Oncology Group study. Cancer. 2005;104(8):1627–1632. doi: 10.1002/cncr.21370. [DOI] [PubMed] [Google Scholar]
- 30.Kouno T, Ando M, Yonemori K, et al. Weekly paclitaxel and carboplatin against advanced transitional cell cancer after failure of a platinum-based regimen. Eur Urol. 2007;52(4):1115–1122. doi: 10.1016/j.eururo.2007.03.078. [DOI] [PubMed] [Google Scholar]
- 31.Soga N, Onishi T, Arima K, Sugimura Y. Paclitaxel carboplatin chemotherapy as a second-line chemotherapy for advanced platinum resistant urothelial cancer in Japanese cases. Int J Urol. 2007;14(9):828–832. doi: 10.1111/j.1442-2042.2007.01831.x. [DOI] [PubMed] [Google Scholar]
- 32.Sternberg CN, Calabro F, Pizzocaro G, Marini L, Schnetzer S, Sella A. Chemotherapy with an every-2-week regimen of gemcitabine and paclitaxel in patients with transitional cell carcinoma who have received prior cisplatin-based therapy. Cancer. 2001;92(12):2993–2998. doi: 10.1002/1097-0142(20011215)92:12<2993::aid-cncr10108>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 33.Fechner G, Siener R, Reimann M, Kobalz L, Albers P. Randomised phase II trial of gemcitabine and paclitaxel second-line chemotherapy in patients with transitional cell carcinoma (AUO Trial AB 20/99) Int J Clin Pract. 2006;60(1):27–31. doi: 10.1111/j.1742-1241.2005.00663.x. [DOI] [PubMed] [Google Scholar]
- 34.Matsumoto K, Irie A, Satoh T, Okazaki M, Iwamura M, Baba S. Gemcitabine and paclitaxel chemotherapy as a second-line treatment for advanced or metastatic urothelial carcinoma. Int J Urol. 2007;14(11):1000–1004. doi: 10.1111/j.1442-2042.2007.01889.x. discussion 04. [DOI] [PubMed] [Google Scholar]
- 35.Kanai K, Kikuchi E, Ohigashi T, et al. Gemcitabine and paclitaxel chemotherapy for advanced urothelial carcinoma in patients who have received prior cisplatin-based chemotherapy. Int J Clin Oncol. 2008;13(6):510–514. doi: 10.1007/s10147-008-0779-x. [DOI] [PubMed] [Google Scholar]
- 36.Suyama T, Ueda T, Fukasawa S, et al. Combination of gemcitabine and paclitaxel as second-line chemotherapy for advanced urothelial carcinoma. Jpn J Clin Oncol. 2009;39(4):244–250. doi: 10.1093/jjco/hyp003. [DOI] [PubMed] [Google Scholar]
- 37.Fahy J, Hellier P, Breillout F, Bailly C. Vinflunine: discovery and synthesis of a novel microtubule inhibitor. Semin Oncol. 2008;35(3 Suppl 3):S3–S5. doi: 10.1053/j.seminoncol.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 38.Bennouna J, Delord JP, Campone M, Nguyen L. Vinflunine: a new microtubule inhibitor agent. Clin Cancer Res. 2008;14(6):1625–1632. doi: 10.1158/1078-0432.CCR-07-2219. [DOI] [PubMed] [Google Scholar]
- 39.Bennouna J, Fumoleau P, Armand JP, et al. Phase I and pharmacokinetic study of the new vinca alkaloid vinflunine administered as a 10-min infusion every 3 weeks in patients with advanced solid tumours. Ann Oncol. 2003;14(4):630–637. doi: 10.1093/annonc/mdg174. [DOI] [PubMed] [Google Scholar]
- 40.Bennouna J, Campone M, Delord JP, Pinel MC. Vinflunine: a novel antitubulin agent in solid malignancies. Expert Opin Investig Drugs. 2005;14(10):1259–1267. doi: 10.1517/13543784.14.10.1259. [DOI] [PubMed] [Google Scholar]
- 41.Johnson P, Geldart T, Fumoleau P, Pinel MC, Nguyen L, Judson I. Phase I study of vinflunine administered as a 10-minute infusion on days 1 and 8 every 3 weeks. Invest New Drugs. 2006;24(3):223–231. doi: 10.1007/s10637-005-3902-0. [DOI] [PubMed] [Google Scholar]
- 42.Teicher BA. Newer cytotoxic agents: attacking cancer broadly. Clin Cancer Res. 2008;14(6):1610–1617. doi: 10.1158/1078-0432.CCR-07-2249. [DOI] [PubMed] [Google Scholar]
- 43.Jordan MA, Horwitz SB, Lobert S, Correia JJ. Exploring the mechanisms of action of the novel microtubule inhibitor vinflunine. Semin Oncol. 2008;35(3 Suppl 3):S6–S12. doi: 10.1053/j.seminoncol.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 44.Kruczynski A, Barret JM, Etievant C, Colpaert F, Fahy J, Hill BT. Antimitotic and tubulin-interacting properties of vinflunine, a novel fluorinated Vinca alkaloid. Biochem Pharmacol. 1998;55(5):635–648. doi: 10.1016/s0006-2952(97)00505-4. [DOI] [PubMed] [Google Scholar]
- 45.Kruczynski A, Hill BT. Vinflunine, the latest Vinca alkaloid in clinical development. A review of its preclinical anticancer properties. Crit Rev Oncol Hematol. 2001;40(2):159–173. doi: 10.1016/s1040-8428(01)00183-4. [DOI] [PubMed] [Google Scholar]
- 46.Lobert S, Ingram JW, Hill BT, Correia JJ. A comparison of thermodynamic parameters for vinorelbine- and vinflunine-induced tubulin self-association by sedimentation velocity. Mol Pharmacol. 1998;53(5):908–915. [PubMed] [Google Scholar]
- 47.Lobert S, Puozzo C. Pharmacokinetics, metabolites, and preclinical safety of vinflunine. Semin Oncol. 2008;35(3 Suppl 3):S28–S33. doi: 10.1053/j.seminoncol.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 48.Etievant C, Barret JM, Kruczynski A, Perrin D, Hill BT. Vinflunine (20’,20’-difluoro-3’,4’-dihydrovinorelbine), a novel Vinca alkaloid, which participates in P-glycoprotein (Pgp)-mediated multidrug resistance in vivo and in vitro. Invest New Drugs. 1998;16(1):3–17. doi: 10.1023/a:1006022811895. [DOI] [PubMed] [Google Scholar]
- 49.Etievant C, Kruczynski A, Barret JM, Tait AS, Kavallaris M, Hill BT. Markedly diminished drug resistance-inducing properties of vinflunine (20’,20’-difluoro-3’,4’-dihydrovinorelbine) relative to vinorelbine, identified in murine and human tumour cells in vivo and in vitro. Cancer Chemother Pharmacol. 2001;48(1):62–70. doi: 10.1007/s002800100275. [DOI] [PubMed] [Google Scholar]
- 50.Kruczynski A, Poli M, Dossi R, et al. Anti-angiogenic, vascular-disrupting and anti-metastatic activities of vinflunine, the latest vinca alkaloid in clinical development. Eur J Cancer. 2006;42(16):2821–2832. doi: 10.1016/j.ejca.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 51.Kruczynski A, Colpaert F, Tarayre JP, Mouillard P, Fahy J, Hill BT. Preclinical in vivo antitumor activity of vinflunine, a novel fluorinated Vinca alkaloid. Cancer Chemother Pharmacol. 1998;41(6):437–447. doi: 10.1007/s002800050764. [DOI] [PubMed] [Google Scholar]
- 52.Hill BT, Fiebig HH, Waud WR, Poupon MF, Colpaert F, Kruczynski A. Superior in vivo experimental antitumour activity of vinflunine, relative to vinorelbine, in a panel of human tumour xenografts. Eur J Cancer. 1999;35(3):512–520. doi: 10.1016/s0959-8049(98)00416-x. [DOI] [PubMed] [Google Scholar]
- 53.Bonfil RD, Russo DM, Binda MM, Delgado FM, Vincenti M. Higher antitumor activity of vinflunine than vinorelbine against an orthotopic murine model of transitional cell carcinoma of the bladder. Urol Oncol. 2002;7(4):159–166. doi: 10.1016/s1078-1439(02)00184-9. [DOI] [PubMed] [Google Scholar]
- 54.Wolf S, Barton D, Kottschade L, Grothey A, Loprinzi C. Chemotherapyinduced peripheral neuropathy: prevention and treatment strategies. Eur J Cancer. 2008;44(11):1507–1515. doi: 10.1016/j.ejca.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 55.Barret JM, Etievant C, Hill BT. In vitro synergistic effects of vinflunine, a novel fluorinated vinca alkaloid, in combination with other anticancer drugs. Cancer Chemother Pharmacol. 2000;45(6):471–476. doi: 10.1007/s002800051021. [DOI] [PubMed] [Google Scholar]
- 56.Hill BT, Barret JM, Fahy J, Kruczynski A. In vitro and in vivo synergistic and additive effects of vinflunine, a novel fluorinated vinca alkaloid currently in phase ii trials, in combination with other anticancer drugs. Proc Am Soc Clin Oncol. 2001;20 [Google Scholar]
- 57.Shnyder SD, Cooper PA, Gyselinck N, Hill BT, Double JA, Bibby MC. Vinflunine potentiates the activity of cisplatin but not 5-fluorouracil in a transplantable murine adenocarcinoma model. Anticancer Res. 2003;23(6C):4815–4820. [PubMed] [Google Scholar]
- 58.Braguer D, Barret JM, McDaid H, Kruczynski A. Antitumor activity of vinflunine: effector pathways and potential for synergies. Semin Oncol. 2008;35(3 Suppl 3):S13–S21. doi: 10.1053/j.seminoncol.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 59.Simoens C, Vermorken JB, Korst AE, et al. Cell cycle effects of vinflunine, the most recent promising Vinca alkaloid, and its interaction with radiation, in vitro. Cancer Chemother Pharmacol. 2006;58(2):210–218. doi: 10.1007/s00280-005-0147-8. [DOI] [PubMed] [Google Scholar]
- 60.Yardley DA, McLeod M, Rubin MS, et al. Final results of a first line multicenter phase II metastatic breast cancer trial of Vinflunine monotherapy and in combination with trastuzumab in HER2+ patients. Cancer Res. 2009;69(Suppl 2):252s–Abst.3148. [Google Scholar]
- 61.Machiels JP, Chollet P, Taleb A, et al. A dose-finding and pharmacokinetic study of I.V. Vinflunine in combination with Doxorubicin as first line treatment of metastatic breast cancer. Cancer Res. 2009;69(Suppl 2):396s–Abst.6124. [Google Scholar]
- 62.Fumoleau P, Cortes-Funes H, Taleb AB, et al. Phase 2 Study of SingleAgent IV Vinflunine as Third-Line Treatment of Metastatic Breast Cancer After Failure of Anthracycline-/Taxane-Based Chemotherapy. Am J Clin Oncol. 2009;32(4):375–380. doi: 10.1097/COC.0b013e31818f2d2f. [DOI] [PubMed] [Google Scholar]
- 63.Paridaens R, Wildiers H, Dalenc F, et al. Vinflunine in combination with trastuzumab: an active combination in the treatment of HER2 positive metastatic breast cancer. Breast Cancer Res Treat. 2007;106(Suppl 1):s70–Abst.1082. [Google Scholar]
- 64.Fumoleau P, Cortes-Funes H, Chan S, Taleb A, Campone M, Pouget JC. Phase II study of i.v. Vinflunine (VFL) as third-line treatment of metastatic breast carcinoma after failure of anthracycline-/taxane-based chemotherapy. Breast Cancer Res Treat. 2006;100(Suppl 1):s279–Abst.6070. [Google Scholar]
- 65.Campone M, Fumoleau P, Roche H, et al. A dose-finding pharmacokinetic study of I.V. Vinflunine (VFL) in combination with Capecitabine (CAPE) as second line treatment of metastatic breast cancer (MBC) Breast Cancer Res Treat. 2006;100(Suppl 1):s279–Abst.6069. [Google Scholar]
- 66.Peacock NW, Spigel DR, Mainwaring MG, et al. Preliminary results of a multicenter phase II trial of vinflunine (with trastuzumab in HER2+ pts) as first-line treatment in metastatic breast cancer. J Clin Oncol. 2007;25(18 Suppl):1043. [Google Scholar]
- 67.Ramlau R, Bennouna J, Tan EH, et al. Phase III study of vinflunine versus docetaxel in patients (pts) with advanced or metastatic non-small cell lung cancer (NSCLC) previously treated with a platinum containing regimen. J Thorac Oncol. 2007;2(8 Suppl 4):s317–Abst.A3-05. [Google Scholar]
- 68.Douillard JY, Coudert B, Gridelli C, et al. Phase III study of IV vinflunine (VFL) versus IV docetaxel (DTX) in patients (pts) with advanced or metastatic non-small cell lung cancer (NSCLC) previously treated with a platinum-containing regimen. EJC Suppl. 2007;5(4):358–Abst.6507. [Google Scholar]
- 69.Stinchcombe TE, Lee CB, Hayes DN, et al. Preliminary safety and efficacy data of a phase II trial of vinflunine and cetuximab in the second-line treatment of patients with advanced non-small cell lung cancer. J Clin Oncol. 2008;26(15 Suppl):19100. [Google Scholar]
- 70.Joppert M, Knapp M, Dakhil SR, Boccia RV, Steis R, Jones CM. A phase II trial of single-agent vinflunine as second-line treatment for advanced non-small cell lung cancer (An International Oncology Network Study, #I-05-009) J Clin Oncol. 2008;26(15 Suppl):19033. [Google Scholar]
- 71.Krzakowski M, Douillard J, Ramlau R, et al. Phase III study of vinflunine versus docetaxel in patients (pts) with advanced non-small cell lung cancer (NSCLC) previously treated with a platinum-containing regimen. J Clin Oncol. 2007;25(18 Suppl):7511. [Google Scholar]
- 72.Lunin SD, Spigel DR, Hainsworth JD, et al. Phase II trial of vinflunine (VFL) in sensitive and refractory relapsed small-cell lung cancer (SCLC): Updated results. J Clin Oncol. 2008;26(15 Suppl):19028. [Google Scholar]
- 73.Peyton JD, Spigel DR, Hainsworth JD, et al. Phase II trial of vinflunine in patients with relapsed small cell lung cancer. J Clin Oncol. 2007;25(18 Suppl):18091. [Google Scholar]
- 74.Meluch AA, Spigel DR, Burris HA, III, et al. Vinflunine (VFL) as salvage chemotherapy in hormone-refractory prostate cancer (HRPC) J Clin Oncol. 2008;26(15 Suppl):16059. [Google Scholar]
- 75.Kaleta R, Chung HC, Park SR, et al. Single agent IV vinflunine (VFL) in the second-line treatment of patients (pts) with advanced gastric cancer (AGC): Initial results of a phase II trial. J Clin Oncol. 2008;26(15 Suppl):15533. [Google Scholar]
- 76.Talbot D, Margery J, Dabouis G, et al. Phase II study of Vinflunine in malignant pleural mesothelioma. J Clin Oncol. 2007;25(30):4751–4756. doi: 10.1200/JCO.2007.12.5641. [DOI] [PubMed] [Google Scholar]
- 77.Olver IN, Byrne MJ, Walpole E, et al. Phase II study of IV vinflunine in patients with chemotherapy naive metastatic malignant melanoma. Eur J Cancer. 2007;43(12):1829–1832. doi: 10.1016/j.ejca.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 78.Margery J, Dabouis G, Dark G, et al. Vinflunine (VFL) in first line treatment of malignant pleural mesothelioma (MPM): Final results of a European phase II study. Lung Cancer. 2006;54(Suppl 1):s47–Abst.193. [Google Scholar]
- 79.Goldstein D, Ackland SP, Bell DR, et al. Phase II study of vinflunine in patients with metastatic renal cell carcinoma. Invest New Drugs. 2006;24(5):429–434. doi: 10.1007/s10637-006-6437-0. [DOI] [PubMed] [Google Scholar]
- 80.Mukohara T, Minami H, Nagai S, et al. Phase I study of i.v. Vinflunine (VFL), a novel microtubule inhibitor, given every three weeks in Japanese patients with solid tumors. Proceedings Annual Meeting American Association of Cancer Research. 2008;49:Abst.228. [Google Scholar]
- 81.Bellmunt J, Theodore C, Demkov T, et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol. 2009;27(27):4454–4461. doi: 10.1200/JCO.2008.20.5534. [DOI] [PubMed] [Google Scholar]
- 82.Culine S, Theodore C, De Santis M, et al. A phase II study of vinflunine in bladder cancer patients progressing after first-line platinum-containing regimen. Br J Cancer. 2006;94(10):1395–1401. doi: 10.1038/sj.bjc.6603118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vaughn DJ, Srinivas S, Stadler WM, et al. Vinflunine in platinum-pretreated patients with locally advanced or metastatic urothelial carcinoma: results of a large phase 2 study. Cancer. 2009;115(18):4110–4117. doi: 10.1002/cncr.24460. [DOI] [PubMed] [Google Scholar]
- 84.Bellmunt J, Delgado FM, George C. Clinical activity of vinflunine in transitional cell carcinoma of the urothelium and other solid tumors. Semin Oncol. 2008;35(3 Suppl 3):S34–S43. doi: 10.1053/j.seminoncol.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 85.Wulfing C, Machiels JP, Richel DJ, et al. A single-arm, multicenter, open-label phase 2 study of lapatinib as the second-line treatment of patients with locally advanced or metastatic transitional cell carcinoma. Cancer. 2009;115(13):2881–2890. doi: 10.1002/cncr.24337. [DOI] [PubMed] [Google Scholar]
- 86.Albers P, Siener R, Hartlein M, et al. Gemcitabine monotherapy as second-line treatment in cisplatin-refractory transitional cell carcinoma –prognostic factors for response and improvement of quality of life. Onkologie. 2002;25(1):47–52. doi: 10.1159/000055202. [DOI] [PubMed] [Google Scholar]
- 87.Pronzato P, Vigani A, Pensa F, Vanoli M, Tani F, Vaira F. Second line chemotherapy with ifosfamide as outpatient treatment for advanced bladder cancer. Am J Clin Oncol. 1997;20(5):519–521. doi: 10.1097/00000421-199710000-00018. [DOI] [PubMed] [Google Scholar]
- 88.Witte RS, Elson P, Bono B, et al. Eastern Cooperative Oncology Group phase II trial of ifosfamide in the treatment of previously treated advanced urothelial carcinoma. J Clin Oncol. 1997;15(2):589–593. doi: 10.1200/JCO.1997.15.2.589. [DOI] [PubMed] [Google Scholar]
- 89.Witte RS, Manola J, Burch PA, Kuzel T, Weinshel EL, Loehrer PJ., Sr Topotecan in previously treated advanced urothelial carcinoma: an ECOG phase II trial. Invest New Drugs. 1998;16(2):191–195. doi: 10.1023/a:1006159525793. [DOI] [PubMed] [Google Scholar]
- 90.Dodd PM, McCaffrey JA, Mazumdar M, et al. Phase II trial of pyrazoloacridine as second-line therapy for patients with unresectable or metastatic transitional cell carcinoma. Invest New Drugs. 2000;18(3):247–251. doi: 10.1023/a:1006477823378. [DOI] [PubMed] [Google Scholar]
- 91.Roth BJ, Manola J, Dreicer R, Graham D, Wilding G. Piritrexim in advanced, refractory carcinoma of the urothelium (E3896): a phase II trial of the Eastern Cooperative Oncology Group. Invest New Drugs. 2002;20(4):425–429. doi: 10.1023/a:1020675017737. [DOI] [PubMed] [Google Scholar]
- 92.Lassiter LK, Tummala MK, Hussain MH, Stadler WM, Petrylak DP, Carducci MA. Phase II open-label study of oral piritrexim in patients with advanced carcinoma of the urothelium who have experienced failure with standard chemotherapy. Clin Genitourin Cancer. 2008;6(1):31–35. doi: 10.3816/CGC.2008.n.005. [DOI] [PubMed] [Google Scholar]
- 93.Winquist E, Vokes E, Moore MJ, Schumm LP, Hoving K, Stadler WM. A Phase II study of oxaliplatin in urothelial cancer. Urol Oncol. 2005;23(3):150–154. doi: 10.1016/j.urolonc.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 94.Akaza H, Naito S, Usami M, Miki T, Miyanaga N, Taniai H. Efficacy and safety of gemcitabine monotherapy in patients with transitional cell carcinoma after Cisplatin-containing therapy: a Japanese experience. Jpn J Clin Oncol. 2007;37(3):201–206. doi: 10.1093/jjco/hym011. [DOI] [PubMed] [Google Scholar]
- 95.Dreicer R, Li S, Manola J, Haas NB, Roth BJ, Wilding G. Phase 2 trial of epothilone B analog BMS-247550 (ixabepilone) in advanced carcinoma of the urothelium (E3800): a trial of the Eastern Cooperative Oncology Group. Cancer. 2007;110(4):759–763. doi: 10.1002/cncr.22839. [DOI] [PubMed] [Google Scholar]
- 96.Beer TM, Goldman B, Nichols CR, et al. Southwest Oncology Group phase II study of irinotecan in patients with advanced transitional cell carcinoma of the urothelium that progressed after platinum-based chemotherapy. Clin Genitourin Cancer. 2008;6(1):36–39. doi: 10.3816/cgc.2008.n.006. [DOI] [PubMed] [Google Scholar]
- 97.Tu SM, Hossan E, Amato R, Kilbourn R, Logothetis CJ. Paclitaxel, cisplatin and methotrexate combination chemotherapy is active in the treatment of refractory urothelial malignancies. J Urol. 1995;154(5):1719–1722. [PubMed] [Google Scholar]
- 98.De Mulder PH, Theodore C, Sella A, et al. Phase II EORTC trial with 5-fluorouracil, cisplatin and interferon-alpha as second-line treatment of advanced transitional cell cancer of the urothelial tract. Ann Oncol. 2000;11(11):1391–1394. doi: 10.1023/a:1026589120989. [DOI] [PubMed] [Google Scholar]
- 99.Krege S, Rembrink V, Borgermann C, Otto T, Rubben H. Docetaxel and ifosfamide as second line treatment for patients with advanced or metastatic urothelial cancer after failure of platinum chemotherapy: a phase 2 study. J Urol. 2001;165(1):67–71. doi: 10.1097/00005392-200101000-00017. [DOI] [PubMed] [Google Scholar]
- 100.Pectasides D, Aravantinos G, Kalofonos H, et al. Combination chemotherapy with gemcitabine and ifosfamide as second-line treatment in metastatic urothelial cancer. A phase II trial conducted by the Hellenic Cooperative Oncology Group. Ann Oncol. 2001;12(10):1417–1422. doi: 10.1023/a:1012599307090. [DOI] [PubMed] [Google Scholar]
- 101.Pagliaro LC, Millikan RE, Tu SM, et al. Cisplatin, gemcitabine, and ifosfamide as weekly therapy: a feasibility and phase II study of salvage treatment for advanced transitional-cell carcinoma. J Clin Oncol. 2002;20(13):2965–2970. doi: 10.1200/JCO.2002.11.114. [DOI] [PubMed] [Google Scholar]
- 102.Dreicer R, Manola J, Schneider DJ, et al. Phase II trial of gemcitabine and docetaxel in patients with advanced carcinoma of the urothelium: a trial of the Eastern Cooperative Oncology Group. Cancer. 2003;97(11):2743–2747. doi: 10.1002/cncr.11413. [DOI] [PubMed] [Google Scholar]
- 103.Di Lorenzo G, Autorino R, Giordano A, et al. FOLFOX-4 in pre-treated patients with advanced transitional cell carcinoma of the bladder. Jpn J Clin Oncol. 2004;34(12):747–750. doi: 10.1093/jjco/hyh132. [DOI] [PubMed] [Google Scholar]
- 104.Shinohara N, Harabayashi T, Suzuki S, et al. Salvage chemotherapy with paclitaxel, ifosfamide, and nedaplatin in patients with urothelial cancer who had received prior cisplatin-based therapy. Cancer Chemother Pharmacol. 2006;58(3):402–407. doi: 10.1007/s00280-005-0175-4. [DOI] [PubMed] [Google Scholar]
- 105.Uhm JE, Lim HY, Kim WS, et al. Paclitaxel with cisplatin as salvage treatment for patients with previously treated advanced transitional cell carcinoma of the urothelial tract. Neoplasia. 2007;9(1):18–22. doi: 10.1593/neo.06661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lin CC, Hsu CH, Huang CY, et al. Gemcitabine and ifosfamide as a second-line treatment for cisplatin-refractory metastatic urothelial carcinoma: a phase II study. Anticancer Drugs. 2007;18(4):487–491. doi: 10.1097/CAD.0b013e3280126603. [DOI] [PubMed] [Google Scholar]
- 107.Han KS, Joung JY, Kim TS, et al. Methotrexate, vinblastine, doxorubicin and cisplatin combination regimen as salvage chemotherapy for patients with advanced or metastatic transitional cell carcinoma after failure of gemcitabine and cisplatin chemotherapy. Br J Cancer. 2008;98(1):86–90. doi: 10.1038/sj.bjc.6604113. [DOI] [PMC free article] [PubMed] [Google Scholar]


