Abstract
Endophytic fungi were isolated from different parts of healthy paddy plants (Oryza sativa). The most common endophytic fungal genus recovered was Fusarium, followed by Aspergillus, Curvularia, Penicillium, Gilmaniella and Arthrobotrys foliicola. Fusarium and Curvularia had higher occurrences in the seeds compared with the other fungi. Aspergillus was recovered mostly from leaf blades and Penicillium from the leaf sheath. Gilmaniella and A. foliicola were isolated only from the roots and leaf blade, respectively. The assemblage of endophytic fungi in healthy tissues of paddy plants may indicate that some of the fungi are possible latent pathogens and some may become saprophytic.
Keywords: Endophyte, Fungi, Paddy
Abstract
Kulat endofit telah dipencilkan daripada bahagian berbeza tanaman padi (Oryza sativa) yang sihat. Genus yang paling lazim diperolehi adalah Fusarium diikuti oleh Aspergillus, Curvularia, Penicillium, Gilmaniella dan Arthrobotrys foliicola. Fusarium dan Curvularia didapati lebih banyak di dalam biji benih. Aspergillus dipencilkan kebanyakannya daripada bilah daun dan Penicillium daripada seludang daun. Gilmaniella dan A. foliicola pula hanya dipencilkan daripada akar dan bilah daun masing-masing. Sekumpulan kulat endofit yang diperolehi daripada tisu-tisu padi yang sihat menunjukkan kemungkinan sebahagian kulat tersebut merupakan patogen pendam dan sebahagian lagi boleh menjadi saprofit.
Endophytic fungi colonise healthy plant tissues but do not always cause noticeable symptoms. This interaction is regarded as mutualistic (Carroll 1988). According to Schulz and Boyle (2005), however, this mutualistic interaction may only be temporary and is subject to change over time. Therefore, endophytic fungi could account for those fungi with an epiphytic phase as well as latent pathogens that live asymptomatically in the host plant for some time in their life (Petrini 1991).
In a mutualistic interaction, an endophyte in plant tissues obtains nutrients and protection from the plant host, while returning metabolites that contribute to host resistance against herbivores, pathogens and various abiotic stresses, thereby enhancing the plant’s fitness in a harsh environment (Saikkonen et al. 1998; Redman et al. 2002). The stability of a mutualistic interaction may change, depending on several factors such as environmental stress, senescence of the plant and the plant defence response to infection (Schulz & Boyle 2005).
There have been numerous studies on endophytic fungi in agricultural crops, such as maize (Fisher et al. 1992), banana (Cao et al. 2002), coffee (Santamaria & Bayman 2005) and wheat (Larran et al. 2007), indicating that endophytic fungi are diverse in various crops. Paddy is one of the most important commercial crops planted in Malaysia, particularly for domestic consumption. There are relatively few studies focused on endophytic fungi colonising healthy paddy tissues. Thus, the objective of the present study was to assess the endophytic fungal assemblages in different parts of apparently healthy paddy plants.
Paddy samples were collected from three locations in Pulau Pinang: Sungai Burung, Balik Pulau; Permatang Pauh, Seberang Perai; Batu Dua, and Sungai Setar, Nibong Tebal. The plants collected were at reproductive stage, and a total of 10 plants were collected at each location. Only healthy plants displaying no disease symptoms or pest infestation were selected. The samples were processed within 48 h after sampling to avoid desiccation of the plant tissues.
For isolation of endophytic fungi, 20 plants, comprising plants from each of the selected locations, were selected. All parts of the paddy plants were thoroughly washed in running tap water for 24 h to remove debris and any soil particles adhered to the surface of the plants. Leaf sheath, leaf blade, stems and roots were cut into small pieces of about 5 mm. For leaf sheath, leaf blade and stems, 40 tissue pieces from each plant part were used for isolation. From the roots, 50 small pieces of tissue were used to isolate endophytic fungi.
The pieces of tissue were surface sterilised by immersion in 75% alcohol for 5 min, then 80% natrium hyphochlorite for 1 min; tissues were then washed in sterile distilled water for 1 min. Plant tissues were plated on the surface of potato dextrose agar (PDA) and peptone chloronitro benzene (PCNB) media. PCNB was used for the isolation of Fusarium species.
The plates were incubated at 27 ± 1°C for 1–10 days, or until mycelia growth from the tissues was observed. Mycelia growing out from the plant tissues were then sub-cultured on PDA to obtain pure culture and used for identification. The endophytic fungi were identified by examining the microscopic and macroscopic structures. Taxonomic fungal manuals of Ellis (1971), Barnett and Hunter (2006) and Leslie and Summerell (2006) were used for identification of the endophytic fungi.
Mycelia sterilia fungi were identified using internal transcribed spacer region of ribosomal DNA (ITS1-5.8S-ITS2). Polymerase chain reaction (PCR) and sequencing procedures, as well as the primer sequences used, were as previously described by White et al. (1990).
A total of 110 fungal isolates were recovered from different parts of the paddy plant. The occurrence of endophytic fungi in different parts of the plant was not tissue specific. From seeds, 40 fungal isolates were recovered. The number of isolates recovered from leaf blade, leaf sheath and stem were 32, 21 and 10, respectively. Only seven isolates were recovered from the roots (Table 1).
Table 1:
Occurrence of endophytic fungi in healthy tissues of paddy.
| Fungi | Plant tissues
|
|||||
|---|---|---|---|---|---|---|
| Seed | Leaf blade | Leaf sheath | Stem | Root | Total number of isolates | |
| Fusarium | 25 | 5 | 5 | 3 | 5 | 43 |
| Aspergillus | 2 | 14 | 4 | 6 | 0 | 26 |
| Curvularia | 13 | 5 | 3 | 0 | 0 | 21 |
| Penicillium | 0 | 6 | 9 | 1 | 1 | 17 |
| Gilmaniella | 0 | 0 | 0 | 0 | 1 | 1 |
| Arthrobotrys foliicola | 0 | 2 | 0 | 0 | 0 | 2 |
|
| ||||||
| 40 | 32 | 21 | 11 | 8 | 110 | |
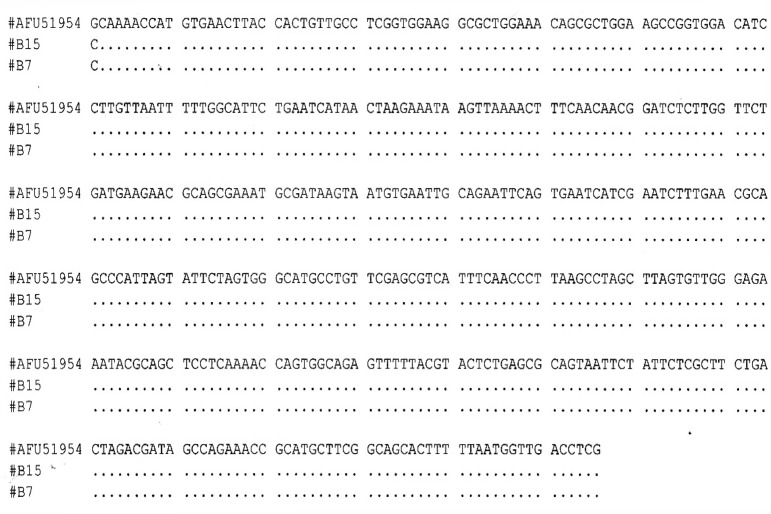
Five genera of fungi were identified based on macroscopic and microscopic structures, and one genus of mycelia sterilia was identified using sequences of the ITS1-5.8S-ITS2 regions. The most common fungal genus recovered was Fusarium, followed by Aspergillus, Curvularia and Penicillium. Gilmaniella was only isolated from the stem. Based on the ITS1-5.8S-ITS2 regions, the two mycelia sterilia isolates recovered from leaf blade were identified as Arthrobotrys foliicola (Fig. 1). The sequences of ITS1-5.8S-ITS2 regions of the 2 isolates showed 100% homology with A. foliicola (AFU51954).
Figure 1:
Sequence alignment of two mycelia sterilia isolates (B15 and B7) with accession AFU51954 (A. foliicola).
Fusarium and Curvularia appeared to have higher occurrences in the seed compared with other fungi. On the other hand, Aspergillus was recovered in higher frequency in the leaf blade and Penicillium in the leaf sheath compared with other fungi. The uncommon endophytic fungi of Gilmaniella and A. foliicola were recovered from the root and leaf blade, respectively.
The endophytic fungi isolated from different parts of paddy could be saprophytic such as Gilmaniella and A. foliicola or potentially pathogenic from the genus Fusarium, Aspergillus, Curvularia and Penicillium. However, these fungal genera are also well-known endophytes in healthy tissues of several plants (Fisher & Petrini 1992; Tian et al. 2004; Geris dos Santos et al. 2003).
Plant pathogenic species of Fusarium have been known to be associated with several types of paddy disease such as bakanae, seedling rot and root rot. The occurrence of Fusarium in paddy plants implies that this fungus is a latent pathogen. Leslie et al. (1990) reported asymptomatic infections by species of Fusarium in maize, sorghum and soybean. Two species of Fusarium, F. equiseti and F. oxysporum have been isolated from healthy paddy plants by Fisher and Petrini (1992).
In the present study, Fusarium was frequently isolated from the seeds. Species of Fusarium have been reported to be one of the most common fungi recovered from paddy grains (Abdel-Hafez et al. 1987), paddy and milled rice (Tonon et al. 1997) and paddy seeds (Pacin et al. 2002). In addition to paddy seed, species of Fusarium have been isolated from soybean seeds (Pacin et al. 2002) and cowpea seeds (Rodrigues & Menezes 2005).
Besides Fusarium, Aspergillus and Penicillium were also among the most common endophytic fungal genera recovered from healthy paddy plant in China (Tian et al. 2004). From healthy leaves and roots of paddy plants, Penicillium chrysogenum and F. oxysporum were frequently isolated (Shankar Naik et al. 2009). Rodolfi et al. (2006) reported that endophytic colonisation of seeds by Penicillium, Fusarium and Aspergillus were detected on more than one Italian rice cultivar. In addition to paddy, endophytic species of Aspergillus and Penicillium were also recovered from banana leaves and roots (Cao et al. 2002) and leaves of Plumeria rubra, a tropical deciduous tree (Suryanarayanan & Thennerasan 2004).
Isolates of Curvularia were mostly recovered from paddy seeds. This genus is a well-known pathogen of paddy seed (Estrada & Sandoval 2004) and is among the mycoflora most frequently isolated from paddy seeds (Tonon et al. 1997; Abdel-Hafez et al. 1987).
Two isolates of mycelia sterilia isolated from the leaf blade were identified as A. foliicola. Species of Arthrobotrys are nematophagous fungi that can exhibit endophytic and free living life styles in rhizophere soils (Lopez-Llorca et al. 2006). Two species of Arthrobotrys have been reported to be endophytic in plants. A. conoides has been recovered from the bark of Crataeva magna (three leave caper) (Nalini et al. 2005), and A. oligospora was shown to endophytically colonise barley roots (Bordallo et al. 2002).
Gilmaniella was isolated from paddy stem. Endophytic Gilmaniella has been isolated from healthy tissues of root, stem, leaves and fruits of Melia azedarach (Geris dos Santos et al. 2003).
Interactions between an endophyte and host may change over time (Saikkonen et al. 1998; Schulz & Boyle 2005). The endophyte may undergo physiological alterations, from a mutualistic to a pathogenic interaction, or vice versa, depending on several factors such as drought, excessive humidity, stress and poor nutrient supply (Millar 1980; Fisher & Petrini 1992). For most endophytic fungi, the types of interactions between the microbe and the host are described as an interaction at a particular point of time or momentary status (Schulz & Boyle 2005). However, an endophytic fungus could act as a latent pathogen in the plant tissues until changes in environmental factors or decline in host defence mechanisms allow the endophyte to become pathogenic (Bayman 2007). Thus, the assemblage of endophytic fungi from paddy plants may indicate that some of the fungi such as Fusarium, Aspergillus, Curvularia and Penicillium are possible latent pathogens, a suggestion put forth by Fisher and Petrini (1992). The other two fungi, Gilmaniella and A. foliicola, could become saprophytic at a later stage of plant growth, but their roles in the paddy plant are not clear and need further study.
In conclusion, six endophytic fungal genera, including Fusarium, Aspergillus, Curvularia, Penicillium, Gilmaniella and A. foliicola, were recovered from different parts of paddy plants, indicating that an assemblage of endophytic fungi occurs in the tissues of the paddy plant.
REFERENCES
- Abdel-Hafez S, II, El-Kady IA, Mazen MB, El-Maghraby OMO. Mycoflora and trichothecene toxins of paddy grains from Egypt. Mycopathologia. 1987;100(2):103–112. doi: 10.1007/BF00467102. [DOI] [PubMed] [Google Scholar]
- Barnett HL, Hunter BB. Illustrated genera of imperfect fungi. 4th ed. Minnesota, USA: American Phytopathological Society Press; 2006. [Google Scholar]
- Bayman P. Fungal endophytes. In: Kubicek CP, Druzhinina IS, editors. The mycota IV: Environmental and microbial relationships. 2nd ed. New York: Springer-Verlag; 2007. [Google Scholar]
- Bordallo JJ, Lopez-Llorca LV, Jansson H-B, Salinas J, Persmark L, Asensio L. Colonization of plant roots by egg-parasitic and nematode trapping fungi. New Phytologist. 2002;154:491–199. doi: 10.1046/j.1469-8137.2002.00399.x. [DOI] [PubMed] [Google Scholar]
- Cao LX, You JL, Zhou SN. Endophytic fungi from Musa acuminata leaves and roots in South China. World Journal of Microbiology and Biotechnology. 2002;18(2):169–171. [Google Scholar]
- Carroll GC. Fungal endophytes in stems and leaves: From latent pathogens to mutualistic symbiont. Ecology. 1988;69:2–9. [Google Scholar]
- Ellis MB. Dematiaceous Hyphomycetes. Kew, England: Commonwealth Agricultural Bureaux; 1971. [Google Scholar]
- Estrada G, Sandoval I. Pathogenicity of Curvularia spp. in rice. Fitosanidad. 2004;8(4):23–26. [Google Scholar]
- Fisher PJ, Petrini O, Lappin Scott HM. The distribution of some fungal and bacterial endophytes in maize (Zea mays L.) New Phytologist. 1992;122:299–305. doi: 10.1111/j.1469-8137.1992.tb04234.x. [DOI] [PubMed] [Google Scholar]
- Fisher PJ, Petrini O. Fungal saprobes and pathogens as endophytes of rice (Oryza sativa L.) New Phytologist. 1992;120:137–143. [Google Scholar]
- Geris dos Santos RM, Rodrigues-Fo E, Rocha WC, Teixeira MFS. Endophytic fungi from Melia azedarach. World Journal of Microbiology and Biotechnology. 2003;19(8):767–770. [Google Scholar]
- Larran S, Perello A, Simon MR, Moreno V. The endophytic fungi from wheat (Triticium aestivum L) World Journal of Microbiology and Biotechnology. 2007;23(4):565–572. [Google Scholar]
- Leslie JF, Pearson CAS, Nelson PE, Tousson TA. Fusarium species from corn, sorghum and soybean fields in the central and eastern United States. Phytopathology. 1990;80:343–350. [Google Scholar]
- Leslie JF, Summerell BA. The Fusarium laboratory manual. Ames, Iowa: Blackwell Publishing Ltd; 2006. [Google Scholar]
- Lopez-Llorca LV, Jansson H–B, Vicente JGM, Salinas J. Nematophagous fungi as root endophytes. In: Schulz BJE, Boyle CJC, Sieber TN, editors. Microbial root endophytes. Berlin: Springer-Verlag; 2006. pp. 191–206. [Google Scholar]
- Millar CS. Infection processes on conifer needles. In: Blakeman JP, editor. Microbial ecology of the phylloplane. London: Academic Press; 1980. pp. 185–209. [Google Scholar]
- Nalini MS, Mahesh B, Tejesvi MV, Prakash HS, Subbaiah V, Kini KR, Shetty HS. Fungal endophytes from the three leaved caper, Crataeva magna (Lour.) (Capparidaceae) Mycopathologia. 2005;159:245–249. doi: 10.1007/s11046-004-5497-y. [DOI] [PubMed] [Google Scholar]
- Pacin AM, Gonzalez HHL, Etcheverry M, Resnik SL, Vivas L, Espin S. Fungi associated with food and feed commodities from Ecuador. Mycopathologia. 2002;156:87–92. doi: 10.1023/a:1022941304447. [DOI] [PubMed] [Google Scholar]
- Petrini O. Fungal endophytes of tree leaves. In: Andrews J, Hirano S, editors. Microbial ecology of leaves. New York: Springer-Verlag; 1991. [Google Scholar]
- Redman RS, Sheehan KB, Stout RG, Rodriguez R, Henson JM. Thermotolerance generated by plant/fungal symbiosis. Science. 2002;298:1581. doi: 10.1126/science.1072191. [DOI] [PubMed] [Google Scholar]
- Rodolfi M, Lorenzi E, Picco AM. Fungal pathogens on Italian rice (Oryza sativa L) seed. 3rd International Seed Health Conference of Seed Pathology Section; Bydgoszcz, Poland. 6–8 September 2006; 2006. pp. 76–77. [Google Scholar]
- Rodrigues AAC, Menezes M. Identification and pathogenic characterization of endophytic Fusarium species from cowpea seeds. Mycopathlogia. 2005;159:79–85. doi: 10.1007/s11046-004-7138-x. [DOI] [PubMed] [Google Scholar]
- Saikkonen K, Faeth SH, Helander M, Sullivan TJ. Fungal endophyes: A continuum of interactions with host plants. Annual Review of Ecology and Systematics. 1998;29:319–343. [Google Scholar]
- Santamaria J, Bayman P. Fungal epiphytes and endophytes of coffee leaves (Coffee arabica) Microbial Ecology. 2005;50(1):1–8. doi: 10.1007/s00248-004-0002-1. [DOI] [PubMed] [Google Scholar]
- Schulz B, Boyle C. Fungal endophyte continuum. Mycological Research. 2005;109:661–686. doi: 10.1017/s095375620500273x. [DOI] [PubMed] [Google Scholar]
- Shankar Naik B, Shashikala J, Krishnamurthy YL. Study on the diversity of endophytic communities from rice (Oryza sativa L.) and their antagonistic activities in vitro. Microbiological Research. 2009;164(3):290–296. doi: 10.1016/j.micres.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Suryanarayanan TS, Thennarasan S. Temporal variation in endophyte assemblages of Plumeria rubra leaves. Fungal Diversity. 2004;15:197–204. [Google Scholar]
- Tian XL, Cao LX, Tan HM, Zaeng QG, Jia YY, Han WQ, Zhao SN. Study on the communities of endophytic fungi and endophytic actinomycetes from rice and their antipathogenic activities in vitro. World Journal of Microbiology and Biotechnology. 2004;20(3):303–309. [Google Scholar]
- Tonon SA, Marucci RS, Jerke G, Garcia A. Mycoflora of paddy and milled rice produced in the region of northeastern Argentina and sourthern Paraguay. International Journal of Food Microbiology. 1997;37(2):231–235. doi: 10.1016/s0168-1605(97)00066-4. [DOI] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: A guide to methods and applications. San Diego: Academic Press Inc; 1990. pp. 315–322. [Google Scholar]