Abstract
Research on natural products has been widely used as a strategy to discover new drugs with potential for applications in complementary medicines because they have fewer side effects than conventional drugs. The aim of the present study was to evaluate the in vitro cytotoxic effects of crude aqueous Catharanthus roseus extract on Jurkat cells and normal peripheral blood mononuclear cells (PBMCs). The aqueous extract was standardised to vinblastine by high performance liquid chromatography (HPLC) and was used to determine cytotoxicity by the MTS [3-(4,5-dimethylthiazol-2-yl)-5(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] assay. DNA fragmentation assay was employed to determine if cell death was due to apoptosis. The results showed that the aqueous extract induced cell death of Jurkat cells at 24, 48 and 72 hours post-treatment in a time- and dose-dependent manner. However, cells treated at 48 and 72 hours produced higher cytotoxic effects with half maximal inhibitory concentration (IC50) values of 2.55 μg/ml and 2.38 μg/ml, respectively. In contrast, the extract induced normal PBMC proliferation, especially after 24 hours treatment with 1000 μg/ml. This result indicates that the C. roseus crude aqueous extract showed differential effects of inhibiting the proliferation of the Jurkat cell line and promoting the growth of PBMCs. These data suggest that the extract may be applicable for modulating the normal and transformed immune cells in leukaemia patients.
Keywords: Catharanthus roseus, Aqueous Extract, Anticancer, Vinblastine, Jurkat Cells
Abstract
Kajian ke atas produk semula jadi telah diaplikasikan secara meluas dalam usaha untuk menemui ubatan terbaru dalam perubatan komplementari disebabkan kebanyakannya menunjukkan kurang kesan sampingan jika dibandingkan dengan perubatan konvensional. Objektif utama kajian terbaru ini adalah untuk menilai kesan toksik in vitro ekstrak akues Catharanthus roseus terhadap sel Jurkat dan sel mononuklear yang normal. Ekstrak tersebut telah dipiawaikan untuk vinblastine dengan menggunakan high performance liquid chromatography (HPLC) dan telah digunakan untuk menentukan kesan toksik ke atas sel seperti di atas menggunakan MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] assay. DNA fragmentation assay telah digunakan untuk menentukan sama ada mekanisma pembunuhan sel disebabkan oleh apoptosis. Keputusan kajian menunjukkan ekstrak tesebut merangsang pembunuhan sel Jurkat pada 24, 48 dan 72 jam setelah diuji bergantung pada masa dan dosis. Namun begitu, sel yang telah diubati pada 48 dan 72 jam telah menghasilkan kesan toksik sel yang lebih tinggi dengan nilai-nilai half maximal inhibitory concentration (IC50) masing-masing 2.55 μg/ml dan 2.38 μg/ml. Sebaliknya, ekstrak tersebut telah merangsang pertumbuhan sel mononuklear normal terutamanya selepas 24 jam diubati pada kepekatan ekstrak 1000 ug/ml. Hasil kajian ini menunjukkan ekstrak akues C. roseus menghasilkan keputusan berbeza di mana ekstrak tersebut telah merencat pertumbuhan sel Jurkat tetapi merangsang pertumbuhan sel mononuklear normal. Ini menunjukkan bahawa ekstrak tersebut mungkin berpotensi untuk diaplikasi dalam memodulasi sel imun yang normal dan berkanser dalam pesakit yang menghidapi kanser leukemia.
INTRODUCTION
A significant development in medical oncology is the use of cytotoxic drugs for cancer chemotherapy. Although these drugs are used to target tumour cells, most of them can also induce genotoxic, carcinogenic and teratogenic effects in non-tumour cells (Chung et al. 1998; Philip 2005). These side effects limit the application of chemotherapeutic agents despite their high efficacy in the killing of target malignant cells. Therefore, the search for alternative or complementary drugs that are effective on cancer cells while showing minimal toxicity to normal cells is an active area of research (Tang et al. 2003). Many of these investigations are plant-based, folkloric medicine from various societies around over the world. Moreover, a report from WHO (World Health Organisation 1996) stated that about 80% of the world population is wholly or partially dependent on plant-based drugs.
The medicinal plant Catharanthus roseus L. G. Don, formerly Vinca rosea L. (Apocynaceae), has been used in traditional medicine by various societies to address diabetes, cancer, hypertension, fever or hemostasis (Don 2003; Renault et al. 1999). C. roseus is of enormous pharmaceutical interest because it contains more than 120 terpenoid indole alkaloids (TIAs), and many of the alkaloids exhibit strong pharmacological activities (Van der Heijden et al. 2004). Previous studies have identified significant active compounds in C. roseus, including vinblastine and vincristine (anticancer), ajmalicine (antihypertensive) and serpentine (sedative) (Van der Heijden et al. 2004; Sottomayor & Ros Barcelo 2005). Vinblastine and vincristine are produced by the plant in small amounts (Tyler 1988) and are commonly used in combination with other drugs for the treatment of cancers, such as lymphomas and leukaemia (Gidding et al. 1999; Van der Heijden et al. 2004).
However, few reports have addressed the effects of C. roseus crude aqueous extract on peripheral blood mononuclear cells (PBMCs). In this work, we analysed one of the anticancer compounds present in the crude extracts, vinblastine to see whether this compound may potentially act towards the cancer cells used, the Jurkat cells (a leukaemic T-cell line). We used commercially available vinblastine for pure compound control. We then followed with the study of the cytotoxic effects of the extract on PBMCs. We showed that C. roseus crude extract significantly inhibited the growth of Jurkat cells but stimulated proliferation and activation of normal PBMCs.
MATERIALS AND METHODS
Plant Material
C. roseus leaves were collected from Kuala Lumpur in October, 2007. The plant was identified by Mr. Adenan Jaafar from the School of Biological Sciences Herbarium at Universiti Sains Malaysia (USM), Pulau Pinang. The reference number of the plant is 10933.
Preparation of Extract
C. roseus leaves were dried in a universal hot air oven (Venticell, Germany) at 40°C before they were homogenised using an electric grinder to obtain a fine powder. 50 g of the powdered leaves were extracted with 1 l distilled water and soaked at 40°C for 24 hours in a shaker water bath. The extract was transferred into 50 ml Falcon tubes before centrifugation at 2000 rpm at 25°C for 15 minutes. The clear supernatant was collected and freeze-dried in a freeze dryer (FDU-1200; Eyela, USA) at −20°C. A working stock of crude aqueous extract, at a concentration of 100 mg/ml, was prepared when 100 mg of extract was dissolved with 1 ml of sterile phosphate buffer saline (PBS). The aqueous extract was filter-sterilised using a 0.2 μm filter (Sartorius, Germany) before being aliquoted into cryovials. The stocks were diluted to various concentrations to be used in the subsequent experiments.
Quantitative Chromatographic Analysis
The extract used in this study was standardised using commercial vinblastine as a standard (Tocris, USA). The HPLC analysis of vinblastine in the C. roseus aqueous extract was performed using a Varian (USA) prostar pump model 240, a Varian prostar autosampler model 410, and a Varian prostar PDA Detector model 335. Sample separation was performed using a reverse-phase Microsorb-MV C18 column (150 x 4.6 mm) with a 5 μm particle size. Vinblastine solutions used for the HPLC analysis were prepared at 36, 40, 44, 48, 52 and 56 μg/ml. Methanol and acetonitrile used for this study were of HPLC grade, and all solvents were filtered and degassed before use.
Cell Line
Jurkat cells [American type culture collection (ATCC) USA] were cultured in suspension using Roswell Park Memorial Institute (RPMI) medium supplemented with 10% foetal calf serum, 1% penicillin-streptomycin and 1% L-glutamine (Gibco, USA). Cells were collected while in the log phase of growth by centrifugation at 1500 rpm for 10 minutes, and the pellet was resuspended in RPMI complete growth medium at a concentration of 4 x 105 cells/ml.
Isolation of PBMCs
This study has been approved by USM’s Ethical Committee. PBMCs were obtained from normal volunteers after informed consent as stipulated by USM’s Ethical Committee guidelines. Preliminary screening was done by adding 20 μl of directly conjugated mouse anti-human CD3, CD4, CD8, CD19, CD16+56 and CD45 (BD Biosciences, USA) to 50 μl of whole blood from volunteer blood donors. The tubes were incubated at room temperature in the dark for 10 minutes. Red blood cells were lysed by adding 450 μl of 1 x BD FACS™ Lysing Solution (BD Biosciences, USA) to each tube for 10 minutes at room temperature. The cells were subsequently analysed by FACS Calibur flow cytometry (BD Biosciences, USA) using Mulltiset software. After the initial screening, 10 ml of the volunteer blood with the normal immune profile was isolated and stored in tubes containing anticoagulants. The blood was then transferred into two 15 ml Falcon tubes (5 ml per tube), diluted with sterile PBS, layered onto Ficoll-Hypague 1.077 g/ml solution and separated by density gradient centrifugation at 1200 rpm for 30 minutes. The buffy coat was then isolated, washed in PBS, counted and suspended at a concentration of 4 x 105 cells/ml.
Viability Assay
Cells were treated with the C. roseus aqueous extract at 1000 μg/ml, 500 μg/ml, 250 μg/ml, 125 μg/ml, 62.5 μg/ml, 31.25 μg/ml, 15.63 μg/ml, 7.81 μg/ml or 3.91 μg/ml in 96-wells plates for 72, 48 or 24 hours. Untreated cells were used as a control. For viability assay of PBMC, the cells treated with phytohaemagglutinin (PHA) were used as a positive control. At the different time intervals, viable cells were determined in triplicate by using the commercial MTS/PMS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carbonxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium/phenazine metho sulfate] kit (Promega, USA). The plates were read at 490 nm using an ELISA reader (Bio Tek, USA). The half maximal inhibitory concentration (IC50) values were determined from the graph as the sample concentration that caused 50% cell death.
DNA Fragmentation
The isolation of fragmented DNA was carried out according to the procedure of Jantova et al. (2003). Jurkat cells at 4 x 105 cells/ml were cultured alone or with C. roseus aqueous extract at 10 μg/ml, 500 μg/ml or 1000 μg/ml for 48 hours at 37°C in a 5% CO2 incubator and then harvested and washed twice with sterile PBS. DNA fragmentation analysis was performed using the AquaPure Genomic DNA Isolation Kit (Bio-Rad, USA) according to the manufacturer’s instruction. Briefly, Jurkat cells (approximately 106) were pelleted and resuspended in 300 μl of genomic DNA lysis buffer. Samples were then incubated at 37°C for 1 hour. Following the lysis, 1.5 μl of RNase (4 mg/ml) was added to the samples, and the tubes were further incubated at 37°C for 1 hour. Samples were allowed to cool at room temperature before the addition of the protein precipitation buffer. After centrifugation, the supernatant was collected and washed once in 100% (v/v) isopropanol and 3 times in 70% (v/v) ethanol. DNA samples were air-dried and resuspended in DNA hydration buffer. After the addition of 1 μl of 5X loading buffer (0.25% bromophenol blue, 40% sucrose), DNA samples was loaded onto a 1% (w/v) Tris-acetate agarose gel. Following electrophoresis at 70 V and 400 mA for 90 minutes, DNA fragmentation was stained with Sybr Green and visualised with a UV transilluminator (Syngene, USA).
Statistical Analysis
All data are expressed as the mean ± SD. Statistical analysis was done using student’s t-test. Dose-response curves between cell viability percentages and extract concentrations were constructed. The cell viability (%) was calculated as follows:
where OD is optical density.
RESULTS
Separation of the Standards
Vinblastine was first used as a standard marker to set the optimal chromatographic conditions. Analysis of the linear calibration curve obtained indicates that the vinblastine was well separated. The relationship between the vinblastine concentration and the peak area was measured using the least square method (R2). The standard calibration curve of vinblastine was y = 12.797x + 0.5433 (R2 = 0.9967). All peaks were detected within the range of 7.01 to 7.05 minutes of retention time.
Analysis of Vinblastine by HPLC
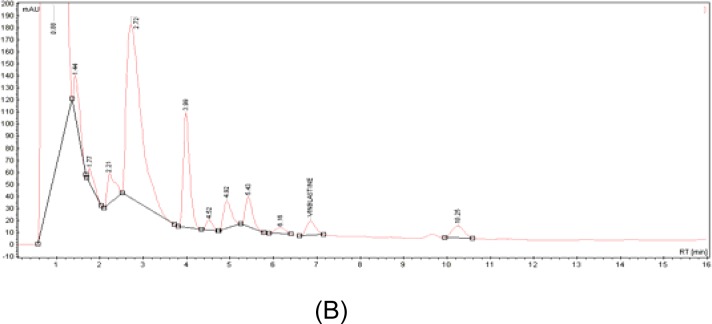
In this study, high performance liquid chromatography (HPLC) was performed for the analysis of vinblastine and other major active compounds in C. roseus aqueous extract. A crude C. roseus aqueous extract was injected into the HPLC system to evaluate the separation efficiencies of the alkaloids [Fig. 1 (B)]. Analyte separation was carried out in less than 11 minutes, and vinblastine was detected at 6.9 minutes of retention time. Based on the equation of the calibration curve obtained, the amount of vinblastine was 0.73 μg in 1.5 mg of aqueous extract, or 0.05% of the sample. In terms of the weight of dried leaves, this amount of vinblastine is equivalent to 0.73 μg/1.5 mg aqueous extract/50 gm of dried leaves, which is 132.47 μg of vinblastine per gram of dried leaves.
Figure 1:
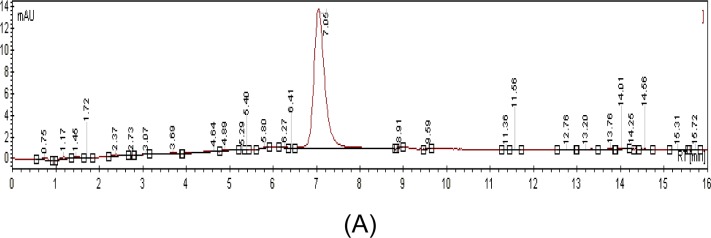
HPLC chromatograms of pure standards (A) and C. roseus crude aqueous extract alkaloids (B). Stationary phase—reverse-phase Microsorb-MV C18 column (150 x 4.6 mm); mobile phase-methanol, phosphate buffer, acetonitrile; flow-rate of mobile phase-0.2 ml/min; detection wavelength-254 nm; injection sample volume-20 μl.
Viability of Jurkat Cells and Normal PBMCs in Response to C. roseus Aqueous Extract
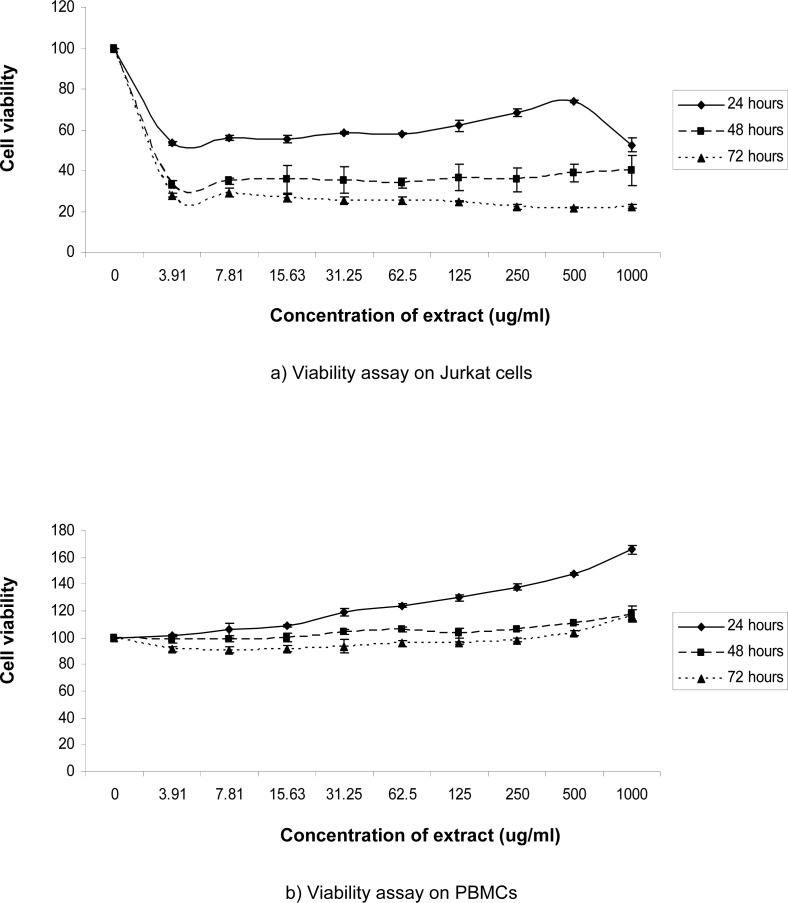
We examined the viability of Jurkat cells and normal PBMCs treated with increasing concentrations of C. roseus aqueous extract (0, 3.91, 7.81, 15.63, 31.25, 62.5, 125, 250, 500 or 1000 μg/ml) for 24, 48 or 72 hours using the MTS assay, as described in Materials and Methods. The extract significantly inhibited the growth of Jurkat cells (p < 0.01) in a dose- and time-dependent manner, especially when treated for 48 and 72 hours, as illustrated in Figure 2 (a) and Figure 3. Cell proliferation consistently decreased, and only 21.84% to 29.00% of the cells survived after 72 hours of incubation. Using an extract concentration of 500 μg/ml, the minimum inhibitory effect was observed after 24 hours, and the maximum inhibitory effect was observed after 72 hours of incubation. Conversely, 1000 μg/ml produced the highest inhibitory effect after 24 hours, but the cells recovered to show the lowest inhibitory effect after 48 hours of incubation with the extract. The IC50 values were determined at 48 hours and 72 hours post-incubation, which were 2.55 μg/ml and 2.38 μg/ml, respectively.
Figure 2:
Cell proliferation by MTS assay in Jurkat cells (a) or normal peripheral blood (b) cultured alone (control) or treated with various concentrations of C. roseus extract for 24, 48 or 72 hours. Results (optical densities) were calculated as the percentage of unexposed control cells. Error bars represents the mean ± SD.
Figure 3:
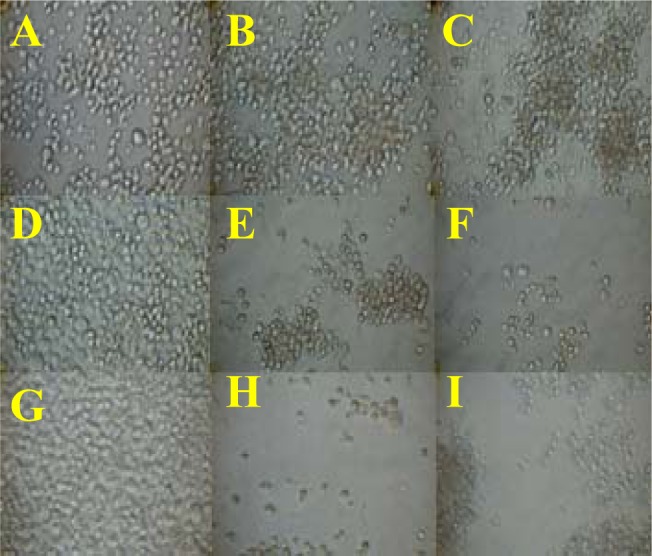
Morphology of Jurkat cells post-treatment. Untreated cells at 24, 48 and 72 hours (A, D, G). Cells treated with 500 μg/ml extract for 24, 48 or 72 hours (B, E, H). Cells treated with 1000 μg/ml extract for 24, 48 or 72 hours (C, F, I).
In this study, we also investigated the effects of C. roseus extract on PBMCs from normal volunteers. The results from our study demonstrate that, compared to the cytotoxic effects using Jurkat cells, the C. roseus extract significantly induced the proliferation of PBMCs (p < 0.01) taken from normal donors, as illustrated in Figure 2 (b). The extract induced the proliferation of normal PBMCs, especially after 24 hours of incubation. Although cell viability started to decrease after 48 hours, we found that the values were still above the untreated control cells. However, cell proliferation was slightly inhibited at 72 hours, specifically at low concentrations of 3.91 to 250 μg/ml. Overall, maximum cell proliferation was observed in PBMCs incubated with 1000 μg/ml of the C. roseus extract, with increases of 165.93% at 24 hours, 117.67% at 48 hours and 116.67% at 72 hours.
C. roseus Aqueous Extract Induces DNA Fragmentation in Jurkat Cells
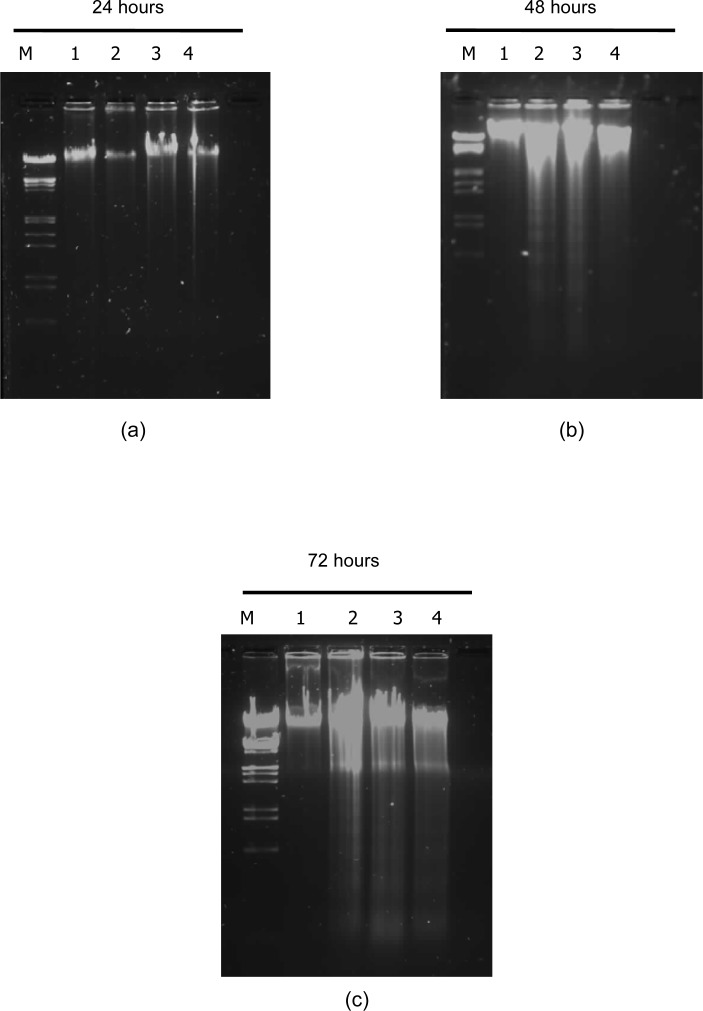
To determine if the C. roseus aqueous extract causes cell death by apoptosis in Jurkat cells, DNA laddering was performed. As shown in Figure 5, the extract could induce apoptosis in a dose- and time-dependent manner, as indicated by significant DNA fragmentation in treated cells, whereas no DNA fragmentation was seen in control cells. Cells treated with extract concentrations of 10, 500 and 1000 μg/ml exhibited significant DNA fragmentation, especially after incubation for 48 and 72 hours [Fig. 5 (b) and (c)].
Figure 5:
DNA expression in Jurkat cells. Jurkat cells were cultured alone or treated with various concentrations of aqueous extract C. roseus for 24 hours (a), 48 hours (b) or 72 hours (c). M-marker (VC Lambda/ EcoRI Hind III), Lane 1-Control cells (untreated), Lane 2-Cells treated with 10 μg/ml, Lane 3-Cells treated with 500 μg/ml, Lane 4-Cells treated with 1000 μg/ml.
DISCUSSION
To our knowledge, not many studies have been done to determine the cytotoxicity of the C. roseus aqueous extract and therefore, we decided to use a double dilution manner ranging from 3.91 to 1000 μg/ml. We also used the same experimental design for both leukaemic Jurkat cells and normal PBMCs to compare the overall growth pattern of the two cell types. Interestingly, our preliminary study suggested that the aqueous extract of C. roseus produced differential effects on Jurkat cells at higher doses of concentrations which were 500 and 1000 μg/ml. These unique inhibitory effects at high doses of extracts may indicate that the cells have the ability to enter the recovery phase at a certain state. In addition, the result suggests that after 24 hours of incubation, more than 50% of Jurkat cells remain viable even at the highest concentration of extract. Therefore, this finding revealed that the Jurkat cells were highly sensitive to the extract after 48 and 72 hours of exposure. The cytotoxic effect may be due to the anticancer compounds present in the aqueous extract of C. roseus such as vinblastine. Presence of this trace amount of vinblastine encouraged us to investigate what are the other active compounds that exist in the extract. Previous study has shown that only a fraction of these chemical entities has been analysed and much less are available commercially (Hisiger & Jolicoeur 2007).
Previous studies showed that pure vinblastine exhibits anti-leukaemic properties in vitro (Caron & Herwood 2007). Vinblastine is one of the first vinca alkaloids with anti-tumour activity to be identified, and it was shown to be able to kill the actively dividing tumour cells by inhibiting progression through mitosis with metaphase arrest (Nobili et al. 2009; Okouneva et al. 2003). The analysis of the extract should be further studied as according to the criteria of the American National Cancer Institute, the IC50 limit to consider a crude extract promising for further purification is lower than 30 μg/ml (Suffness & Pezzuto 1990).
In this study, the results of cell viability assays and morphological analyses (Fig. 3) showed that C. roseus crude aqueous extract significantly (p < 0.01) killed Jurkat cells in a time- and dose-dependent manner. The most common indicators of apoptosis include morphologic changes examined by light or electron microscopy and DNA fragmentation detected by colorimetric assay or agarose gel electrophoresis (Mangan et al. 1993). In our study, we evaluated the ability of the extract to induce DNA fragmentation by gel electrophoresis (Fig. 4). In each case, DNA fragmentation was characterised by oligonucleosomal size fragments of about 180–200 base pairs (bp), a well-known feature indicative of apoptosis (Compton 1992).
Figure 4:
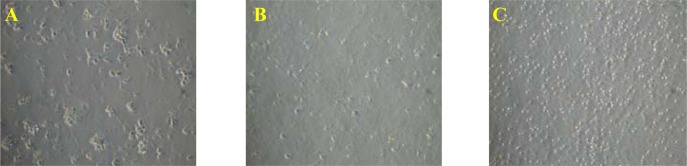
Morphology of normal peripheral blood mononuclear cells after 48 hours post-treatment. Cells treated with phytohaemagglutinin (PHA) were used as a positive control (A), untreated cells (B) and treated with 1000 μg/ml of extract (C).
Results from this study demonstrate the potential use of C. roseus crude aqueous extract at very low doses to inhibit the proliferation of Jurkat cells. The DNA fragmentation result shows that the cytotoxicity effect of the extract is due to apoptosis. However, we do not know which compound or component of the extract triggered apoptosis. In addition, chemical interactions between the major active compounds in the C. roseus extract may act in concert to inhibit Jurkat cells proliferation. A number of studies have documented the more significant and interesting inhibitory effects of crude extract on cell growth as compared to the purified compounds (Kuete et al. 2008; Kang et al. 2005).
The results from this study indicate that the extract is able to induce the proliferation of PBMC. This population of cells comprise of at least 80% T cell, 10% B cell and 10% natural killer (NK) cells (Abbas et al. 2000). It is unclear whether the extract acts directly or indirectly on the various types of cells in this population. It may be possible that the extract acts equally on all types of cells found in PBMC on specific population of the cells. It has been shown that different types of mitogens act differently on lymphocytes, for example PHA-acts specifically to induce the proliferation of T cell while Staphylococcus protein A (Sp A) is specific to B cell (Stites et al. 1987). However, the result from this study indicates that there is a uniform increase in the cell population rather than distinct formation of clumps (Fig. 4). This suggests that C. roseus extract maybe acting as a universal activator of immune cells proliferation rather than acting on specific subpopulation of cells as seen with known mitogen, such as PHA or Sp A.
Our results indicate that the extract may contain compounds that specifically induce cell death in Jurkat cells but on the other hand induce cell proliferation in normal cells. This result concurs with a number of similar studies (Okonogi et al. 2007; Nobili et al. 2009). The growth-promoting effect of the extract on normal white blood cells indicates that it may contain compounds with mitogenic activity. HPLC analysis shows that there are at least 12 different components in the C. roseus extract used in this study. Which of these compounds possess mitogenic activity is not known and requires further bioassay of each fraction. There might be an interaction between subsets of cells in the PBMC consisting of lymphocytes, plasma cells, monocytes and mast cells. The extract could be useful as a natural source of nutraceutical for preventive medicine since a good cancer-preventive agent should show inhibitory activity towards cancer cell growth but harmless to human PBMC (Okonogi et al. 2007).
However, Jurkat cells may have lost the ability to respond to the mitogenic activity of the extract. The changes associated with the transformed state of the cells may increase their susceptibility to the cytotoxic effects of the C. roseus extract. Further investigations on the compounds of C. roseus extract and their effect on Jurkat cells are currently going on in our laboratory to further assess their usefulness.
CONCLUSION
These results indicate that the crude aqueous extract of C. roseus shows differential effects of inhibiting the proliferation of Jurkat cells and promoting normal peripheral blood immune cells proliferation. This finding suggests that the extract may be efficacious in patients with this type of cancer.
Acknowledgments
This research was supported by a MAKNA (Majlis Kanser National) grant. We would like to thank Siti Razila Abd Razak, Tarmizi Abu Baker, Adilah Abdul Khalil and Siti Salwa Zulkifli for their cooperation and technical expertise.
REFERENCES
- Abbas AK, Lichtman AH, Pober JS. Cellular and molecular immunology. 4th ed. Philadelphia, USA: W.B. Saunders Company; 2000. [Google Scholar]
- Caron JM, Herwood M. Vinblastine, a chemotherapeutic drug, inhibits palmitoylation of tubulin in human leukemic lymphocytes. Chemotherapy. 2007;53(1):51–58. doi: 10.1159/000098419. [DOI] [PubMed] [Google Scholar]
- Chung KT, Wong TY, Wei CI, Huang YW, Lin Y. Tannins and human health: A review. Critical Reviews in Food Science and Nutrition. 1998;38(6):421–464. doi: 10.1080/10408699891274273. [DOI] [PubMed] [Google Scholar]
- Compton MM. A biochemical hallmark of apoptosis: Internucleosomal degradation of the genome. Cancer and Metastasis Reviews. 1992;11(2):105–119. doi: 10.1007/BF00048058. [DOI] [PubMed] [Google Scholar]
- Don G. Catharanthus roseus. In: Ross IA, editor. Medicinal plants of the world: Chemical constituents, traditional and modern uses, vol 1. Totowa, New Jersey, USA: Humana Press; 2003. pp. 175–196. [Google Scholar]
- Gidding CEM, Kellie SJ, Kamps WA, de Graaf SSN. Vincristine revisited. Critical Reviews in Oncology/Hematology. 1999;29(3):267–287. doi: 10.1016/s1040-8428(98)00023-7. [DOI] [PubMed] [Google Scholar]
- Hisiger S, Jolicoeur M. Analysis of Catharanthus roseus alkaloids by HPLC. Phytochemistry Reviews. 2007;6(2–3):207–234. [Google Scholar]
- Jantova S, Cipak L, Cernakova M, Kostalova D. Effect of berberine on proliferation, cell cycle and apoptosis in HeLa and L1210 cells. Journal of Pharmacy and Pharmacology. 2003;55(8):1143–1149. doi: 10.1211/002235703322277186. [DOI] [PubMed] [Google Scholar]
- Kang JX, Liu J, Wang J, He C, Li FP. The extract of huanglian, a medicinal herb, induces cell growth arrest and apoptosis by upregulation of interferon-β and TNF-α in human breast cancer cells. Carcinogenesis. 2005;26(11):1934–1939. doi: 10.1093/carcin/bgi154. [DOI] [PubMed] [Google Scholar]
- Kuete V, Ngameni B, Simo CC, Tankeu RK, Ngadjui B, Meyer JJM, Lall N, Kuiate JR. Antimicrobial activity of the crude extracts and compounds from Ficus chlamydocarpa and Ficus cordata (Moraceae) Journal of Ethnopharmacology. 2008;120(1):17–24. doi: 10.1016/j.jep.2008.07.026. [DOI] [PubMed] [Google Scholar]
- Mangan DF, Mergenhagen SE, Wahl SM. Apoptosis in human monocytes: Possible role in chronic inflammatory diseases. Journal of Periodontology. 1993;64:461–466. [PubMed] [Google Scholar]
- Nobili S, Lippi D, Witort E, Donnini M, Bausi L, Mini E, Cappaccioli S. Natural compounds for cancer treatment and prevention. Pharmacological Research. 2009;59(6):365–378. doi: 10.1016/j.phrs.2009.01.017. [DOI] [PubMed] [Google Scholar]
- Okonogi S, Duangrat C, Anuchpreeda S, Tachakittirungrod S, Chowwanapoonpohn S. Comparison of antioxidant capacities and cytotoxicities of certain fruit peels. Food Chemistry. 2007;103(3):839–846. [Google Scholar]
- Okouneva T, Hill BT, Wilson L, Jordan MA. The effects of vinflunine, vinorelbine, and vinblastine on centromere dynamics. Molecular Cancer Therapeutics. 2003;2(5):427–436. [PubMed] [Google Scholar]
- Philip PA. Experience with docetaxel in the treatment of gastric cancer. Seminars in Oncology. 2005;32(Supp. 4):24–38. doi: 10.1053/j.seminoncol.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Renault JH, Nuzillard JM, Crouérour GL, Thépenier P, Zèches-Hanrot M, Le Men-Olivier L. Isolation of indole alkaloids from Catharanthus roseus by centrifugal partition chromatography in the pH-zone refining mode. Journal of Chromatography A. 1999;849(2):421–431. doi: 10.1016/s0021-9673(99)00495-1. [DOI] [PubMed] [Google Scholar]
- Sottomayor M, Ros Barceló A. The Vinca alkaloids: From biosynthesis and accumulation in plant cells, to uptake, activity and metabolism in animal cells. In: Rahman A, editor. Studies in natural products chemistry (bioactive natural products) The Netherlands: Elsevier Science Publishers; 2005. pp. 813–857. [Google Scholar]
- Stites DP, Stobo JD, Wells JV. Basic and clinical immunology. California: Appleton and Lange; 1987. p. 295. [Google Scholar]
- Suffness M, Pezzuto JM. Assays related to cancer drug discovery. In: Hostettmann K, editor. Methods in plant biochemistry: Assays for bioactivity, vol 6. London: Academic Press; 1990. pp. 71–133. [Google Scholar]
- Tang W, Hemm I, Bertram B. Recent development of antitumor agents from Chinese herbal medicines, part I. Low molecular compounds. Planta Medica. 2003;69(2):97–108. doi: 10.1055/s-2003-37718. [DOI] [PubMed] [Google Scholar]
- Tyler VE. Medicinal plant research: 1953–1987. Planta Medica. 1988;54(2):95–100. doi: 10.1055/s-2006-962359. [DOI] [PubMed] [Google Scholar]
- Van der Heijden R, Jacobs DI, Snoeijer W, Hallard D, Verpoorte R. The Catharanthus alkaloids: Pharmacognosy and biotechnology. Current Medicinal Chemistry. 2004;11(5):607–628. doi: 10.2174/0929867043455846. [DOI] [PubMed] [Google Scholar]
- World Health Organisation . WHO guideline for the assessment of herbal medicines. WHO Expert Committee on specification for pharmaceutical preparation. Technical Report series no. 863. Geneva: WHO; 1996. [Google Scholar]