Abstract
Genetic engineering is a powerful tool for the improvement of plant traits. Despite reported successes in the plant kingdom, this technology has barely scratched the surface of the Melastomataceae family. Limited studies have led to some optimisation of parameters known to affect the transformation efficiency of these plants. The major finding of this study was to optimise the presence of selected enhancers [e.g., monosaccharides (D-glucose, D-galactose and D-fructose), tyrosine, aluminium chloride (AICI3) and ascorbic acid] to improve the transformation efficiency of Tibouchina semidecandra. Agrobacterium tumefaciens strain LBA4404 harbouring the disarmed plasmid pCAMBIA1304 was used to transform shoots and nodes of T. semidecandra. Different concentrations of the transformation enhancers were tested by using green fluorescent protein (GFP) as a reporter. The results obtained were based on the percentage of GFP expression, which was observed 14 days post-transformation. A combination of 120 μM galactose and 100 μM tyrosine supplemented with 600 μM AICI3 in the presence of 15 mg/l ascorbic acid gave the highest percentage of positive transformants for T. semidecandra shoots. Whereas 60 μM galactose and 50 μM tyrosine with 200 μM AICI3 in the presence of 15 mg/l ascorbic acid was optimum for T. semidecandra nodes. The presence of the hygromycin phosphotransferase II (hptII) transgene in the genomic DNA of putative T. semidecandra transformants was verified by PCR amplification with specific primers.
Keywords: Monosaccharide, Tyrosine, Aluminium Chloride, Ascorbic Acid, GFP Transient Expression
Abstract
Kejuruteraan genetik adalah satu peralatan canggih untuk memperbaiki ciri tumbuhan. Walaupun dilaporkan berjaya dalam alam tumbuhan, teknologi ini hanya mencalar sedikit permukaan famili Melastomataceae. Kajian terhad telah dilakukan untuk memperolehi keputusan yang terbaik bagi parameter yang diketahui mempengaruhi kecekapan transformasi tumbuhan ini. Hasil utama kajian ini adalah penemuan keadaan yang terbaik dengan kehadiran perangsang yang tertentu [contohnya, monosakarida (D-glukosa, D-galaktosa dan D-fruktosa), tirosina, aluminium klorida (AICI3) dan asid askorbik] dalam meningkatkan kecekapan transformasi Tibouchina semidecandra. Agrobacterium tumefaciens stren LBA4404 dengan plasmid pCAMBIA1304 telah digunakan dalam transformasi keratan pucuk and tunas ketiak T. semidecandra. Pelbagai kepekatan perangsang transformasi telah dikaji dengan menggunakan green fluorescent protein (GFP) sebagai penanda. Keputusan yang diperolehi adalah berdasarkan kepada peratusan ekspresi GFP yang diperhatikan pada hari ke-14 selepas transformasi dilakukan. Kombinasi 120 μM galaktosa dan 100 μM tirosina berserta dengan 600 μM AICI3 dan kehadiran 15 mg/l asid askorbik telah memberi peratus kejadian transformasi yang tertinggi bagi keratan pucuk T. semidecandra. Sedangkan 60 μM galaktosa dan 50 μM tirosina berserta dengan 200 μM AICI3 dan kehadiran 15 mg/l asid askorbik adalah terbaik bagi keratan tunas ketiak T. semidecandra. Kehadiran hygromycin phosphotransferase II (hptII) transgen di dalam DNA genomik bagi putatif T. semidecandra transforman telah dikaji dengan amplifikasi PCR menggunakan primer yang spesifik.
Tibouchina semidecandra (Schrank & Mart. ex DC.) Cogn. belongs to the Melastomataceae (Abdullah & Yong 2007) family, and Melastomataceae spp. has great potential as an ornamental plant. Locally known as ‘senduduk biru’, T. semidecandra, produces attractive purplish blue flowers at a very young age and blooms continuously, which is characteristic of tropical ornamental plants. Nurseries in Malaysia and other southeast Asian countries propagate and sell them as landscaping plants for borders and foundation planting. However, uncontrolled growth of the plants, limited colours and limited lifespan of each individual flower have reduced their commercial value (Yong et al. 2009). To improve the quality of the plants and/or develop new varieties, genetic transformation is recommended because it has opened new avenues to the modification of characteristics, such as flower colour (Fukusaki et al. 2004), fragrance (Zuker et al. 2002), longevity (Mitiouchkina & Dolgov 2000), shape and size (Giovannini et al. 1999). The most effective transformation technique is probably Agrobacterium-mediated transformation. Agrobacterium-mediated transformation was the method of interest in this study using T. semidecandra as the target plant species. To date, the only reports of genetic manipulation carried out on T. semidecandra are two studies by Yong et al. on system optimisation (2006) and on transformation of dihydroflavonol-4-reductase (DFR) genes (2009).
Plant transformation mediated by the soil-borne pathogen Agrobacterium tumefaciens was first reported in the early 1980s (Gelvin 2003). Since then, Agrobacterium-mediated transformation has been extensively used to genetically modify dicotyledonous as well as monocotyledonous plants such as tobacco (Slavov et al. 2005), torenia (Li et al. 2007), tomato (Sui et al. 2007), barley (Shrawat et al. 2007) and rice (Ozawa 2009). The efficacy of transformation is highly dependent on many factors such as the bacterial strain, plant species and the type of tissue explant used. The key factors in the method are a ‘super-binary’ vector and the addition of acetosyringone to the co-cultivation medium as an enhancer. General parameters such as pre-culture time, co-cultivation time, immersion time, bacterial concentration, wound type and acetosyringone concentration were shown to influence the transformation efficiency by Yong et al. (2006), and the results obtained were based on the percentage of green fluorescent protein (GFP) gene expression, which was observed three days post-transformation.
In this study, instead of costly acetosyringone, different concentrations of D-monosaccharides, tyrosine, aluminium chloride (AICI3) and ascorbic acid were tested as enhancers to improve the transformation efficiency of T. semidecandra. These groups of compounds were expected to be able to replace the function of acetosyringone as transformation enhancers, as reported by previous researchers (Shimoda et al. 1990: Sarker et al. 2003; Okamoto et al. 2005; Abdullah & Yong 2007). Expression of the GFP gene, a useful non-invasive and non-destructive marker, was used to evaluate the transformation parameters. The optimised conditions have potential for use in the integration of desirable commercially important genes into this plant species to improve its quality and value as an ornamental plant.
In vitro-generated plantlets of T. semidecandra from Plant Biotechnology Laboratory, Faculty of Biotechnology and Biomolecular Sciences, Universiti Putra Malaysia, Selangor were used as sources of explants. The plantlets were multiplied and maintained on half-strength MS basal medium (Murashige & Skoog 1962) under a 16-h light/8-h dark photoperiod at 25 ± 2°C. Shoots (2 leaf stage) and nodes (all 3 nodes counting from the apex) from 6- to 8-week old plantlets showing high regeneration ability were used as explant material for transformation. All experiments were carried out with 3 replicates per treatment and 20 explants per replicate.
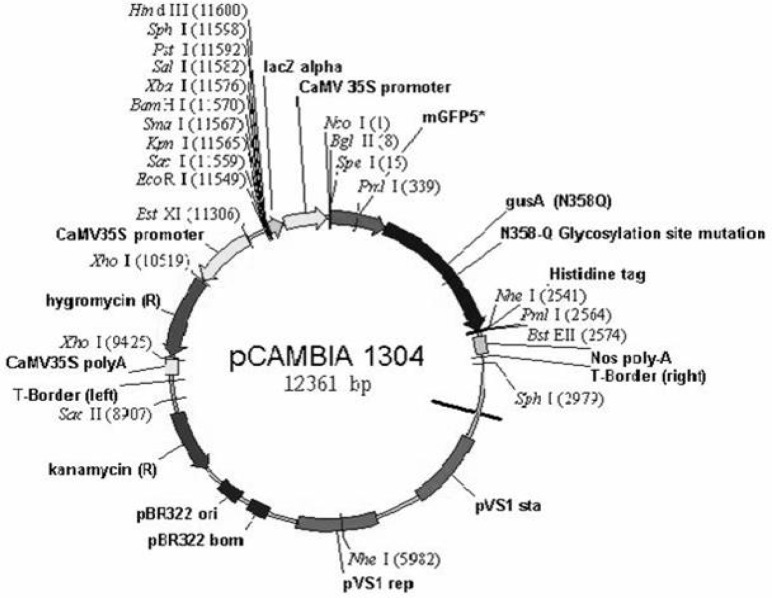
The binary vector pCAMBIA1304 (CSIRO, Australia) harbouring the reporter mgfp5 driven by the CaMV 35S promoter (as illustrated in Fig. 1) was used. The plasmid also contains hygromycin phosphotransferase II (hptII) (the coding region of the hptII gene for hygromycin resistance in plant systems) as a selectable marker. The plasmid DNA was prepared from Escherichia coli using the alkaline lysis method (Sambrook et al. 1989) and mobilised into A. tumefaciens LBA4404 (Yong et al. 2006) prior to transformation of T. semidecandra. The plasmid yield was determined by a spectrophotometer (Pharmacia LBK-U1-trospec III, LKB Biochrom Ltd., England).
Figure 1:
Plasmid map of pCAMBIA1304.
Explants of T. semidecandra as described above were prepared and pre-cultured for three days on half-strength MS basal medium prior to being transferred into Agrobacterium suspension (OD600 0.8) with the addition of monosaccharide and tyrosine. The bacterial suspension and explants were mixed and gently shaken at 150 rpm to ensure that all the explants were fully submerged (Yong et al. 2006). After an hour of immersion, the explants were blotted dry on sterile filter paper and subsequently transferred to the co-cultivation medium supplemented with AICI3 and ascorbic acid for three days. The effects of the selected transformation enhancers were tested in combination, where addition of 30 μM glucose and 50 μM tyrosine into Agrobacterium suspension together with 200 μM AICI3 and 85 μM (15 mg/l) ascorbic acid in the co-cultivation medium were treated as the control. In this study, different concentrations of monosaccharides (0, 30, 60, 90, 120 and 150 μM), tyrosine (0, 50, 100, 150 and 200 μM) and AICI3 (0, 200, 400, 600 and 800 μM) were assessed, as was the presence of ascorbic acid. The concentration ranges for these compounds were based on previous studies carried out by Shimoda et al. (1990), Sarker et al. (2003), Okamoto et al. (2005) and Abdullah and Yong (2007). The cultures were incubated at 25 ± 2ºC under a 16-h light/8-h dark photoperiod. After co-cultivation for 3 days, the explants were washed by gentle shaking for 30 min in Petri dishes containing liquid culture medium 5 times to remove the bacteria (Puchooa 2004) before being transferred to bacterial elimination medium (half strength MS containing 100 mg/l cefotaxime). The bacterial elimination treatment was repeated every three days to remove the bacteria completely.
All parameters were optimised by screening for transient green flourescent protein (GFP) expression using a fluorescence stereomicroscope (Leica MZFL III, Leica Microsystems Inc., Bannockburn, IL, USA) equipped with a GFP2 filter set. GFP observations were carried out daily and targeted to the newly regenerated shoots. The results obtained were based on the highest GFP expression at 14 days post-transformation, when the Agrobacterium was completely eliminated. The data were analysed using one-way ANOVA, and the differences were assessed using Tukey’s multiple comparison test. The statistical analyses were performed using SPSS 17.0 (SPSS Inc., USA) at the 5% level.
Newly regenerated shoots from randomly selected GFP-positive transformants at 14 days post-transformation were subjected to genomic DNA extraction and amplification of the hptII transgene by PCR with specific primers (hpt1: 5′-TAG GAGGGCGTGGATATGTC-3′ and hpt2: 5′-TAC ACAGCCATCGGTCCAGA-3′) as suggested by Zou et al. (2004) using standard protocols (Sambrook & Russell 2001), which provided an amplification product of ∼800 bp. PCR was performed using a Mastercycler Gradient (Eppendorf, Germany) machine. The amplifications were carried out in 25 μl reaction volumes containing 100 ng genomic DNA, PCR buffer (10 mM Tris-Cl, 50 mM KCl and 0.8% Nonidet P40), dNTP mix (200 μM each), 1.5 mM MgCl2, 15 pmole of each primer and 5 U taq DNA polymerase. The PCR was carried out with an initial denaturation step of 5 min at 94°C, followed by 35 cycles of denaturation (94°C, 30 s), annealing (55°C, 30 s) and elongation (72°C, 30 s) and a final extension step of 10 min at 72°C.
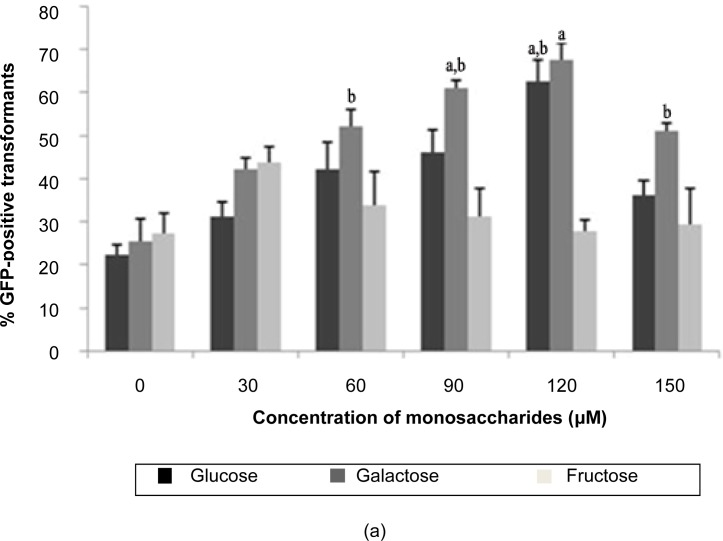
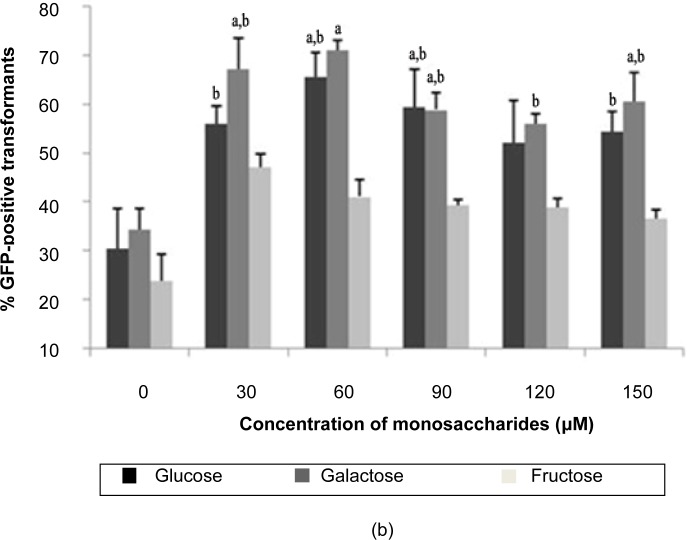
The optimisation of T. semidecandra explant transformation efficiency with monosaccharide (D-glucose, D-galactose and D-fructose) treatment is presented in Figure 2. Among the different concentrations of the 3 types of monosaccharide, 120 μM and 60 μM galactose gave the highest percentages of GFP expression for both shoot (67.78%) and node (61.11%) explants. Based on this observation, the trends of GFP expression for glucose and galactose treatments increased simultaneously with increases in their respective monosaccharide concentrations, up to 120 μM for shoots and 150 μM for nodes, although the trend observed for the nodes fluctuated. The results indicated that the GFP expression profile for fructose treatment was different from that of glucose and galactose, where fructose above the concentration of 30 μM decreased GFP expression in both shoot and node explants. This phenomenon may be due to the structural differences between the different functional carbonyl groups of these monosaccharides; glucose and galactose contain an aldehyde group, whereas fructose contains a keto group. A previous study reported that the aldehyde monosaccharide form in aldoses can markedly enhance the expression of vir genes in Agrobacterium for T-DNA transfer into plant cells (Cangelosi et al. 1990; Shimoda et al. 1990). The key molecule that allows Agrobacterium to sense environmental conditions favourable for T-DNA transfer is the VirA protein (Lee et al. 1996). For optimal vir gene induction, the periplasmic domain of VirA is a sensor of a variety of monosaccharides on the cell walls of plants, including arabinose, galactose, galacturonic acid, glucose, glucuronic acid, mannose, fucose, cellobiose and xylose (Ankenbauer & Nester 1990). In this study, the addition of monosaccharides significantly enhanced the transformation efficiency of T. semidecandra, and galactose was found to be the most favourable monosaccharide utilised by Agrobacterium in this process.
Figure 2:
Percentage of GFP expression observed 14 days post-transformation with different concentrations of monosaccharides: (a) shoots, (b) nodes.
Notes: Error bars indicate standard deviations (n = 3). Different letters indicate value that are significantly different (p ≤ 0.05).
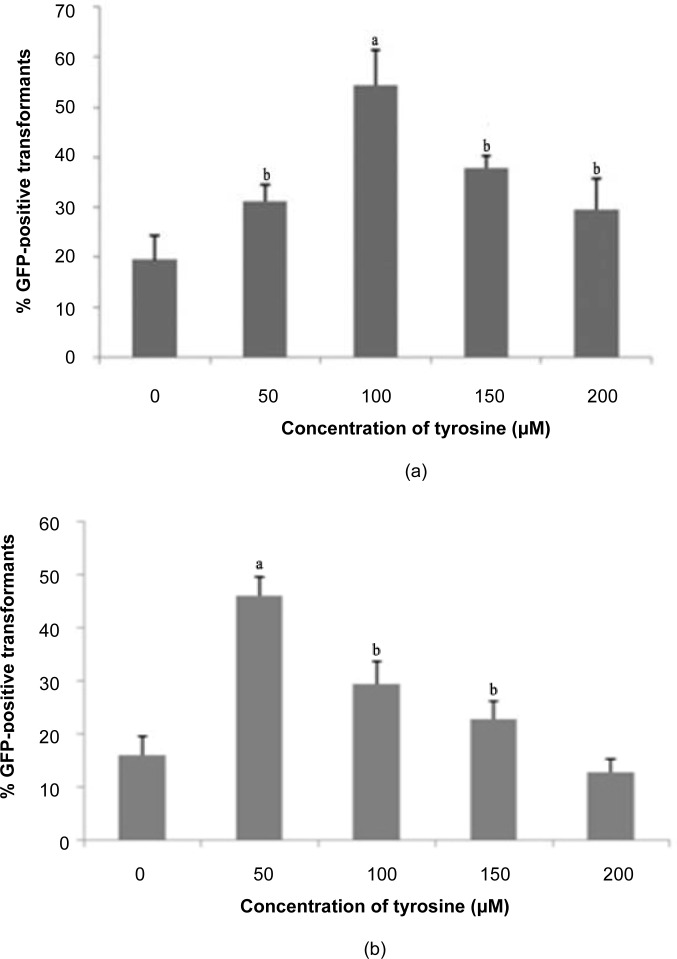
The highest GFP expression was observed with 100 μM tyrosine treatment for shoot explants (54.45%) and 50 μM tyrosine treatment for the nodes (46.11%), as presented in Figure 3. Tyrosine can be used as an alternative transformation enhancer due to its structure, which is similar to acetosyringone (Baker et al. 2005). In previous research carried out by Sarker et al. (2003), transformed explants were co-cultivated on tyrosine to enhance transformation efficiency and the plant regeneration process. Both acetosyringone and tyrosine contain an aromatic benzene structure similar to phenolic compounds present in plants that are responsible for Agrobacterium vir gene induction (Joubert et al. 2002). However, using tyrosine for transformation is more economical than using acetosyringone. While supplementation with phenolic compounds did enhance transformation efficiency, the degree of influence was dependent upon its concentration. Guivarc’h et al. (1993) reported that more than 75 μM acetosyringone rarely promoted gene transfer, although levels up to 200 μM are not considered to be significantly toxic to Agrobacterium cells. In addition, the decreased transformation efficiency observed above the optimum level of tyrosine may be associated with the accumulation of avirulent mutants, as indicated by Fortin et al. (1992).
Figure 3:
Percentage of GFP expression observed 14 days post-transformation with different concentrations of tyrosine: (a) shoots, (b) nodes.
Notes: Error bars indicate standard deviations (n = 3). Different letters indicate values that are significantly different (p ≤ 0.05).
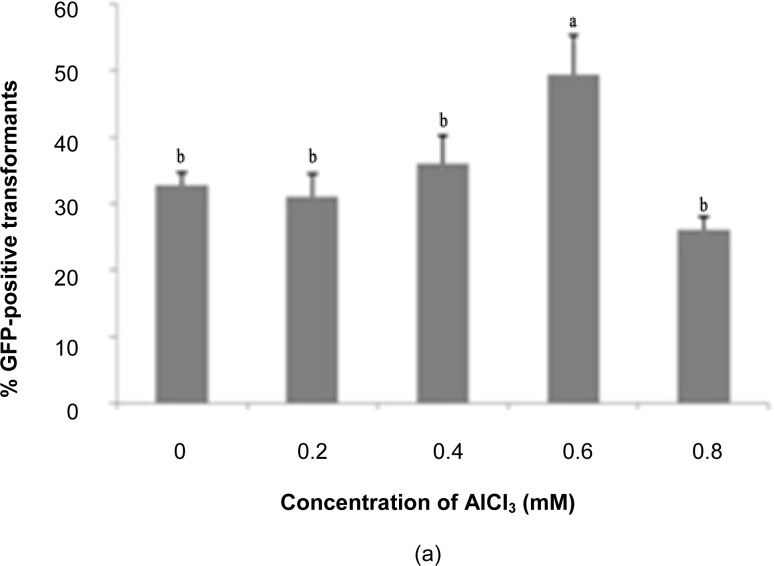
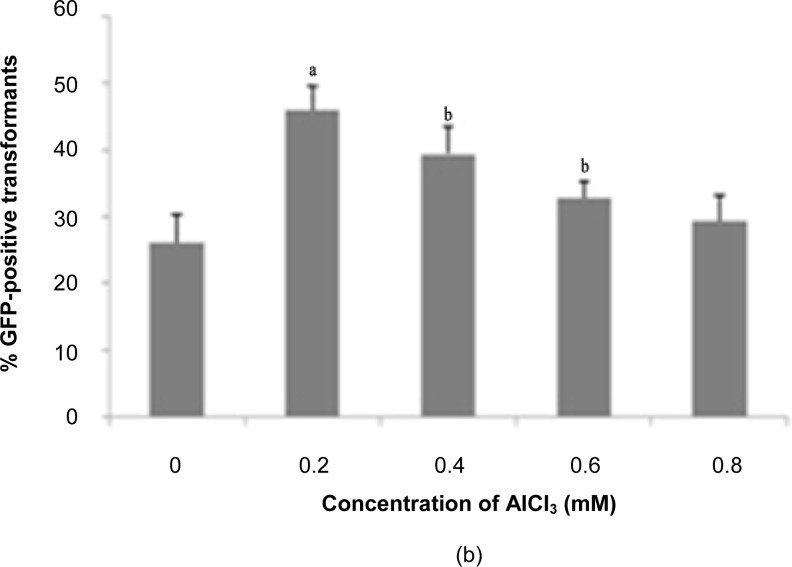
The effect of AlCl3 on the transformation efficiency of T. semidecandra explants is demonstrated in Figure 4. Supplementation with 600 μM AlCl3 gave the highest GFP expression for shoot explants (49.44%), while 200 μM AlCl3 was the optimum level for nodes (46.11%). Addition of AlCl3 to the co-cultivation medium notably facilitates the processes of replication, transcription and translation of the transgenic protein in plant cells (Davey et al. 2005). Aluminium toxicity is often a primary factor affecting crop productivity, but the growth of Melastomataceae spp. was reported to improve when the growth medium was supplemented with aluminium at 500 μM (Watanabe & Osaki 2002; Watanabe et al. 2005). Jansen et al. (2002) surveyed members of this family and found at least 127 species (including Tibouchina sp.) with the capacity to accumulate aluminium. However, above tolerable levels, excess aluminium may cause toxicity to plants via inhibition of cell elongation and cell division, leading to tissue stunting accompanied by reduced water and nutrient uptake (Samac & Tesfaye 2003). This is evidenced by decreased explant survival rates above the optimum levels of aluminium. The mechanisms of aluminium toxicity and tolerance in plants were specifically discussed by Delhaize and Ryan (1995).
Figure 4:
Percentage of GFP expression observed 14 days post-transformation with different concentrations of AICI3: (a) shoots, (b) nodes.
Notes: Error bars indicate standard deviations (n = 3). Different letters indicate values that are significantly different (p ≤ 0.05).
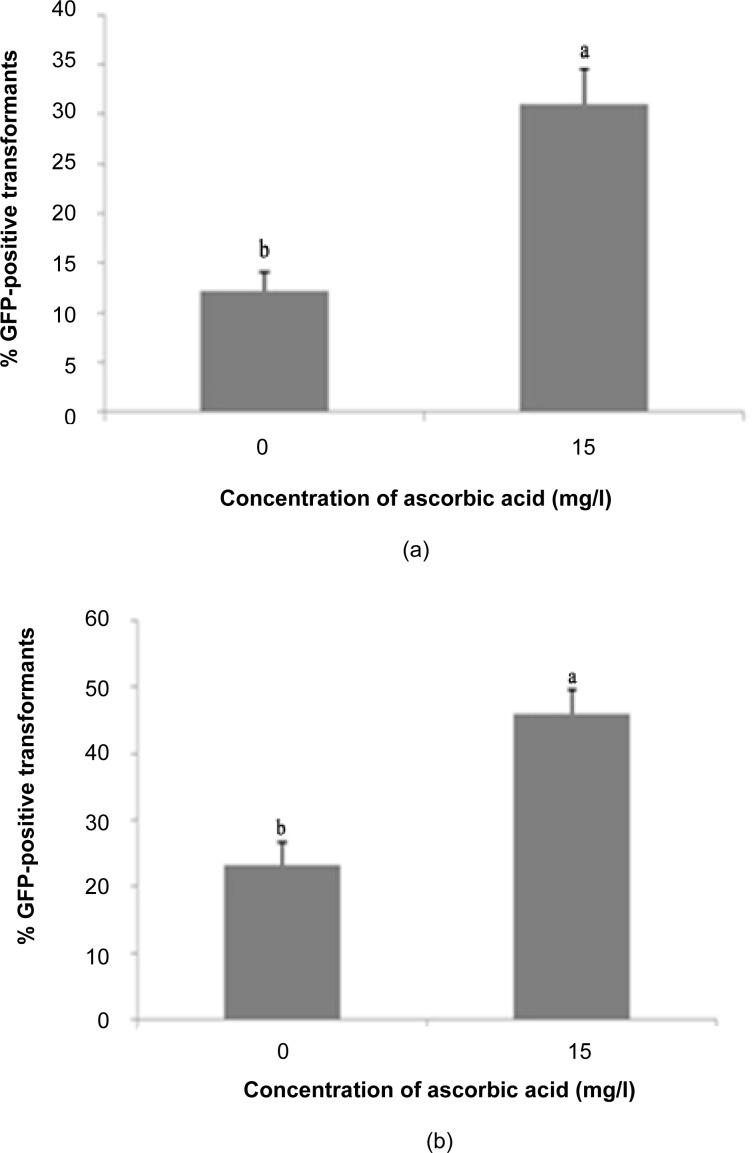
With the exclusion of ascorbic acid, the percentage of GFP expression in transformed shoots decreased by about 60%, with only 12.22% positive expression compared to 31.11% with ascorbic acid treatment (Fig. 5). For transformed nodes, the percentage of GFP expression increased about 50% (from 23.33% to 46.11%) with the addition of ascorbic acid to the co-cultivation medium. Tissue browning is apparently a part of the plant defence machinery, as this makes a dead cell barrier around wounded sites that protects plants from further spreading of the injury (Okamoto et al. 2005). Thus, plants with high browning activity are recalcitrant to Agrobacterium-mediated transformation because their defence responses are often induced by Agrobacterium infection (Pu & Goodman 1992; Hansen 2000). Application of reducing compounds, such as ascorbic acid, cysteine and dithiothreitol, has contributed to the establishment of Agrobacterium-mediated transformation of some crop plants (Perl et al. 1996; Enriquez-Obregon et al. 1999; Frame et al. 2002), probably due to suppression of tissue browning. Inclusion of ascorbic acid in the post-transformation culture medium decreases the cell death rate in explants by inhibiting hypersensitive responses to damage generated during tissue manipulation. A previous study by Enriquez-Obregon et al. (1997) reported that reducing compounds were able to decrease the hypersensitivity reaction in cut zones of sugarcane meristematic explants, and they subsequently improved the competence of the plant tissue for Agrobacterium-mediated gene transfer.
Figure 5:
Percentage of GFP expression observed 14 days post-transformation for ascorbic acid treatment: (a) shoots, (b) nodes.
Notes: Error bars correspond to standard deviation (n = 3). Different letters indicate values are significantly different (p ≤ 0.05).
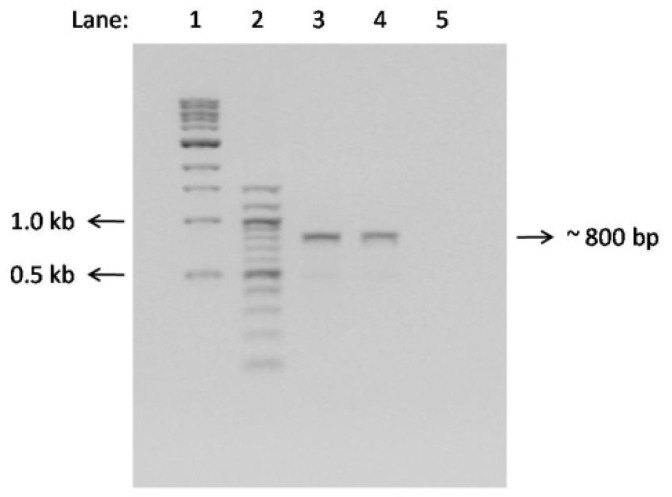
The result obtained from the PCR analysis revealed that ∼800 bp of the hptII transgene was successfully amplified from putative transformants randomly selected from newly regenerated shoots (Fig. 6). Analyses of regenerated shoots from putative transformants are recommended to reduce the chimerism rate in the future recovery of transgenic plants (Ahn et al. 2007). Untransformed plants were used as controls, and no bands were amplified from them in the PCR analyses. The hptII gene was co-integrated with the GFP gene in the selected putative transformants. Co-transformation of the mgfp5 and hptII genes indicated that there was no breakage of T-DNA during the transformation process. The Agrobacterium-mediated delivery of foreign DNA into target tissue was found to be superior to microprojectile bombardment, which tended to promote DNA breakage and subsequently reduce the transformation efficiency (Yong et al. 2009).
Figure 6:
PCR analysis of the hptII transgene in GFP-positive transformants; lane 1, 2 = molecular weight markers (1: 1-kb DNA ladder; 2: 100-bp DNA ladder), lane 3, 4 = genomic DNA samples of GFP-positive transformants, lane 5 = untransformed plants (control).
The efficiency of plant transformation is dependent on multiple factors, including transformation enhancers that stimulate the activity of the vir gene in Agrobacterium to enable the transfer of T-DNA into plant cells. In this study, selected enhancers with significant effects on transformation efficiency as reported by previous researchers were optimised for T. semidecandra transformation. The concentrations of monosaccharides, tyrosine, AlCI3 were optimised for the transformation of T. semidecandra explants, as was the presence of ascorbic acid. However, further molecular studies including Southern and Northern blot analyses are highly recommended to demonstrate the integration and functionality of the transgene in the new host. With the optimised parameters, transformation of economically important genes that improve the quality of T. semidecandra is feasible. Furthermore, the plant must be in a healthy condition in order for its cells to undergo gene expression. It is important to have a good understanding of basic transformation, and more parameters should be optimised in order to obtain the best protocol for plant transformation.
Acknowledgments
The authors wish to thank the Ministry of Science, Technology and Innovation Malaysia for funding the research. Special thanks are also due to Prof. Dr. Maziah Mahmood for providing the plasmid pCAMBIA1304 for the research.
REFERENCES
- Abdullah JO, Yong WTL. Melastomataceae: Inherent economical values substantiating potential transgenic studies in the family. Transgenic Plant Journal. 2007;1:237–243. [Google Scholar]
- Ahn YJ, Vang L, McKeon TA, Chen GQ. High-frequency plant regeneration through adventitious shoot formation in castor (Ricinus communis L.) In vitro Cellular & Developmental Biology-Plant. 2007;43(1):9–15. [Google Scholar]
- Ankenbauer RG, Nester EW. Sugar-mediated induction of Agrobacterium tumefaciens virulence genes: Structural specificity and activities of monosaccharides. Journal of Bacteriology. 1990;172(11):6442–6446. doi: 10.1128/jb.172.11.6442-6446.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker CJ, Mock NM, Whitaker BD, Roberts DP, Rice CP, Deahl KL, Aver’yanov AA. Involvement of acetosyringone in plant-pathogen recognition. Biochemical and Biophysical Research Communications. 2005;328(1):130–136. doi: 10.1016/j.bbrc.2004.12.153. [DOI] [PubMed] [Google Scholar]
- Cangelosi GA, Ankenbauer RG, Nester EW. Sugars induce the Agrobacterium virulence genes through a periplasmic binding protein and a transmembrane signal protein. Proceedings of the National Academy of Sciences of the United States of America. 1990;87(17):6708–6712. doi: 10.1073/pnas.87.17.6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey MR, Anthony P, Power JB, Lowe KC. Plant protoplasts: Status and biotechnological perspectives. Biotechnology Advances. 2005;23(2):131–171. doi: 10.1016/j.biotechadv.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Delhaize E, Ryan PR. Aluminium toxicity and tolerance in plants. Plant Physiology. 1995;107(2):315–321. doi: 10.1104/pp.107.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enriquez-Obregon GA, Prieto-Samsonov DL, de la Riva GA, Perez MI, Selman-Housein G, Vazquez-Padron RI. Agrobacterium-mediated Japonica rice transformation: A procedure assisted by an antinecrotic treatment. Plant Cell, Tissue and Organ Culture. 1999;59:159–168. [Google Scholar]
- Enriquez-Obregon GA, Vazquez-Padron RI, Prieto-Samsonov DL, Parez M, Selman-Housein G. Genetic transformation of sugarcane by Agrobacterium tumefaciens using antioxidants compounds. Biotecnologia Aplicada. 1997;14:169–174. [Google Scholar]
- Fortin C, Nester EW, Dion P. Growth inhibition and loss of virulence in cultures of Agrobacterium tumefaciens treated with acetosyringone. Journal of Bacteriology. 1992;174(17):5676–5685. doi: 10.1128/jb.174.17.5676-5685.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame BR, Shou H, Chikwamba RK, Zhang Z, Xiang C, Fonger TM, Pegg SEK, et al. Agrobacteriumm tumefaciens-mediated transformation of maize embryos using a standard binary vector system. Plant Physiology. 2002;129:13–22. doi: 10.1104/pp.000653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukusaki E, Kawasaki K, Kajiyama S, An C, Suzuki K, Tanaka Y, Kobayashi A. Flower color modulations of Torenia hybrida by downregulation of chalcone synthase genes with RNA interference. Journal of Biotechnology. 2004;111:229–240. doi: 10.1016/j.jbiotec.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Gelvin SB. Agrobacterium-mediated plant transformation: The biology behind the “gene-jockeying” tool. Microbiology and Molecular Biology Reviews. 2003;67(1):16–37. doi: 10.1128/MMBR.67.1.16-37.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannini A, Zottini M, Morreale G, Spena A, Allavena A. Ornamental traits modification by rol genes in Osteospermum ecklonis transformed with Agrobacterium tumefaciens. In vitro Cellular & Developmental Biology-Plant. 1999;35:70–75. [Google Scholar]
- Guivarc’h A, Caissard JC, Brown S, Marie D, Dewitte W, Van Onckelen H, Chriqui D. Localization of target cells and improvement of Agrobacterium-mediated transformation efficiency by direct acetosyringone pretreatment of carrot root discs. Protoplasma. 1993;174:10–18. [Google Scholar]
- Hansen G. Evidence for Agrobacterium-induced apoptosis in maize cells. Molecular Plant-Microbe Interactions. 2000;13(6):649–657. doi: 10.1094/MPMI.2000.13.6.649. [DOI] [PubMed] [Google Scholar]
- Jansen S, Watanabe T, Smets E. Aluminium accumulation in leaves of 127 species in Melastomataceae, with comments on the Order Mrytales. Annals of Botany. 2002;90(1):53–64. doi: 10.1093/aob/mcf142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joubert P, Beaupere D, Lelievre P, Wadouachi A, Sangwan RS, Sangwan-Norreel BS. Effects of phenolic compounds on Agrobacterium vir genes and genetransfer induction-a plausible molecular mechanism of phenol binding protein activation. Plant Science. 2002;162(5):733–743. [Google Scholar]
- Lee YW, Jin S, Sim WS, Nester EW. The sensing of plant signal molecules by Agrobacterium: Genetic evidence for direct recognition of phenolic inducers by the VirA protein. Gene. 1996;179(1):83–88. doi: 10.1016/s0378-1119(96)00328-9. [DOI] [PubMed] [Google Scholar]
- Li HQ, Kang PJ, Li ML, Li MR. Genetic transformation of Torenia fournieri using the PMI/mannose selection system. Plant Cell, Tissue and Organ Culture. 2007;90(1):103–109. [Google Scholar]
- Mitiouchkina TY, Dolgov SV. Modification of chrysanthemum plant and flower architecture by rolC gene from Agrobacterium rhizogenes introduction. ISHS Acta Horticulturae. 2000;508:163–169. [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays for tobacco tissue culture. Physiologia Plantarum. 1962;15:473–497. [Google Scholar]
- Okamoto S, Tsuruda K, Yamaguchi N, Matsuo T, Nakamura Y. Effects of reducing compounds on Agrobacterium-mediated transformation of sweet potato. ISHS Acta Horticulturae. 2005;682:411–418. [Google Scholar]
- Ozawa K. Establishment of a high efficiency Agrobacterium-mediated transformation system of rice (Oryza sativa L.) Plant Science. 2009;176:522–527. doi: 10.1016/j.plantsci.2009.01.013. [DOI] [PubMed] [Google Scholar]
- Perl A, Lotan O, Abu-Abied M, Holland D. Establishment of an Agrobacterium-mediated transformation system for grape (Vitis vinifera L.): The role of antioxidants during grape-Agrobacterium interactions. Nature Biotechnology. 1996;14(5):624–628. doi: 10.1038/nbt0596-624. [DOI] [PubMed] [Google Scholar]
- Puchooa D. Expression of green fluorescent protein gene in litchi (Litchi chinensis Sonn.) tissues. Journal of Applied Horticulture. 2004;6(1):11–15. [Google Scholar]
- Pu X, Goodman RN. Induction of necrogenesis by Agrobacterium tumefaciens on grape explants. Physiological and Molecular Plant Pathology. 1992;41(4):241–254. [Google Scholar]
- Samac DA, Tesfaye M. Plant improvement for tolerance to aluminium in acid soils-a review. Plant Cell, Tissue and Organ Culture. 2003;75(3):189–207. [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: A laboratory manual. New York: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sambrook J, Russell DW. In vitro amplification of DNA by the polymerase chain reaction. In: Sambrook J, Fritsch EF, Maniatis T, editors. Molecular cloning: A laboratory manual. 3rd ed. Vol. 2. New York: Cold Spring Harbor Laboratory; 2001. pp. 8.1–8.113. [Google Scholar]
- Sarker RH, Biswas A, Mustafa BM, Mahbub S, Hoque MI. Agrobacterium-mediated transformation of lentil (Lens culinaris Medik.) Plant Tissue Culture. 2003;13(1):1–12. [Google Scholar]
- Shimoda N, Toyoda-Yamamoto A, Nagamine J, Usami S, Katayama M, Sakagami Y, Machida Y. Control of expression of Agrobacterium vir genes by synergistic actions of phenolic signal molecules and monosaccharides. Proceedings of The National Academy of Sciences of the United States of America. 1990;87(17):6684–6688. doi: 10.1073/pnas.87.17.6684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrawat AK, Becker D, Lorz H. Agrobacterium tumefaciens-mediated genetic transformation of barley (Hordeum vulgare L.) Plant Science. 2007;172(2):281–290. [Google Scholar]
- Slavov S, Valkov V, Batchvarova R, Atanassova S, Alexandrova M, Atanassov A. Chlorsulfuron resistant transgenic tobacco as a tool for broomrape control. Transgenic Research. 2005;14(3):273–278. doi: 10.1007/s11248-004-8081-9. [DOI] [PubMed] [Google Scholar]
- Sui N, Li M, Zhao SJ, Li F, Liang H, Meng QW. Overexpression of glycerol-3-phosphate acyltransferase gene improves chilling tolerance in tomato. Planta. 2007;226(5):1097–1108. doi: 10.1007/s00425-007-0554-7. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Jansen S, Osaki M. The beneficial effect of aluminium and the role of citrate in Al accumulation in Melastoma malabathricum. New Phytologist. 2005;165(3):773–780. doi: 10.1111/j.1469-8137.2004.01261.x. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Osaki M. Role of organic acids in aluminium accumulation and plant growth in Melastoma malabathricum. Tree Physiology. 2002;22:785–792. doi: 10.1093/treephys/22.11.785. [DOI] [PubMed] [Google Scholar]
- Yong WTL, Abdullah JO, Mahmood M. Optimization of Agrobacterium-mediated transformation parameters for Melastomataceae spp. using green fluorescent protein (GFP) as a reporter. Scientia Horticulturae. 2006;109(1):78–85. [Google Scholar]
- Yong WTL, Abdullah JO, Mahmood M. Agrobacterium-mediated transformation of Melastoma malabathricum and Tibouchina semidecandra with sense and antisense dihydroflavonol-4-reductase (DFR) genes. Plant Cell, Tissue and Organ Culture. 2009;96(1):59–67. [Google Scholar]
- Zou L, Qi D, Li P, Wang S, Li S, Jiang H. A study on transformation of the antisense Waxy gene into rice mediated by Agrobacterium. Molecular Plant Breeding. 2004;2:765–770. [Google Scholar]
- Zuker A, Tzfira T, Ben-Meir H, Ovadis M, Shklarman E, Itzhaki H, Forkmann G, et al. Modification of flower color and fragrance by antisense suppression of the flavanone 3-hydroxylase gene. Molecular Breeding. 2002;9(1):33–41. [Google Scholar]