Abstract
The diversity and the feeding guilds of birds in three different habitats (secondary forest, oil palm plantation and paddy field) were investigated in riparian areas of the Kerian River Basin (KRB), Perak, Malaysia. Point-count observation and mist-netting methods were used to determine bird diversity and abundance. A total of 132 species of birds from 46 families were recorded in the 3 habitats. Species diversity, measured by Shannon’s diversity index, was 3.561, 3.183 and 1.042 in the secondary forest, the paddy field and the oil palm plantation, respectively. The vegetation diversity and the habitat structure were important determinants of the number of bird species occurring in an area. The relative abundance of the insectivore, insectivore-frugivore and frugivore guilds was greater in the forest than in the monoculture plantation. In contrast, the relative abundance of the carnivore, granivore and omnivore guilds was higher in the plantation. The results of the study show that the conversion of forest to either oil palm plantation or paddy fields produced a decline in bird diversity and changes in the distribution of bird feeding guilds.
Keywords: Secondary Forest, Oil Palm Plantation, Paddy Field, Feeding Guild, Habitat Types, Riparian Area
Abstract
Kajian kepelbagaian dan kumpulan pemakanan burung di tiga habitat berbeza (hutan sekunder, kelapa sawit dan sawah padi) telah dijalankan di kawasan riparian Lembangan Sungai Kerian, Perak, Malaysia. Tinjauan melalui kaedah point-count dan pemasangan jaring kabut (mist-netting) telah digunakan untuk menentukan kepelbagaian burung dan kelimpahannya. Sebanyak 132 spesies burung daripada 46 famili telah direkodkan di 3 habitat tersebut. Kepelbagaian spesies diukur melalui indeks kepelbagaian Shannon’s dengan catatan masing-masing sebanyak 3.561, 3.183 dan 1.042 di hutan sekunder, sawah padi dan kelapa sawit. Kepelbagaian tumbuhan dan struktur habitat merupakan penentu utama kehadiran bilangan spesies burung di sesuatu kawasan. Kelimpahan relatif kumpulan pemakanan insektivor, insektivor-frugivor dan frugivor adalah lebih banyak di kawasan hutan berbanding di kawasan penanaman monokultur. Sebaliknya, kelimpahan relatif kumpulan pemakanan karnivor, grainivor dan omnivor adalah tinggi di kawasan penanaman. Hasil kajian menunjukkan, perubahan kawasan hutan kepada kawasan kelapa sawit atau sawah padi menghasilkan penurunan spesies burung dan perubahan taburan kumpulan pemakanan burung.
INTRODUCTION
Many forested areas worldwide have been converted to agricultural uses (Rappole & McDonald 1994; Danielsen & Heegaard 1995; Dobson et al. 1997; McMorrow & Talip 2001; Zhijun & Young 2003; Aratrakorn et al. 2006). As a result, the loss of habitats used by wildlife for shelter, breeding and feeding has become a major problem. Habitat loss can threaten wildlife populations and can eventually lead to extinction. Deforestation also causes changes in animal feeding guilds because of alterations in the structure of the food web. The diversity of fruit trees decreases in modified forested areas (Harris & Pimm 2004). This decrease produces changes in the distribution of feeding guilds (Gray et al. 2007).
The most threatened groups of fauna are those that are “totally dependent” on forest. Species tolerant to habitat change, the “survivor” groups, are less likely to decline (Harris & Pimm 2004). The totally dependent species have a low tolerance for habitat change. The factors responsible for this low tolerance include the relative inability of these species to colonise required habitat or to adapt to new habitat, as well as changes in dietary guilds (Lim & Sodhi 2004). Birds were selected for this study because they are best suited for studies of the patterns of change in feeding guilds. They are tolerant of habitat change, and they show a wide range of feeding guilds (Johns 1991).
To date, studies of avian feeding guilds in different habitats resulting from land conversion have not been conducted (Gray et al. 2007). The study of avian feeding guilds is important for understanding the complexity of ecosystem structure and for providing updated information on each type of habitat in the ecosystem. In Malaysia, the human demands for food, raw materials and residential areas have produced a loss of natural vegetation. Buildings and monocultures, such as oil palm plantations and paddy fields, have replaced the natural vegetation. Good management practices should be implemented to sustain both ecosystem stability and human demand.
This study examined three habitat types: secondary forest, oil palm plantation and paddy fields. The study areas were located along the Kerian River, Perak, Malaysia. Each habitat represented the dominant form of land use along a different section of the river. The secondary forest occurred in the upstream area, the oil palm plantations were along the middle section of the stream and the paddy fields were located in the downstream area. These three habitats were also selected to show how changes in species can occur if a forested area is converted to oil palm plantation or to paddy fields. The relative abundance of each avian feeding guild was expected to differ between natural vegetation and monoculture as the avian species diversity declined with habitat change (Waltert et al. 2004). Thus, the occurrence of different feeding guilds should differ among the three habitat types.
Natural vegetation in a forested area includes a wide range of trees of different species, sizes and heights. This diverse vegetation creates several canopy layers. Different avian species inhabit different niches in each storey. For example, the upper storey is mainly occupied by warbler species, whereas the ground layer is occupied by babbler (Timaliidae) species (Robson 2008). This pattern is different from that occurring in monoculture areas, such as oil palm plantations and paddy fields. Both of these habitats are dominated by single plant species. The size and the height of trees in the monoculture areas are uniform and therefore offer only limited variation in the canopy layer. Moreover, the number of feeding niches is small, and the available niches can only be occupied by certain species. For these reasons, the number of species in monoculture areas is less than that found in natural vegetation.
The objectives of this study were to identify the variation in diversity and the changes in the feeding guilds of bird species that resulted from the conversion of a forested area to a monoculture. We hypothesised that the diversity and the percentage of feeding guilds (e.g., carnivore, insectivore, frugivore and granivore) would change between natural vegetation (represented by secondary forest) and monoculture (oil palm and paddy field) owing to differences in the vegetation characteristics, the level of human disturbance and the factors affecting shelter, breeding and food availability.
MATERIALS AND METHODS
Study Site
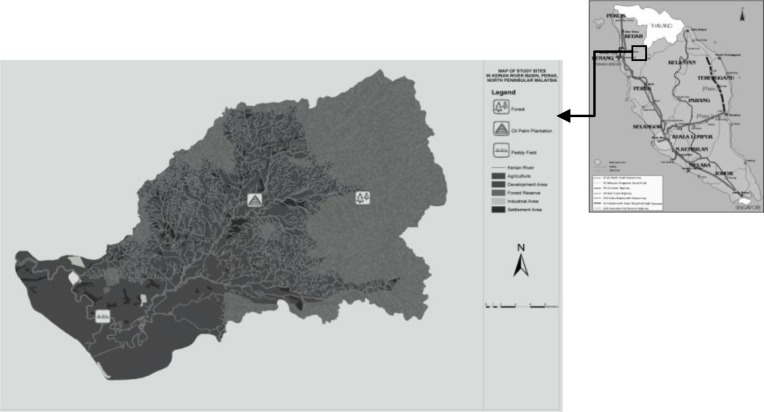
The Kerian River Basin (KRB) is located in northern Peninsular Malaysia (Fig. 1). It forms the boundary between Kedah and Perak. The Kerian River passes through three major districts: Bandar Baharu in Kedah, and Kerian and Larut Matang in Perak. Secondary forest is found primarily in the upper part of the KRB, whereas oil palm plantations and paddy fields are among the chief agricultural plantations found in the KRB. Three habitat types were sampled: secondary forest (upstream), oil palm plantation (middle section of the stream) and paddy field (downstream).
Figure 1:
Study area in the Kerian River Basin on the border of Perak and Kedah states.
Secondary forest
The secondary forest was located near the upper riparian areas of the KRB (5.2631° N, 100.8217° E) at 180 m above sea level. The study site of 19.62 ha was located within 600 ha of secondary forest area. The information given by villagers indicated that this forest was logged over 20 years ago. The vegetation in the forest is dominated by trees belonging to the family Dipterocarpaceae and by the stemless palm, bertam (Eugeissona tristis). Several other habitat types, such as rubber plantations and orchards, were located 20 km from the study site. An intact area of primary forest was adjacent to the secondary forest. Wild species inhabiting the primary forest frequently visited the secondary forest, especially during the fruiting season. These species included tiger (Panthera tigris), wild boar (Sus scrofa), sun bear (Helarctos malayanus), spectacled leaf monkey (Trachypithecus obscurus) and elephant (Elephas maximus). We identified the presence of these animals either from observations or pugmarks.
Oil palm plantation
The study site was located in the middle riparian area of the KRB (5.23° N, 100.6885° E and 20 m above sea level). It covered 18 ha within 550 ha of oil palm plantation. The 10-year-old plantation was converted from forested areas and was surrounded by roads and human settlements. Wild boars and buffalo (Bubalus bubalis) were commonly seen at the study site. Shrubs and weeds formed most of the ground vegetation. Workers were frequently seen harvesting oil palm fruits and applying fertilizer to the plants. The oil palm fruits were harvested every two weeks.
Paddy field
The paddy field site was located in the downstream section of the KRB (5.0949° N, 100.5307° E) in Bandar Baharu district, Kedah. The study site covered 19 ha and included more than 10 paddy plots. The paddy field was planted approximately 70 days before the first month of the sampling period. The crop was growing during the sampling period. The sampling period ended at the start of the harvesting season. During the growth of the paddy, pesticide and herbicides were used to prevent insects and weeds from harming the growth of the crop. In each paddy plot, a barn owl box was provided by the Department of Agriculture as a biological pest control measure.
Sampling: Bird community sampling methods
From February through April 2009, samples were collected monthly in each habitat type over four consecutive days. Mist-netting (132 hours of netting effort) was conducted only in the secondary forest and the oil palm plantation, whereas point-count surveys (24 individual surveys) were performed in each of the three habitat types. The use of both methods in combination was expected to increase the number of bird species recorded (Zakaria & Rajpar 2010).
Bird point count surveys
A bird census was taken by the observer (the author) using the point-count distance-sampling method (Gibbons et al. 1996; Zakaria & Rajpar 2010). The species and number of individuals were recorded. The birds heard or seen were counted at 15 points separated by a distance of 150 m in the secondary forest and oil palm plantation and by a distance of 200 m in the paddy field (Gibbons et al. 1996). A different observation interval was chosen for the paddy field because the birds could be directly observed at a considerably greater distance in that habitat. An observation time of approximately 10 minutes at each stop allowed the detection of birds that were difficult to see, that seldom vocalised or that tended to fly rapidly. Precautions were taken to avoid recounting the same individuals at a given point. The surveys were conducted from 0700 h to 1000 h, during the period of greatest bird activity. No surveys were made on rainy or windy days. Only birds belonging to the families Accipitrinae, Apodidae, Hemiprocnidae and Hirundinae were recorded in flight. These observations were necessary because birds belonging to these families were rarely found perching on trees. The birds were identified based on Robson (2008). Each species was assigned to a feeding guild according to Wells (1999, 2007).
Mist-netting
Mist-netting was important because it served to confirm the bird species recorded during the point-count surveys and because it could capture individuals of wary species. In the secondary forest and the oil palm plantation, 10 mist nets were placed in areas where birds were frequently observed and heard during the point counts. Mist-netting was not used in the paddy field because the birds could be observed in open areas. Most of the birds occurring in the paddy field were easily observed and identified. The net locations were changed for each sampling visit. The nets were left open from 0700 h to 1800 h. They were checked frequently (every two hours) to ensure that the birds remained alive and in good condition. The captured birds were placed in cloth bags and subsequently measured. For each bird, the body weight, length of the tarsus, length of the wings, length of the mandible and total length were recorded using the procedures in Robson (2008).
RESULTS
Birds Observed
A total of 132 species of birds from 46 families were identified through the point-count-survey (Table 1). The values of Shannon’s diversity index were 3.561 for the secondary forest, 3.183 for the paddy field and 1.042 for the oil palm plantation. During the sampling period, unique bird species (those found only in one habitat type) were recorded most frequently in the secondary forest (53%). Unique bird species were less frequent in the paddy field (32%) and the oil palm plantation (15%). Other bird species were commonly seen in more than one habitat type. Jaccard’s coefficient indicated that similar bird species were present in the oil palm plantation and in the paddy field, whereas the secondary forest was the habitat in which most unique bird species occurred (Fig. 2).
Table 1:
Bird species and feeding guilds in three different habitat types in the Kerian River Basin (February–April 2009).
| Family | Scientific name | Habitat type
|
Feeding guild | ||
|---|---|---|---|---|---|
| SF | OP | PF | |||
| Ardeidae | Egretta garzetta | − | − | + | Car |
| Ixobrychus sinensis | − | − | + | Car | |
| Nycticorax nycticorax | − | − | + | Car | |
| Butorides striata | − | + | + | Car | |
| Ardeola baccus | − | + | + | Car | |
| Bubulcus coromandus | − | − | + | Car | |
| Ardeola speciosa | − | + | + | Car | |
| Ardea purpurea | − | − | + | Car | |
| Ardea alba | − | − | + | Car | |
| Mesophoyx intermedia | − | − | + | Car | |
| Ixobrychus cinnamomeus | − | − | + | Car | |
| Egretta sacra | − | − | + | Car | |
| Accipitrinae | Pernis ptilorhynchus | + | − | − | Car |
| Haliaeetus leucogaster | + | − | − | Car | |
| Spilornis cheela | + | + | + | Car | |
| Aviceda leuphotes | − | + | − | Car | |
| Elanus caeruleus | − | − | + | Car | |
| Haliastur indus | − | − | + | Car | |
| Phasianidae | Gallus gallus | − | + | + | Omn |
| Rallidae | Amaurornis phoenicurus | − | − | + | Omn |
| Gallicrex cinerea | − | − | + | Omn | |
| Vanellidae | Vanellus cinereus | − | − | + | Car |
| Columbidae | Columba liva | − | − | + | Gra |
| Treron vernans | + | − | + | Fru | |
| Streptopelia chinensis | − | + | + | Gra | |
| Geopelia striata | − | − | + | Gra | |
| Tringinae | Actitis hypoleucos | − | − | + | Car |
| Tringa stagnatilis | − | − | + | Car | |
| Cuculidae | Zanclostomus curvirostris | + | − | − | Ins |
| Cacomantis merulinus | + | − | − | Ins-fru | |
| Cacomantis sonneratti | + | − | − | Ins-fru | |
| Surniculus lugubris | − | + | − | Fru | |
| Eudynamys scolopaceus | − | + | + | Ins | |
| Rhopodytes diardi | − | + | − | Ins | |
| Centropus bengalensis | − | − | + | Ins | |
| Rhinortha chlorophaeus | − | + | − | Ins | |
| Rhopodytes tristis | − | + | − | Ins | |
| Tytonidae | Tyto alba | − | − | + | Car |
| Strigidae | Otus lettia | − | + | − | Car |
| Apodidae | Ketupa ketupu | − | + | − | Car |
| Aerodramus fuciphaga | + | − | − | Ins | |
| Aerodramus maximus | + | − | − | Ins | |
| Apus pacificus | + | − | + | Ins | |
| Apus affinis | + | + | + | Ins | |
| Collocalia esculenta | + | − | − | Ins | |
| Rhaphidura leucopygialis | + | − | − | Ins | |
| Hemiprocnidae | Hemiprocne longipennis | + | − | − | Ins |
| Alcedinidae | Pelargopsis capensis | − | + | + | Car |
| Halcyon smyrnensis | − | + | + | Car | |
| Halcyon coromanda | + | − | − | Car | |
| Cexy erithacus | + | − | − | Car | |
| Alcedo meninting | − | + | − | Car | |
| Meropidae | Merops philippinus | − | − | + | Ins |
| Merops viridis | + | − | − | Ins | |
| Prionopidae | Tephrodornis gularis | + | − | − | Ins |
| Bucerotidae | Buceros rhinoceros | + | − | − | Omn |
| Aceros undulates | + | − | − | Fru | |
| Megalaimidae | Megalaima australis | + | − | − | Fru |
| Megalaima mystacophanos | + | − | − | Fru | |
| Megalaima chrysopogon | + | − | − | Ins-fru | |
| Megalaima lineata | + | − | − | Ins-fru | |
| Picidae | Meiglyptes tukki | − | + | − | Ins |
| Meiglyptes tristis | + | − | − | Ins | |
| Dinopium javanense | + | − | − | Ins | |
| Picoides moluccensis | + | − | − | Ins-fru | |
| Micropternus brachyurus | − | + | − | Ins | |
| Picus puniceus | + | − | − | Ins | |
| Eurylaimidae | Cymbirhynchus macrorhynchos | − | + | − | Ins |
| Hirundinidae | Hirundo rustica | − | − | + | Ins |
| Hirundo tahitica | − | + | + | Ins | |
| Pittidae | Pitta moluccensis | − | + | − | Car |
| Campephagidae | Pericrocotus divaricatus | + | − | − | Ins |
| Pericrocotus speciosus | + | − | − | Ins-fru | |
| Coracina fimbriata | + | − | − | Ins | |
| Lalage nigra | − | − | + | Ins-fru | |
| Aegithinidae | Aegithina tiphia | + | + | + | Ins-fru |
| Chloropseidae | Chloropsis sonnerati | + | − | − | Ins-fru |
| Chloropsis cynopogon | + | − | − | Ins-fru | |
| Pycnonotidae | Pycnonotus brunneus | + | + | − | Ins-fru |
| Pycnonotus plumosus | + | + | − | Fru | |
| Pycnonotus atriceps | + | − | − | Ins-fru | |
| Pycnonotus goaivier | + | + | + | Ins-fru | |
| Pycnonotus simplex | + | − | − | Ins-fru | |
| Pycnonotus finlaysoni | + | − | − | Ins-fru | |
| Pycnonotus squamatus | + | − | − | Fru | |
| Pycnonotus cyaniventris | + | − | − | Fru | |
| Pycnonotus flaviventris | + | − | − | Ins-fru | |
| Criniger bres* | + | − | − | Ins-fru | |
| Dicruridae | Dicrurus annectans | + | − | − | Ins |
| Dicrurus annectans* | + | − | − | Ins-fru | |
| Corvidae | Corvus splendens | − | − | + | Sca |
| Corvus macrorhynchos | − | − | + | Sca | |
| Oriolidae | Oriolus chinensis | − | + | + | Ins-fru |
| Irenidae | Irena puella | + | − | − | Ins-fru |
| Timaliidae | Stachyris poliocephala* | + | − | − | Ins |
| Malacocincla malaccensis* | + | − | − | Ins | |
| Stachyris erythroptera* | + | − | − | Ins | |
| Macronus gularis | + | − | − | Ins | |
| Pelloerneum ruficeps* | + | − | − | Ins | |
| Erpornis zantholeuca* | + | − | − | Ins | |
| Sylviidae | Orthotomus sutorius | − | + | + | Ins-fru |
| Orthotomus atrogularis | + | + | − | Ins | |
| Muscicapidae | Muscicapa dauurica | + | − | − | Ins |
| Cyornis tickelliae | + | − | − | Ins-fru | |
| Ficedula zanthopygia | + | − | + | Ins-fru | |
| Copsychus saularis | + | + | + | Ins | |
| Copsychus malabaricus | + | + | − | Ins | |
| Ficedula sp. | − | + | − | Ins | |
| Luscinia cyane | + | − | − | Ins | |
| Luscinia cyane* | + | − | − | Ins | |
| Cyornis tickelliae* | − | + | − | Ins | |
| Rhipiduridae | Rhipidura javanica | − | + | + | Ins |
| Monarchidae | Terpsiphone paradisi | + | − | − | Ins |
| Motacillidae | Motacilla cinerea | + | − | − | Ins |
| Laniidae | Lanius cristatus | + | − | + | Ins |
| Lanius tigrinus | + | − | − | Ins | |
| Sturnidae | Aplonis panayensis | − | + | + | Fru |
| Acridotheres fuscus | − | + | + | Ins-fru | |
| Acridotheres tristis | − | + | + | Omn | |
| Nectariniidae | Arachnothera longirostra | + | + | − | Ins-nec |
| Arachnothera flavigaster | + | − | − | Ins-nec | |
| Arachnothera chrysogenys | + | − | − | Ins-nec | |
| Arachnothera affinis | + | − | − | Ins-nec | |
| Cinnyris jugularis | − | − | + | Ins-nec | |
| Dicaeidae | Dicaeum percussus | + | − | − | Fru |
| Dicaeum trigonostigma | + | − | − | Fru | |
| Dicaeum cruentatum | + | − | + | Fru | |
| Dicaeum maculatus | + | − | − | Fru | |
| Passeridae | Passer montanus | − | − | + | Ins-gra |
| Ploceidae | Ploceus philippinus | − | − | + | Ins-gra |
| Estrildidae | Lonchura punctulata | − | − | + | Gra |
| Lonchura striata | + | − | + | Gra | |
| Lonchura maja | − | − | + | Gra | |
| Lonchura atricapilla | − | − | + | Gra | |
| Phylloscopidae | Phylloscopus borealis | + | − | − | Ins |
| Cisticoladiae | Cisticola juncidis | − | − | + | Ins |
| Prinia flaviventris | + | − | + | Ins | |
Notes: Car=carnivore; Omn=omnivore; Ins=insectivore; Nec=nectarivore; Fru=frugivore; Gra=grainivore; Sca= scavenger;
species missed by the point count method.
Figure 2:
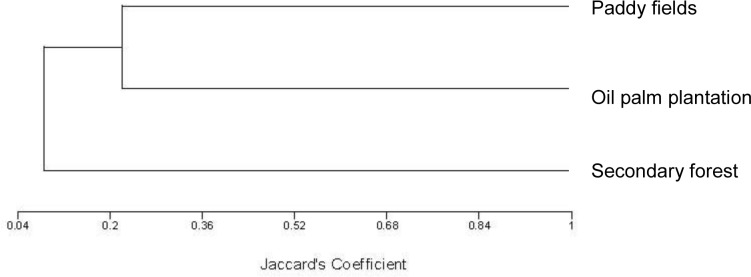
Dendrogram of Jaccard’s coefficient of similarity between selected habitat types based on bird species composition.
A total of 399 individuals, belonging to 72 bird species and 30 families, were found in the secondary forest. The most abundant birds observed in the secondary forest were bulbuls (26.65%), followed by treeswifts (19.77%), flycatchers, robins and sharma (7.10% each), and minivets and iora (6.01% each). The bulbuls, belonging to nine species, fed on small berries, insects, small invertebrates and geckos. Whiskered treeswifts (Hemiprocne comate) and Grey-rumped treeswifts (Hemiprocne longipennis) were usually seen individually or in a large flock perching on branches of tall trees. The Whiskered treeswifts were often seen perching at the tip of a branch, from which they would make frequent sallies. Flycatchers, robins, sharma, minivets and iora were usually spotted flying in medium-high forest strata (approximate height 15 m). Birds of the family Pycnonotidae (9 species) were recorded most often from the secondary forest, followed by Apodidae (6 species) and Timaliidae (5 species).
In the oil palm plantation, 248 individuals, belonging to 49 species and 30 families, were recorded. 86% of all the total bird species occurring in the oil palm plantation were also found either in the secondary forest or the paddy field. The four most abundant bird families observed were Pycnonotidae (21.59%), Muscicapidae (19.12%), Sturnidae (17.95%) and Halcyonidae (9.49%). The family Pycnonotidae was represented by the Yellow-vented bulbul (Pycnonotus goiavier), with 44 individuals. This species was usually seen hunting for insects within shrub areas. The Oriental magpie robin (Copsychus saularis), a member of the Muscicapidae, was the second-commonest species (35 individuals). These birds, known locally as murai kampung, were easily detected by their repeated songs. The largest bird family recorded from the oil palm plantation was Cuculidae (5 species), followed by Ardeidae (3 species) and Pycnonotidae (3 species).
In the paddy field, 1277 individuals, belonging to 59 species and 30 families, were recorded. Flocks of birds were commonly seen during the census. The most-abundant bird groups were herons and egrets (29.09% each), followed by starlings and mynas (10.15% each), swallows (7.86%), sparrows (7.53%), munias (7.18%) and bulbuls (5.07%). These bird groups, exemplified by the Purple heron (Ardea purpurea), the Little heron (Butorides striata) and the Eastern cattle egret (Bulbulcus coromandus), fed primarily on small mammals and various aquatic animals. The starlings, mynas and sparrows were associated with fruiting trees, houses and open areas, respectively. These species were commonly seen in large flocks of up to 20 to 25 individuals. Swallows and munias were commonly observed in the paddy plots. The largest bird family recorded from the paddy field was Ardeidae (12 species), followed by Columbidae (4 species) and Estrildidae (4 species).
Mist-netting
In the secondary forest, 53 birds, belonging to 26 species and 10 families, were mist-netted (Table 2). The Pycnonotidae (8 species; 50 individuals, 47.17%), Timaliidae (6 species; 18 individuals, 16.98%) and Muscicapidae (3 species; 8 individuals, 7.55%) were the dominant families. Most of the individuals caught using mist nets belonged to these three families. The Megalaimidae, Chloropseidae, Dicruridae and Estrildidae were the rarest families, with only one bird captured for each species (1.89%).
Table 2:
Bird species captured by mist-netting in secondary forest and oil palm plantation, Kerian River, Perak (February–April 2010).
| Families | Species | Secondary forest | Oil palm plantation | ||
|---|---|---|---|---|---|
|
| |||||
| No. of individuals | % | No. of Individual | % | ||
| Strigidae | Collared-scops owl | – | – | 1 | 1.17 |
| Alcedinidae | Blue-eared kingfisher | – | – | 6 | 13.04 |
| Halcyonidae | White-throated kingfisher | – | – | 5 | 10.87 |
| Megalaimidae | Red-crowned barbet | 1 | 1.89 | – | – |
| Picidae | Buff-necked woodpecker | – | – | 1 | 1.17 |
| Pittidae | Blue-winged pitta | – | – | 1 | 4.35 |
| Chloropseidae | Lesser green leafbird | 1 | 1.89 | – | – |
| Pycnonotidae | Cream-vented bulbul | 2 | 3.77 | – | – |
| Black-headed bulbul | 6 | 13.11 | – | – | |
| Olive-winged bulbul | 2 | 3.77 | 9 | 19.57 | |
| Red-eyed bulbul | 8 | 15.09 | 1 | 1.17 | |
| Stripe-throated bulbul | 3 | 5.66 | – | – | |
| Spectacled bulbul | 1 | 1.89 | – | – | |
| Yellow-vented bulbul | 1 | 1.89 | 5 | 10.87 | |
| Grey-cheeked bulbul | 1 | 1.89 | – | – | |
| Dicruridae | Crow-billed drongo | 1 | 1.89 | – | – |
| Timaliidae | Grey-headed babbler | 1 | 1.89 | – | – |
| Short-tailed babbler | 2 | 3.77 | – | – | |
| Puff-throated babbler | 2 | 3.77 | – | – | |
| Chesnut-winged babbler | 2 | 3.77 | – | – | |
| Pin-striped tit babbler | 1 | 1.89 | – | – | |
| White-bellied epornis | 1 | 1.89 | – | – | |
| Cisticoladiae | Dark-necked tailorbird | 3 | 5.66 | – | – |
| Common tailorbird | – | – | 1 | 1.17 | |
| Muscicapidae | Yellow-rumped flycatcher | 1 | 1.89 | – | – |
| Tickell’s blue flycatcher | – | – | 1 | 1.17 | |
| Siberian blue robin | 2 | 3.77 | – | – | |
| Oriental magpie robin | 1 | 1.89 | 8 | 17.39 | |
| White-rumped shama | – | – | 1 | 1.17 | |
| Rhipiduridae | Pied fantail | – | – | 1 | 1.17 |
| Laniidae | Brown shrike | – | – | 1 | 1.17 |
| Sturnidae | Jungle myna | – | – | 1 | 1.17 |
| Nectariniidae | Little spiderhunter | 5 | 5.66 | 1 | 1.17 |
| Brown-throated sunbird | – | – | 1 | 1.17 | |
| Dicacidae | Orange-bellied flowerpecker | 2 | 3.77 | – | – |
| Yellow-breasted flowerpecker | 1 | 1.89 | – | – | |
| Scarlet-backed flowerpecker | 2 | 3.77 | – | – | |
| Estrildidae | White-rumped munia | 1 | 1.89 | – | – |
|
| |||||
| Total of individual | 106 | 100 | 46 | 100 | |
A total of 46 birds, belonging to 17 species and 12 families, were mist-netted in the oil palm plantation. The dominant family was the Pycnonotidae (3 species; 15 individuals, 31.61%), followed by the Muscicapidae (3 species; 10 individuals, 19.73%) and the Nectariniidae (2 species; 2 individuals, 2.34%). The six rarest families were the Strigidae, Picidae, Pittidae, Rhipiduridae, Laniidae and Sturnidae. Only one individual of each of these species was captured (1.17%).
These results show that mist-netting is more important in a habitat with complex vegetation than in a monoculture plantation. This difference is clear from the finding that eight species found in the secondary forest were not recorded through observation and were only identified through mist-netting. In contrast, most of the birds mist-netted in the oil palm plantation were also seen through observation, with the exception of one species, Tickell’s blue flycatcher (Cyornis tickelliae). Furthermore, the study confirmed that mist-netting was not necessary in the paddy field because the areas were open and contained no obstructions that prevented the birds from being observed.
Feeding Guilds
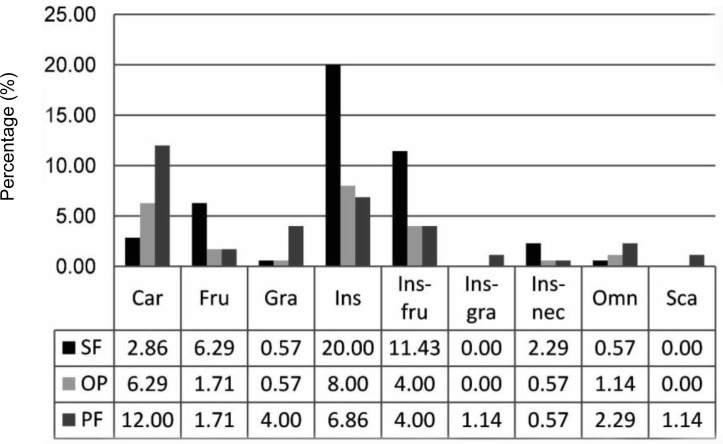
Nine types of feeding guilds were identified in the study areas: the carnivore, omnivore, insectivore, insectivore-frugivore, insectivore-granivore, insectivore-nectivore, frugivore, granivore and scavenger guilds (Fig. 3). The secondary forest was dominated by insectivorous species (20%), followed by insectivore-frugivore (11.43%) and frugivorous species (6.29%). The families represented in these three most-frequent feeding guilds were primarily the Apodidae, Picidae, Pycnonotidae, Timaliidae and Muscicapidae. Granivorous and omnivorous species (0.57%) were the feeding guilds recorded least often in the secondary forest. The insectivore-granivore and scavenger guilds were absent from the secondary forest.
Figure 3:
Relative abundance of different avian feeding guilds in three different habitat types: secondary forest (SF), oil palm plantation (OP) and paddy field (PF).
Notes: Car=carnivore; Fru=frugivore; Gra=granivore; Ins=insectivore; Omn=omnivore; Sca=scavenger
In the oil palm plantation, insectivores (8%) were the most-frequent feeding guild, followed by carnivores (6.29%) and insectivore-frugivores (4%). Granivore and insectivore-nectarivore (0.57%) were the least-frequent feeding guilds in the oil palm plantation. The insectivore-granivore and scavenger guilds did not occur in the oil palm plantation.
In the paddy field, carnivorous species (12%) were dominant, followed by granivores (4%) and insectivore-frugivores (4%). Most of the carnivorous species belonged to the families Ardeidae and Acciptrinae. The insectivore-nectarivore guild (0.59%) was the rarest feeding guild in the paddy field.
DISCUSSION
Bird Diversity
The results of the study indicate that the bird diversity was greater in the forest than in the oil palm plantation or the paddy field. This result supports the conclusion of Waltert et al. (2004) that the number of bird species declines if forested areas are converted into agricultural areas. Two major consequences of the conversion of forest to commercial plantations have been identified: a decrease in bird richness and an increase in the number of wide-ranging, adaptable species (Aratrakorn et al. 2006).
The vegetation diversity and structure of a habitat are important determinants of the bird communities that occur in that habitat. Secondary forest is structurally complex, offers more niches, and has higher levels of plant and insect diversity. It can therefore support more bird species (Miller et al. 2004). Fewer species can occur in oil palm plantations and paddy fields because these habitats are structurally simpler and offer fewer but different feeding niches (Fitzherbert et al. 2008; Koh & Wilcove 2008; Fujioka & Yoshida 2001).
More than 50% of the bird species were found exclusively in the secondary forest. They were categorised as forest-dependent bird species (Wells 1999). Secondary forests have several forest strata, composed of tree species whose number, size and composition varies among the strata. The observations reported by this study show that different bird species occupied different forest strata. Most raptors, such as the Crested serpent eagle (Spilornis cheela) and the Changeable hawk eagle (Spizaetus cirrhatus), inhabited the emergent layer. The canopy layer was dominated by species such as the Asian fairy bluebird (Irena puella), Gold-whiskered barbet (Megalaima chrysopogon), Blue-eared barbet (Megalaima australis) and Rhinoceros hornbill (Buceros rhinoceros). These species usually inhabited the upper storey of the forest, where they commonly perched, foraged and nested. In the middle storey of the forest, the Oriental magpie robin (Copsychus saularis) and all of the Pycnonotidae recorded from the habitat were frequently observed and captured during the sampling period. Most of the babbler and prinia species were found frequently in the lower storey. These bird species are difficult to observe but can be identified from their calls.
Most of the birds found in the oil palm plantation were common species, adaptable to unfavourable environments. The oil palm plantation is categorised as an unfavourable environment owing to the low availability of food sources and the frequent disturbance caused by humans, especially during the fruit harvest (Aratrakorn et al. 2006). The results of the study demonstrated that bird species were fewest in number and least diverse in the oil palm plantation, compared with the secondary forest and the paddy field. Approximately 76% of the birds found in the secondary forest were not observed in the oil palm plantation. This finding is comparable with the results of other studies conducted in southern Peninsular Malaysia (Peh et al. 2005, 2006; Koh & Wilcove 2008). Woodpeckers, barbets, leafbirds and babblers are examples of groups that were well represented in the forest but absent or poorly represented in the plantation areas. These species are sensitive to habitat change and adapt poorly to disturbed environments (Wells 1999, 2007).
Paddy fields were the other type of monoculture present along most of the lower Kerian River. Many species of water birds and land birds were consistently found in this wetland habitat. These birds formed an avifauna whose composition was different from that of the bird assemblages found in the secondary forest or the oil palm plantation. Previous studies suggest that the manmade habitat of paddy fields is very well suited for water birds and land birds as a place to forage, breed and find shelter (Zou et al. 2006; Razafimanjato et al. 2007; Takahashi & Ohkawara 2007; Wood et al. 2010). Farming activities and paddy-growing seasons are considered to be the main factors that influence bird use and occurrence (Kelly et al. 2008; Ibáñez et al. 2010; King et al. 2010).
Mist-netting
In the secondary forest, the point-count method and mist-netting together revealed a total of 80 species. Of these species, 72 (90%) were detected by the point-count method and 26 species (32.5%) were captured by mist-netting. Eight of these 80 species (10%) were not detected by the point-count method, and 54 (67.5%) were not captured by mist-netting. Only 18 species (22.5%) were detected by both methods.
In the oil palm plantation, the point-count method and mist-netting together detected a total of 50 species. The point-count method detected 49 of these species (98%). Seventeen (34%) of the 50 species were captured by mist-netting. One species (2%) was not detected by the point-count method, whereas 33 of the species (66%) were not captured by mist-netting. Sixteen species (32%) were detected by both methods.
The findings of this study confirm the conclusions of Zakaria and Rajpar (2010) that the point-count method produces better results and is more efficient than mist-netting. In the secondary forest, mist-netting identified additional species not detected by the point-count method. However, the point-count method succeeded in detecting most species in that habitat. This result supports the conclusion by Derlindati and Caziani (2005), and Wang and Finch (2002) that mist-netting helps to increase the detection of bird species in complex vegetation structure. Our study showed that if the vegetation structure is relatively simple, the point-count method is able to detect almost all of the birds present in the habitat. For example, the point-count method failed to detect only one species present in the oil palm plantation.
Feeding Guilds
Variation in vegetation structure influences the distribution of bird feeding guilds (Pearman 2002). The secondary forest, the oil palm plantation and the paddy fields furnished food sources suitable for different kinds of bird feeding guilds. The insectivore, insectivore-frugivore and frugivore species decreased as the forest was converted to a monoculture plantation, whereas the carnivore, granivore and omnivore species increased. This finding is comparable with those of other studies that have found a positive relationship between the abundance of insectivores and relatively undisturbed habitat. Furthermore, the abundance of carnivores and granivores was shown to be significantly higher in disturbed environments (de Iongh & Van Weerd 2006; Chettri et al. 2005; Zakaria et al. 2005; Fujioka & Yoshida 2001; Lombardini et al. 2001; Sekercioglu et al. 2002).
A habitat having dense vegetation, i.e., one in which the density of trees is higher and their basal areas are greater, is very suitable for insects (Chettri et al. 2005). Insects are strongly favoured by moist conditions and dense foliage (Erwin 2001). Thus, insectivorous birds were abundant in the secondary forest, where their food was abundant. The observed abundance of insectivore-frugivores and frugivores in the secondary forest indicates that fruits were sufficiently available in this habitat. Most of the insectivore-frugivore species belonged to the Pycnonotidae. This group of birds is adaptable to the seasonal availability of fruits. They can shift to insectivory and are therefore good colonising species (Zakaria et al. 2005). Frugivores feed on a variety of fruits widely available within the secondary forest. Fruit selection by frugivorous birds is influenced by colour (Gautier-Hion et al. 1985), seed size and number (Levey 1987a), nutrient content (Levey 1987b; Herrera 1982) and fruiting arrangement (Levey et al. 1984; Denslow & Moermond 1982).
The presence of carnivorous species in the oil palm plantation was primarily influenced by the availability of their food sources. This finding is consistent with the results of a study in southern Portugal showing that the number of carnivorous species or predators increased in proportion to the number of prey (Lourenço & Sergio 2006). Prey species such as shrews, snakes and rats are among the food sources available in oil palm plantations. Previous studies have found that shrews are the preferred prey of many raptors worldwide (Bose & Guidali 2001; McGhie 2001; Lovari et al. 1976; Glue 1974). Moreover, the noise made by shrews and their territorial behaviour make them vulnerable to predation and tend to attract predators. Omnivorous bird species were the next most-abundant species in the oil palm plantation. The Yellow-vented bulbul (Pycnonotus goiavier), the Jungle myna (Acridotheres fuscus) and the Common myna (Aplonis tristis) represented common bird species whose diets included a broad range of food items. These birds consume a variety of animals and plants. In the oil palm plantation, the omnivorous birds prefer the pericarp of the oil palm fruit, insects such as Dermaptera, Hymenoptera, Isoptera, Orthoptera and Coleoptera, and leeches and snails (Arshad et al. 2000). Their wide dietary range allows them to adapt if their preferred food sources become scarce.
Water birds of various species have been found in paddy fields in a number of countries worldwide, including Korea, Japan, China, the United States, Indonesia and India (Fujioka et al. 2010; Pierluissi et al. 2010; Elphick 2008; Fasola et al. 2004). Birds belonging to aquatic families were common or abundant at the study’s paddy field site in Bandar Baharu, Kedah. Among the primary food items in the diets of these birds were small invertebrates, fish, snakes and rodents. Stafford et al. (2010) indicate that the diets of most water birds found in paddy fields consist of benthic and surface-dwelling invertebrates and aquatic vertebrates. In addition, paddy fields offer many food sources that attract large numbers of carnivorous species, e.g., members of the familes Ardeidae, Accipitrinae, Tytonidae and Strigidae. Insectivorous species were also common in the paddy field because the area is a temporary wetland in which many insect species breed (Kato et al. 1952) and complete their life cycles. The abundant insects in the paddy field support the insectivorous species. Immediately prior to the harvesting season, the rice ripens and attracts granivorous species, such as the White-rumped munia (Lonchura striata) and the Scaly-breasted munia (Lonchura punctulata). The dry fields and the grain remaining after harvesting were used for feeding by many bird species, primarily granivores (Maeda 2001).
CONCLUSION
The conversion of forest to oil palm plantation or to paddy fields causes changes in bird diversity and variation in the distribution of avian feeding guilds. Secondary forest continues to represent an important habitat for forest-dependent birds, whereas oil palm plantations are inhabited by common species. Paddy fields potentially provide a habitat that sustains various types of water birds. Because the existing data on birds in Malaysian paddy fields are insufficient, further studies of birds in paddy fields should be conducted.
Acknowledgments
This study was funded by Universiti Sains Malaysia (USM) Research Grant (815019). Transportation was furnished by the School of Biological Sciences, USM. The first author was partially supported by a Graduate Fellowship Scheme from the Institute for Graduate Studies, USM.
REFERENCES
- Aratrakorn S, Thunhikorn S, Donald PF. Changes in bird communities following conversion of lowland forest to oil palm and rubber plantations in southern Thailand. Bird Conservation International. 2006;16(1):71–82. [Google Scholar]
- Arshad MI, Zakaria M, Sajap AS, Ismail A. Food and feeding habits of red junglefowl. Pakistan Journal of Biological Sciences. 2000;3(6):1024–1026. [Google Scholar]
- Bose M, Guidali F. Seasonal and geographic differences in the diet of the barn owl in an agro-ecosystem in northern Italy. Journal of Raptor Research. 2001;35(3):240–246. [Google Scholar]
- Chettri N, Deb DC, Sharma E, Jackson R. The relationship between bird communities and habitat. Mountain Research and Development. 2005;25(3):235–243. [Google Scholar]
- Danielsen F, Heegaard M. Impact of logging and plantation development on species diversity: A case study from Sumatra. In: Sandbukt O, editor. Management of Tropical Forests: Towards an Integrated Perspective. Oslo, Norway: Centre for Development and the Environment, University of Oslo; 1995. pp. 73–92. [Google Scholar]
- de Iongh HH, van Weerd M. The use of avian guilds for the monitoring of tropical forest disturbance by logging. Wageningen, Netherlands: Tropenbos International; 2006. p. 17. [Google Scholar]
- Denslow JS, Moermond TC. The effect of accessibility on rates of fruit removal from tropical shrubs: An experimental study. Oecologia. 1982;54(2):170–176. doi: 10.1007/BF00378389. [DOI] [PubMed] [Google Scholar]
- Derlindati EJ, Caziani SM. Using canopy and understory mist nets and point counts to study bird assemblages in Chaco forests. The Wilson Bulletin. 2005;117(1):92–99. [Google Scholar]
- Dobson AP, Bradshaw AD, Baker AJM. Hopes for the future: Restoration ecology and conservation biology. Science. 1997;277(5325):515–522. [Google Scholar]
- Elphick CS. Landscape effects on waterbird densities in California rice fields: Taxonomic differences, scale-dependence, and conservation implications. Waterbirds. 2008;31(1):62–69. [Google Scholar]
- Erwin TL. Tropical forests: Their richness in Coleoptera and other arthropod species. In: Chazdon RL, Whitmore TC, editors. Foundations of tropical forest biology: Classic papers with commentaries. Chicago: University of Chicago Press; 2001. [Google Scholar]
- Fasola M, Galeotti P, Dai N, Dong Y, Zhang Y. Large numbers of breeding egrets and herons in China. Waterbirds. 2004;27(1):126–128. [Google Scholar]
- Fitzherbert EB, Struebig MJ, Morel A, Danielsen F, Brühl CA, Donald PF, Phalan B. How will oil palm expansion affect biodiversity? Trends in Ecology and Evolution. 2008;23(10):538–545. doi: 10.1016/j.tree.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Fujioka M, Lee DS, Kurechi M, Yoshida H. Bird use of rice fields in Korea and Japan. Waterbirds. 2010;33(1):8–29. [Google Scholar]
- Fujioka M, Yoshida H. The potential and problems of agricultural ecosystems for birds in Japan. Global Environmental Research. 2001;5(2):151–161. [Google Scholar]
- Gautier-Hion A, Duplantier JM, Quris R, Feer F, Sourd C, Decaux JP, et al. Fruit characters as a basic of fruit choice and seed dispersal in a tropical forest vertebrate community. Oecologia. 1985;65(3):324–337. doi: 10.1007/BF00378906. [DOI] [PubMed] [Google Scholar]
- Gibbons DW, Hill D, Sutherland WJ. Birds. In: Suntherland WJ, editor. Ecogical census techniques. NY, USA: Cambridge University Press; 1996. pp. 243–248. [Google Scholar]
- Glue DE. Food of the barn owl in Britain and Ireland. Bird Study. 1974;21(3):200–210. [Google Scholar]
- Gray MA, Baldauf SL, Mayhew PJ, Hill JK. The response of avian feeding guilds to tropical forest disturbance. Conservation Biology. 2007;21(1):133–141. doi: 10.1111/j.1523-1739.2006.00557.x. [DOI] [PubMed] [Google Scholar]
- Harris GM, Pimm SL. Bird species' tolerance of secondary forest habitats and its effects on extinction. Conservation Biology. 2004;18(6):1607–1616. [Google Scholar]
- Herrera CM. Seasonal variation in the quality of fruits and diffuse coevolution between pants and avian dispersers. Ecology. 1982;63(3):773–785. [Google Scholar]
- Ibáñez C, Curcó A, Riera X, Ripoll I, Sánchez C. Influence on birds of rice field management practices during the growing season: A review and an experiment. Waterbirds. 2010;33(1):167–180. [Google Scholar]
- Johns AD. Responses of Amazonian rain forest birds to habitat modification. Journal of Tropical Ecology. 1991;7(4):417–437. [Google Scholar]
- Kato M, Matsuda T, Yamashita Z. Associative ecology of insects found in the paddy field cultivated by various planting forms. The Sciences Reports of the Tohoku University, 4th Series (Biology) 1952;19:291–301. [Google Scholar]
- Kelly JP, Stralberg D, Etienne K, McCaustland M. Landscape influence on the quality of heron and egret colony sites. Wetlands. 2008;28(2):257–275. [Google Scholar]
- King S, Elphick CS, Guadagnin D, Taft O, Amano T. Effects of landscape features on waterbird use of rice fields. Waterbirds. 2010;33(1):151–159. [Google Scholar]
- Koh LP, Wilcove DS. Is oil palm agriculture really destroying tropical biodiversity? Conservation Letters. 2008;1(2):60–64. [Google Scholar]
- Levey DJ. Seed size and fruit-handling techniques of avian frugivores. The American Naturalist. 1987a;129(4):471–485. [Google Scholar]
- Levey DJ. Sugar-tasting ability and fruit selection in tropical fruit-eating birds. The Auk. 1987b;104(2):173–179. [Google Scholar]
- Levey DJ, Moermond TC, Denslow JS. Fruit choice in neotropical birds: The effect of distance between fruits on preference patterns. Ecology. 1984;65(3):844–850. [Google Scholar]
- Lim HC, Sodhi NS. Responses of avian guilds to urbanisation in a tropical city. Landscape and Urban Planning. 2004;66(4):199–215. [Google Scholar]
- Lombardini K, Bennetts RE, Tourenq C. Foraging success and foraging habitat use by cattle egrets and little egrets in the Camargue, France. Condor. 2001;103:38–44. [Google Scholar]
- Lourenco R, Sergio F. The food habits of Eurasian Eagle-owls in southern Portugal. Journal of Raptor Research. 2006;40(4):297–300. [Google Scholar]
- Lovari S, Renzoni A, Fondi R. The predatory habits of the barn owl (Tyto alba Scopoli) in relation to the vegetation cover. Bolletino di Zoologie. 1976;43(1–2):173–191. [Google Scholar]
- Maeda T. Patterns of bird abundance and habitat use in rice fields of the Kanto Plain, central Japan. Ecological Research. 2001;16(3):569–585. [Google Scholar]
- McGhie H. Diet of barn owl in East Ross and East Ness. Scottish Birds. 2001;22(2):92–103. [Google Scholar]
- McMorrow J, Talip MA. Decline of forest area in Sabah, Malaysia: Relationship to state policies, land code and land capability. Global Environmental Change. 2001;11(3):217–230. [Google Scholar]
- Miller JR, Dixon MD, Turner MG. Response of avian communities in large-river floodplains to environmental variation at multiple scales. Ecological Applications. 2004;14(5):1394–1410. [Google Scholar]
- Pearman PB. The scale of community structure: Habitat variation and avian guilds in tropical forest understory. Ecological Monographs. 2002;72(1):19–39. [Google Scholar]
- Peh KSH, de Jong J, Sodhi NS, Lim SLH, Yap CAM. Lowland rainforest avifauna and human disturbance: Persistence of primary forest birds in selectively logged forests and mixed-rural habitats of southern Peninsular Malaysia. Biological Conservation. 2005;123(4):489–505. [Google Scholar]
- Peh KSH, Sodhi NS, de Jong J, Sekercioglu CH, Yap CAM, Lim SLH. Conservation value of degraded habitats for forest birds in southern Peninsular Malaysia. Diversity and Distributions. 2006;12(5):572–581. [Google Scholar]
- Pierluissi S, King SL, Kaller MD. Waterbird nest density and nest survival in rice fields of Southwestern Louisiana. Waterbirds. 2010;33(3):323–330. [Google Scholar]
- Rappole JH, McDonald MV. Cause and effect in population declines of migratory birds. The Auk. 1994;111(3):652–660. [Google Scholar]
- Razafimanjato G, Sam TS, Thorstrom R. Waterbird monitoring in the Antsalova region, western Madagascar. Waterbirds. 2007;30(3):441–447. [Google Scholar]
- Robson C. A field guide to the birds of South-East Asia. London: New Holland Publisher Ltd; 2008. [Google Scholar]
- Sekercioglu Ç H, Ehrlich PR, Daily GC, Aygen D, Goehring D, Sandí RF. Disappearance of insectivorous birds from tropical forest fragments. Proceedings of the National Academy of Sciences. 2002;99(1):263–267. doi: 10.1073/pnas.012616199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford JD, Kaminski RM, Reinecke KJ. Avian foods, foraging and habitat conservation in world rice fields. Waterbirds. 2010;33(1):133–150. [Google Scholar]
- Takahashi M, Ohkawara K. Breeding behavior and reproductive success of Grey-headed Lapwing Vanellus cinereus on farmland in central Japan. Ornithological Science. 2007;6(1):1–9. [Google Scholar]
- Waltert M, Mardiastuti A, Muhlenberg M. Effects of land use on bird species richness in Sulawesi, Indonesia. Conservation Biology. 2004;18(5):1339–1346. [Google Scholar]
- Wang Y, Finch DM. Consistency of mist netting and point counts in assessing landbird species richness and relative abundance during migration. The Condor. 2002;104(1):59–72. [Google Scholar]
- Wells DR. The birds of the Thai-Malay Peninsular: Passerines. Vol. 2. London: Academic Press; 2007. [Google Scholar]
- Wells DR. The birds of the Thai-Malay Peninsular: Non-passerines. Vol. 1. London: Academic Press; 1999. [Google Scholar]
- Wood C, Qiao Y, Li P, Ding P, Lu B, Xi Y. Implications of rice agriculture for wild birds in China. Waterbirds. 2010;33(1):30–43. [Google Scholar]
- Zakaria M, Leong PC, Yusuf ME. Comparison of species composition in three forest types: Towards using bird as indicator of forest ecosystem health. Journal of Biological Sciences. 2005;5(6):734–737. [Google Scholar]
- Zakaria M, Rajpar MN. Bird species composition and feeding guilds based on point count and mist netting methods at the Paya Indah Wetland Reserve, Peninsular Malaysia. Tropical Life Sciences Research. 2010;21(2):7–26. [PMC free article] [PubMed] [Google Scholar]
- Zhijun W, Young SS. Differences in bird diversity between two swidden agricultural sites in mountainous terrain, Xishuangbanna, Yunnan, China. Biological Conservation. 2003;110(2):231–243. [Google Scholar]
- Zou F, Yang Q, Dahmer T, Cai J, Zhang W. Habitat use of waterbirds in coastal wetland on Leizhou Peninsula, China. Waterbirds. 2006;29(4):459–464. [Google Scholar]