Abstract
Experiments were carried out in the laboratory and greenhouse to determine the growth inhibitory effects of Grassohopper’s cyperus (Cyperus iria L.) on the seedlings of 5 Malaysian rice varieties namely MR211, MRQ74, MR220, MR84 and MR232. Three concentrations of the aqueous extract of the weed (12.5, 25.0 and 50.0 g/l) and weed debris (5, 10 and 20 g dry debris/1000 g soil) were used to test the allelopathic effect of C. iria on the growth of the rice plants. The weed leaf, stem and root extracts reduced the growth of the rice seedlings and showed selective activity in the varieties. The C. iria leaf and stem extracts showed comparatively higher growth inhibitory effects than those from the root. The weed extract caused more reduction in the root length of the rice plant compared to the shoot length. Among the rice varieties tested, MR232 was found to be more susceptible to the weed inhibitory effect. The leaf extract of C. iria at full strength caused root and shoot reduction of MR232 by 88.1% and 73.1% respectively (compared to the control). In most cases the fresh weight of the rice seedlings were more affected than the plant height. Weed debris caused significant reduction of leaf chlorophyll content in all the rice varieties tested with the exception of MR211. The chlorophyll content of MR232 was greatly affected by the weed debris which caused reduction of 36.4% compared to the control. The inhibitory effects of weed extracts and debris on rice growth parameters were found to be concentration dependent.
Keywords: Allelopathy, Cyperus iria, Aqueous Extract, Debris, Chlorophyll
Abstract
Kajian telah dijalankan di dalam makmal dan rumah hijau bagi menentukan kesan perencatan pertumbuhan Cyperus iria pada anak benih bagi 5 varieti padi Malaysia iaitu MR211, MRQ74, MR220, MR84 dan MR232. Tiga kepekatan ekstrak akues rumpai (12.5, 25.0 dan 50.0 g/l) dan sarap rumpai (5, 10 dan 20 g sarap kering/1000 g tanah) telah digunakan bagi menguji kesan alelopati bagi C. iria pada pertumbuhan pokok padi. Ekstrak daun, batang dan akar rumpai merencat pertumbuhan anak benih padi dan menunjukkan aktiviti memilih bagi semua varieti. Ekstrak daun dan batang C. iria menunjukkan kesan perencatan pertumbuhan yang tinggi berbanding dengan ekstrak akar. Ekstrak rumpai menyebabkan pengurangan yang lebih terhadap panjang akar berbanding dengan panjang pucuk bagi pokok padi. Di antara varieti padi yang diuji, didapati C. iria lebih memberikan kesan perencatan kepada MR232. Ekstrak C. iria pada kepekatan tertinggi menyebabkan pengurangan pada MR232 sebanyak 88.1% dan 73.1% pada akar dan pucuk masing-masing (perbandingan dengan kawalan). Di dalam kebanyakan kes, berat segar bagi anak benih padi lebih banyak dipengaruhi oleh ketinggian pokok. Sarap rumpai menyebabkan pengurangan yang signifikan bagi kandungan klorofil daun bagi semua varieti beras yang diuji kecuali pada MR211. Kandungan klorofil bagi MR232 sangat dipengaruhi oleh sarap rumpai yang menyebabkan pengurangan sebanyak 36.4% berbanding dengan kawalan. Kesan perencatan bagi ekstrak rumpai dan sarapnya kepada parameter pertumbuhan padi didapati bergantung kepada kepekatan.
INTRODUCTION
Weed infestation is a major problem limiting the growth and yield of the rice crop (Bhatt & Tewari 2006). In rice cultivation, control of weeds is one of the important procedures adopted to ensure good crop yield. In rice, the loss of yield due to weed infestation is higher than the combined yield loss caused by insect pests and diseases (Isley 1960). The direct-seeded (DS) culture has become increasingly popular in rice cultivation. The scarcity of rural labour coupled with escalating production costs constitute the main reason for the shift to the DS rice culture and its rapid and eventual adoption as opposed to the previous transplanting method (Azmi & Baki 2006). The main constraint in the DS culture is weed infestation. Extensive use of the direct seeding method of rice culture from the year 2000 onwards has resulted in Cyperus iria L. becoming a serious weed in rice fields (Azmi & Baki 2002). It is an annual herbaceous sedge, is tufted, tall and spreads by seeds. C. iria has also been reported to be present in transplanted rice fields (Mian et al. 2007). It is an extremely invasive weed, responsible for yield reduction of economically important crops particularly rice (Holm et al. 1977). It has been reported that, infestation with C. iria throughout the crop growth period caused 64% reduction in rice yield (Dhammu & Sandhu 2002). Weeds compete with cultivated species for space, light, water, nutrients and other growth requirements and are certainly the major sources accounting for the adverse effects on crop growth and yield (Sharma et al. 1986; Patterson 1981). In addition to competition with rice plant for nutrients, some weeds have growth inhibitory effects on the rice plant (Ohigashi et al. 1997; Yamamoto et al. 1999; Siddique & Ismail 2009). Research on various aspects of C. iria has been documented: Manandhar et al. (2007) reported that, C. iria may contain some active allelochemicals; identification of which would help in understanding the crop-weed relationship and the appropriate measures that need to be taken for proper weed management. C. iria contains a high concentration of juvenile hormone (JH) III, which plays important biological role(s) in the plant mechanism perhaps through plant-insect, plant-plant or other interactions (Jacqueline et al. 1999). In the context of research findings on other aspects, only limited information is available on C. iria’s growth inhibitory effect on the rice plant. Therefore, the objective of this study was to investigate the growth inhibitory effects of C. iria in vitro and in greenhouse conditions on five rice varieties commonly grown in Malaysia.
MATERIALS AND METHODS
C. iria plants were collected from the Tanjung Karang rice growing area in Selangor, Malaysia. The weed plants were washed, separated into leaf, stem and root, air-dried at room temperature (27±3°C) for 72 hours, ground by a commercial blender and kept in the laboratory at room temperature. Rice seed of varieties MR211, MRQ74, MR220, MR84 and MR232 were obtained from the MARDI Research Station at Seberang Perai, Malaysia.
In vitro Effect of Aqueous Extracts of the Leaves, Stems and Roots of C. iria on Rice Seedlings
10 g each of air dried plant parts (leaf, stem or root) were placed separately in flasks containing 200 ml of distilled water and shaken for 48 hr at room temperature (27±3°C) by an orbital shaker (160 rpm). The extracts were strained through 4 layers of cheese cloth and then through 2 layers of Whatman no-2 filter paper to remove solid material. The filtrate was centrifuged at 4000 rpm for 15 min. The supernatant was collected and filtered through a 0.22 μm membrane filter paper. The stock solution was stored at 4°C until further use. Four concentrations of the aqueous extract were used in the experiment: full strength (50.0 g/l), half strength (25.0 g/l), quarter strength (12.5 g/l) and control (0 g/l). Dilutions were made using distilled water just before use. Rice seeds were surface sterilised (with 0.5% sodium hypochlorite for 15 min) and 10 seeds of each variety namely MR220, MRQ74, MR211, MR84 and MR232 were placed in separate Petri dishes lined with 9 cm diameter Whatman no-2 filter paper. 5 ml of weed extract were used to wet each filter paper. Four replicates were used for each concentration. The Petri dishes were then incubated at 30°C (12 h photoperiod) and checked daily. They were kept moist by adding the specific extract and distilled water when necessary. The root and shoot length of the seedlings were recorded after 7 days. Root length and shoot length of the seedlings were measured and expressed as a percentage of the control.
Bioassay of the Weed Debris
To determine the effect of weed debris, whole dried weed plants (leaf, stem and root) were cut into pieces and ground by a commercial blender and stored at 4°C until they were to be used. Debris of four concentrations (0, 5, 10 and 20 g dry debris) were mixed separately with 1000 g soil (46.5% sand, 23.5% clay, 30% silt; N=0.36 mg/100 g soil, P=5.50 mg/100 g soil, K=0.70 mg/100 g soil) and placed in black polybags (height 12 cm x diameter 10 cm) which were punched with holes. For control, similar bags were filled with soil but without debris. Twenty seeds of each rice variety were sown in separate bags and watered regularly. The bags were kept in the greenhouse (temperature: 25°C–38°C, light density: 780±250 μEm−2s−2 and relative humidity: 55%). After 7 days the seedlings were thinned to 10 per bag. Plants were harvested 2 weeks after sowing. Plant height (above ground part) and seedling fresh weight were recorded and expressed as percentage of the control.
Chlorophyll Extraction and Measurement
Twenty rice seedlings each were grown separately in black polybags (height 12 cm x diameter 10 cm). The bags contained soil that was treated with the four different concentrations of debris as described above. Fully expanded rice leaves were collected randomly 2 weeks after sowing. The fresh leaves were cut into small pieces; 100 mg were placed in a test tube containing 10 ml acetone (80%) and then kept in a refrigerator at 4°C overnight. The mixture was strained through glass wool. After centrifugation (5000 rpm) for 10 min at room temperature (25°C), the supernatant was withdrawn and absorbance was recorded at 663 and 645 nm using the double beam spectrophotometer (Hitachi U-2000, Hitachi Ltd., Tokyo, Japan). The amount of chlorophyll extracted was calculated using the following formula (Arnon 1949):
Statistical Analysis
All experiments were conducted using the completely randomised design with 4 replications. The experimental data was subjected to the analysis of variance and means were compared using the Duncan Multiple range test at the 5% level of significance. The statistical analysis was done using the SPSS/PC version 11.5 software (SPSS Inc., Chicago, USA).
RESULTS AND DISCUSSION
Aqueous Extracts
Root growth
The weed leaf aqueous extract showed inhibitory effects on seedling root growth for all the rice varieties. It caused significant root reduction in MR211, MRQ74, MR220, MR84 and MR232 by 32%, 74%, 73%, 65% and 88% respectively at full strength compared to the control (Table 1). The stem extracts reduced the root growth of MRQ74, MR220 and MR232 plants significantly based on different concentration level. It reduced the root growth of MR232 by 84% (compared to the control), followed by the other varieties at full strength. The root length of MR232 was reduced significantly with increasing concentrations of the weed root extract. Root reduction of MR232 was 49% (compared to the control), followed by the other 3 rice varieties with the exception of MR84. The results showed that the extracts from different parts of the weed (leaf, stem and root) exerted different degrees of inhibition on root growth among the rice varieties. Quantities of allelochemicals vary in different plant tissues and under different phenological and environmental conditions (Putnam & Duke 1978; Ismail & Kumar 1996). The various responses may be due to the selectivity of allelochemicals for the target varieties (Inderjit & Duke 2003). Besides the selectivity of allelochemicals, the tested varieties may also have demonstrated selectivity. For example, with respect to growth parameters, different rice and wheat cultivars responded differently to Echinochloa colona (Siddique & Ismail 2010) and sunflower (Kamal & Asghari 2008) allelopathy respectively. Olofsdotter et al. (2002) reported that different rice cultivars showed different degree of tolerance to phenolic acid. The C. iria leaf extracts enhanced the root growth of MR211 at quarter strength and MRQ74 at quarter and half strengths. But these extracts significantly reduced the root growth of the same varieties at full strength. Many studies showed that allelochemicals stimulated growth at lower concentrations and caused growth inhibition at higher concentrations (Ismail & Chong 2009; Leather & Einhellig 1988; Mandal 2001).
Table 1:
Effect of C. iria extracts (leaf, stem and root) on the seedling root length (% of the control) of five rice varieties.
| Rice varieties | Extract concentration (g/l)
|
|||
|---|---|---|---|---|
| Control (0) | Quarter (12.5) | Half (25.0) | Full (50.0) | |
| Leaf extract | ||||
| MR211 | 100.0b | 107.8a | 93.0c | 68.3d |
| MRQ74 | 100.0a | 103.1a | 104.8a | 25.6b |
| MR220 | 100.0a | 61.7b | 59.8b | 26.8c |
| MR84 | 100.0a | 93.4ab | 73.4b | 34.8c |
| MR232 | 100.0a | 66.7b | 65.4b | 11.9c |
| Stem extract | ||||
| MR211 | 100.0a | 103.8a | 108.0a | 64.6b |
| MRQ74 | 100.0a | 82.3b | 84.9b | 64.0c |
| MR220 | 100.0a | 72.7b | 74.0b | 58.0c |
| MR84 | 100.0b | 108.1a | 96.5b | 90.6c |
| MR232 | 100.0a | 57.1b | 52.2b | 15.7c |
| Root extract | ||||
| MR211 | 100.0ab | 107.0a | 90.7b | 71.4c |
| MRQ74 | 100.0b | 108.4a | 96.7b | 89.7c |
| MR220 | 100.0a | 77.6a | 73.6a | 49.8b |
| MR84 | 100.0a | 133.6a | 109.5a | 119.8a |
| MR232 | 100.0a | 81.69b | 56.81c | 51.5c |
Note: Means within rows followed by same alphabet are not significantly different (p>0.05).
Shoot growth
Aqueous leaf extracts of C. iria at full strength showed inhibitory effects on seedling shoot growth for all the rice varieties tested. The leaf extract caused significant shoot reduction of MR211, MRQ74, MR220, MR84 and MR232 by 14%, 35%, 61%, 61% and 73% respectively compared to the control (Table 2). The shoot growth of the rice seedlings decreased progressively when exposed to increasing concentrations of the aqueous extract of the weed stem. At full strength the stem extract of C. iria showed a pattern of inhibition of MR232 similar to that of the leaf extract. The shoot growth of MRQ74 and MR220 was not adversely affected by the root extract, rather it was stimulated. The aqueous extract of C. iria root caused shoot reduction of MR232 by 27% (compared to the control) and the reduction started at half strength. Similar to the root growth, the shoot growth of MR232 was greatly reduced by the leaf, stem and root extracts of the weed. Increasing the weed extract concentration caused further reduction of the shoot and root length. The magnitude of allelopathic interactions is dependent on the concentration and chemical stability of the active inhibitory compounds as well as the plant's tolerance to such compounds and their microbial metabolites (Phillips et al. 1980).
Table 2:
Effect of C. iria extracts (leaf, stem and root) on the seedling shoot length (% of the control) of five rice varieties.
| Rice varieties | Extract concentration (g/l)
|
|||
|---|---|---|---|---|
| Control (0) | Quarter (12.5) | Half (25.0) | Full (50.0) | |
| Leaf extract | ||||
| R211 | 100.0a | 104.6a | 96.8a | 85.9b |
| MRQ74 | 100.0a | 104.7a | 101.7a | 64.6b |
| MR220 | 100.0a | 76.4b | 53.2c | 38.6d |
| MR84 | 100.0a | 93.1a | 67.8b | 39.4c |
| MR232 | 100.0a | 78.4b | 67.3b | 26.9c |
| Stem extract | ||||
| MR211 | 100.0a | 96.7a | 93.0a | 81.4a |
| MRQ74 | 100.0a | 97.7a | 100.8a | 73.1b |
| MR220 | 100.0a | 88.6a | 71.9b | 55.1c |
| MR84 | 100.0a | 98.4a | 66.6b | 52.2b |
| MR232 | 100.0a | 69.7b | 54.6b | 51.0b |
| Root extract | ||||
| MR211 | 100.0a | 106.7a | 105.1a | 86.9b |
| MRQ74 | 100.0a | 123.0b | 112.0ab | 105.7a |
| MR220 | 100.0a | 110.8b | 108.3ab | 114.4b |
| MR84 | 100.0a | 104.9a | 101.1a | 88.6b |
| MR232 | 100.0a | 103.2a | 86.9b | 72.9c |
Note: Means within rows followed by same alphabet are not significantly different (p>0.05).
Weed Debris
Table 3 shows the inhibitory effect of the weed debris (incorporated into the soil) on plant height, and seedling fresh weight. Irrespective of the varieties, plant height and fresh weight of rice seedlings decreased with increasing concentration of weed debris. The plant height and fresh weight reduction of the rice seedlings tested started at quarter strength. Rice variety MR232 was seen to be more affected than the other varieties with respect to the inhibitory effect of the weed on fresh weight and plant height. The plant height and fresh weight of MR232 was reduced significantly by 41% and 53% respectively, of the control by the weed debris, followed by the other varieties. It has been reported that debris of some allelopathic plants retarded the growth of bioassay species (Ismail & Chong 2009; Randall et al. 1989).
Table 3:
Effect of the debris of C. iria, on seedling plant height and fresh weight (% of control) of five rice varieties.
| Rice varieties | Debris concentration (g/kg)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Control (0) | Quarter (5) | Half (10) | Full (20) | Control (0) | Quarter (5) | Half (10) | Full (20) | |
|
| ||||||||
| Plant height | Fresh weight | |||||||
| MR211 | 100.0a | 81.8b | 72.8b | 69.7b | 100.0a | 74.7b | 73.5b | 67.5b |
| MRQ74 | 100.0a | 85.0b | 71.7c | 67.0d | 100.0a | 73.1b | 66.9b | 64.6b |
| MR220 | 100.0a | 80.8b | 74.4b | 73.5b | 100.0a | 72.9b | 66.0b | 63.6b |
| MR84 | 100.0a | 68.1b | 66.8b | 66.8b | 100.0a | 70.4b | 62.4bc | 57.1c |
| MR232 | 100.0a | 65.0b | 65.2b | 58.9c | 100.0a | 59.1b | 53.1b | 47.0c |
Note: Means within rows followed by same letter are not significantly different (p>0.05).
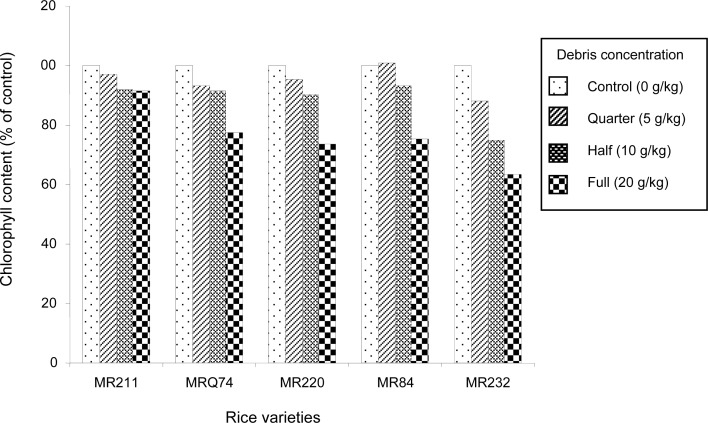
Chlorophyll Content
Figure 1 shows the inhibitory effect of C. iria debris on the chlorophyll content of rice leaves. The weed debris at full strength significantly reduced the chlorophyll content of MRQ74, MR220, MR84 and MR232 by 22.6%, 26.3%, 24.7% and 36.4% respectively, compared to the control. The variety MR232 showed the highest reduction in the chlorophyll content. Significant reduction of the chlorophyll content was observed in this variety even at quarter strength (5 g debris/1000 g soil). The degree of chlorophyll content reduction in MR232 increased as the debris concentration increased. Decrease in chlorophyll content by the presence of allelochemicals has been reported by Baziramakenga et al. (1994), Patterson (1981) and Zeng et al. (2001). Since chlorophyll content is closely related to plant dry matter production (Buttery & Buzzell 1977), reduction in leaf chlorophyll content would cause decreased photosynthesis and hence total plant growth.
Figure 1:
Inhibitory effect of the debris of C. iria on the leaf chlorophyll content (% of control) of five rice varieties.
Results of the study showed that, in addition to its competitive ability, C. iria has inhibitory effects on the growth factors of rice plants and among the 5 varieties tested; it was found that MR232 is the most sensitive to its inhibition. More work has to be done in order to discover whether there are multi dimensional inhibitory effects of this weed on rice plants.
Acknowledgments
We would like to thank Centre for Graduate Management, Universiti Kebangsaan Malaysia for providing fellowship research grant to the second author.
REFERENCES
- Arnon DI. Copper enzymes in isolated chloroplasts: Polyphenoloxidase in Beta vulgaris. Plant Physiology. 1949;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azmi M, Baki BB. Weed flora landscapes and innovative management in direct seeded culture. Proceedings of the Second International Rice Congress; New Delhi, India. 9–13 October.2006. [Google Scholar]
- Azmi M, Baki BB. Impact of continuous direct seeding rice culture on weed species diversity in the Malaysian rice ecosystem. Proceedings of the Regional Symposium on Environment and Natural Resources; Kuala Lumpur, Malaysia. 10–11 April; 2002. pp. 61–67. [Google Scholar]
- Baziramakenga R, Simard RR, Leroux GD. Effects of benzoic and cinnamic acids on growth, mineral composition and chlorophyll content of soybean. Journal of Chemical Ecology. 1994;20(11):2821–2833. doi: 10.1007/BF02098391. [DOI] [PubMed] [Google Scholar]
- Bhatt MD, Tewari A. Losses in growth and yield attributes due to weed composition in transplanted paddy in Terai region. Scientific World. 2006;4(4):99–101. [Google Scholar]
- Buttery BR, Buzzell RI. The relationship between chlorophyll content and rate of photosynthesis in soybean. Canadian Journal of Plant Science. 1977;57(1):1–5. [Google Scholar]
- Dhammu HS, Sandhu KS. Critical period of Cyperus iria L. competition in transplanted rice. 13th Australian Weeds Conference: Weeds “Threats now and forever?”; Perth, Western Australia. 8–13 September.2002. [Google Scholar]
- Holm LG, Plucknett DL, Pancho JV, Herberger JP. The world's worst weeds: Distribution and biology. Honolulu: University Press of Hawaii; 1977. pp. 240–242. [Google Scholar]
- Inderjit, Duke SO. Ecophysiological aspects of allelopathy. Planta. 2003;217(4):529–539. doi: 10.1007/s00425-003-1054-z. [DOI] [PubMed] [Google Scholar]
- Isley D. Weed identification and control in North Central States. Ames, Iowa USA: Iowa State University Press; 1960. pp. 28–39. [Google Scholar]
- Ismail BS, Chong TV. Allelopathic effects of Dicranopteris linearis debris on common weeds of Malaysia. Allelopathy Journal. 2009;23(2):277–286. [Google Scholar]
- Ismail BS, Kumar A. Effects of aqueous extracts and residues decomposition of Mikania micrantha H.B.K. on selected crops. Allelopathy Journal. 1996;3(2):195–206. [Google Scholar]
- Jacqueline CB, Walter G, Tobe S. Insect juvenile hormone III in the sedge, Cyperus iria L. Distribution and possible biological significance. 1999. http://www.iupac.org/symposia/proceedings/phuket97/bede.html© (15 August 2010).
- Kamal J, Asghari B. Effects of sunflower (Helianthus annuus L.) extracts on wheat (Triticum aestivum L.) and physicochemical characteristics of soil. African Journal of Biotechnology. 2008;7(22):4130–4135. [Google Scholar]
- Leather GR, Einhellig FA. Bioassay of naturally occurring allelochemicals for phytotoxicity. Journal of Chemical Ecology. 1988;14(10):1821–1828. doi: 10.1007/BF01013479. [DOI] [PubMed] [Google Scholar]
- Manandhar S, Shrestha BB, Lekhak HD. Weeds of paddy field at Kirtikpur, Kathmandu. Scientific World. 2007;5(5):100–106. [Google Scholar]
- Mandal S. Allelopathic activity of root exudates from Leonurus sibiricus L. (Raktodrone) Weed Biology and Management. 2001;1(3):170–175. [Google Scholar]
- Mian MAK, Matin MA, Hossain MA. Occurance of weed species in transplanted aman rice field as affected by cultivar. Bangladesh Journal of Botany. 2007;36(1):89–92. [Google Scholar]
- Ohigashi H, Harada J, Tadachika S, Nobuhiro H, Premasthira C, Asakawa Y, Yoshiharu F, Sakashita S. Allelochemicals of gooseweed, Sphenoclea zeylanica. Abstracts of the international union of pure and applied chemistry. International Conference Biodiversity and Bioresources-Conservation and Utilization; Phuket, Thailand. 23–27 November, 157.1997. [Google Scholar]
- Olofsdotter M, Rebulanan M, Madrid A, Dali W, Navarez D, Olk DC. Why phenolic acids are unlikely primary allelochemicals in rice. Journal of Chemical Ecology. 2002;28(1):229–242. doi: 10.1023/a:1013531306670. [DOI] [PubMed] [Google Scholar]
- Patterson DT. Effects of allelopathic chemicals on growth and physiological responses of soybean (Glycine max) Weed Science. 1981;29(1):53–59. [Google Scholar]
- Phillips RE, Blevins RL, Thomas GW, Frye WW, Phillips SH. No-tillage agriculture. Science. 1980;208(4448):1108–1113. doi: 10.1126/science.208.4448.1108. [DOI] [PubMed] [Google Scholar]
- Putnam AR, Duke WB. Allelopathy in agroecosystems. Annual Review of Phytopathology. 1978;16:431–451. [Google Scholar]
- Randall H, White A, Worsham D, Blum U. Allelopathic potential of legume debris and aqueous extracts. Weed Science. 1989;37(5):674–679. [Google Scholar]
- Sharma HL, Singh CM, Tripathi B. Response of transplanted rice to nitrogen fertilization under different weed management practices. International Journal of Pest Management. 1986;32(2):108–110. [Google Scholar]
- Siddique AB, Ismail BS. Allelopathic effects of Fimbristylis miliaceae on rice plants. Proceedings of the 16th Asian Agricultural Symposium (AAS) and 1st International Symposium on Agriculture Technology (ISAT); Bangkok, Thailand. 25–27 August; 2010. pp. 72–75. [Google Scholar]
- Siddique AB, Ismail BS. Assessment of allelopathic potential of goose weed (Sphenoclea zeylanica) on selected commonly used rice varieties in Malaysia. Prosiding Kolokium Siswazah Ke-9, Fakulti Sains dan Teknologi; Universiti Kebangsaan Malaysia, Bangi. 24–25 June; 2009. pp. 150–152. [Google Scholar]
- Yamamoto T, Yokotani-Tomita K, Kosemura S, Yamamura K, Yamada, Hasegawa K. Allelopathic substance exuded from a serious weed, germinating barnyard grass (Echinochloa crus-galli L.), roots. Journal of Plant Growth Regulation. 1999;18(2):65–67. doi: 10.1007/pl00007050. [DOI] [PubMed] [Google Scholar]
- Zeng RS, Luo SM, Shi YH, Shi MB, Tu CY. Physiological and biochemical mechanism of allelopathy of secalonic acid F on higher plants. Agronomy Journal. 2001;93(1):72–79. [Google Scholar]