Abstract
Fat contained within skeletal muscle is strongly associated with obesity, type 2 diabetes mellitus, and metabolic syndrome. Physical inactivity may be a risk factor for greater fat infiltration within skeletal muscle during growth.
PURPOSE
We sought to examine the relationship between physical activity and skeletal muscle fat content of the calf and thigh in girls.
METHODS
Data from 464 girls, aged 8–13 years, was used to examine the relationship between physical activity and skeletal muscle fat content of the calf and thigh. Calf and thigh muscle density (mg/cm3), an index of skeletal muscle fat content, was assessed at the 66% tibia and 20% femur sites relative to the respective distal growth plates of the non-dominant limb using peripheral quantitative computed tomography (pQCT). Physical activity level was classified by past year physical activity questionnaire (PYPAQ) score.
RESULTS
Muscle densities of the calf and thigh were inversely correlated with percent total body fat (r =−0.37 and−0.48, P values < 0.001) and total body fat mass (r =−0.33 and−0.40, P values < 0.001). Multiple linear regression with physical activity, ethnicity, maturity offset, and muscle cross-sectional area as independent variables showed that physical activity was independently associated with muscle densities of the calf (β = 0.14, P = 0.002) and thigh (β = 0.15, P < 0.001). Thus, lower physical activity was associated with higher skeletal muscle fat content.
CONCLUSION
Our results suggest that a lower level of physical activity may lead to excess skeletal muscle fat content of the calf and thigh in girls.
Keywords: MUSCLE QUALITY, EXERCISE, FEMALE, YOUTH, PQCT
Introduction
The incidence of type 2 diabetes mellitus (T2DM) increased in children and adolescents in the United States by 10-fold from 1982 to 1994 (29), paralleling the abrupt increase in the prevalence of childhood obesity (27). Along with intra-abdominal obesity, a consistent body of evidence suggests that the pathogenesis of insulin resistance in obesity and T2DM is associated with greater fat content within skeletal muscle in adults (14, 24) and children (32), even after adjusting for total body adiposity. Recent studies in children with disabilities that limit physical activity (17, 22), and otherwise healthy adults (9, 23), have suggested a link between physical inactivity and skeletal muscle fat content. Furthermore, prospective studies in adults have reported that regular physical activity prevents excess accumulation of fat within skeletal muscle (12, 30, 33). Taken together, these findings suggest that lower physical activity may be partly responsible for the link between greater skeletal muscle fat content and insulin resistance. Given that sedentary behavior is a risk factor for the development of childhood obesity, insulin resistance, and T2DM (27), it is imperative to better understand the relationship between physical activity and skeletal muscle fat content in youth.
Few studies in youth have examined the association of physical activity with skeletal muscle fat content. Johnson and colleagues (17) recently reported that children with quadriplegic cerebral palsy have greater skeletal muscle fat content than healthy children, which may be related to their lower level of physical activity. These findings are consistent with another study in boys, aged 9–12 years, with other disabilities that limit physical activity (22). Taken together, these studies suggest that lower physical activity contributes to greater skeletal muscle fat content, even in children and adolescents. Whether lower physical activity is associated with skeletal muscle fat content in otherwise healthy youth remains unclear.
The purpose of this study was to examine the association between physical activity and skeletal muscle fat content of the calf and thigh in girls. A unique feature of the study was the use of peripheral quantitative computed tomography (pQCT) because of its ability to differentiate tissues based on attenuation characteristics, which are directly related to tissue composition and density (13, 19). Controlled studies in sedentary obese and T2DM groups have demonstrated that lower muscle density (mg/cm3), which can be assessed with pQCT, is a valid measure of greater skeletal muscle fat content (13, 19). Based on observations that lower physical activity is associated with greater skeletal muscle fat content in children with disabilities (17, 22) and otherwise healthy adults (9, 23), we hypothesized that lower physical activity would be associated with higher skeletal muscle fat content in girls.
Methods
Participants
Baseline data were analyzed for 464 girls, aged 8–13 years, who were participants in the “Jump-In: Building Better Bones” study (5-7). Girls who were in school grade 4 or 6 were recruited from 14 elementary and 4 middle schools around Tucson, Arizona. Exclusion criteria included learning disabilities (identified by schools) that made it impossible to complete questionnaires or otherwise unable to comply with assessment protocols; medications known to affect bone, medical conditions, or a disability that limited participation in physical exercise as defined by the Committee on Sports Medicine and Fitness (1); excluded (or excused) from participation in physical education; and the inability to read and understand English. The protocol was approved by the University of Arizona Human Subjects Protection Committee and the study was conducted in accordance with the Helsinki Declaration. All guardians and girls provided written informed consent.
Covariates
The study population and methods for obtaining measures of body mass, height, trunk height, leg length, and non-dominant femur and tibia lengths have been described in detail previously (6). Coefficients of variation (CVs) for tibia and femur lengths were 0.51% and 0.33%, respectively (n = 464). Guardians completed a questionnaire that inquired about participant ethnicity and race. Maturity was assessed from self-report of breast development (Tanner stages) using a questionnaire that presents illustrations of stages of development that has been validated (26). Tanner staging is common in developmental studies, but its ability to accurately assess maturation is limited (36). Thus, we also used an alternate index of maturation (maturity offset), based on estimated years from peak height velocity (PHV) using an equation developed by Mirwald and colleagues (25) which was derived from data from a six-year longitudinal study in boys and girls (3). In Mirwald’s sample, the maturity offset equation for girls explained 89% of the variance in years from PHV (25).
Soft tissue composition
Soft tissue composition was assessed at the 66% tibia (calf) and 20% femur (thigh) sites relative to the respective distal growth plates of the non-dominant limb using pQCT (XCT 3000; STRATEC Medizintechnik GmbH, Pforzheim, Germany, Division of Orthometrix; White Plains, NY). A detailed description of the scanner and our protocol has been published (6). Briefly, scout scans were performed to locate the distal growth plates, with the scanner programmed to subsequently find the sites of interest. Scanner speed was set at 25 mm/second. Slice thicknesses were 2.3 mm and voxel sizes were set at 0.4 mm. Image processing and calculation of bone parameters were performed using the Stratec software package (Version 6.0). Contour mode 3 (−101 mg/cm3) and Peel mode 2 (40 mg/cm3) were used to separate adipose (<40 mg/cm3) and muscle/bone (≥40 mg/cm3), respectively. Images were subsequently filtered with a 7 × 7 image filter that clearly defined the edge of the muscle and eliminated all bone above 120 mg/cm3. Further details on image processing, calculations, and analysis, including descriptions of Contour and Peel modes are published elsewhere (37). We have previously used this technique to report the relationship between skeletal muscle fat content and bone strength in our sample of girls (6). Soft tissue parameters obtained at the calf and thigh regions included muscle cross-sectional area (MCSA, mm2) and muscle density (mg/cm3). CVs estimated from 29 subjects scanned twice within the same day after subject repositioning, calculated as described by Glüer (10) for muscle density (mg/cm3) and muscle cross-sectional area (MCSA, mm2) were 0.9% and 1.4%, respectively, at the calf site (6); CVs for the same parameters at the thigh were 0.4% and 1.3%, respectively (6). Total body mass, total body fat mass, percent total body fat, and total body lean mass were obtained from whole body dual energy X-ray absorptiometry (DXA) scans using the GE Lunar Prodigy (software version 5.60.003) fan-beam densitometer (GE Lunar Corp, Madison, WI, USA). Subjects were positioned following standard GE/Lunar protocols. DXA CVs in our laboratory estimated from 261 women scanned twice within the same day after subject repositioning, expressed as a percent of mean BMD, were ± 1.8%, ± 2.4%, ± 2.4%, and ± 0.8% for lumbar spine, femoral neck, trochanter, and total body BMD, respectively (11). The CV for repeated measures of percent total body fat was 2.8%.
Physical activity
The past year physical activity questionnaire (PYPAQ) (5, 7) was used to collect information about the average duration and frequency of physical activity participation. The PYPAQ has been validated in adolescents (2). Total PYPAQ score was computed using the validated equation from Shedd et al. (35): PYPAQ score = ∑1−n [duration (minutes/session) × frequency (days/week) × intensity (METs (31)) × load (peak strain score (15))], where n was the number of activities a subject reported during the past year. Average daily time spent in moderate-to-vigorous physical activity (MVPA, min/day) was determined from the duration and frequency of each at least moderate-intensity activity reported. The average daily time spent in MVPA (min/day) was used to calculate the proportion of girls who achieved U.S. Centers for Disease Control and Prevention physical activity recommendations for children and adolescents of 60 minutes of MVPA per day (38).
Statistical analysis
Data were checked for outliers and normality using histograms and all variables were tested for skewness and kurtosis. Bivariate correlations were computed using Pearson’s r for continuous and Spearman’s rho for categorical variables to examine relationships between maturity, ethnicity, anthropometric characteristics, and percent total body fat and muscle density, MCSA, and total body lean and fat masses. Multiple linear regression was used to examine the independent associations between physical activity and muscle densities of the calf and thigh, after controlling for ethnicity, maturity offset, and MCSA (Model 1). Regression analyses were then repeated after substituting MCSA with total body lean mass (Model 2). MCSA and total body lean mass were not included in the same model to protect against collinearity. MCSA and total body lean mass were used as covariates to determine whether the association between physical activity and skeletal muscle fat content was independent of muscle size and total body lean mass. Body mass and height were excluded to protect against collinearity with MCSA and total body lean mass. Ethnicity (non-Hispanic = 0; Hispanic = 1) and maturity offset were included as covariates in both models to adjust for the potential confounding effects of ethnicity and physical maturation on skeletal muscle fat content. Prior to multiple linear regression analyses, all variables were checked for normality, linearity, and homoscedasticity using residual plots. Postestimation procedures showed that none of the final regression models had any substantial collinearity that would affect the results of the conclusions. Quartiles were subsequently used to divide the sample into four physical activity groups (fourths). Muscle densities of the two middle fourths of physical activity were similar; thus, we collapsed these groups into a single group and used ANCOVA, after adjusting for the same covariates included in the regression models described above, to determine whether there were differences in muscle densities of the calf and thigh between the middle group (average of the middle 2 fourths) and the lowest and highest groups of physical activity. Bonferroni post hoc tests were used to adjust for multiple comparisons. All analyses were also performed within maturity offset and Tanner stage maturity categories [maturity offset < 0 years from PHV (PRE) and ≥ 0 years from PHV (POST); and Tanner stage I (prepubertal), Tanner stage II–III (early pubertal), Tanner stage IV–V (late pubertal)]. A significance level of P = 0.05 was used in all tests. All analyses were performed using The Statistical Package for the Social Sciences for Windows, Version 18.0 (SPSS, Chicago, IL, USA).
Results
Descriptive characteristics are shown in Table 1. Sample ethnicity was 23% Hispanic and 77% non-Hispanic. Sample race was 88% white, 7% Asian, 3% black or African American, 0.5% Native American or Alaska Native, 0.5% Native Hawaiian or other Pacific Islander, and 1.0% other. Based on U.S. National Center for Health Statistics/Centers for Disease Control percentiles for body mass index (BMI, kg/m2) (20), 3.0% of the sample was underweight (BMI <5th percentile), 73.8% of the sample was healthy weight (BMI 5th–85th percentile), 15.1% of the sample was overweight (BMI 85th–95th percentile), and 8.1% of the sample was obese (BMI >95th percentile). Tanner stage distributions for the total sample were 33% prepubertal (stage I, n = 155), 60% early pubertal (stages II–III, n = 280), and 7% late pubertal (stages IV–V, n = 30). Maturity offset values indicated that girls were on average 1.1 years prior to PHV, with a range from 3.2 years prior to PHV to 1.4 years post PHV.
TABLE 1.
Sample descriptive characteristics.
| Characteristics | Total sample (n = 464) |
|---|---|
| Age (yr) | 10.7 ± 1.1 |
| Menarche (%; Post) | 10 |
| Tanner stage (%; 1/2/3/4/5) | 33/33/27/5/2 |
| Maturity offset (yr) | −1.1 ± 1.0 |
| Body mass (kg) | 39.2 ± 10.4 |
| Height (cm) | 144.3 ± 9.8 |
| BMI (kg/m2) | 18.6 ± 3.4 |
| Trunk height (cm) | 75.5 ± 4.8 |
| Leg length (cm) | 68.8 ± 5.7 |
| Femur length (cm) | 34.0 ± 3.0 |
| Tibia length (cm) | 33.2 ± 2.8 |
| Lean mass (kg) | 25.7 ± 5.1 |
| Fat mass (kg) | 11.2 ± 6.2 |
| Total percent body fat (%) | 27.8 ± 8.4 |
| Calf muscle density (mg/cm3) | 78.9 ± 1.2 |
| Thigh muscle density (mg/cm3) | 76.3 ± 1.5 |
| Physical activity score | 5135.0 ± 4905.7 |
Values are presented as % or mean ± SD.
As expected, muscle densities of the calf and thigh were inversely correlated with percent total body fat (r =−0.37 and−0.48, P values < 0.001) and total body fat mass (r =−0.33 and−0.40, P values < 0.001). Unadjusted bivariate correlations (Pearson’s r) showed that physical activity was significantly correlated with muscle densities of the calf (r = 0.13, P = 0.004) and thigh (r = 0.16, P = 0.001). Muscle densities of the thigh and calf were not significantly associated with MCSA (r =−0.06 and 0.07, P values > 0.05) or total body lean mass (r =−0.01 and 0.02, P values > 0.05). There were moderate to strong correlations between maturity (maturity offset), anthropometric characteristics, percent total body fat and MCSA and total body lean and fat masses (Table 2). Lower correlations were found between maturity, anthropometric characteristics, percent total body fat and muscle densities of the calf and thigh (Table 2). Ethnicity was not significantly (P values > 0.05) correlated with muscle density, MCSA, or total body lean and fat masses.
TABLE 2.
Bivariate correlations between maturity, ethnicity, anthropometric characteristics, percent body fat and pQCT measures of skeletal muscle fat content and MCSA of the calf and thigh and total body lean and fat masses in girls.
| Physical activity |
Maturity | Ethnicity | Height | Body mass | Body fat (%) | |
|---|---|---|---|---|---|---|
| Calf muscle density (mg/cm3) | 0.13* | −0.01 | −0.12 | 0.02 | −0.21* | −0.37* |
| Thigh muscle density (mg/cm3) | 0.16* | −0.03 | −0.09 | 0.01 | −0.23* | −0.48* |
| Calf MCSA (mm2) | 0.16* | 0.80* | −0.02 | 0.72* | 0.88* | 0.40* |
| Thigh MCSA (mm2) | 0.17* | 0.70* | 0.01 | 0.65* | 0.78* | 0.38* |
| Total body lean mass (kg) | 0.15* | 0.89* | −0.01 | 0.89* | 0.85* | 0.23* |
| Total body fat mass (kg) | 0.01 | 0.56* | 0.06 | 0.47* | 0.91* | 0.91* |
pQCT = peripheral quantitative computed tomography; MCSA = muscle cross-sectional area (mm2).
Significant, P < 0.05; Pearson’s r for continous and Spearman’s rho for categorical variables.
Multiple linear regression analyses with physical activity, ethnicity, maturity offset, and MCSA as independent variables showed that physical activity was independently associated with muscle densities of the calf (β = 0.14, P = 0.002) and thigh (β = 0.15, P < 0.001) (Table 3, Model 1). Similarly, physical activity was independently associated with muscle density of the calf (β = 0.14, P = 0.003) and thigh (β = 0.16, P < 0.001) when ethnicity, maturity offset, and total body lean mass were covariates (Table 3, Model 2). Thus, higher physical activity was significantly associated with less skeletal muscle fat content of the calf and thigh. Multiple linear regression analyses also showed an inverse relationship between Hispanic ethnicity and muscle densities of the calf (Model 1: β =−0.126, P = 0.006; Model 2: β = to−0.127, P = 0.006) and thigh (Model 1: β =−0.091, P = 0.046; Model 2: β =−0.089, P = 0.053), indicating that Hispanic ethnicity was independently associated with higher skeletal muscle fat content. Of the total variance in calf and thigh skeletal muscle fat content explained by each full regression model, the percent explained by physical activity was 43–65% (Table 3). Analyses within maturity categories [maturity offset < 0 years from PHV (PRE) and ≥ 0 years from PHV (POST); and Tanner stage I (prepubertal), Tanner stages II–III (early pubertal), Tanner stages IV–V (late pubertal)] gave similar results and did not markedly change the magnitude or direction of the observed relationships between physical activity and muscle density (data not shown).
TABLE 3.
Multiple linear regression models with pQCT measures of calf and thigh skeletal muscle fat content as dependent variables in girls.
| Model 1 | Physical activity |
Ethnicity |
Maturity |
MCSA |
|||||
|---|---|---|---|---|---|---|---|---|---|
| β | P | Variance | β | P | β | P | β | P | |
| Calf muscle density | 0.142 | 0.002 | 64.6% | −0.126 | 0.006 | −0.001 | 0.985 | −0.045 | 0.564 |
| Thigh muscle density | 0.153 | <0.001 | 42.8% | −0.091 | 0.046 | −0.205 | 0.002 | 0.213 | 0.002 |
|
| |||||||||
| Model 2 |
Physical activity
|
Ethnicity
|
Maturity
|
TBLM
|
|||||
| β | P | Variance | β | P | β | P | β | P | |
|
| |||||||||
| Calf muscle density | 0.138 | 0.003 | 62.7% | −0.127 | 0.006 | −0.034 | 0.734 | 0.003 | 0.976 |
| Thigh muscle density | 0.162 | <0.001 | 61.2% | −0.089 | 0.053 | −0.258 | 0.011 | 0.232 | 0.021 |
Standardized β coefficients and P values are presented for independent predictors of pQCT measures of calf and thigh skeletal muscle fat content (muscle density, mg/cm3). Of the total variance in calf and thigh skeletal muscle fat content explained by each full regression model, the percent explained by physical activity is presented. pQCT = peripheral quantitative computed tomography; MCSA = muscle cross-sectional area (mm2); TBLM = total body lean mass (kg).
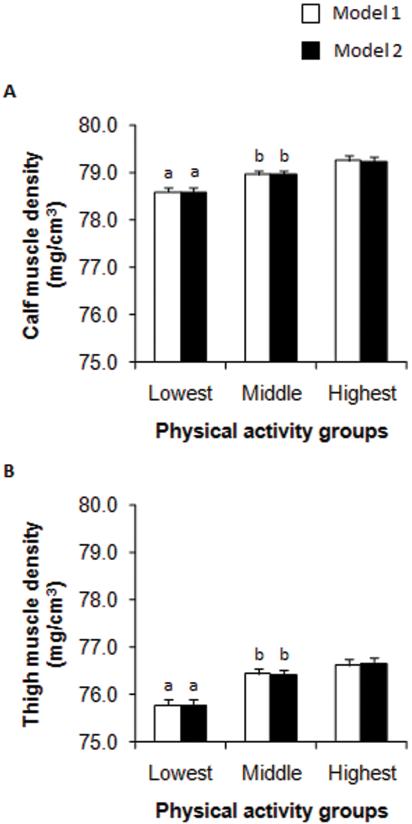
Comparisons of muscle densities across the lowest, middle (average of the middle 2), and highest groups of physical activity were performed using ANCOVA, after adjusting for ethnicity, maturity offset, and MCSA (Figure 1, Model 1). Calf muscle density was 1.7% (P < 0.001) lower in the lowest compared with the highest group of physical activity. Similarly, muscle density of the thigh was 1.8% (P < 0.001) lower in the lowest compared with the highest group of physical activity. After substituting MCSA with total body lean mass (Figure 1, Model 2), muscle densities of the calf and thigh were 1.6% (P < 0.001) and 1.9% (P < 0.001), respectively, lower in the lowest compared with the highest group of physical activity. Taken together, these results indicate that a lower level of physical activity was significantly associated with greater skeletal muscle fat content of the calf and thigh, independent of maturity, ethnicity, MCSA, and total body lean mass. Girls in the lowest, middle, and highest groups of physical activity averaged 9.1 ± 5.3, 33.4 ± 10.8, and 96.2 ± 32.5 minutes of MVPA per day, respectively. Twenty five percent of girls achieved the U.S. Centers for Disease Control and Prevention recommendation for children and adolescents of 60 minutes MVPA per day (38).
FIGURE 1.
Estimated marginal means (±SE) for calf (A) and thigh (B) muscle density (mg/cm3) for the lowest, average of the middle two, and highest fourths of physical activity in girls. Group differences were evaluated by ANCOVA. Model 1 = ethnicity, maturity offset, and muscle cross-sectional area. Model 2 = ethnicity, maturity offset, and total body lean mass. Bonferroni post hoc tests were used to adjust for multiple comparisons. a Significantly (P < 0.001) different from highest group. b Significantly (P < 0.01) different from lowest group.
Discussion
We used pQCT in a large sample of girls, 8–13 years of age, to examine the relationship between physical activity and skeletal muscle fat content. To our knowledge, this is the first study to investigate this relationship in girls. Our data demonstrate that lower physical activity is associated with higher skeletal muscle fat content, independent of maturity, ethnicity, muscle size, and total body lean mass. These findings suggest that a lower level of physical activity contributes to greater fat infiltration within skeletal muscle in healthy girls as early as the peri-pubertal years.
Observations that lower physical activity is associated with greater skeletal muscle fat content in children with disabilities that limit physical activity (17, 22) and otherwise healthy adults (9, 23) have generated interest in better understanding the relationship between physical activity and skeletal muscle fat content. Johnson and colleagues (17) used magnetic resonance imaging (MRI) to assess skeletal muscle fat content of the midthigh in 24 children (12/group, 5–14 years old) and found that children with quadriplegic cerebral palsy had greater skeletal muscle fat content than healthy children, which was significantly associated with their lower level of physical activity. The findings of Johnson and colleagues (17) are consistent with findings from another study in boys, 9–12 years of age, with low muscle mass and disabilities that limited physical activity participation (22). Until now, an association between lower physical activity and skeletal muscle fat content has not been shown previously in otherwise healthy youth. Importantly, our findings are consistent with the studies in children with disabilities that limited participation in physical activity (17, 22), and indicate that lower physical activity is associated with higher skeletal muscle fat content in healthy girls. Our findings are also consistent with a study by Manini and colleagues (23) in which 6 men and 12 women (19–28 years of age) underwent MRI assessments of skeletal muscle fat content of the thigh and calf after a 4 week control period and then again after 4 weeks of unilateral limb suspension to reduce physical activity. In that study, 4 weeks of lower limb suspension resulted in increases in skeletal muscle fat content of the thigh and calf of 14.5% and 20%, respectively, suggesting that reduced physical activity leads to marked increases in skeletal muscle fat content (23). Our results are also consistent with findings from a study by Gilsanz and colleagues (9) who showed in 90 postpubertal females (16–22 years of age) that physical inactivity was independently associated with skeletal muscle fat content of the midthigh, assessed by CT. Finally, our findings are consistent with studies in older adults (12, 30, 33) that have demonstrated that reduced physical activity is associated with increased skeletal muscle fat content, assessed by CT. In toto, our data and data from previous studies across a wide age range (9, 12, 17, 22, 23, 30, 33) suggest that a lower level of physical activity is a risk factor for greater fat infiltration within skeletal muscle.
The U.S. Centers for Disease Control and Prevention recommend that children and adolescents engage in at least 60 minutes of at least moderate-intensity physical activity per day (38). Twenty five percent of girls in our sample achieved this recommendation. While we found no differences between the highest fourth (25%) of physical activity and the average of the middle two fourths of physical activity, the lowest fourth of physical activity had significantly higher skeletal muscle fat content, supporting the notion that a lower level of physical activity and sedentary behavior may be important risk factors for greater skeletal muscle fat content. Although we did not measure sedentary behavior, the amount of time spent in sedentary behaviors is undoubtedly related to a lower level of physical activity and increased risk of metabolic syndrome, T2DM, and heart disease (8, 16). Future studies should examine the independent association between sedentary time and skeletal muscle fat content.
Fat compartments within skeletal muscle are dynamic; they can be depleted during exercise and used for storage during periods of elevated energy availability (34). Regular physical activity is associated with rapid depletion and repletion of skeletal muscle fat compartments, which contributes to greater insulin sensitivity in skeletal muscle (14). In contrast, sedentary behavior results in down-regulation of fat oxidation enzymes and subsequently, results in a lower capacity for fat oxidation (34). Consequently, sedentary obese and obese T2DM individuals have a diminished ability to utilize skeletal muscle fat stores during exercise (4, 18), which contributes to the development of insulin resistance. Thus, regular turnover of skeletal muscle fat stores during exercise and subsequent recovery periods may improve insulin sensitivity.
The present study was not without limitations. First, the cross-sectional design makes it impossible to establish a causal relationship between lower physical activity and greater skeletal muscle fat content. Secondly, there is well-known difficulty in assessing physical activity via self-report questionnaires in children and adolescents. We acknowledge that this approach is susceptible to errors, although we attempted to minimize the limitations of administering self-report questionnaires in youth by encouraging guardian assistance with physical activity recall and by limiting recall to past year activities. Nonetheless, any misclassifications of physical activity would have likely lead to an underestimation of the true association between lower physical activity and higher skeletal muscle fat content. Another potential limitation is that pQCT cannot directly measure the lipid content of skeletal muscle. However, controlled studies in obese sedentary and T2DM groups have demonstrated that lower muscle density is associated with greater skeletal muscle lipid content (13, 19). Furthermore, pQCT and CT have both been used in a number of previous studies (9, 12, 14, 24, 30, 33) to differentiate tissues of the midthigh and calf regions based on attenuation characteristics, which are directly related to tissue composition and density (13, 19). Our study obtained a single slice at the 66% tibia and 20% femur sites relative to the respective distal growth plates of the non-dominant leg. These regions have a smaller depot of skeletal muscle adipose tissue than at the midthigh, a potential limitation, although relatively strong correlations between skeletal muscle fat content of the midthigh and calf have been reported using MRI (21). While MRI has better contrast resolution than pQCT, its high cost and higher radiation prevents its use in large samples. pQCT, because of its relative low cost, fast speed, and low radiation dose, has promise for application in future large-scale studies. These features make pQCT uniquely suited to safely estimate skeletal muscle fat content of the calf and thigh. Future applications of this technique should help to further clarify the relationship between physical activity and skeletal muscle fat content. Lastly, we acknowledge that the total variance in skeletal muscle fat content explained by the full regression models was low. Nevertheless, of the total variance in skeletal muscle fat content explained by each full regression model, the percent explained by physical activity was relatively large (43–65%) and more variance in skeletal muscle fat content was explained by physical activity compared to the other variables (maturity, ethnicity, MCSA, lean mass) included in the models. The significant relationship between inactivity and skeletal muscle fat content at the young age of these girls underscores the importance of physical activity and the need for future studies, including prospective observational studies, with a more direct measure of muscle triglyceride, and intervention studies, to test whether skeletal muscle fat content would be reduced with physical activity and the dose required to do so.
In conclusion, our results indicate that lower physical activity is significantly associated with higher skeletal muscle fat content in otherwise healthy girls, independent of maturity, ethnicity, muscle size, and total body lean mass. Importantly, this finding is consistent with available data in children with disabilities that limit physical activity (17, 22), healthy adults (9, 23), and older adults (12, 30, 33), and suggests that the relationship between lower physical activity and greater skeletal muscle fat content begins, in females at least, as early as the peri-pubertal years. Interventions designed to incorporate physical activity into the lives of sedentary girls are a critical need.
ACKNOWLEDGEMENTS
We appreciate the participation and support of principals, teachers, parents and students from the schools in the Catalina Foothills and Marana School Districts. We also wish to thank the radiation technicians, program coordinators, and all other members of the Jump-In Study team for their contributions. The project described was supported by Award Number HD-050775 (SG) from the National Institute of Child Health and Human Development. JF is supported by NIH NIGMS T32 GM-08400: Graduate Training in Systems and Integrative Physiology. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Child Health and Human Development or the National Institutes of Health. The results of the present study do not constitute endorsement by ACSM.
Grant Support: NIH: HD050775
Footnotes
DICLAIMERS: All authors have no conflict of interest
Clinical Trials #: NCT00729378; Registration Date: 07/17/2008
REFERENCES
- 1.American Academy of Pediatrics Medical conditions affecting sports participation. Pediatrics. 2001;107(5):1205–9. doi: 10.1542/peds.107.5.1205. [DOI] [PubMed] [Google Scholar]
- 2.Aaron DJ, Kriska AM, Dearwater SR, et al. Reproducibility and validity of an epidemiologic questionnaire to assess past year physical activity in adolescents. Am J Epidemiol. 1995;142(2):191–201. doi: 10.1093/oxfordjournals.aje.a117618. [DOI] [PubMed] [Google Scholar]
- 3.Bailey DA, McKay HA, Mirwald RL, Crocker PR, Faulkner RA. A six-year longitudinal study of the relationship of physical activity to bone mineral accrual in growing children: The University of Saskatchewan Bone Mineral Accrual Study. J Bone Miner Res. 1999;14(10):1672–9. doi: 10.1359/jbmr.1999.14.10.1672. [DOI] [PubMed] [Google Scholar]
- 4.Blaak EE, van Aggel-Leijssen DP, Wagenmakers AJ, Saris WH, van Baak MA. Impaired oxidation of plasma-derived fatty acids in type 2 diabetic subjects during moderate-intensity exercise. Diabetes. 2000;49(12):2102–7. doi: 10.2337/diabetes.49.12.2102. [DOI] [PubMed] [Google Scholar]
- 5.Farr JN, Blew RM, Lee VR, Lohman TG, Going SB. Associations of physical activity duration, frequency, and load with volumetric BMD, geometry, and bone strength in young girls. Osteoporos Int. 2011;22(5):1419–30. doi: 10.1007/s00198-010-1361-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farr JN, Funk JL, Chen Z, et al. Skeletal muscle fat content is inversely associated with bone strength in young girls. J Bone Miner Res. doi: 10.1002/jbmr.414. (Epub ahead of print 4 May 2011). DOI: 10.1002/jbmr.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farr JN, Lee VR, Blew RM, Lohman TG, Going SB. Quantifying bone-relevant activity and its relation to bone strength in girls. Med Sci Sports Exerc. 2011;43(3):476–83. doi: 10.1249/MSS.0b013e3181eeb2f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ford ES, Kohl HW, Mokdad AH, Ajani UA. Sedentary behavior, physical activity, and the metabolic syndrome among U.S. adults. Obes Res. (3rd) 2005;13(3):608–14. doi: 10.1038/oby.2005.65. [DOI] [PubMed] [Google Scholar]
- 9.Gilsanz V, Kremer A, Mo AO, Wren TA, Kremer R. Vitamin D status and its relation to muscle mass and muscle fat in young women. J Clin Endocrinol Metab. 2010;95(4):1595–601. doi: 10.1210/jc.2009-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gluer CC, Blake G, Lu Y, et al. Accurate assessment of precision errors: how to measure the reproducibility of bone densitometry techniques. Osteoporos Int. 1995;5(4):262–70. doi: 10.1007/BF01774016. [DOI] [PubMed] [Google Scholar]
- 11.Going S, Lohman T, Houtkooper L, Metcalfe L, Flint-Wagner H, Blew R, Stanford V, Cussler E, Martin J, Teixeira P, Harris M, Milliken L, Figueroa-Galvez A, Weber J. Effects of exercise on bone mineral density in calcium-replete postmenopausal women with and without hormone replacement therapy. Osteoporos Int. 2003;14:637–43. doi: 10.1007/s00198-003-1436-x. [DOI] [PubMed] [Google Scholar]
- 12.Goodpaster BH, Chomentowski P, Ward BK, et al. Effects of physical activity on strength and skeletal muscle fat infiltration in older adults: a randomized controlled trial. J Appl Physiol. 2008;105(5):1498–503. doi: 10.1152/japplphysiol.90425.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol. 2000;89(1):104–10. doi: 10.1152/jappl.2000.89.1.104. [DOI] [PubMed] [Google Scholar]
- 14.Goodpaster BH, Krishnaswami S, Resnick H, et al. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care. 2003;26(2):372–9. doi: 10.2337/diacare.26.2.372. [DOI] [PubMed] [Google Scholar]
- 15.Groothausen J, Siemer H, Kemper G, Twisk J, Welten D. Influence of peak strain on lumbar bone mineral density: an analysis of 15-year physical activity in young males and females. Pediatr Exerc Sci. 1997;9(2):159–73. [Google Scholar]
- 16.Hu FB, Li TY, Colditz GA, Willett WC, Manson JE. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA. 2003;289(14):1785–91. doi: 10.1001/jama.289.14.1785. [DOI] [PubMed] [Google Scholar]
- 17.Johnson DL, Miller F, Subramanian P, Modlesky CM. Adipose tissue infiltration of skeletal muscle in children with cerebral palsy. J Pediatr. 2009;154(5):715–20. doi: 10.1016/j.jpeds.2008.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelley DE, Simoneau JA. Impaired free fatty acid utilization by skeletal muscle in non-insulin-dependent diabetes mellitus. J Clin Invest. 1994;94(6):2349–56. doi: 10.1172/JCI117600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelley DE, Slasky BS, Janosky J. Skeletal muscle density: effects of obesity and non-insulin-dependent diabetes mellitus. Am J Clin Nutr. 1991;54(3):509–15. doi: 10.1093/ajcn/54.3.509. [DOI] [PubMed] [Google Scholar]
- 20.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000;8(314):1–27. [PubMed] [Google Scholar]
- 21.Larson-Meyer DE, Smith SR, Heilbronn LK, et al. Muscle-associated triglyceride measured by computed tomography and magnetic resonance spectroscopy. Obesity (Silver Spring) 2006;14(1):73–87. doi: 10.1038/oby.2006.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leroy-Willig A, Willig TN, Henry-Feugeas MC, et al. Body composition determined with MR in patients with Duchenne muscular dystrophy, spinal muscular atrophy, and normal subjects. Magn Reson Imaging. 1997;15(7):737–44. doi: 10.1016/s0730-725x(97)00046-5. [DOI] [PubMed] [Google Scholar]
- 23.Manini TM, Clark BC, Nalls MA, et al. Reduced physical activity increases intermuscular adipose tissue in healthy young adults. Am J Clin Nutr. 2007;85(2):377–84. doi: 10.1093/ajcn/85.2.377. [DOI] [PubMed] [Google Scholar]
- 24.Miljkovic-Gacic I, Gordon CL, Goodpaster BH, et al. Adipose tissue infiltration in skeletal muscle: age patterns and association with diabetes among men of African ancestry. Am J Clin Nutr. 2008;87(6):1590–5. doi: 10.1093/ajcn/87.6.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mirwald RL, Baxter-Jones AD, Bailey DA, Beunen GP. An assessment of maturity from anthropometric measurements. Med Sci Sports Exerc. 2002;34(4):689–94. doi: 10.1097/00005768-200204000-00020. [DOI] [PubMed] [Google Scholar]
- 26.Morris NM, Udry RJ. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc. 1980;9(3):271–80. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- 27.Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999-2000. JAMA. 2002;288(14):1728–32. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- 28.Phillips DI, Caddy S, Ilic V, et al. Intramuscular triglyceride and muscle insulin sensitivity: evidence for a relationship in nondiabetic subjects. Metabolism. 1996;45(8):947–50. doi: 10.1016/s0026-0495(96)90260-7. [DOI] [PubMed] [Google Scholar]
- 29.Pinhas-Hamiel O, Dolan LM, Daniels SR, et al. Increased incidence of non-insulin-dependent diabetes mellitus among adolescents. J Pediatr. 1996;128(5 Pt 1):608–15. doi: 10.1016/s0022-3476(96)80124-7. [DOI] [PubMed] [Google Scholar]
- 30.Prior SJ, Joseph LJ, Brandauer J, et al. Reduction in midthigh low-density muscle with aerobic exercise training and weight loss impacts glucose tolerance in older men. J Clin Endocrinol Metab. 2007;92(3):880–6. doi: 10.1210/jc.2006-2113. [DOI] [PubMed] [Google Scholar]
- 31.Ridley K, Ainsworth BE, Olds TS. Development of a compendium of energy expenditures for youth. Int J Behav Nutr Phys Act. 2008;5:1–8. doi: 10.1186/1479-5868-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roemmich JN, Clark PA, Walter K, et al. Pubertal alterations in growth and body composition. V. Energy expenditure, adiposity, and fat distribution. Am J Physiol Endocrinol Metab. 2000;279(6):E1426–36. doi: 10.1152/ajpendo.2000.279.6.E1426. [DOI] [PubMed] [Google Scholar]
- 33.Ryan AS, Nicklas BJ, Berman DM, Dennis KE. Dietary restriction and walking reduce fat deposition in the midthigh in obese older women. Am J Clin Nutr. 2000;72(3):708–13. doi: 10.1093/ajcn/72.3.708. [DOI] [PubMed] [Google Scholar]
- 34.Shaw CS, Clark J, Wagenmakers AJ. The effect of exercise and nutrition on intramuscular fat metabolism and insulin sensitivity. Annu Rev Nutr. 2010;30:13–34. doi: 10.1146/annurev.nutr.012809.104817. [DOI] [PubMed] [Google Scholar]
- 35.Shedd KM, Hanson KB, Alekel DL, et al. Quantifying leisure physical activity and its relation to bone density and strength. Med Sci Sports Exerc. 2007;39(12):2189–98. doi: 10.1249/mss.0b013e318155a7fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sherar LB, Baxter-Jones AD, Mirwald RL. Limitations to the use of secondary sex characteristics for gender comparisons. Ann Hum Biol. 2004;31(5):586–93. doi: 10.1080/03014460400001222. [DOI] [PubMed] [Google Scholar]
- 37.Stratec XCT 3000 manual, software version 6.0 Pforzheim. Germany: 2004. pp. 46–65. [Google Scholar]
- 38.U.S. Department of Health and Human Services . Physical Activity and Health: A Report of the Surgeon General. Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; Atlanta, GA: 1996. pp. 85–259. [Google Scholar]