Abstract
Background
Using available communication technologies, clinicians may offer timely support to family caregivers in managing symptoms in patients with advanced cancer at home.
Aim
To assess the effects of an online symptom reporting system on caregiver preparedness, physical burden, and negative mood.
Design
A pooled analysis of two randomized trials (NCT00214162 and NCT00365963) was conducted to compare caregiver outcomes at 6 and 12 months after intervention between two randomized, unblinded groups using General Linear Mixed Modeling. Caregivers in one group (Comprehensive Health Enhancement Support System-Only) were given access to an interactive cancer communication system, the Comprehensive Health Enhancement Support System. Those in the other group (Comprehensive Health Enhancement Support System + Clinician Report) received access to Comprehensive Health Enhancement Support System plus an online symptom reporting system called the Clinician Report. Clinicians of patients in the Comprehensive Health Enhancement Support System + Clinician Report group received e-mail alerts notifying them when a symptom distress was reported over a predetermined threshold.
Setting/Participants
Dyads (n=235) of advanced-stage lung, breast, and prostate cancer patients and their adult caregivers were recruited at five outpatient oncology clinics in the United States.
Results
Caregivers in the Comprehensive Health Enhancement Support System + Clinician Report group reported less negative mood than those in the Comprehensive Health Enhancement Support System-Only group at both 6 months (p=0.009) and 12 months (p=0.004). Groups were not significantly different on caregiver preparedness or physical burden at either time point.
Conclusions
This study provides new evidence that by using an online symptom reporting system, caregivers may experience less emotional distress due to the Clinician Report’s timely communication of caregiving needs in symptom management to clinicians.
Keywords: Caregivers, palliative care, communication barriers, signs and symptoms, eHealth, cancer
Introduction
In the United States, an estimated 4.6 million people served as family caregivers for cancer patients in 2009.1 Caregivers, or carers, are family or friends who offer unpaid support to patients. As advanced cancer treatments prolong patients’ lives, symptom management becomes the major responsibility of family caregivers.2,3 Cancer symptoms such as pain and fatigue are significantly associated with caregiver physical burden (e.g., sleep disorders and fatigue) and emotional problems (e.g., anxiety and depression).4–6 Physical and emotional challenges are common among cancer caregivers.7 As patient symptoms worsen, caregivers may experience more burdens and less confidence to manage patients’ care.8,9 Prepared and supported caregivers may cope better with adversity10,11 and subsequently experience less burden.12–14 Unfortunately, many caregivers facing advanced cancer are neither prepared nor supported, particularly in managing patient symptoms at home.9,15–17
The Needs for Timely Communication
Clinicians could cooperate with caregivers in managing patient symptoms. However, many clinicians (1) feel pressured to spend less visit time with patients/families, (2) avoid discussions of psychosocial issues, and (3) tend to underestimate patient’s/family’s needs.18–22 To overcome these barriers, nurses in the Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments (SUPPORT) repeatedly communicated caregivers’ and patients’ concerns, including pain control, to physicians.23 Yet, physician behavior did not change. More recent studies with similar communication approaches reported mixed results—one showed no improvement on caregiver depression24 while the other showed reduced caregiver distress.25 Moreover, clinician-initiated communication is pre-scheduled and may not occur when caregivers and patients need help the most. Through timely communication, clinicians may help caregivers be prepared in managing symptom changes over time.26–30 A more proactive and feasible intervention that ensures timely caregiver-initiated communications may overcome these barriers.
eHealth Solutions
Information technology may help improve the effectiveness, feasibility, and timeliness of clinician communication. Growing evidence demonstrates that electronic patient reported outcome (ePRO) systems are feasible, well-received by both patients and clinicians, and equivalent to original paper-based systems.31–36 Patients typically log into ePRO systems via Internet-enabled computers or smartphones, and complete electronic report forms. Reported outcomes are transmitted to their clinicians or integrated into the electronic medical records. Moderately to significantly improved patient symptoms and quality of life were reported.37–40 Several ePRO system developments are underway in the U.S. and Europe.39,41,42 The ePRO systems may facilitate timely interaction between patients, caregivers, and clinicians. While current ePRO systems are designed for patients, some43–45 argued that caregivers are in a good (possibly better) position to assess patient condition. Systematic development and evaluation of systems that involve caregivers are needed.
Clinicians Report in Comprehensive Health Enhancement Support System
An ePRO system, the Clinician Report (CR), was designed specifically for caregivers caring for advanced-stage cancer patients, built upon a non-commercial, web-based system, the Comprehensive Health Enhancement Support System (CHESS),46,47 which already offered patients a means of tracking their symptoms. Current CHESS modules include caregiver and patient tracking of patient status and caregiver reporting of his/her own status, via a ‘Check-in’ service. The CR delivers this information to the clinicians. With the CR’s alert function, concerns about patient symptoms and caregiving needs may be more likely to reach the clinicians’ attention, potentially leading them to address these issues in a timely manner. Caregivers, with immediate support from clinicians, may be better prepared to manage patient symptoms. As the literature supports, uncontrolled cancer symptoms and side effects lead to increased caregiving physical burden and negative mood. Conversely, well-managed patient symptoms and side effects may reduce caregiving physical burden and negative mood. We examined whether caregivers with access to the CR are better prepared, and experience less physical burden and less negative mood than those without.
Methods
Participants
Between September 2004 and April 2007, 235 patients with advanced-stage breast, prostate, or lung cancer and their caregivers (family/friends) were jointly recruited. Eligible breast cancer patients were women with metastatic, recurrent or metastatic inflammatory breast cancer, or a chest wall recurrence following mastectomy. Prostate cancer patients were eligible if they had hormone refractory or metastatic prostate cancer. Eligible lung cancer patients included those in stage IIIA, IIIB, or IV. Depending on disease statuses, patients were receiving standard care including curative or palliative treatment. Patients may or may not have had a hospitalization during the course of the treatment, but our intervention was targeted to the outpatient setting. Eligible caregivers were at least 18 years old and were identified by patients as their primary source of physical, emotional, and/or financial support. Caregivers were predominantly female (64.2%) and an average of 56 years old; caregivers’ average educational attainment fell between ‘some college course’ and ‘associate degree’ (Table 1). In total, 55.8% of patients were female. Patients’ average age was 63 years old. Most caregivers (69.3%) were spouse/partners. At baseline, caregivers were fairly comfortable with using the Internet, with the average between ‘a medium amount’ and ‘quite a bit’ of comfort.
Table 1.
Demographics and dependent variables by group at pretest
| Categorical demographic variables | CHESS-Only
|
CHESS + CR
|
||
|---|---|---|---|---|
| N | n (%) | N | n (%) | |
| Cancer type: | 107 | 110 | ||
| Breast cancer | 45 (42) | 44 (40) | ||
| Prostate cancer | 30 (28) | 34 (31) | ||
| Lung cancer | 32 (30) | 32 (29) | ||
| Patient gender (female) | 107 | 60 (56) | 110 | 61 (56) |
| Caregiver gender (female) | 107 | 71 (66) | 110 | 69 (63) |
| Caregiver relationship to patient (spouse) | 107 | 75 (70) | 110 | 75 (68) |
| Caregiver race: | 107 | 110 | ||
| White | 100 (93) | 100 (91) | ||
| Non-White | 5 (5) | 9 (8) | ||
| Not reported a | 2 (2) | 1 (1) | ||
| Caregiver annual household income: | 107 | 110 | ||
| Below US$40,000 | 35 (33) | 35 (32) | ||
| US$40,001 – US$80,000 | 37 (35) | 36 (33) | ||
| US$80,001 and Over | 26 (24) | 28 (25) | ||
| Not Reported a | 9 (8) | 11 (10) | ||
|
| ||||
| Continuous demographic variables | N | M (SD) | N | M (SD) |
|
| ||||
| Patient age a | 105 | 62.53 (9.63) | 109 | 62.73 (11.00) |
| Caregiver age a | 105 | 55.73 (13.02) | 107 | 56.36 (13.39) |
| Caregiver education a,b | 106 | 3.96 (1.58) | 109 | 3.67 (1.52) |
| Caregiver Internet comfort (1–4) a | 102 | 2.57 (1.26) | 107 | 2.36 (1.37) |
| Caregiver-reported patient’s ESAS (1–90) a | 94 | 27.75(16.82) | 101 | 28.13(15.90) |
|
| ||||
| Primary outcomes | N | M (SD) | N | M (SD) |
|
| ||||
| Physical burden (0–4) a | 103 | 1.40 (0.81) | 109 | 1.32 (0.75) |
| Preparedness (0–4) a | 106 | 2.84 (0.63) | 108 | 2.71 (0.69) |
| Negative mood (0–4) a | 103 | 0.85 (0.68) | 107 | 0.91 (0.79) |
ESAS: Edmonton Symptom Assessment System; CHESS: Comprehensive Health Enhancement Support System; CR: Clinician Report; SD: standard deviation.
One caregiver in CHESS-Only group and one caregiver in the CHESS + CR group did not return pretest survey.
On the education scale, 3 indicates “Some college coursework” and 4 indicates “Associate or technical degree.”
Recruitment
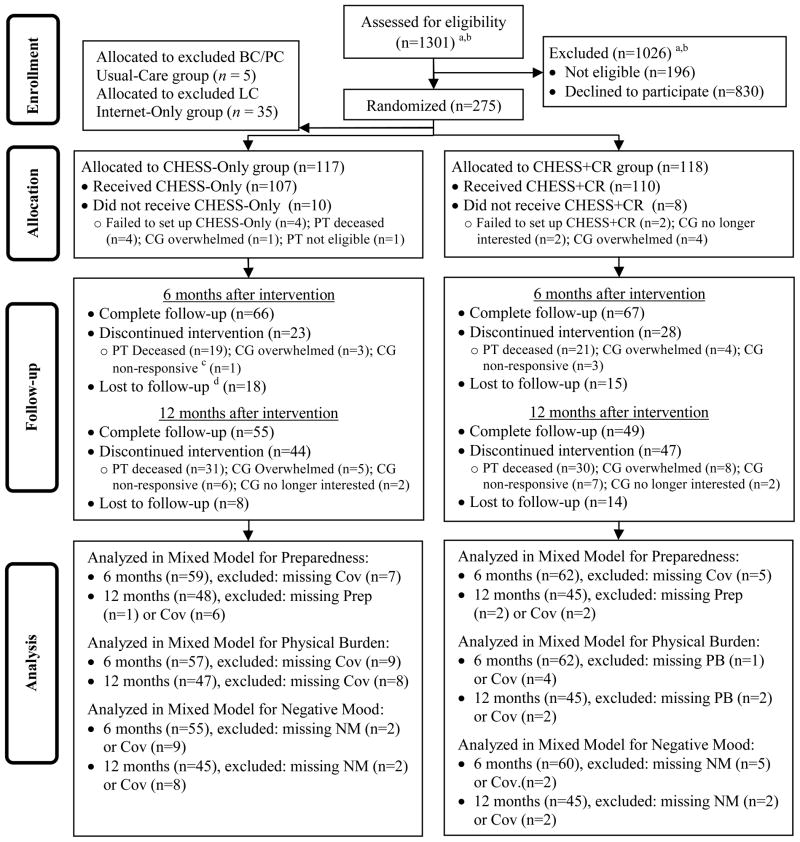
Recruitment sites were five cancer centers in the Northeastern, Midwestern, and Southwestern United States. At an outpatient clinic visit, the physician or nurse invited the patient and caregiver (if present) to learn about the project. A site enrollment coordinator then talked with interested people about the study and the consent process. Both the patient and a caregiver had to agree to be in the study. To boost enrollment a one-time invitation was mailed to 561 breast and prostate cancer patients in the State of Wisconsin, identified through Medicaid records. Participants were recruited into one of two randomized trials. One trial enrolled breast and prostate cancer patients and caregivers, the other lung cancer patients. Both studies originally were designed with three randomization groups: (1) Usual-Care (the breast/prostate cancer study) or Internet-Only (the lung study), (2) CHESS-Only, and (3) CHESS + CR. Due to low recruitment rates for both studies, each study was changed to include only two groups. The breast/prostate cancer study retained the CHESS-Only and the CHESS + CR groups. The lung cancer study retained the Internet-Only and the CHESS + CR groups. The 5 breast/prostate cancer dyads in the Usual-Care group and the 37 lung cancer dyads in the CHESS-Only group continued the intervention. This analysis excludes the Usual-Care and the Internet-Only groups but includes the 37 lung cancer dyads in the CHESS-Only group. We sequentially selected a subsample of 37 lung cancer dyads in the CHESS + CR group to match the number of those in the CHESS-Only group from the same recruitment period. Caregiver, patients, and their clinicians were not blinded regarding group assignments. The Internet-Only group in the lung cancer study was not included in this analysis. Approximately 95 caregivers per group were needed to achieve 0.80 power with two-tailed alpha of 0.05 in a planned analysis to detect an effect size of 0.35σ between CHESS-Only and CHESS + CR groups at the two different time periods. This paper reports on a total of 117 dyads in the CHESS-Only group and 118 in the CHESS + CR group across the two studies (Figure 1).
Figure 1.
Flow diagram
PT: patients; CG: caregivers; Cov: covariates; Prep: preparedness; PB: physical burden; NM: negative mood; CHESS: Comprehensive Health Enhancement Support System; CR: Clinician Report; BC: Breast cancer; PC: Prostate cancer; LC: Lung cancer.
“CG nonresponsive” means those caregivers who did not respond to previous posttest surveys and to project administrators’ contact. Because of their nonresponse, the project administrators decided to discontinue their Internet and CHESS access. “Lost to follow up” means those whose current posttest surveys were not returned.
a In total, 561 breast and prostate cancer dyads received mailed invitations, and the others were approached at the clinic.
b Because one lung cancer recruitment data have missing recruitment dates, approximate recruitment numbers were calculated by multiplying total recruitment numbers by the proportion of selected dyads in this study over total dyads recruited from this site: 28 (13 not eligible) × 57/90 =18 (8 not eligible).
Intervention
Both groups received access to the CHESS website, which included information, communication, and coaching resources addressing advanced cancer and caregiving needs (Table 2). At initial login to CHESS and then every 7 days, caregivers and patients completed a Check-in, asking questions about their needs and patient symptoms from the modified Edmonton Symptom Assessment System (ESAS)48 and Eastern Cooperative Oncology Group Performance Status49. They could write questions to be addressed by the clinicians in the next visit. Caregivers reported caregiving burden50 and preparedness51. This Check-in allows users to track patient symptom status, monitoring decline or improvement. In addition for the CHESS + CR group, CHESS included the CR that summarized the information provided by patients and caregivers at Check-in and made it available online to the clinicians (Figure 2). Details of the CR development and initial clinician reactions to its use are described in elsewhere.52 Clinicians could access the CR via CHESS anytime. However, any caregiver- or patient-reported ESAS symptom rated at a threshold of 7 or higher on a 0- to 10-scale automatically generated an email alerting the clinician to review the report immediately. Clinicians also received an e-mail alert to review reports 2 days before a scheduled clinic visit, regardless of the ESAS rating. Therefore, the critical distinction between two groups was—while the CHESS-Only group responded to the same Check-in questions as the CHESS + CR group, for the CHESS-Only group, there was no communication of this information to the clinicians.
Table 2.
CHESS services
| Information services | |
| Frequently Asked Questions (FAQs) | Brief answers to many common and important questions about the physical, emotional, practical, social, and spiritual aspects of the disease. |
| Instant Library | Links to hundreds of relevant full-text articles from the scientific and popular press. |
| Resource Directory | Descriptions of local and national services and ways to contact them. |
| Web links | Connections to other high-quality websites, as determined by our expert clinicians. |
| Cancer News | Summaries of recent cancer-related news and research. |
| Personal Stories | First-person narratives of patient and caregiver experiences with their health crisis. |
| Caregiver Tips | Brief tips on a variety of topics developed by experts or added by CHESS users. |
| Communication services | |
| Discussion Groups | Limited-access bulletin boards monitored by a professional facilitator. Separate groups exist for patients, caregivers, and bereaved caregivers. |
| Ask an Expert | One-on-one confidential question-and-answer service. A cancer information specialist responds to questions within 48 h. |
| Personal Web page | Place for users to set up their own bulletin board and interactive calendar with their family and friends to share updates and messages, and request help (e.g., transportation to clinic visits, meals, errands). |
| Clinician Report | Summaries of health status reported to the clinical team in three ways:
|
| Coaching/training services | |
| Health Status | Data about the patient’s health, including graphs illustrating changes, created by prompting the user to enter data. |
| Decision Aids | Structured analysis to help patients and caregivers learn about options, clarify values, understand consequences, and implement decisions. |
| Easing Distress | Cognitive-Behavioral Therapy principles help users identify emotional distress and use coping techniques to manage it. |
| Healthy Relating | Instruction on techniques to increase closeness and decrease conflict.
|
| Action Plan | Guidance to help users plan behavior changes by identifying goals, resources, and ways to overcome obstacles. |
CHESS, Comprehensive Health Enhancement Support System
Figure 2.
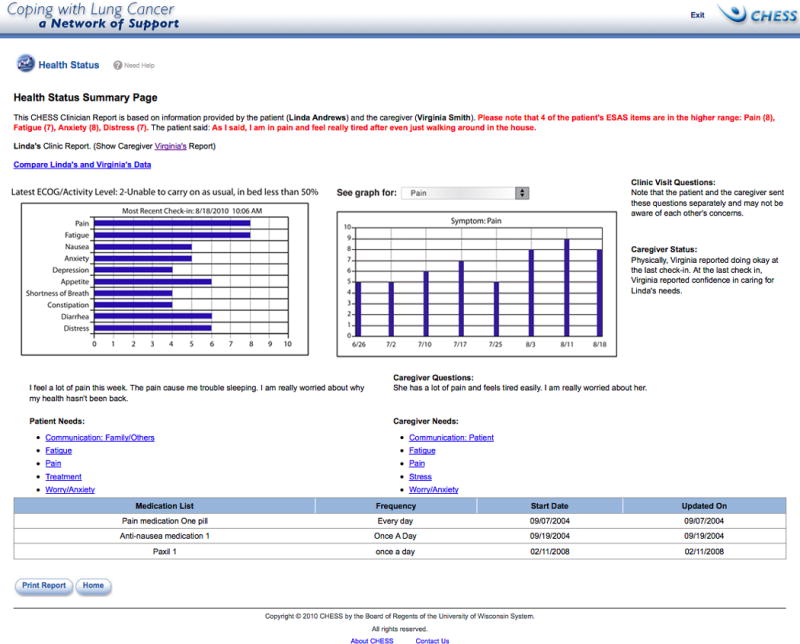
Clinician Report screen shot
CHESS, Comprehensive Health Enhancement Support System; ESAS: Edmonton Symptom Assessment System; ECOG: Eastern Cooperative Oncology Group.
Procedures
Both studies were approved by the Institutional Review Boards at recruitment sites. Oncology clinicians were consented, agreeing to receive reports. After patients and caregivers completed the consent and pretest, dyads were randomly assigned to the CHESS-Only or CHESS + CR group (1:1 ratio) with assignment determined at the University of Wisconsin by a random number generator. Each site used a separate randomization schedule. Randomization was blocked by caregiver-patient relationship (spouse/partner vs. nonspouse/partner) and race (Caucasian vs. non-Caucasian). All participants received a laptop with Internet access if needed plus CHESS login information. For those with Internet access, Internet cost was reimbursed while in the study. Computers and user manuals were mailed to the caregivers. Technical support was available by telephone. CHESS staff provided training on using CHESS via telephone or in clinic. Although caregivers and patients were enrolled as dyads, caregivers were the target population in both studies. Patients were offered access but were not required to use CHESS or complete surveys. Breast and prostate cancer dyads had access to the intervention for 12 months; lung cancer dyads had a 24-month intervention period. This analysis utilized the first 12-month data for all three cancer types. Caregivers in both studies were surveyed every two months after receiving the intervention. Breast and prostate cancer caregivers were paid US$20 for each survey completed, while lung cancer participants were able to keep the laptop after study. The 6- and 12-month posttests were hypothesized in the study protocol as the targeted outcome points because 6 months would allow participants enough time to gain benefits from using the systems while 12 months would allow us to study the lasting effect of the Intervention. Study protocols are available upon request.
Measures
Caregiver characteristics
Caregiver demographic characteristics were assessed at pretest. Caregivers’ comfort in using the Internet was assessed at pretest with a Likert-type item, ‘How comfortable are you using the Internet?’, with response choices from 0 (not at all) to 4 (extremely). Caregiver-perceived patient symptom distress was measured at pretest and posttest using the modified ESAS48 on a 0- to 10-point scale (10 indicating the highest symptom burden). Based on oncologists’ feedback, we replaced three items in the original ESAS (i.e., activity, drowsy, and wellbeing) with three commonly occurring cancer symptoms (i.e., fatigue, constipation, and diarrhea). The ESAS scale score was calculated by summing scores of these nine symptoms.
Caregiver preparedness
The four-item caregiver Preparedness scale, a subscale of Family Care Inventory51, was used to assess preparedness for caregiving tasks. Caregivers were asked to indicate, on a 5-point scale, their agreement with the statement from 0 (strongly disagree) to 4 (strongly agree). These items are as follows: I am confident that I can… (1) take care of the patient’s physical needs, (2) take care of the patient’s emotional needs, (3) find out about and set up services for the patient, and (4) cope with the stress of caregiving. Scale scores are calculated as means across items. The scale has high internal consistency measured using Cronbach’s α (pretest: α =0.79, 6-month posttest: α =0.85, and 12-month posttest: α =0.85).
Caregiver physical burden
The four-item caregiver Physical Burden scale, a subscale of the Caregiver Burden Inventory50, was used to assess the impact of caregiving on caregivers’ health. Caregivers were asked to indicate, on a 5-point scale, their agreement with the statement from 0 (strongly disagree) to 4 (strongly agree). These items are as follows: (1) I’m getting enough sleep (reverse coded), (2) my health has suffered, (3) I am physically tired, and (4) caregiving has made me physically sick. Scale scores are calculated as means across items. The scale has high internal consistency (pretest: α =0.78, 6-month posttest: α =0.85, and 12-month posttest: α =0.83).
Caregiver negative mood
A subset of negative mood items from the Shortened Version Profile of Mood States (SV-POMS)53 was used to assess caregiver negative mood. To reduce participant survey burden, we further shortened the SV-POMS. Items were selected to be representative of the three negative mood sub-scales: (1) Tension-Anxiety (tense, on edge, uneasy, nervous, anxious), (2) Anger-Hostility (annoyed, angry, grouchy, furious, bitter), and (3) Depression-Dejection (discouraged, helpless, hopeless, sad, unhappy, worthless). Using data from a previous CHESS study54, selected items demonstrated reliability with similar overall scale α compared to the original measures. The internal consistency for the depression sub-scale was α = 0.94 for the original SV-POMS and 0.92 for the CHESS version. Caregivers were asked to indicate, on a 5-point scale, the level of negative mood from 0 (not at all) to 4 (extremely). Scale scores are calculated as means across items. The scale has high internal consistency (pretest: α =0.95, 6-month posttest: α =0.96, and 12-month posttest: α =0.96).
Data Cleaning
All data were doubled-entered. Little’s Missing Completely at Random (MCAR) Test55 showed that the item missingness for all outcome variables was missing completely at random at each time point. Therefore, the scale scores were calculated if at least 75% of items were answered, in order to minimize case deletion due to item missingness.
Some caregivers did not provide data at posttests due to unreturned surveys or officially dropping out. To assess the possible impact of the dropout data, we used pattern mixture modeling56 and determined that no additional adjustments were necessary.
Data Analysis
To assess the potential effect of sample characteristics on outcome missingness due to discontinued intervention and lost to follow-up, we compared baseline outcome measures and demographics between caregivers who completed the 6- or 12-month survey versus those who did not. Hypothesis testing was done using General Linear Mixed Model procedure in SPSS 17.57 We examined differences between the CHESS-Only and CHESS + CR group at 6 and 12 months on three outcomes—caregiver preparedness, physical burden, and negative mood. We tested the effects of group, time, and group by time interaction, while controlling for caregiver age, education, gender, caregiver’s comfort of using the Internet, caregiver-reported patient ESAS at 6 and 12 months, and pretest levels of the dependent variables. Group comparisons at 6 and 12 months were conducted using estimated marginal means. Covariates were selected based on their likelihood of influencing caregiver outcomes. Research has shown that age and gender correlate with caregiver distress.58,59 Education and comfort using the Internet may influence a caregiver’s ability to use and benefit from the CR. As mentioned earlier, patient symptom distress is highly correlated to caregiver outcomes.
Results
Baseline demographic characteristics
Tests revealed that caregivers who completed posttests at 6 or 12 months reported lower patient symptom distress at pretest (p=0.04 and 0.03, respectively) than those who did not. However, dependent variables, age, gender, education level, and Internet comfort at baseline were not significantly different between these groups. Tests also showed no significant differences between treatment groups at baseline on demographics, covariate, or dependent variables.
Intervention effects at 6- and 12-month follow-up
General Linear Mixed Model analyses were used to test for effects on three caregiver outcomes—preparedness, physical burden, and negative mood—at 6- and 12-month follow-up. Compound symmetry covariance structure of the repeated measure was used for a better model fit based on the likelihood test and Bayesian Information Criteria (BIC)60. No main effect of the group assignment was found for caregiver preparedness, F(1, 118.98) = 0.42, p = 0.52, or physical burden, F(1, 107.60) = 0.03, p = 0.86. However, a significant group main effect was found for caregiver negative mood, F(1, 113.74) = 11.04, p = 0.001. Contrast tests comparing groups at each time point showed no significant differences between groups for either preparedness or physical burden, though caregivers in the CHESS + CR group were found to experience less negative mood than those in the CHESS-Only Group at both 6 (p = 0.009) and 12 months (p = 0.004) (Table 3).
Table 3.
Group comparison of estimated marginal means between groups at 6 and 12 months
| Outcome variables | Months since intervention | Estimated marginal means
|
Mean difference, ΔM (SE) | P | 95% CI of mean difference | |
|---|---|---|---|---|---|---|
| CHESS-Only, M (SE) | CHESS + CR, M (SE) | |||||
| Caregiver preparedness | 6 | 2.83a (0.07) | 2.91 (0.07) | −0.08 (0.09) | 0.42 | −0.26, 0.11 |
| 12 | 2.79 (0.07) | 2.82 (0.08) | −0.03 (0.10) | 0.78 | −0.24, 0.18 | |
| Caregiver physical burden | 6 | 1.22 (0.09) | 1.22 (0.08) | 0.003 (0.12) | 0.98 | −0.23, 0.24 |
| 12 | 1.22 (0.09) | 1.27 (0.09) | −0.04 (0.13) | 0.73 | −0.30, 0.21 | |
| Caregiver negative mood | 6 | 0.88 (0.07) | 0.62 (0.07) | 0.26 (0.10) | 0.009 | 0.07, 0.45 |
| 12 | 0.93 (0.08) | 0.61 (0.08) | 0.32 (0.11) | 0.004 | 0.10, 0.53 | |
CHESS: Comprehensive Health Enhancement Support System; CR: Clinician Report; SE: standard error; CI: confidence interval.
Estimated marginal means after controlling the following covariates: caregiver age, caregiver highest education level completed, caregiver gender, Internet comfort, outcome variables at pretest, and Edmonton Symptom Assessment System at the testing month.
Discussion
This study provided randomized trials of an online symptom reporting system designed to facilitate timely communication between clinicians and caregivers caring for advanced-stage cancer patients. In that sense, CR may increase the opportunity for cooperation between clinicians and caregivers. Through receiving timely alerts and accessing the CR, clinicians may help to manage uncontrolled symptoms and offer support to caregivers immediately. The study found that caregivers with access to the CR reported less negative mood than those without. In an earlier article specifically focused on the CR, we found that high patient symptom distress was improved significantly for those with the CR relative to those without.61 This may explain the effects of negative mood. The CR may enable earlier intervention because clinicians receive automatic e-mail alerts triggered by high patient symptom distress that might otherwise have gone unreported and unattended. With the feeling that patient symptoms are in control, caregivers may experience less emotional distress.9,25,62
However, preparedness and physical burden were not significantly different between those with and without the CR. Both groups can access the same CHESS services which have improved patient self-efficacy in other trials46,54. As such, the CR may not have affected physical burden and preparedness much because the comprehensive resources offered within CHESS may have helped with these outcomes in both groups to some extent. However, another alternative explanation may offer more insight. The CR—as a communication tool—neither tells the clinicians how to better support caregivers nor magically allocates clinicians’ time and resources to do so. Previous ePRO studies showed that patient self-reported outcomes have led to more frequent discussion of symptoms during clinic visits, more symptom issues addressed by clinicians, and majority (84%) of the alerts responded within 24 h.37,38,40 The clinicians, under a tight schedule, may more likely address patient symptom burden than take time helping caregivers cope.15 Future studies should track clinicians’ actual responses to caregivers’ needs, which may help to better understand the findings.
The small difference in negative mood (Table 3)—about 0.6 for CHESS + CR group and 0.9 for CHESS-Only group—may draw concerns regarding the clinical relevance of the effect. To address this concern, we compared the results to another study that reported subscales of SV-POMS for a healthy versus a cancer population. The CHESS + CR caregivers’ perceived levels of negative mood were found to be comparable to that reported by the healthy population whereas the CHESS-Only caregivers experienced similar levels as cancer patients undergoing chemotherapy.53 Therefore, the improvement in the CHESS + CR group compared to the CHESS-Only group is statistically significant and may be clinically meaningful as well.
Several limitations are noteworthy here. First, those caregivers reporting lower patient symptom burden at pretest were more likely to complete their 6- and 12-month follow-up surveys. Our results may underestimate the potential impact of CHESS + CR on those who may benefit the most. Secondly, in our study, about 26% (29/110) of CHESS + CR caregivers and 21% (22/107) of CHESS-Only caregivers did not use the Check-in. However, the small amount of nonusers does not offer sufficient power for sub-group analyses. Future studies acquiring larger sample size may be needed to explore further the effects of amount of system use.63 Moreover, the majority of patients in our sample are well-educated Caucasians. This may limit the ability to generalize the results to other ethnic populations. Although a digital divide may still exist64, recent studies have highlighted benefits of information and communication technologies to both the underserved population65,66 and the hospitals providing care to them67. Finally, because CR is integrated in CHESS our study could not assess how CR alone would influence caregiver outcomes.
The needs for quality, empirical, and longitudinal research in the development and testing of interventions to support caregivers have been well documented.7,68,69 Despite the limitations, this study provides new evidence testing an ePRO system designed specifically for cancer caregivers. Moreover, whereas a previous nursing communication study reported outcomes at 10 weeks after intervention25, our study showed a similar but longer (1 year) effect of improved negative mood. The results are likely generalizable to populations facing other cancers because this study included both gender-specific (breast and prostate) and gender-neutral (lung) cancers, as well as cancers with long-term and short-term median survival periods. The timely communication of caregiver reported information—through the CR—seems to help reduce caregiver’s negative mood. Future system development to overcome barriers identified here may better assist clinicians to support caregivers caring for advanced-stage cancer patients.
Acknowledgments
Funding
This research was made possible through grant funding from the National Cancer Institute (1 P50 CA095817-01A1) and National Institute of Nursing Research (RO1 NR008260-01).
Footnotes
Trail Registration
ClinicalTrials.gov Identifiers: NCT00214162 and NCT00365963
Conflict of interest
The Authors declare that there is no conflict of interest.
Contributor Information
Ming-Yuan Chih, Department of Industrial and Systems Engineering, University of Wisconsin-Madison, Madison, WI, USA; Center for Health Enhancement Systems Studies, University of Wisconsin-Madison, Madison, WI, USA.
Lori L DuBenske, School of Medicine and Public Health, University of Wisconsin-Madison, Madison, WI, USA; Center for Health Enhancement Systems Studies, University of Wisconsin-Madison, Madison, WI, USA.
Robert P Hawkins, School of Journalism and Mass Communication, University of Wisconsin-Madison, Madison, WI, USA; Center for Health Enhancement Systems Studies, University of Wisconsin-Madison, Madison, WI, USA.
Roger L Brown, School of Nursing, University of Wisconsin-Madison, Madison, WI, USA.
Susan K Dinauer, Center for Health Enhancement Systems Studies, University of Wisconsin-Madison, Madison, WI, USA.
James F Cleary, School of Medicine and Public Health, University of Wisconsin-Madison, Madison, WI, USA.
David H Gustafson, Department of Industrial and Systems Engineering, University of Wisconsin-Madison, Madison, WI, USA; Center for Health Enhancement Systems Studies, University of Wisconsin-Madison, Madison, WI, USA.
References
- 1.The National Alliance for Caregiving and AARP. [accessed 8 June 2011];Caregiving in the US. 2009 2009 http://immn.org/nac/pdf/research/Caregiving_in_the_US_2009_full_report.pdf. [Google Scholar]
- 2.Glajchen M. The emerging role and needs of family caregivers in cancer care. J Support Oncol. 2004;2:145–55. [PubMed] [Google Scholar]
- 3.Haley WE. Family caregivers of elderly patients with cancer: understanding and minimizing the burden of care. J Support Oncol. 2003;1:25–9. [PubMed] [Google Scholar]
- 4.Sharpe L, Butow P, Smith C, et al. The relationship between available support, unmet needs and caregiver burden in patients with advanced cancer and their carers. Psychooncology. 2005;14:102–14. doi: 10.1002/pon.825. [DOI] [PubMed] [Google Scholar]
- 5.Bevans M, Sternberg EM. Caregiving burden, stress, and health effects among family caregivers of adult cancer patients. JAMA. 2012;307:398–403. doi: 10.1001/jama.2012.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Northouse LL, Williams A-L, Given B, et al. Psychosocial Care for Family Caregivers of Patients With Cancer. J Clin Oncol. 2012:30. doi: 10.1200/JCO.2011.39.5798. [DOI] [PubMed] [Google Scholar]
- 7.Stenberg U, Ruland CM, Miaskowski C. Review of the literature on the effects of caring for a patient with cancer. Psychooncology. 2010;19:1013–25. doi: 10.1002/pon.1670. [DOI] [PubMed] [Google Scholar]
- 8.Grunfeld E, Coyle D, Whelan T, et al. Family caregiver burden: results of a longitudinal study of breast cancer patients and their principal caregivers. CMAJ. 2004;170:1795–801. doi: 10.1503/cmaj.1031205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Northouse LL, Mood DW, Montie JE, et al. Living with prostate cancer: patients’ and spouses’ psychosocial status and quality of life. J Clin Oncol. 2007;25:4171–7. doi: 10.1200/JCO.2006.09.6503. [DOI] [PubMed] [Google Scholar]
- 10.Archbold PG, Stewart BJ, Greenlick MR, et al. Mutuality and preparedness as predictors of caregiver role strain. Res Nurs Health. 1990;13:375–84. doi: 10.1002/nur.4770130605. [DOI] [PubMed] [Google Scholar]
- 11.Gaugler JE, Linder J, Given CW, et al. Family cancer caregiving and negative outcomes: the direct and mediational effects of psychosocial resources. J Fam Nurs. 2009;15:417–44. doi: 10.1177/1074840709347111. [DOI] [PubMed] [Google Scholar]
- 12.Scherbring M. Effect of caregiver perception of preparedness on burden in an oncology population. Oncol Nurs Forum. 2002;29:E70–6. doi: 10.1188/02.ONF.E70-E76. [DOI] [PubMed] [Google Scholar]
- 13.Schumacher KL, Stewart BJ, Archbold PG. Mutuality and preparedness moderate the effects of caregiving demand on cancer family caregiver outcomes. Nurs Res. 2007;56:425–33. doi: 10.1097/01.NNR.0000299852.75300.03. [DOI] [PubMed] [Google Scholar]
- 14.Sherwood PR, Given BA, Given CW, et al. The influence of caregiver mastery on depressive symptoms. J Nurs Scholarsh. 2007;39:249–55. doi: 10.1111/j.1547-5069.2007.00176.x. [DOI] [PubMed] [Google Scholar]
- 15.Mitnick S, Leffler C, Hood VL. Family caregivers, patients and physicians: ethical guidance to optimize relationships. J Gen Intern Med. 2010;25:255–60. doi: 10.1007/s11606-009-1206-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamayo GJ, Broxson A, Munsell M, et al. Caring for the caregiver. Oncol Nurs Forum. 2010;37:E50–7. doi: 10.1188/10.ONF.E50-E57. [DOI] [PubMed] [Google Scholar]
- 17.Docherty A, Owens A, Asadi-Lari M, et al. Knowledge and information needs of informal caregivers in palliative care: a qualitative systematic review. Palliat Med. 2008;22:153–71. doi: 10.1177/0269216307085343. [DOI] [PubMed] [Google Scholar]
- 18.Gottschalk A, Flocke SA. Time spent in face-to-face patient care and work outside the examination room. Ann Fam Med. 2005;3:488–93. doi: 10.1370/afm.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waitzkin H. Information giving in medical care. J Health Soc Behav. 1985;26:81–101. [PubMed] [Google Scholar]
- 20.Fallowfield L, Jenkins V. Effective communication skills are the key to good cancer care. Eur J Cancer. 1999;35:1592–7. doi: 10.1016/s0959-8049(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 21.Marvel MK, Epstein RM, Flowers K, et al. Soliciting the patient’s agenda: have we improved? JAMA. 1999;281:283–7. doi: 10.1001/jama.281.3.283. [DOI] [PubMed] [Google Scholar]
- 22.Hudson PL, Aranda S, Kristjanson LJ. Meeting the supportive needs of family caregivers in palliative care: challenges for health professionals. J Palliat Med. 2004;7:19–25. doi: 10.1089/109662104322737214. [DOI] [PubMed] [Google Scholar]
- 23.Connors AF, Dawson NV, Desbiens NA, et al. A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT). The SUPPORT Principal Investigators. JAMA. 1995;274:1591–8. [PubMed] [Google Scholar]
- 24.Kozachik SL, Given CW, Given BA, et al. Improving depressive symptoms among caregivers of patients with cancer: results of a randomized clinical trial. Oncol Nurs Forum. 2001;28:1149–57. [PubMed] [Google Scholar]
- 25.Given B, Given CW, Sikorskii A, et al. The impact of providing symptom management assistance on caregiver reaction: results of a randomized trial. J Pain Symptom Manage. 2006;32:433–43. doi: 10.1016/j.jpainsymman.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 26.DuBenske LL, Wen KY, Gustafson DH, et al. Caregivers’ differing needs across key experiences of the advanced cancer disease trajectory. Palliat Support Care. 2008;6:265–72. doi: 10.1017/S1478951508000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cooley ME, Short TH, Moriarty HJ. Symptom prevalence, distress, and change over time in adults receiving treatment for lung cancer. Psychooncology. 2003;12:694–708. doi: 10.1002/pon.694. [DOI] [PubMed] [Google Scholar]
- 28.Arora NK. Interacting with cancer patients: the significance of physicians’ communication behavior. Soc Sci Med. 2003;57:791–806. doi: 10.1016/s0277-9536(02)00449-5. [DOI] [PubMed] [Google Scholar]
- 29.Osse BHP, Vernooij-Dassen MJFJ, Schadé E, et al. Problems experienced by the informal caregivers of cancer patients and their needs for support. Cancer Nurs. 2006;29:378–88. doi: 10.1097/00002820-200609000-00005. quiz 389–90. [DOI] [PubMed] [Google Scholar]
- 30.Ong LM, de Haes JC, Hoos AM, et al. Doctor-patient communication: a review of the literature. Soc Sci Med. 1995;40:903–18. doi: 10.1016/0277-9536(94)00155-m. [DOI] [PubMed] [Google Scholar]
- 31.Basch E, Artz D, Dulko D, et al. Patient online self-reporting of toxicity symptoms during chemotherapy. J Clin Oncol. 2005;23:3552–61. doi: 10.1200/JCO.2005.04.275. [DOI] [PubMed] [Google Scholar]
- 32.Abernethy AP, Herndon JE, Wheeler JL, et al. Feasibility and acceptability to patients of a longitudinal system for evaluating cancer-related symptoms and quality of life: pilot study of an e/Tablet data-collection system in academic oncology. J Pain Symptom Manage. 2009;37:1027–38. doi: 10.1016/j.jpainsymman.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 33.Carpenter JS, Rawl S, Porter J, et al. Oncology outpatient and provider responses to a computerized symptom assessment system. Oncol Nurs Forum. 2008;35:661–9. doi: 10.1188/08/ONF.661-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gwaltney CJ, Shields AL, Shiffman S. Equivalence of electronic and paper-and-pencil administration of patient-reported outcome measures: a meta-analytic review. Value Health. 2008;11:322–33. doi: 10.1111/j.1524-4733.2007.00231.x. [DOI] [PubMed] [Google Scholar]
- 35.Cox A, Illsley M, Knibb W, et al. The acceptability of e-technology to monitor and assess patient symptoms following palliative radiotherapy for lung cancer. Palliat Med. 2011;25:675–81. doi: 10.1177/0269216311399489. [DOI] [PubMed] [Google Scholar]
- 36.Gustafson DH, Shaw BR, Isham A, et al. An E-Health Solution for People with Alcohol Problems. Alcohol Res Health. 2011;33:327–37. [PMC free article] [PubMed] [Google Scholar]
- 37.Cleeland CS, Wang XS, Shi Q, et al. Automated symptom alerts reduce postoperative symptom severity after cancer surgery: a randomized controlled clinical trial. J Clin Oncol. 2011;29:994–1000. doi: 10.1200/JCO.2010.29.8315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruland CM, Holte HH, Røislien J, et al. Effects of a computer-supported interactive tailored patient assessment tool on patient care, symptom distress, and patients’ need for symptom management support: a randomized clinical trial. J Am Med Inform Assoc. 2010;17:403–10. doi: 10.1136/jamia.2010.005660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kearney N, McCann L, Norrie J, et al. Evaluation of a mobile phone-based, advanced symptom management system (ASyMS) in the management of chemotherapy-related toxicity. Support Care Cancer. 2009;17:437–44. doi: 10.1007/s00520-008-0515-0. [DOI] [PubMed] [Google Scholar]
- 40.Velikova G, Booth L, Smith AB, et al. Measuring quality of life in routine oncology practice improves communication and patient well-being: a randomized controlled trial. J Clin Oncol. 2004;22:714–24. doi: 10.1200/JCO.2004.06.078. [DOI] [PubMed] [Google Scholar]
- 41.Cella D, Yount S, Rothrock N, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care. 2007;45:S3–S11. doi: 10.1097/01.mlr.0000258615.42478.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaasa S, Loge JH, Fayers P, et al. Symptom assessment in palliative care: a need for international collaboration. J Clin Oncol. 2008;26:3867–73. doi: 10.1200/JCO.2007.15.8881. [DOI] [PubMed] [Google Scholar]
- 43.Kutner JS, Bryant LL, Beaty BL, et al. Symptom distress and quality-of-life assessment at the end of life: the role of proxy response. J Pain Symptom Manage. 2006;32:300–10. doi: 10.1016/j.jpainsymman.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 44.Elliott MN, Beckett MK, Chong K, et al. How do proxy responses and proxy-assisted responses differ from what Medicare beneficiaries might have reported about their health care? Health Serv Res. 2008;43:833–48. doi: 10.1111/j.1475-6773.2007.00820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meeker MA, Finnell D, Othman AK. Family caregivers and cancer pain management: a review. J Fam Nurs. 2011;17:29–60. doi: 10.1177/1074840710396091. [DOI] [PubMed] [Google Scholar]
- 46.Gustafson DH, Hawkins R, McTavish F, et al. Internet-Based Interactive Support for Cancer Patients: Are Integrated Systems Better? J Commun. 2008;58:238–57. doi: 10.1111/j.1460-2466.2008.00383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DuBenske LL, Gustafson DH, Shaw BR, et al. Web-based cancer communication and decision making systems: connecting patients, caregivers, and clinicians for improved health outcomes. Med Decis Making. 2010;30:732–44. doi: 10.1177/0272989X10386382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bruera E, Kuehn N, Miller MJ, et al. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care. 1991;7:6–9. [PubMed] [Google Scholar]
- 49.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55. [PubMed] [Google Scholar]
- 50.Novak M, Guest C. Application of a multidimensional caregiver burden inventory. Gerontologist. 1989;29:798–803. doi: 10.1093/geront/29.6.798. [DOI] [PubMed] [Google Scholar]
- 51.Archbold PG, Stewart BJ, Geeenlick MR, et al. The clinical assessment of mutuality and preparedness in family caregivers to frail older people. In: Funk SG, Tornquist EM, Champagne MT, et al., editors. Key Aspects of Elder Care: Managing Falls, Incontinence, and Cognitive Impairment. New York: Springer; 1992. pp. 328–39. [Google Scholar]
- 52.Dubenske LL, Chih MY, Dinauer S, et al. Development and implementation of a clinician reporting system for advanced stage cancer: initial lessons learned. J Am Med Inform Assoc. 2008;15:679–86. doi: 10.1197/jamia.M2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dilorenzo TA, Bovbjerg DH, Montgomery GH, et al. The application of a shortened version of the profile of mood states in a sample of breast cancer chemotherapy patients. Br J Health Psychol. 1999;4:315–25. [Google Scholar]
- 54.Gustafson DH, McTavish FM, Stengle W, et al. Use and Impact of eHealth System by Low-income Women With Breast Cancer. J Health Commun. 2005;10 (Suppl 1):195–218. doi: 10.1080/10810730500263257. [DOI] [PubMed] [Google Scholar]
- 55.Little RJA. Modeling the Drop-Out Mechanism in Repeated-Measures Studies. J Am Stat Assoc. 1995;90:1112. [Google Scholar]
- 56.Hedeker D, Gibbons RD. Application of random-effects pattern-mixture models for missing data in longitudinal studies. Psychol Methods. 1997;2:64–78. [Google Scholar]
- 57.SPSS Statistics for Windows, Rel. 17.0.0. Chicago: SPSS Inc; 2008. [Google Scholar]
- 58.Given BA, Given CW, Kozachik S. Family support in advanced cancer. CA Cancer J Clin. 2001;51:213–31. doi: 10.3322/canjclin.51.4.213. [DOI] [PubMed] [Google Scholar]
- 59.Gilbar O. Gender as a predictor of burden and psychological distress of elderly husbands and wives of cancer patients. Psychooncology. 1999;8:287–94. doi: 10.1002/(SICI)1099-1611(199907/08)8:4<287::AID-PON385>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 60.Schwarz G. Estimating the Dimension of a Model. Ann Stat. 1978;6:461–4. [Google Scholar]
- 61.Dubenske L, Gustafson D, Chih MY, et al. Alerting Clinicians to Caregiver’s Ratings of Cancer Patient Symptom Distress: A Randomized Comparison of the Clinician Report (CR) Service. Psychooncology. 2010;19:S112. [Google Scholar]
- 62.Miaskowski C, Kragness L, Dibble S, et al. Differences in mood states, health status, and caregiver strain between family caregivers of oncology outpatients with and without cancer-related pain. J Pain Symptom Manage. 1997;13:138–47. doi: 10.1016/s0885-3924(96)00297-7. [DOI] [PubMed] [Google Scholar]
- 63.Connell AM. Employing complier average causal effect analytic methods to examine effects of randomized encouragement trials. Am J Drug Alcohol Abuse. 2009;35:253–9. doi: 10.1080/00952990903005882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Talukdar D, Gauri DK. Home Internet Access and Usage in the USA: Trends in the Socio-Economic Digital Divide. CAIS. 2011;28:7. [Google Scholar]
- 65.Gustafson DH, McTavish FM, Stengle W, et al. Reducing the digital divide for low-income women with breast cancer: a feasibility study of a population-based intervention. J Health Commun. 2005;10 (Suppl 1):173–93. doi: 10.1080/10810730500263281. [DOI] [PubMed] [Google Scholar]
- 66.Gustafson DH, Shaw BR, Isham A, et al. Explicating an evidence-based, theoretically informed, mobile technology-based system to improve outcomes for people in recovery for alcohol dependence. Subst Use Misuse. 2011;46:96–111. doi: 10.3109/10826084.2011.521413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jha AK, DesRoches CM, Shields AE, et al. Evidence of an emerging digital divide among hospitals that care for the poor. Health Aff (Millwood) 2009;28:w1160–70. doi: 10.1377/hlthaff.28.6.w1160. [DOI] [PubMed] [Google Scholar]
- 68.Grande G, Stajduhar K, Aoun S, et al. Supporting lay carers in end of life care: current gaps and future priorities. Palliat Med. 2009;23:339–44. doi: 10.1177/0269216309104875. [DOI] [PubMed] [Google Scholar]
- 69.Harding R, List S, Epiphaniou E, et al. How can informal caregivers in cancer and palliative care be supported? An updated systematic literature review of interventions and their effectiveness. Palliat Med. 2012;26:7–22. doi: 10.1177/0269216311409613. [DOI] [PubMed] [Google Scholar]