Abstract
Studies in humans suggest that leukocyte telomere length may act as a marker of biological aging. We investigated whether individuals in the Nicoya region of Costa Rica, known for exceptional longevity, had longer telomere length than those in other parts of the country. After controlling for age, age squared, rurality, rainy season and gender, mean leukocyte telomere length in Nicoya was substantially longer (81 base pairs, p<0.05) than in other areas of Costa Rica, providing evidence of a biological pathway to which this notable longevity may be related. This relationship remains unchanged (79 base pairs, p<0.05) after statistically controlling for nineteen potential biological, dietary and social and demographic mediators. Thus the difference in mean leukocyte telomere length that characterizes this unique region does not appear to be explainable by traditional behavioral and biological risk factors. More detailed examination of mean leukocyte telomere length by age shows that the regional telomere length difference declines at older ages.
Keywords: telomere length, Costa Rica, aging, biomarkers, socioeconomic, longevity
1. Introduction
Telomeres are repetitive canonical sequences of DNA at the ends of chromosomes that, together with associated proteins, protect chromosome ends and also prevent the degradation of coding regions of DNA that would otherwise result from the inability of DNA replication enzymes to copy the end of a DNA strand. Although the functional importance of telomeres has been understood for decades, their implication in the human aging process has begun to emerge more recently. Shorter telomeres were first found to occur at older ages (Lee and others 2002), and more recent work has shown associations with chronic disease (Brouilette and others 2007) and mortality, independent of age (Bakaysa and others 2007; Cawthon and others 2003; Honig and others 2006). However, associations with mortality have not been consistent, especially for individuals at older ages (Boonekamp and others 2013; Mather and others 2011). Furthermore, the social and economic determinants of telomere length are not yet clear. There is some evidence that shorter telomeres are associated with chronic stress (Epel and others 2004), as well as lower socioeconomic position, less educational attainment and unemployment (Batty and others 2009; Cherkas and others 2006; Steptoe and others 2011), with a suggestion that associations are stronger with earlier life measures of socioeconomic position, such as education (Needham and others 2013; Steptoe and others 2011; Surtees and others 2012).
The Nicoyan Peninsula region in Costa Rica has recently been characterized as a region with exceptionally high longevity (Buettner 2010). Mortality rates of elderly people in Nicoya may be 29% lower than in the rest of Costa Rica according to a five-year follow up of a population-based sample of close to 3,000 Costa Ricans aged 60 years and over (Rosero-Bixby and Dow 2012), which means two or three years of additional life expectancy at age 60 -- an extraordinary result given the already high life expectancy of elderly Costa Ricans in general (Rosero-Bixby 2008). Yet reasons for the Nicoyan mortality advantage –and even the exact figures of health and morbidity levels in Nicoya -- are not known, in part because of the recentness of the discovery of this area as a potential hot-spot of high longevity. The present article aims at filling the knowledge gap about health in Nicoya by studying a marker that is considered an indicator of biological ageing.
In the current study we examine leukocyte telomere length (LTL) in a nationally representative population-based study of Costa Ricans. Understanding whether LTL in Nicoya differs from other Costa Rican populations after controlling for age will offer insights both into the potential role of telomeres in aging, as well as offer clues to the possible biological mechanisms through which Nicoyans have an exceptionally long life expectancy. This examination of LTL provides the first biomarker analysis of the underlying biology of why elderly Nicoyans have unusually high survival. To our knowledge, telomere length has not been examined among any of the geographically defined, notably long-living populations in the world (e.g. Sardinia, Loma Linda, Okinawa) (Buettner 2010; Cockerham and Yamori 2001).
2. Materials and methods
2.1 Study sample
We studied LTL in a sub-sample of 612 elderly individuals drawn from the nationally representative Costa Rican Study on Longevity and Healthy Aging (CRELES). CRELES is a longitudinal study based on a national sample of 2,827 residents of Costa Rica aged 60 and older in 2005, with oversampling of the oldest old (Rosero Bixby and others 2010). The CRELES sample was selected quasi-randomly from the 2000 census database using a multi-stage sampling design and complemented with a 100% sample of quasi-centenarians of the Nicoya region that rendered 91 additional participants in this longitudinal study. The ability of the CRELES sample to provide unbiased descriptions of characteristics of elderly Costa Ricans and of their mortality has been assessed elsewhere (Rosero Bixby and others 2010; Rosero-Bixby and Dow 2009). The “Committee on Science and Ethics” of the University of Costa Rica approved the study, and all participants provided written informed consent.
We randomly drew a sub-sample of CRELES data for assaying telomere length, selecting approximately 200 individuals in each of three different age strata: 60-75, 76-94, and 95 and over, thus implicitly oversampling older individuals. We also forced the subsample to have approximately 100 Nicoyans in the youngest and oldest age groups.1 All analyses control for age to account for the nature of the sampling.
Fasting blood samples were taken in CRELES during two waves of household visits: the first between November 2004 and September 2006 and the second between October 2006 and July 2008. In our subsample of 612 individuals, 365 had DNA samples taken at two time points. The mean length of time between interviews among this longitudinal subsample was 598 days, with a minimum time between samples of 365 days and maximum time of 903 days. The most common reason for individuals having only one LTL measurement was death before the second wave visit (55%). An additional 27% had measurement only in one of the waves because the fasting blood sample was not possible to draw in the field, DNA was not possible to extract from the blood cells, or the DNA concentration was not appropriate for measuring telomeres. The remaining 18% of individuals with only one measurement are part of common attrition causes of longitudinal studies, mostly lost of follow up. The correlation of LTL between samples was 0.57. This was substantially less than another analysis that found a correlation of 0.92 between waves that were 5.8 years apart (Chen and others 2011), however this study was in a middle aged population where LTL may be more stable over time. In addition, the lower correlation in our study may be due to measurement error that is inherent to the quantitative PCR telomere length assay.
2.2 Study sample characteristics
We obtained exact ages using participants’ national identity card, double-checked against the national database of births. Other demographic covariates were obtained through in-person interview. Self-assessed economic situation was determined through self-report of household conditions as “Excellent”, ”Very good”, “Good”, ”Average/Normal”, or “Bad”, with the first three categories combined for analysis. Household wealth was based on a simple count of ten goods and conveniences in the household, ranging from running water and a toilet to having a clothes washer and a car. The three categories of wealth we use in our analysis are high when all ten goods are in the household, medium for seven to nine goods and poor for less than seven goods. Educational attainment was categorized into three groups based on the distribution in this population: less than three years of education, from three to six years of education (primary school comprises six grades), or at least one year of secondary school. BMI was calculated from measured weight and height. Obesity was defined as BMI greater than or equal to 30. We use obesity because of the highly non-linear relationship between BMI and health (Flegal and others 2007). We used knee height as a proxy for early life environment as it is a marker of nutrient intake during gestation and early childhood (Wadsworth and others 2002). Knee height was measured once in 258 participants and twice in 714 participants. If knee height was measure twice the average was used. High glycosylated Hemoglobin was defined as >=6.5%. High trigylcerides was defined as >=200 mg/dl. Sitting systolic and diastolic blood pressure were measured twice during the interview and the average reading was used. High systolic blood pressure was defined as 140 mmHg and above, and high diastolic blood pressure 90 mmHg and above. Diet was measured using an abbreviated food frequency questionnaire of 27 tracer foods, and macronutrient summary measures were imputed from this using a prediction equation validated from a Costa Rican coronary health study that contained a full food frequency questionnaire (Rosero Bixby and others 2010).
2.3 Telomere length assay
To measure mean LTL, quantitative PCR (qPCR) assay was used to determine the relative ratio of telomere repeat copy number to single-copy gene copy number (t/s ratio), with the analyses using the average of two assays per DNA extraction sample. The lab was blind to the sample characteristics, and samples were assayed without association between date of survey or geographic origin. The inter-assay coefficient of variation for LTL was 3.7%. The coefficient of variation was not based on control samples run on each plate. Each sample has its coefficient of variation based on the two runs and the coefficient of variation reported is the average coefficient of variation of all samples. The coefficient of variation here only considers the precision of the analytic step, without assessing the pre-analytical steps. While both qPCR and Southern Blot methods have been shown to have highly reproducible results, the inter-assay coefficient of variation for Southern blot is lower (Aviv and others 2011). However, qPCR was preferable for our study because of the smaller amount of DNA required for the assay (Kimura and others 2010). Twenty-three individuals did not have sufficient DNA quantity to perform the assays, leaving an assayed sample of 977. Individuals without sufficient DNA quantity did not differ significantly by any demographic characteristics. The equation for conversion from T/S ratio to base pairs used was base pairs = 3274 + 2413*(T/S) based on prior work (Farzaneh-Far and others 2010a). It is important to note that this conversion ratio is likely to differ between labs, and even between assays within the same lab, and thus exact base pair values we report should be used as an approximation of actual telomere length. While this does not impact the internal validity of the analyses presented here, the same base pair length calculation cannot be used for other studies. The assays were conducted in the Blackburn Laboratory at the University of California, San Francisco.
2.4 Statistical methods
We used ordinary least squares regression models to examine predictors of LTL. All standard errors reported are hetroskedasticity robust, using Stata 12. Analysis also accounted for the clustered nature of the sample, since some individuals were measured twice, by specifying individual-level random effects. The demographic description of the population shown in Table 1 includes one observation per individual (n=581), as does the unadjusted comparison of telomere length by demographic characteristics and region shown in Table 2. Age standardization for Nicoya in Table 1 was performed using the demographic or health variable as a dependent variable in a regression analysis controlling for 5-year age category as a factor and Nicoya. Difference and p-value of difference were estimated based on the coefficient for Nicoya in the regression model. Subsequent regression analyses (shown in Table 3 and 4) include all panel observations where LTL was assayed. We also controlled all of our regression analyses for rainy season (May-October), since in our data rainy season is associated with telomere length, and there was moderate correlation between regional sampling and season. The plotted smoothed associations between telomere length and age and change in telomere length and age shown in Figures 1 and 2 were from generalized additive models using regression splines and did not control for other covariates (Wood 2006). We performed quantile regression analysis (Koenker 2000; Koenker and Hallock 2001) (Table 4) in order to examine whether the differences in LTL between Nicoya and the rest of Costa Rica are similar across levels of LTL. Whereas our multiple regression models compared the mean level of LTL between Nicoyans and individuals living in the rest of Costa Rica, the quantile regression models compared LTL at the 10th, 20th ... 90th percentiles. In this manner, we determined if differences in LTL by region were primarily among individuals with short LTL (e.g. 10th and 20th percentiles), with long LTL (e.g. 80th and 90th percentiles) or across the full distribution of LTL.
Table 1.
Sample Characteristics (percents or means) by Costa Rica (except Nicoya) and Nicoya
| Costa Rica (except Nicoya) (n=372) Raw | Nicoya (n=209) Raw | Age-Standardizeda | Age Standardized Difference (p-value) | |
|---|---|---|---|---|
| Age | ||||
| 60-74 (n=195) | 30% | 40% | ||
| 75-94 (n=180) | 43% | 9% | ||
| 95+ (n=206) | 27% | 51% | ||
| Male | 47% | 46% | 44% | 0.66 |
| Rural | 41% | 63% | 68% | <0.001 |
| Live alone | 14% | 11% | 11% | 0.38 |
| Less than weekly child contact 15% | 9% | 7% | 0.01 | |
| Education | ||||
| <3 years (n=277) | 47% | 49% | 52% | 0.34 |
| 3-6 years (n=259) | 44% | 46% | 47% | 0.54 |
| >=7 years (n=45) | 10% | 4% | 4% | 0.002 |
| Self-assessed economic condition | ||||
| Bad (n=107) | 20% | 16% | 14% | 0.07 |
| Normal (n=274) | 45% | 51% | 52% | 0.13 |
| Good (n=200) | 35% | 33% | 35% | 0.91 |
| Household Wealth | ||||
| Poor (n=210) | 23% | 60% | 60% | <0.001 |
| Medium (n=312) | 63% | 37% | 46% | <0.001 |
| High (n=58) | 14% | 3% | 2% | <0.001 |
| Calories per day (kcal/day) | 2054 | 2023 | 2031 | 0.76 |
| Protein (gram/day) | 67 | 66 | 66 | 0.63 |
| Saturated fat (grams/day) | 26 | 28 | 27 | 0.24 |
| Omega-3 (grams/day) | 1.8 | 1.7 | 1.7 | 0.33 |
| Omega-6 (grams/day) | 15 | 14 | 14 | 0.04 |
| Glycemic Load | 76 | 76 | 76 | 0.19 |
| Obese | 14% | 18% | 19% | 0.12 |
| Knee Height (cm) | 48.7 | 49.4 | 49.5 | <0.001 |
| Systolic bp (mm Hg) | 145 | 141 | 142 | 0.23 |
| Diastolic bp (mm Hg) | 81 | 78 | 79 | 0.08 |
| Triglycerides (mg/dL) | 153 | 137 | 143 | 0.19 |
| Glycosylated Hemoglobin (%) | 5.8 | 6.2 | 6.2 | 0.001 |
| C-reactive protein (mg/L) | 7.1 | 6.9 | 6.8 | 0.74 |
| Current Smoker | 6% | 7% | 8% | 0.45 |
Age standardization of Nicoya was performed to the general Costa Rican sample, using the demographic or health characteristic as a dependent variable in a regression analysis controlling for 5-year age category as a factor and Nicoya.
Table 2.
Mean Telomere Length (standard error) by Sample Characteristics and Region
| Costa Rica (except Nicoya) (n=372) | Nicoya (n=209) | Difference in means (95% CI) | |
|---|---|---|---|
| Full Population | 5136 (20) | 5265 (31) | 130 (61, 198) |
| Age | |||
| 60-74 | 5279 (35) | 5436 (50) | 157 (40, 274) |
| 75-94 | 5129 (30) | 5303 (93) | 174 (3, 345) |
| 95+ | 5005 (35) | 5131 (39) | 127 (24, 229) |
| Gender | |||
| Male | 5113 (31) | 5222 (44) | 110 (5, 215) |
| Female | 5156 (24) | 5300 (42) | 144 (54, 234) |
| Urban | 5116 (23) | 5245 (51) | 128 (33, 224) |
| Rural | 5164 (35) | 5277 (38) | 113 (10, 215) |
| Live Alone | 5134 (52) | 5125 (90) | −9 (−204, 186) |
| Do not live alone | 5136 (21) | 5282 (32) | 146 (73, 220) |
| No Weekly contact with child | 5089 (53) | 5133 (96) | 44 (−166, 253) |
| At least weekly contact with child | 5143 (21) | 5278 (32) | 134 (62, 208) |
| Education | |||
| <3 years | 5111 (28) | 5299 (45) | 188 (88, 288) |
| 3-6 years | 5154 (31) | 5258 (43) | 103 (1, 207) |
| >=7 years | 5173 (52) | 4947 (120) | −225 (−468, 18) |
| Self-assessed economic condition | |||
| Bad | 5130 (47) | 5254 (90) | 124 (−58, 308) |
| Normal | 5181 (31) | 5264 (43) | 83 (−18, 184) |
| Good | 5080 (29) | 5270 (50) | 190 (84, 297) |
| Household Wealth | |||
| Poor | 5139 (42) | 5255 (42) | 116 (−4, 237) |
| Medium | 5132 (25) | 5307 (47) | 175 (73, 277) |
| High | 5146 (47) | 5173 (160) | 27 (−270, 325) |
| Obese | 5148 (49) | 5308 (74) | 161 (−8, 330) |
| Not obese | 5134 (21) | 5255 (34) | 122 (46, 197) |
| High systolic blood pressure | 5151 (34) | 5285 (63) | 133 (4, 264) |
| Not high systolic blood pressure | 5126 (17) | 5177 (25) | 51 (−6, 108) |
| High diastolic blood pressure | 5143 (21) | 5221 (32) | 79 (6, 151) |
| Not high diastolic blood pressure | 5117 (22) | 5170 (33) | 53 (−22, 128) |
| High Triglycerides | 5183 (46) | 5318 (79) | 136 (−45, 317) |
| Not high trigylcerides | 5124 (22) | 5257 (33) | 134 (59, 208) |
| High Glycosylated Hemoglobin | 5105 (43) | 5210 (45) | 105 (−19, 229) |
| Not high glycosylated hemoglobin | 5141 (22) | 5289 (39) | 148 (65, 230) |
| High C-reactive protein | 5135 (19) | 5181 (28) | 46 (−17, 110) |
| Not high C-reactive protein | 5126 (25) | 5222 (41) | 96 (6, 186) |
| Current Smoker | 5021 (41) | 5288 (117) | 267 (54, 480) |
| Not a current smoker | 5143 (21) | 5263 (32) | 120 (48, 192) |
Table 3.
Regression coefficients and standard errors of predictors of telomere length.a
| Model 1 | Model 2 | Model 3b | Model 4b | Model 5 | |
|---|---|---|---|---|---|
| Nicoyan | 81 (31)* | 52 (33) | 83 (32)** | 111 (34)* | 79 (35)* |
| Age | −51 (18)** | −52(18)** | −52 (18)** | −64 (18)** | −65 (18)** |
| Age-squared | 0.2 (0.1)* | 0.3 (0.1)* | 0.3 (0.1)* | 0.3 (0.1)* | 0.3 (0.1)* |
| Male | −64 (29)* | −65 (29)* | −62 (29)* | −94 (39)* | −101 (40)* |
| Rural | 40 (29) | 23 (29) | 33 (30) | 44 (31) | 29 (32) |
| Rainy season | −75 (25)** | −80 (25)** | −75 (25)** | −62 (26)* | −65 (26)* |
| Potential social mediators | |||||
| Do not live Alone | 69 (47) | 91 (50) | |||
| At least weekly contact with child | 28 (45) | 30 (49) | |||
| Education | |||||
| 3-6 years | −2.8 (29) | 24 (33) | |||
| >=7 years | −26 (57) | 12 (58) | |||
| Self-Assessed Economic Condition | |||||
| Poor (versus high) | −19 (40) | −14 (43) | |||
| Medium (versus high) | 32 (27) | 32 (29) | |||
| Household Wealth | |||||
| Poor (versus high) | 105 (51)* | 153 (55)* | |||
| Medium (versus high) | 29 (40) | 54 (42) | |||
| Potential dietary mediators | |||||
| Calories per day (kcal/day) | 0.04 (0.07) | −0.01 (0.07) | |||
| Protein intake (gram/day) | 0.75 (1.6) | 2.2 (1.7) | |||
| Saturated fat intake (grams/day) | −2.2 (1.8) | −2.5 (1.9) | |||
| Omega-3-Fatty Acid intake (grams/day) | −21 (27) | −19 (29) | |||
| Omega-6-Fatty Acid intake (grams/day) | −2.4 (3.7) | −1.0 (4.2) | |||
| Glycemic Load | −3.4 (4.7) | 0.26 (5.0) | |||
| Potential biological and behavioral mediators | |||||
| Obese | −37 (39) | −48 (40) | |||
| Knee Height (cm) | 8.5 (6.1) | 9.5 (5.9) | |||
| Systolic blood pressure (mm Hg) | 3.5 (0.85)** | 3.4 (0.87)** | |||
| Diastolic blood pressure (mm Hg) | −5.4 (1.6)** | −5.5 (1.6)** | |||
| Triglycerides (mg/dl) | −0.13 (0.18) | −0.14 (0.18) | |||
| Glycosylated Hemoglobin (%) | −8.6 (9.4) | −8.5 (9.6) | |||
| C-reactive protein (mg/L) | 2.2 (1.8) | 2.5 (1.8) | |||
| Smoking | −32 (51) | −40 (55) | |||
| R-squared | 0.15 | 0.16 | 0.15 | 0.15 | 0.17 |
| N | 977 | 960 | 976 | 869 | 850 |
Standard errors are shown in parentheses and are robust standard errors that account for clustered sample by individual.
indicates statistically signficiant different of p<0.05
indicates statistically signficiant different of p<0.01
Table 4.
Regression coefficients for Nicoya residence at deciles of telomere length
| Percentile of LTL | Model 1 | Model 2 | Model 3 | Model4 | Model 5 |
|---|---|---|---|---|---|
| 10th | 31 (35) | 7 (28) | 42 (30) | 33 (32) | 49 (35) |
| 20th | 37 (25) | 32 (33) | 36 (27) | 58 (26)* | 32 (38) |
| 30th | 38 (25) | 25 (28) | 51 (22)* | 83 (33)* | 67 (39) |
| 40th | 60 (25)* | 33 (23) | 60 (31)* | 98 (31)** | 70 (36) |
| 50th | 54 (29) | 39 (29) | 44 (23) | 95 (33)** | 53 (43) |
| 60th | 69 (32)* | 51 (35) | 40 (35) | 88 (32)** | 64 (40) |
| 70th | 63 (39) | 39 (42) | 93 (39) | 116 (51)* | 95 (42)* |
| 80th | 101 (39)* | 63 (49) | 94 (40)* | 193 (41)** | 127 (50)* |
| 90th | 195 (73)** | 123 (80) | 152 (75)* | 200 (73)** | 171 (85)* |
| N | 977 | 960 | 976 | 873 | 850 |
Standard errors are shown in parentheses. All models control for the same covariates as shown previously in Table 3, coefficients not shown.
indicates p<0.05
indicates p<0.01.
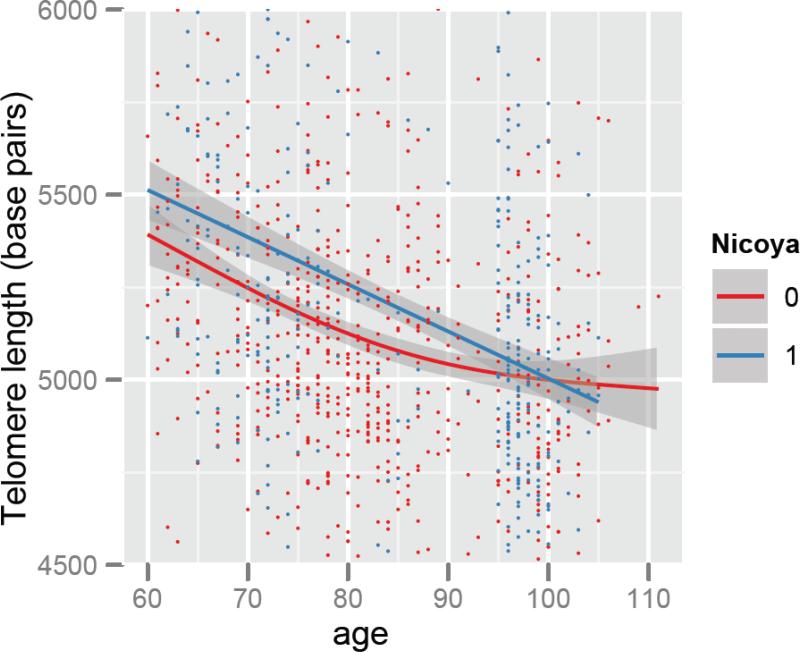
Figure 1.
Telomere length (in base pairs) by age in Costa Rica (Red) as compared to Nicoya (Blue). Solid line shows the regression based smoothed association of telomere length with age. Shaded area shows 95% confidence intervals around the point estimate of the association. Dots on the plot show individual values.
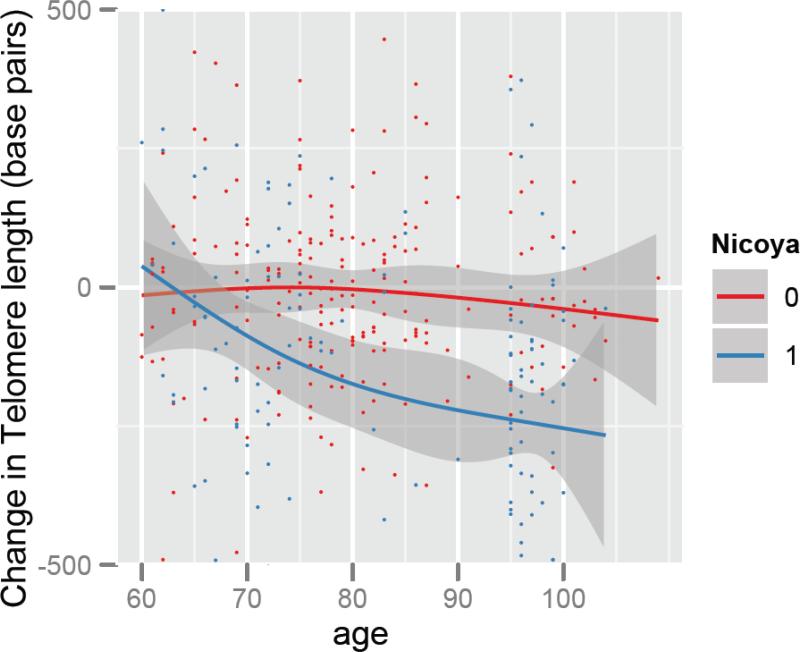
Figure 2.
Yearly Rate of change of Telomere length (in base pairs) by age in Costa Rica (Red) as compared to Nicoya (Blue). Solid line shows the regression based smoothed association of Change in telomere length with age. Shaded area shows 95% confidence intervals around the point estimate of the association. Dots on the plot show individual values.
3. Results
3.1 Description of the Sample
Table 1 provides a description of the sample of individuals living in all regions of Costa Rica except Nicoya as compared to individuals residing in the Nicoya region. Due to our strategy of oversampling older individuals and Nicoyans age 95 and above, the samples have a greater proportions of individuals in these categories than would be expected from a representative population distribution. Therefore, to make Nicoya data comparable, Table 1 also includes percents or means age-adjusted to the full Costa Rican sample. P-values of tests for statistically significant differences of the age-adjusted percents or means are shown in the last column. Nicoyans more commonly live in a rural area and have more frequent contact with children. On average, Nicoyans have lower levels of education and also lower household wealth. Cardiovascular disease risk factors were generally similar, other than Nicoyans having a higher median percent of glycosylated hemoglobin. Knee height was significantly higher in Nicoya.
3.2 Average telomere length by region and individual characteristics
Table 2 displays a description of mean telomere length by region and differences between region by individual characteristics. The purpose of this table is first to describe factors that may differ between regions, as they are not adjusted for potentially confounding factors. Mean unadjusted LTL is significantly longer in Nicoya than elsewhere in Costa Rica (130 base pairs; 95% CI: 61, 198).
The finding of longer LTL in Nicoyans is generally consistent across the examined demographic and health-related characteristics, with a few exceptions, notably, variables measuring isolation and economic circumstances. Among individuals who live alone (11% of Nicoyans and 14% elsewhere in Costa Rica), there is only a 9 base pair difference (95% CI: −186, 204) in LTL between Nicoya and elsewhere in Costa Rica, and among individuals with no weekly contact with a child (9% of Nicoyans and 15% elsewhere in Costa Rica), there is only a 44 base pair difference in LTL (95% CI: −166, 253). For those with the worst self-assessed economic conditions and for those with the highest household wealth, there were not significant differences between Nicoya and the rest of Costa Rica.
3.3 Telomere length by age
In order to more precisely compare mean LTL by age between Nicoya and elsewhere in Costa Rica, Figure 1 presents regional cross-sectional data of mean LTL by exact age. In both regions, shorter telomere length is associated on average with a difference of 10 base pairs per year of age. As shown in Figure 1, at most ages, Nicoyans possess longer LTL. However, at ages above around 90, the statistically significant Nicoyan telomere length advantage disappears, as the telomere lengths in the two groups overlap and there is a continuing linear decline with age in Nicoya, whereas the decline with age is less elsewhere in Costa Rica (p<0.05 for interaction of age and Nicoya).
The narrowing of the difference in LTL over time between Nicoyans and other Costa Ricans shown in Figure 1 could have two non-mutually exclusive explanations. First, the observed differences could be explained by differences in the rate of telomere length change by age between Nicoya and the rest of Costa Rica. A second explanation is that greater mortality of individuals with shorter telomere lengths in the rest of Costa Rica could result in selection that would cause the appearance of slowing declines in telomere length with age in the rest of Costa Rica. To distinguish between these two explanations, we examined annual rate of telomere length change within the subset of our population who were measured at two time points (n=365) (Figure 2). In the general Costa Rican population sample, annual mean change in LTL is −72 base pairs (95% CI: −97 to −47) per year. However, Nicoyans on average have a significantly greater annual decline in LTL than other Costa Ricans (−168 base pairs (95% CI −211,−125) vs. −10 base pairs (95% CI −38,18)). Figure 2 shows this relationship by age, and this longitudinal analysis shows that, furthermore, the difference in the yearly decrease in telomere length between Nicoyans and the rest of Costa Ricans varies with age (p<0.01 of interaction term of Nicoya and Age with telomere change as the dependent variable). This difference between the yearly rate of change in telomere length by age in Nicoya as compared to the rest of Costa Rica appears to be the driving factor underlying the narrowing of the gap in LTL over time in Figure 1, rather than it being primarily due to mortality selection.
Notably, these differences in change by age differ markedly by region, as shown in the non-linear relationship between change and age in Nicoya (Figure 2). The marked differences in LTL and change occur up to the age of about 90, as shown by the non-overlapping confidence intervals (Figure 2). Due to sampling, data on the Nicoyan region is sparse between the age of 85 and 95. However, at ages of 95 and above, the LTL and change in cross sectional telomere length with age do not differ substantially, and the difference in LTL and change by age are similar between Nicoya and the rest of Costa Rica (Figure 1), even as the change per year remains greater among Nicoyans (Figure 2). For those aged 95 years and older, given the similar telomere lengths in the Costa Rica and Nicoyan groups, a further interesting finding was that, at least for these older individuals, the significant difference between the two groups in the longitudinal measure, yearly rate of LTL change, is not explained by baseline telomere lengths differing between the two groups.
3.4 Telomere length by region controlling for confounding factors and mediators
Table 3 presents the results from ordinary least squares regression models examining the strength of association between individual characteristics and cross-sectional telomere length controlling for potentially confounding variables. After controlling for age, age-squared, gender, rurality and rainy season (model 1) individuals living in Nicoya have a mean LTL that is 81 base pairs longer than individuals living in the rest of Costa Rica (p<0.05).
A second goal of our analysis is to examine whether the association between living in Nicoya and telomere length remains after controlling for potential social and economic mediators (model 2), dietary mediators (model 3) or biomarker (model 4) mediators. After controlling for potential social mediators of the relationship between Nicoya and mean LTL, there was a moderate attenuation in the Nicoya coefficients, decreasing from 81 to 52 base pairs. Among the examined mediating variables only household wealth had a statistically significant relationship with LTL, where those living in the poorest households had average model-based LTL of 105 base pairs longer. Model 3 controlled for potential dietary mediators, and the relationship of LTL and residence in Nicoya remained unchanged at 83. After controlling for potential biological mediators in Model 4, Nicoya LTL is greater by 111 base pairs, as compared to 81 in the base model, indicative of worse levels of biological risk factors in Nicoya that also are associated with LTL. Among biological risk factors, higher systolic blood pressure was associated with longer LTL, and higher diastolic blood pressure was associated with shorter LTL. Finally, model 5 combines all potential social, dietary, behavioral and biological mediators, and shows no attenuation (compared to Model 1 without these controls) of the association between residence in Nicoya and mean LTL, which remains substantial and nearly identical (79 base pairs) and significant (p<0.05), despite controlling for 19 potentially mediating factors. While as shown in the last line of Table 3 sample size differed slightly due to missing values for some mediators, models run on the subset of observations with no missing values (n=850) showed no notable difference in coefficients.
3.5 Sensitivity analyses for age specification and quantiles of distribution
Given the strong confounding effects of age and the potential for non-linear associations between age and telomere length, we fit additional models with indicator variables for each single year of age in addition to the other covariates in models 1 through 5. Comparing the Nicoyan effect estimate in models with age and age squared as compared to each year separately, the parameter estimate of LTL for Nicoya decreased only slightly from 81 to 74, in model 2 it changed from 52 to 46, in model 3 it changed from 83 to 75 and in model 4 it changed from 111 to 100. In model 5 the parameter estimate for Nicoya also decreased only slightly from 79 to 71, with the R-squared increasing from 0.17 to 0.21. These sensitivity analyses suggest that our primary results were not due to possible misspecification of the functional form of the relationship between age and mean LTL.
Finally, we also fit quantile regression models for deciles of the distribution of LTL in order to examine whether the Nicoyan differences varied at different levels of LTL. For the model only controlling for basic demographic characteristics (model 1), there were differences between Nicoya and the rest of Costa Rica primarily near the median and above deciles, with the difference particularly marked at the 90th percentile. The difference between Nicoya and the rest of Costa Rica at the 90th percentile of LTL were also significantly different in models three, four and five, consistent with the results of our primary mean regression models presented in Table 3. Thus while we qualitatively observe greater LTL differences by region among those with the longest telomeres, the nature of these differences are generally similar to those observed in our primary mean based models.
4.0 Discussion
Our principal study finding is that Nicoyans have longer telomeres than non-Nicoyans, and these differences are not explained by basic demographic covariates nor by potential mediators including social factors, diet and biomarkers capturing known underlying health. These findings provide a potential biological basis for older Nicoyans’ exceptional longevity. This study is a further step to uncovering measures of different apparent rates of biological aging, as measured by telomere length, in a region of exceptional longevity.
Despite considering 19 potentially mediating factors, based on work elsewhere on determinants of LTL, the present study did not determine the underlying reason for the on average longer LTL in Nicoyans. The as yet undetermined underlying causes of this difference could be due to either: 1) inaccurate measures of factors that we examined in our study (i.e. measurement error), or 2) the existence of critical factors that we did not measure and thus could not examine, which are the primary limitations of our study. One category of factors that we considered as a potential explanation for the differences was dietary, including a measure of Omega-3 Fatty Acid Levels, which has previously been shown to be associated with mean LTL in the United States (Farzaneh-Far and others 2010b). We had a priori theorized this as a plausible difference between Nicoyans and individuals living in the rest of Costa Rica due to the close proximity of much of the population to the coastline in Nicoya and the active fishing culture and industry. However, control for a basic number of dietary components, including Omega-3 Fatty Acids, did not attenuate the association of Nicoyan residence with telomere length. While we do not find support for the hypothesis that diet explains the longer telomere length in Nicoya, this lack of findings could be due to the well acknowledged difficulty of measuring dietary intake in large population based studies (Willett 1998), and specifically the imputed nature of our dietary indicators. Early life levels of nutrition, as captured in part by knee height, also did not result in attenuation of the Nicoyan difference in LTL.
A second area of substantial inquiry in the literature on social determinants of telomere length is the association of high levels of chronic life stress with shorter telomere length (Entringer and others 2011; Epel and others 2004; Humphreys and others 2011; O'Donovan and others 2011). We do not have measures of chronic stress in our study, so could not directly examine whether this could explain differences between Nicoya and the result of Costa Rica. Independent of work on telomere length, anthropological studies have examined social and cultural differences between Nicoya and the rest of Costa Rica. While investigation was based on small sample sizes, Nicoyans had greater psychological attachment to the family than individuals in the capital of Costa Rica, San Jose (Rosabal-Coto 2004). It is possible that these close families ties may have buffered against detrimental effects of life stress that have been found to be associated with shorter telomere length. The tighter family structure may also offer more opportunities for material support through kinship and family networks. No studies have examined social support and telomere length, although a recent study found that having more ambivalent social relationships (with both high positive and negative aspects) was associated with shorter telomere length (Uchino and others 2012). Among all of the potential mediators examined, social and economic mediators had the most impact attenuating the association between Nicoya and LTL (Table 3, model 2). Among mediators in this model, the only one significantly associated with LTL was living with poor household wealth, where LTL was 105 base pairs longer (p<0.05). While in industrialized countries higher socioeconomic position indicators have generally been found to be associated with longer telomere length (Needham and others 2013), Costa Rica is notable for socioeconomic differences that are in many cases different than the United States (Rehkopf and others 2010a), and socioeconomic relationships vary by context and time (Kunitz 2007). The exact nature of the wealth measure, and the categories being compared, needs to be considered. For example, to be in the highest wealth category, having all ten items is required, which includes owning a car, which may not translate into longer telomere length among this cohort of individuals in Nicoya. A second mediator was “living alone,” where individuals living alone had LTL that was shorter by 69 base pairs, although this association was not statistically significant (p>0.05). However, it is descriptively interesting to note that among those individuals living alone, there was little difference in mean LTL between Nicoyans and non-Nicoyans (Table 2), consistent with the benefits of living in Nicoya being more accessible to those individuals with social contacts.
Finally, a third category of potential reasons for differences in LTL between Nicoya and the rest of Costa Rica is a differential regional distribution of genetic polymorphisms that impact telomere length. We have no data on polymorphisms in genes that contribute toward LTL, so are not able to answer this question directly. While the extent to which telomere length is genetically determined remains uncertain, a replicated GWAS identified TERC region loci was associated with an ~75 base-pair difference in telomere length (Codd and others 2010), although another GWAS study failed to find any significant loci (Prescott and others 2011). There could be potential genetic differences (and gene-environment interactions) due to either different historical patterns of migration, or to early life mortality selection if associated with genes related to telomere length. While this remains a potential explanation of our findings, a prior study comparing those living in Nicoya to the rest of Costa Rica did not find any differences in polymorphisms of genes related to mortality (ApoE, ACE, IL-6, TNFα, APOA5) (Poulain M, et al., unpublished analysis).
Strengths of the current analysis include the source of the population – the CRELES sample is a well-characterized representative sample of the population of Costa Rica over the age of 60. A further strength of our analysis is a telomere length assay that was performed blinded to sample characteristics and by individuals with extensive assay experience. Our population-based sample was of a relatively large sample size as compared to other studies of telomere length (Mather and others 2011). We also had measures of important cardiovascular risk biomarkers for inclusion in models as potential biological mediators. This study took special care to have the correct ages of individuals by obtaining the information from the identity card and double-checking the data with the birth certificate in the civil registration system. It is highly unlikely that age-exaggeration errors, which often hamper studies of elderly people in developing regions, impacted our estimates. The strong age effects observed among Nicoyans compared to other Costa Ricans would not likely be present if reportedly older Nicoyan individuals were actually younger.
While not the emphasis of our analysis, the coefficients of association between biomarkers and telomere length are consistent with prior literature for diastolic blood pressure (inverse association) (Fitzpatrick and others 2007), although other studies have found no association (Epel and others 2009). In contrast to the literature, we find a statistically significant direct association with systolic blood pressure where worse levels of systolic blood pressure (higher) are associated with longer telomere length. Future work should examine the robustness of this finding in models focused on blood pressure, including potential non-linearities (Rehkopf and others 2010b) in association with telomere length.
Our analysis of the association of age with telomere length examined a subset of individuals with longitudinal measures of LTL in order to determine whether the age dependent differences between Nicoya and the rest of Costa Rica were due to actual differences in change by age or an artifact of sample composition (due to higher mortality among individuals in the rest of Costa Rica with shorter mean telomere length). Our analyses of the subsample suggest that this change is not due to an artifact of mortality selection, but due to greater decreases in LTL with age in Nicoyans. As with the overall telomere length differences between Nicoya and the rest of Costa Rica, this difference in age related telomere change remains unexplained. Nevertheless, our analysis of the longitudinal changes within individuals suggests that it is unlikely to be an artifact of sample selection or regression (Chen and others 2011).
There have been few studies with multiple time point measures of telomere length within the same individuals, but our results are generally consistent with these prior findings (Aviv and others 2009; Chen and others 2011; Ehrlenbach and others 2009; Epel and others 2009; Farzaneh-Far and others 2010a; Nordfjall and others 2009). We found that among the demographic group with on average longer telomeres (Nicoyans), there were generally shorter average LTL found over time, and that this was greater among older individuals (Figure 2). Consistent with this, prior work in the United States found that the strongest predictor of shorter telomeres at a later time was baseline telomere length, where longer telomeres were associated with shorter lengths over time (Farzaneh-Far and others 2010a). Nevertheless, the extent to which increases in LTL are due to chance is still not fully understood.
Compared to prior work investigating longevity in Nicoya, our findings are the first to examine whether this region is associated with a putative biological marker of aging, and our results suggest future consideration of this may be useful for understanding the true causes of why individuals live longer in Nicoya, which could in turn produce basic fundamental knowledge about successful aging more generally.
Highlights.
We examine leukocyte telomere length in a population-based sample of Costa Ricans.
We test whether telomere length is longer in the high longevity Nicoya region.
We find longer telomere length among Nicoyans, even after controlling for age.
This difference persists after statistical control for predictors of telomere length.
The Nicoya telomere difference decreases at older age due to more rapid shortening.
Acknowledgments
We would like to acknowledge the work of CRELES collaborating investigators: Ericka Méndez, Guido Pinto, Hannia Campos, Kenia Barrantes, Floribeth Fallas, Gilbert Brenes, and Fernando Morales. CRELES informatics and support staff: Daniel Antich, Aaron Ramírez, Jeisson Hidalgo, Juanita Araya, and Yamileth Hernández. CRELES Field workers: José Solano, Julio Palma, Jenny Méndez, Maritza Aráuz, Mabelyn Gómez, Marcela Rodríguez, Geovanni Salas, Jorge Vindas and Roberto Patiño. DNA extraction from blood cells: Gabriel Aguilar under supervision of Jorge Azofeifa and Alejandro Leal from the School of Biology of the University of Costa Rica. We also acknowledge Lynn Fang and Kyle Lapham from the University of California, San Francisco for performing the telomere length assays. We would also like to thank Philip Grant for helpful comments on an earlier version of the manuscript.
Funding
The CRELES project (Costa Rican Longevity and Healthy Aging Study) is a longitudinal study of the Universidad de Costa Rica, carried on by the Centro Centroamericano de Población in collaboration with the Instituto de Investigaciones en Salud, with the support of the Wellcome Trust Foundation (grant N. 072406). Principal Investigator: Luis Rosero-Bixby. Co-principal investigators: Xinia Fernández and William H. Dow.
The funding sources had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Abbreviations
- LTL
leukocyte telomere length
- CRELES
Costa Rican Study on Longevity and Healthy Aging
- BMI
body mass index
- GWAS
genome wide association study
- bp
blood pressure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
DHR, WHD and LRB designed the study and analyzed the data. JL and EHB performed the assays. DHR, WHD, LRB, JL, ESE and EHB interpreted result and wrote the manuscript.
We assigned a uniformly distributed random number to each individual with stored DNA in CRELES, after sorting the individuals in each stratum with this random number, we selected the first 100 non-Nicoyans in the age group 60-75, the first 100 Nicoyans in this age group, the first 200 in the age bracket 76-94, and the first 100 non-Nicoyans aged 95 or more; all 112 Nicoyans aged 95 or more were included in the subsample.
Conflicts of Interest
None.
References
- Aviv A, Chen W, Gardner JP, Kimura M, Brimacombe M, Cao X, Srinivasan SR, Berenson GS. Leukocyte telomere dynamics: longitudinal findings among young adults in the Bogalusa Heart Study. Am J Epidemiol. 2009;169:323–329. doi: 10.1093/aje/kwn338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv A, Hunt SC, Lin J, Cao X, Kimura M, Blackburn E. Impartial comparative analysis of measurement of leukocyte telomere length/DNA content by Southern blots and qPCR. Nucleic Acids Res. 2011;39:e134. doi: 10.1093/nar/gkr634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakaysa SL, Mucci LA, Slagboom PE, Boomsma DI, McClearn GE, Johansson B, Pedersen NL. Telomere length predicts survival independent of genetic influences. Aging Cell. 2007;6:769–774. doi: 10.1111/j.1474-9726.2007.00340.x. [DOI] [PubMed] [Google Scholar]
- Batty GD, Wang Y, Brouilette SW, Shiels P, Packard C, Moore J, Samani N, Ford I. Socioeconomic status and telomere length: the West of Scotland Primary Prevention Study. J Epidemiol Community Health. 2009 doi: 10.1136/jech.2009.088427. [DOI] [PubMed] [Google Scholar]
- Boonekamp JJ, Simons MJ, Hemerik L, Verhulst S. Telomere length behaves as biomarker of somatic redundancy rather than biological age. Aging Cell. 2013;12:330–332. doi: 10.1111/acel.12050. [DOI] [PubMed] [Google Scholar]
- Brouilette SW, Moore JS, McMahon AD, Thompson JR, Ford I, Shepherd J, Packard CJ, Samani NJ. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet. 2007;369:107–114. doi: 10.1016/S0140-6736(07)60071-3. [DOI] [PubMed] [Google Scholar]
- Buettner D. The Blue Zones: Lessons for Living Longer From the People Who've Lived the Longest. National Geographic Society; Washington, D.C.: 2010. [Google Scholar]
- Cawthon RM, Smith KR, O'Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- Chen W, Kimura M, Kim S, Cao X, Srinivasan SR, Berenson GS, Kark JD, Aviv A. Longitudinal versus cross-sectional evaluations of leukocyte telomere length dynamics: age-dependent telomere shortening is the rule. J Gerontol A Biol Sci Med Sci. 2011;66:312–319. doi: 10.1093/gerona/glq223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkas LF, Aviv A, Valdes AM, Hunkin JL, Gardner JP, Surdulescu GL, Kimura M, Spector TD. The effects of social status on biological aging as measured by white-blood-cell telomere length. Aging Cell. 2006;5:361–365. doi: 10.1111/j.1474-9726.2006.00222.x. [DOI] [PubMed] [Google Scholar]
- Cockerham WC, Yamori Y. Okinawa: an exception to the social gradient of life expectancy in Japan. Asia Pac J Clin Nutr. 2001;10:154–158. doi: 10.1111/j.1440-6047.2001.00232.x. [DOI] [PubMed] [Google Scholar]
- Codd V, Mangino M, van der Harst P, Braund PS, Kaiser M, Beveridge AJ, Rafelt S, Moore J, Nelson C, Soranzo N, Zhai G, Valdes AM, Blackburn H, Mateo Leach I, de Boer RA, Kimura M, Aviv A, Goodall AH, Ouwehand W, van Veldhuisen DJ, van Gilst WH, Navis G, Burton PR, Tobin MD, Hall AS, Thompson JR, Spector T, Samani NJ. Common variants near TERC are associated with mean telomere length. Nat Genet. 2010;42:197–199. doi: 10.1038/ng.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlenbach S, Willeit P, Kiechl S, Willeit J, Reindl M, Schanda K, Kronenberg F, Brandstatter A. Influences on the reduction of relative telomere length over 10 years in the population-based Bruneck Study: introduction of a well-controlled high-throughput assay. Int J Epidemiol. 2009;38:1725–1734. doi: 10.1093/ije/dyp273. [DOI] [PubMed] [Google Scholar]
- Entringer S, Epel ES, Kumsta R, Lin J, Hellhammer DH, Blackburn EH, Wust S, Wadhwa PD. Stress exposure in intrauterine life is associated with shorter telomere length in young adulthood. Proc Natl Acad Sci U S A. 2011;108:E513–518. doi: 10.1073/pnas.1107759108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Merkin SS, Cawthon R, Blackburn EH, Adler NE, Pletcher MJ, Seeman TE. The rate of leukocyte telomere shortening predicts mortality from cardiovascular disease in elderly men. Aging (Albany NY) 2009;1:81–88. doi: 10.18632/aging.100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzaneh-Far R, Lin J, Epel E, Lapham K, Blackburn E, Whooley MA. Telomere length trajectory and its determinants in persons with coronary artery disease: longitudinal findings from the heart and soul study. PLoS One. 2010a;5:e8612. doi: 10.1371/journal.pone.0008612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzaneh-Far R, Lin J, Epel ES, Harris WS, Blackburn EH, Whooley MA. Association of marine omega-3 fatty acid levels with telomeric aging in patients with coronary heart disease. Jama. 2010b;303:250–257. doi: 10.1001/jama.2009.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick AL, Kronmal RA, Gardner JP, Psaty BM, Jenny NS, Tracy RP, Walston J, Kimura M, Aviv A. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. American Journal of Epidemiology. 2007;165:14–21. doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA : the journal of the American Medical Association. 2007;298:2028–2037. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- Honig LS, Schupf N, Lee JH, Tang MX, Mayeux R. Shorter telomeres are associated with mortality in those with APOE epsilon4 and dementia. Ann Neurol. 2006;60:181–187. doi: 10.1002/ana.20894. [DOI] [PubMed] [Google Scholar]
- Humphreys J, Epel ES, Cooper BA, Lin J, Blackburn EH, Lee KA. Telomere Shortening in Formerly Abused and Never Abused Women. Biol Res Nurs. 2011 doi: 10.1177/1099800411398479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M, Stone RC, Hunt SC, Skurnick J, Lu X, Cao X, Harley CB, Aviv A. Measurement of telomere length by the Southern blot analysis of terminal restriction fragment lengths. Nat Protoc. 2010;5:1596–1607. doi: 10.1038/nprot.2010.124. [DOI] [PubMed] [Google Scholar]
- Koenker R. Galton, Edgeworth, Frisch, and prospects for quantile regression in econometrics. Journal of Econometrics. 2000;95:347–374. [Google Scholar]
- Koenker R, Hallock KF. Quantile regression. J Econ Perspect. 2001;15:143–156. [Google Scholar]
- Kunitz SJ. The health of populations : general theories and particular realities. Oxford University Press; Oxford ; New York: 2007. [Google Scholar]
- Lee WW, Nam KH, Terao K, Yoshikawa Y. Age-related telomere length dynamics in peripheral blood mononuclear cells of healthy cynomolgus monkeys measured by Flow FISH. Immunology. 2002;105:458–465. doi: 10.1046/j.1365-2567.2002.01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather KA, Jorm AF, Parslow RA, Christensen H. Is telomere length a biomarker of aging? A review. The journals of gerontology Series A, Biological sciences and medical sciences. 2011;66:202–213. doi: 10.1093/gerona/glq180. [DOI] [PubMed] [Google Scholar]
- Needham BL, Adler N, Gregorich S, Rehkopf D, Lin J, Blackburn EH, Epel ES. Socioeconomic status, health behavior, and leukocyte telomere length in the National Health and Nutrition Examination Survey, 1999-2002. Soc Sci Med. 2013;85:1–8. doi: 10.1016/j.socscimed.2013.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordfjall K, Svenson U, Norrback KF, Adolfsson R, Lenner P, Roos G. The individual blood cell telomere attrition rate is telomere length dependent. PLoS Genet. 2009;5:e1000375. doi: 10.1371/journal.pgen.1000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan A, Epel E, Lin J, Wolkowitz O, Cohen B, Maguen S, Metzler T, Lenoci M, Blackburn E, Neylan TC. Childhood trauma associated with short leukocyte telomere length in posttraumatic stress disorder. Biol Psychiatry. 2011;70:465–471. doi: 10.1016/j.biopsych.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott J, Kraft P, Chasman DI, Savage SA, Mirabello L, Berndt SI, Weissfeld JL, Han J, Hayes RB, Chanock SJ, Hunter DJ, De Vivo I. Genome-wide association study of relative telomere length. PLoS One. 2011;6:e19635. doi: 10.1371/journal.pone.0019635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehkopf DH, Dow WH, Rosero-Bixby L. Differences in the association of cardiovascular risk factors with education: a comparison of Costa Rica (CRELES) and the USA (NHANES). J Epidemiol Community Health. 2010a;64:821–828. doi: 10.1136/jech.2009.086926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehkopf DH, Krieger N, Coull B, Berkman L. Biologic Risk Markers for Coronary Heart Disease: Nonlinear Associations With Income. Epidemiology. 2010b;21:38–46. doi: 10.1097/EDE.0b013e3181c30b89. [DOI] [PubMed] [Google Scholar]
- Rosabal-Coto M. Parental belief systems, conflict resolution strategies, and cultural orgientation in the mother-child interactive context: a comparative study of two Costa Rican samples. Department of Cultural and Developmental Psychology. University of Osnabruck; Osnabruck: 2004. [Google Scholar]
- Rosero Bixby L, Fernandez X, Dow WH. CRELES: Costa Rican Longevity and Healthy Aging Study, 2005 (Costa Rica Estudio de Longevidad y Envejecimiento Saludable: Sampling and Methods No. ICPSR26681-v2. Inter-university Consortium for Political and Social Research; Ann Arbor, MI: 2010. [Google Scholar]
- Rosero-Bixby L. The Exceptionally High Life Expectancy of Costa Rican Nonagenarians. Demography. 2008;45:673–691. doi: 10.1353/dem.0.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosero-Bixby L, Dow WH. Surprising SES Gradients in mortality, health, and biomarkers in a Latin American population of adults. J Gerontol B Psychol Sci Soc Sci. 2009;64:105–117. doi: 10.1093/geronb/gbn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosero-Bixby L, Dow WH. Predicting mortality with biomarkers: a population-based prospective cohort study for elderly Costa Ricans. Popul Health Metr. 2012;10:11. doi: 10.1186/1478-7954-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Butcher L, Lin J, Brydon L, Kivimaki M, Marmot M, Blackburn E, Erusalimsky JD. Educational attainment but not measures of current socioeconomic circumstances are associated with leukocyte telomere length in healthy older men and women. Brain Behav Immun. 2011;25:1292–1298. doi: 10.1016/j.bbi.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Surtees PG, Wainwright NW, Pooley KA, Luben RN, Khaw KT, Easton DF, Dunning AM. Educational attainment and mean leukocyte telomere length in women in the European Prospective Investigation into Cancer (EPIC)-Norfolk population study. Brain, behavior, and immunity. 2012;26:414–418. doi: 10.1016/j.bbi.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Uchino BN, Cawthon RM, Smith TW, Light KC, McKenzie J, Carlisle M, Gunn H, Birmingham W, Bowen K. Social relationships and health: Is feeling positive, negative, or both (ambivalent) about your social ties related to telomeres? Health Psychol. 2012 doi: 10.1037/a0026836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsworth M, Hardy R, Paul A, Marshall S, Cole TG. Leg and trunk length at 43 years in relation to childhood health, diet and family circumstances; evidence from the 1946 national birth cohort. Int J Epidemiol. 2002;31:383–390. [PubMed] [Google Scholar]
- Willett WC. Nutritional Epidemiology. Oxford University Press; New York: 1998. [Google Scholar]
- Wood S. Generalized Additive Models: An Introduction with R. Chapman & Hall/CRC; Boca Raton: 2006. [Google Scholar]