Abstract
Objective
Obesity is a significant contributing factor to endometrial cancer risk. We previously demonstrated that estrogen-induced endometrial proliferation is enhanced in the context of hyperinsulinemia and insulin resistance. In this study we investigate whether pharmacologic agents that modulate insulin sensitivity or normalize insulin levels will diminish the proliferative response to estrogen.
Study Design
Zucker fa/fa obese rats and lean controls were used as models of hyperinsulinemia and insulin resistance. Insulin levels were depleted in ovariectomized rats following treatment with Streptozotocin (STZ), or modulated by metformin treatment. The number of BrdU incorporated cells, estrogen dependent proliferative and anti-proliferative gene expression, and activation of mTOR and Erk1/2 MAPK signaling were studied. A rat normal endometrial cell line RENE1 was used to evaluate the direct effects of metformin on endometrial cell proliferation and gene expression in vitro.
Results
STZ lowered circulating insulin levels in obese rats and decreased the number of BrdU labeled endometrial cells even in the presence of exogenous estrogen. Treatment with the insulin-sensitizing drug metformin attenuated estrogen-dependent proliferative expression of c-myc and c-fos in the obese rat endometrium compared to untreated controls and was accompanied by inhibition of phosphorylation of the insulin and IGF1 receptors (IRβ/IGF1R) and ERK1/2. In vitro studies indicated metformin inhibited RENE1 proliferation in a dose dependent manner.
Conclusion
These findings suggest that drugs that modulate insulin sensitivity, such as metformin, hinder estrogen-mediated endometrial proliferation. Therefore, these drugs may be clinically useful for the prevention of endometrial cancer in obese women.
Keywords: Obesity, estrogen, insulin resistance
Introduction
Overweight and obesity not only increase the risk of a variety of chronic illnesses, including cardiovascular disease and type 2 diabetes, but also are known risk factors for a variety of cancer types 1, 2, 3. Among all cancers, increasing body mass index is most strongly associated with endometrial cancer risk, with greater than 50% of all endometrial cancers attributable to obesity 4. While hyperestrogenism associated with obesity is a significant contributor to the development of endometrial cancer, other factors, including hyperinsulinemia, contribute to its pathogenesis and progression.
We previously evaluated the effect of obesity-associated insulin resistance and hyperinsulinemia on estrogen-associated endometrial proliferation in a rat model. Specifically, we showed that the expression of the pro-proliferative genes was increased while the expression of anti-proliferative genes were inhibited in the endometrium of estrogen-treated obese, insulin-resistant rats as compared to lean controls 5. These data suggested that insulin potentiates estrogen-regulated endometrial proliferation in the context of obesity.
To address the effects of insulin modulation as a chemopreventive strategy for endometrial cancer, circulating insulin levels and insulin levels were manipulated in obese female Zucker rats using the drugs streptozotocin (STZ) and metformin, both in the presence and absence of estrogen. Like obese humans, the Zucker rat model develops insulin resistance, hyperinsulinemia and ultimately, non-insulin dependent diabetes 6, 7.
STZ, a glucosamine-nitrosourea compound, has been used to treat cancer of the pancreatic islets of Langerhans in humans. It is extremely toxic to the beta-cells of the pancreas, inhibiting insulin production, and therefore has limited clinical utility. However, this drug can be used to permanently reduce circulating insulin levels in laboratory animals. Metformin, a biguanide drug commonly used to treat type 2 diabetes, has recently been demonstrated to exert chemopreventive and anti-proliferative effects for a variety of cancers 8, 9, 10. Metformin inhibits cell growth both by insulin and non-insulin dependent mechanisms. Metformin increases insulin receptor sensitivity, increases insulin uptake, thereby reducing systemic insulin levels. Metformin also inhibits cell proliferation by activating the growth inhibitory AMPK, which counteracts signaling through both the PI3K/AKT and MAPK pathways downstream of the insulin and IGF1 receptors.
The overall goal of these studies is to provide preclinical data to determine the ability of insulin sensitizing drugs to attenuate estrogen-induced endometrial proliferation and serve as chemopreventive agents for endometrial cancer in obese individual.
Materials and Methods
Cell Lines
RENE1, a Sprague-Dawley rat normal endometrial cell line was purchased from Sigma-Aldrich (St. Louis, MO).
Cell Proliferation assay
RENE1 cells were treated with metformin or vehicle for 72 hours and cell proliferation was evaluated using MTT assay as previously described 11.
Western Blot
The effect of metformin on cell signaling pathways was evaluated by Western blot analysis. RENE1 cells were plated in 6 well plates at 2×105/well. Following 24 hours, cells were treated by metformin (5mM in culture medium) for 72 hours. Cells were lysed in Protein Extraction Reagent (ThermoScientific, Rockford, IL). Equal amounts of protein for each treatment group were resolved by SDS–PAGE and transferred onto PVDF membranes, and probed for phospho-AMPK (T172), phospho-Erk1/2 (Thr202/Tyr204), phospho-S6 protein (Cell signaling technology, Danvers, MA,) or β-actin (Sigma, St Louis, MO) followed by HRP-conjugated secondary antibody, as per manufacturer’s instructions. When necessary, PVDF membranes were stripped (62.5 mM Tris-HCl, PH 6.8, 2% SDS and 100 mM 2-mercaptoethanol)at 50°C for 30 minutes), washed twice in TBST then reprobed.
Animals Care and Use
All animal experiments were conducted in compliance with federal guidelines and approved by the Institutional Animal Care and Use Committee. Mature (5-weeks-old) female Zucker fa/fa rats and their lean littermates were purchased from Harlan Laboratories (Indianaopolis, IN). After one week of acclimation, animals were ovariectomized, and held for 5 days to clear endogenous ovarian hormones.
Streptozotocin (STZ) Treatment
Obese and lean animals were randomized to 4 treatment groups (n=6 per group): vehicle, estradiol (Innovative Research of America, Sarasota, FL), STZ (Sigma, St. Louis, MO), and estradiol plus STZ. Estradiol (10 g/kg body weight/day, for 2 weeks) was administrated by subcutaneously implanting the timed releasing estradiol pellets two weeks before the end of the experiment, while in control group animals were implanted with the placebo pellets. Seven days after estradiol administration, animals were injected intraperitoneally with either vehicle (citrate buffer) or STZ (45 mg/kg). Seven days after STZ administration, animals were sacrificed for tissue collection.
Metformin Treatment
Obese and lean rats were randomized to 3 treatment groups (17 to 26 animals per group): vehicle alone, estradiol, and estradiol plus metformin. Metformin (300 mg/kg body weight/day in 1% methyl-cellulose solution) was administrated by daily oral gavage for three weeks. Control animals received vehicle alone. Estradiol (40 g/kg body weight/day, for 3 days) was administrated intraperitoneally for the last three days of the experiment. Control animals received saline alone. Animals were sacrificed and uteri were collected for histochemical evaluation and RNA isolation.
Plasma glucose level and insulin level detection
Three to five rats from each treatment group were fasted overnight, and were subjected to an oral glucose tolerance test (GTT) 5. Plasma glucose concentrations were tested with the Ascensia Contour Blood Glucose Monitoring System (Bayer Health Care, New York, NY). Insulin levels were by ELISA (Insulin Ultrasensitive EIA kit, ALPCO Diagnostics, Salem, NH).
Immunohistochemistry
All rats were injected intraperitoneally with BrdU at a dose of 100 mg/kg body weight ninety minutes before sacrifice. Fresh uterine tissues were collected and fixed in 10% neutral-buffered formalin, and processed for paraffin embedding. BrdU immunostaining was performed using BrdU in-situ detection kit (BD Biosciences, San Diego, CA). The slides were counterstained with Mayer’s hematoxylin for 1 min. The total number of BrdU-stained nuclei per 200 endometrial cells was counted in 10 randomly selected fields (200×).
Immunohistochemical analysis of rat uterine tissue was performed using Ki67 (BD Biosciences, San Diego, CA), phospho-IGF1R (Tyr1131)/Insulin Receptor β (Tyr1146), phospho-S6 ribosomal protein (Ser235/236), phospho-ERK1/2 (Thr202/Tyr204), phospho-Acetyl-CoA carboxylase (Ser79) (pACC), and cleaved caspase-3 (Asp175) (Cell Signaling, Danvers, MA), as per manufacturers’ instructions. The sections were counterstained with Mayer’s hematoxylin. The average number of positively Ki67 or Caspase-3 stained cells in 5-10 high-power microscopic fields were counted per slide, and calculated as: 200×(numbers of stained endometrial cell/total endometrial cells). For all other markers, staining was scored based on intensity as negative or weak (0 or 1+), versus positive or strong (2+ or 3+).
RNA isolation and real-time quantitative reverse transcription-polymerase chain reaction (RT-qPCR)
Total RNA was extracted from frozen endometrial tissue using Tri-reagent (as described previously) 12. For each transcript, specific PCR primer pairs and a dual fluorochrome-labeled hybridization probe (Hydrolysis probe) were designed using Primer Express (Applied Biosystems, Carlsbad, CA) or Beacon Designer (Premier Biosoft Intl, Palo Alto, CA) (Supplemental table 1). All real-time RT-qPCR reactions were set up using liquid handling robotics 5. Samples, controls and 5-log standard curves were run on 384-well plates using an Applied Biosystems 7900 qPCR instrument under the following conditions: 95°C for 2 min followed by 40 cycles of 95°C-12 sec and 60°C-30 sec. Data was analyzed using SDS version 2.4 software post-run using auto baseline and manual threshold settings and was normalized to 18SrRNA levels.
Statistical Analysis
Statistical analyses were performed using SAS version 9.1 statistical software (SAS Institute Inc., Cary, NC) and STATA/SE version 10.1 statistical software (Santa Corp. LP, College Station, TX). For BrdU, Ki67, Caspase-3, and RT-qPCR test, one-way ANOVA was used to compare treatment groups. Tests were made using log transformed measurements. For other immunohistochemical tests, Fisher’s exact tests were used in place of logistic regression models. A significance level of 0.05 was used to judge statistical significance.
Results
Direct effects of metformin on endometrial cell growth in vitro
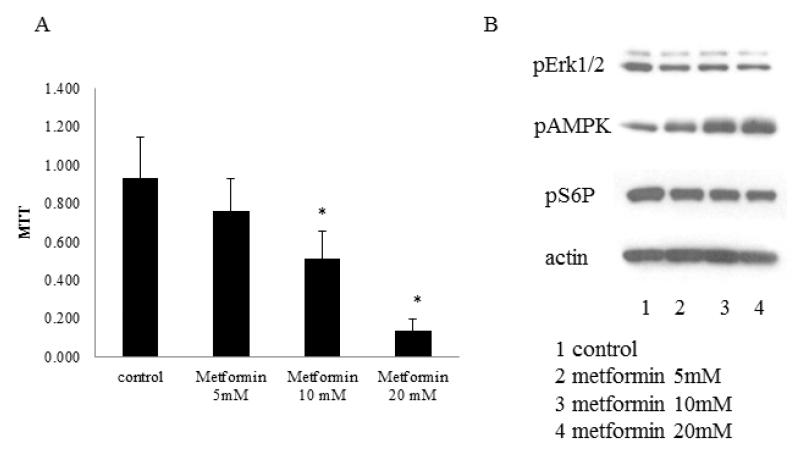
We examined the direct effects of metformin on endometrial cell proliferation and gene expression in vitro, using the normal rat endometrial cell line, RENE1 13. This in vitro evaluation also permitted the direct analysis of several concentrations of metformin on endometrial cell proliferation by MTT. RENE1 proliferation was inhibited in a dose dependent manner after 3 days of metformin (p<0.001; Figure 1A).
Figure 1.
Metformin modulates endometrial cell line proliferation and signal transduction.
The effects of metformin on endometrial cell proliferation was determined by MTT test (A), and signaling activation was tested by western blot (B). RENE1 cells were plated in 96 well plates and allowed to adhere for 24 hours. Cells were then treated with 5, 10, 20mM metformin for a further 72 hours.
The effect of metformin on growth promoting and inhibitory pathways were evaluated by western blot using activation-specific antibodies (Figure 1B). Metformin inhibited phosphorylation of pERK1/2 and S6R protein, while promoting AMPK phosphorylation. Overall, these studies suggest that metformin can inhibit endometrial proliferation, in part as a consequence of its ability to directly modulate pro- and anti-proliferative pathways.
Proliferative effect of estrogen under low insulin conditions
We confirmed the effect of STZ in lowering serum insulin levels using an oral glucose tolerance test (Supplemental data 1A). Low dose β-toxin STZ treatment decreased obese rat serum insulin level (p=0.0107 vs. obese control) at all-time points after glucose challenge, but showed no effect in lean rats (p=0.9519). STZ administration significantly increased serum glucose level in both lean (p<0.0001) and obese rats (p<0.0001).
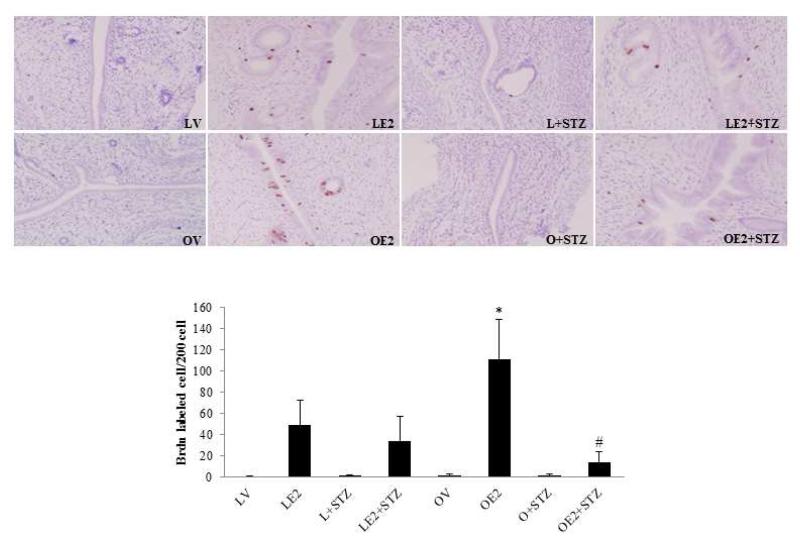
BrdU incorporation and Ki67 immunohisotchemical staining confirmed the proliferative effects of estrogen under low insulin conditions (Figure 2). Estradiol treatment increased BrdU incorporation in both lean (48.8±23.8 vs. 0.3±0.5) and obese (111.1± 37.7 vs. 1.7±1.2) endometrium. The number of estrogen-induced, BrdU-labeled endometrial cells was 2.3 fold higher in obese animals as compare to that observed in lean rats (111.1 ± 37.7 vs. 48.8±23.8, p<0.001). STZ treatment decreased BrdU incorporation in both estrogen-treated lean rat endometrium (34.1±23.2 vs. 48.8±23.8) and obese rat endometrium (14.0±10.1 vs. 111.1± 37.7). In obese rat endometrium, the proliferative effect of estrogen was antagonized by STZ treatment. BrdU incorporation was significantly decreased in obese rats treated with estradiol plus STZ when compared with rats treated with estrogen alone (p<0.0001). Ki67 staining validates these findings (data not shown), and supports the observation that a reduction in circulating insulin, blunts the effects of proliferative effects of estrogen in the endometrium.
Figure 2.
Effect of STZ on endometrial cell proliferation in Zucker obese and lean rats.
The proliferative effect of estrogen on the endometrium is reported as a ratio of BrdU labeled endometrial cells per 200 endometrial epithelial cells. BrdU (100 mg/kg) was injected intraperitoneally into rats 90 minutes before they were sacrificed. Uterine tissue sections were stained with anti-BrdU antibody. The average number of positively stained cells in 10 high-power microscopic fields were counted for each slide (n=3-6). * p<0.001, Lean E2 vs. Obese E2; # p<0.0001, Obese E2 vs. Obese E2+STZ.
Effect of metformin therapy on rat endometrial proliferation
Metformin decreased serum glucose levels. At 45 minutes following a glucose challenge, glucose and insulin levels were significantly higher in obese rats compared with lean rats (p=0.0176). Treatment with metformin decreased serum glucose in obese rats as compared with the non-treated group (Supplemental data 2), however metformin did not significantly decrease circulating insulin levels in this obese animal model during the 3-week treatment period. This is perhaps not surprising, as metformin has been shown to decrease gluconeogenesis in the liver, with no demonstrated impact on insulin synthesis by the pancreas. Instead, metformin has been shown to increase insulin sensitivity and uptake, which contributes to a modest decrease in circulating insulin levels after prolonged use. Indeed, a reduction in circulating insulin was observed in mice fed a high-fat diet, following 8-10 weeks of metformin therapy. Levels observed in metformin treated versus untreated animals mice approached, but did not reach statistical significance, as reflected by C-peptide levels, a surrogate marker for insulin 14.
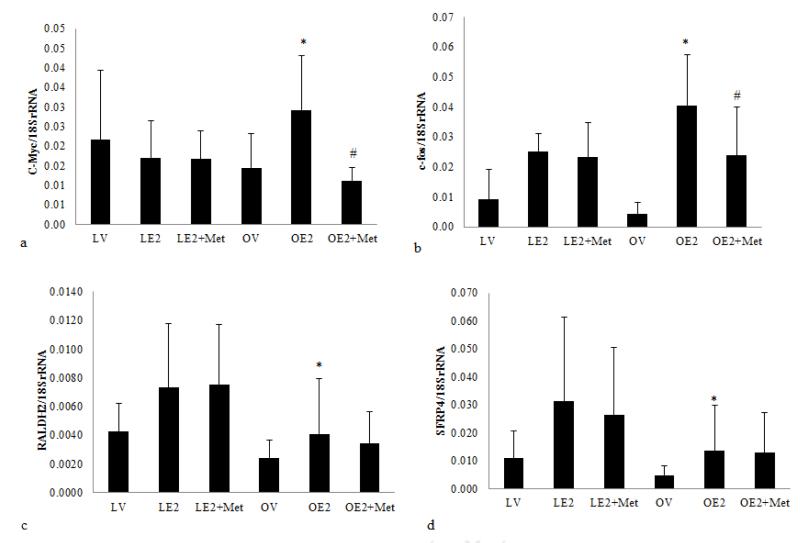
We examined the effect of metformin on the expression of genes associated with estrogen-mediated endometrial proliferation.5. In the normal physiologic state, estrogen induces both growth stimulatory (c-myc, c-fos) and growth inhibitory (RALDH2 and sFRP4) pathways. The result is controlled, balanced endometrial growth. We have already shown that estradiol treatment augments transcription of the pro-proliferative gene c-myc in the obese rat endometrium as compared to the lean rat endometrium. Conversely, the growth inhibitory genes, RALDH2, and SFRP4, whose transcription is induced by estrogen in the endometrium of lean rats, are attenuated in obese rats. In this study, we further demonstrate the induction of c-fos transcription in estrogenized obese rat endometrium compared to lean controls (0.04±0.017 vs.0.025±0.010, p<0.025, Figure 3A). We anticipate these transcriptional changes reflect the changes in insulin and IGF1 levels associated with obesity.
Figure 3.
The effect of metformin on estrogen induced endometrial proliferation in obese and lean Zucker rats.
(3A) Effect of metformin on pro-proliferative and anti-proliferative genes expression in the rat endometrium. The transcript level of c-myc (a), c-fos (b), RALDH2 (c), and SFRP4 (d) in the endometrium from ovariectomized Zucker fa/fa rats and their lean littermates was tested by RT-qPCR. Each sample was assayed in triplicate plus a -RT control. The number of transcript molecules in 20 ng total RNA were normalized to and expressed as the percentage of 18SrRNA (n = 17-20). * p<0.025, Lean E2 vs. obese E2; # p<0.001, obese E2 vs. obese E2+metformin.
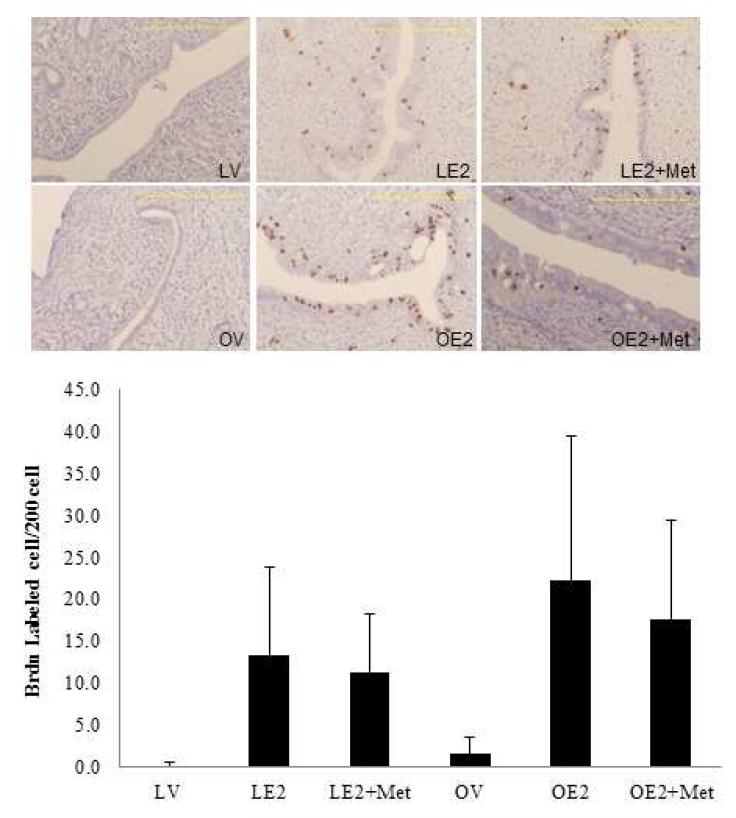
(B) Effect of metformin on endometrial cell proliferation in Zucker obese and lean rats. The proliferative effect of estrogen on the endometrium is reported as a ratio of BrdU labeled endometrial cells per 200 endometrial epithelial cells. BrdU (100mg/kg) was injected intraperitoneally into rats 90 minutes before they were sacrificed. Uterine tissue sections were stained with anti-BrdU antibody. The average number of positively stained cells in 10 high-power microscopic fields were counted per slide (n=17-26/group). Scale bar=200 μm.
To address the effect of metformin on proliferation via estrogen-induced gene expression, we compared the mRNA level of c-myc, c-fos, SFRP4 and RALDH2 transcripts in metformin and vehicle treated rat endometrium. Metformin treatment significantly decreased transcript levels for both c-myc (0.011±0.003 vs. 0.029±0.014, p<0.001) and c-fos (0.024±0.016 vs. 0.040±0.017, p<0.001) in the estrogenized obese rat endometrium, as compared to untreated obese animals. No significant effect was observed in lean rat endometrium (Fig. 3A). Interestingly, expression of the antiproliferative, RALDH2 and SFRP4 genes, in estrogenized obese rat endometrium were not significantly affected by metformin (Figure 3A). Overall, these data suggest that metformin treatment attenuates the transcription of a subset of estrogen-induced pro-proliferative genes, but does not significantly promote the expression of estrogen-induced, growth inhibitory genes in the endometrium of obese rats.
The effect of metformin on endometrial cell proliferation was evaluated by both BrdU and Ki67 staining. Three days of treatment with estradiol versus control-treatment induced endometrial proliferation in both lean (13.48±10.5 vs. 0.1±0.4) and obese (22.3±17.2 vs. 1.6±2.1) rats (Figure 3B). Significant endometrial proliferation was observed in obese animals as compared to lean animals, in response to estrogen (22.3±17.2 vs. 13.4±10.5, p=0.056). Metformin therapy did not significantly alter estrogen-mediated endometrial proliferation when compared to controls in both lean (11.3±6.9 vs. 13.4±10.5) and obese rats (17.6±4.7 vs. 22.3±17.2; data not shown).
While metformin inhibits the transcription of growth promoting genes, c-myc and c-fos in the endometrium of obese, estrogen treated rats, the levels of the growth inhibitory genes were seemingly unaffected within the time frame of this experiment. Furthermore, given the lack of short-term effects resulting from a 3 week course of metformin on circulating insulin levels, we hypothesize that the overall effect on endometrial proliferation as measured by Ki67 and BrdU incorporation are not yet fully apparent. As reflected by the trend of reduced BrdU incorporation in obese, estrogen treated rats following treatment with metformin (p = 0.056), we expect the antiproliferative effects of metformin on endometrial tissue may become more pronounced over time.
Effect of metformin on endometrial cell apoptosis
To address the possibility that metformin may induce apoptosis, rather than inhibit proliferation in the obese rat endometrium, we tested endometrial cell apoptosis by caspase 3 staining. Metformin treatment did not produce a significant increase in caspase 3 staining in obese rat endometrium when compared with untreated obese rat endometrium (Supplemental data 3).
Effect of metformin on Insulin/IGF signaling
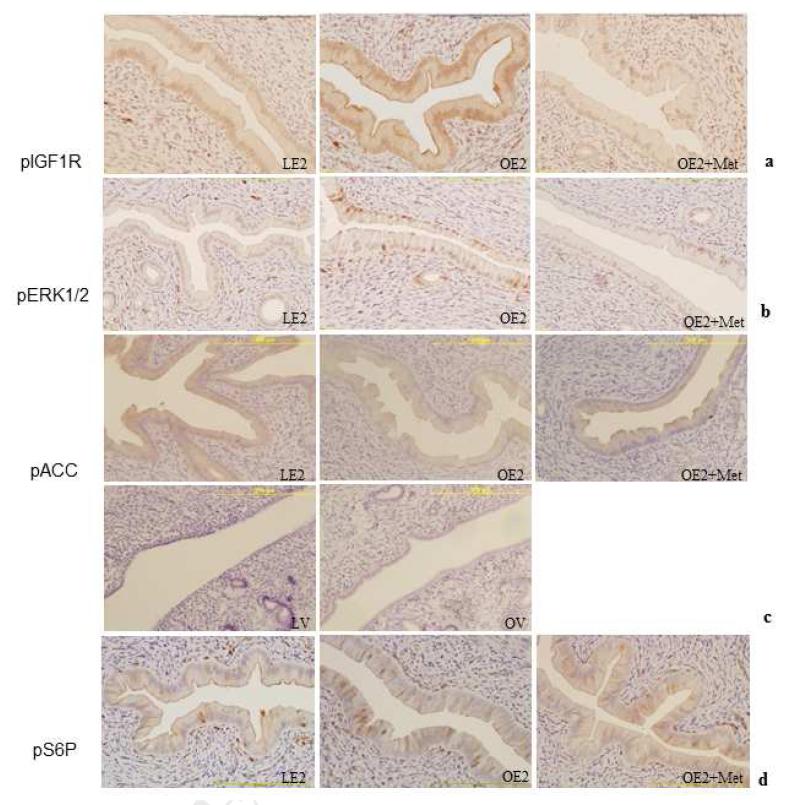
Hyperinsulinemia in the obese rat can contribute to elevated IGFI levels and activation of the IGF-IR. The effect of metformin on IGFI and insulin signaling in rat endometrial tissue was determined by immunohistochemical staining for phospho-IGF1 Receptor (Tyr-1131)/Insulin Receptor (Tyr-1146). These sites represent one of the early sites of IGF1R and IR autophosphorylation, which is required for full receptor tyrosine kinase activation.
Metformin treatment significantly inhibited IGF1R/IRβ activation in obese rat endometrium.. Phospho-IGF1R/IRβ staining was significantly weaker in obese rat treated with metformin as compared to those treated with estrogen alone (31% vs. 92%, 4/13 vs 12/13 positive samples; p<0.025; Figure 4A). These findings suggest that metformin may regulate IGF1R/IR activity by modulating receptor autophosphorylation.
Figure 4.
Metformin modulates endometrial cell signal transduction.
Phosphorylation of IGF1R, ERK1/2 MAPK, and S6 protein, was determined by immunohistochemical staining. Uterine tissue sections were stained with antibody against phospho-IGF1R (a), phospho-ERK1/2 MAPK (b), phospho-Acc (c), and phospho-S6 protein (d). To evaluate differential expression levels, weakest staining was counted as weak (0, or 1+), and a greater than 10% increase in the staining intensity was counted as strong (2+, or 3+) (n=11-13/group). Scale bar=200 μm.
Effect of metformin on MAPK activation
We evaluated MAPK pathway activation as a downstream reflection of IGF/IR signaling. Phospho-ERK1/2 was significantly elevated in estrogenized obese rats (8/13) versus lean rats (2/13); (62% vs 17%; p<0.05), indicating estradiol had a pronounced effect on MAPK signaling in obese rats. Administration of metformin significantly inhibited ERK1/2 phosphorylation in obese rat endometrium compared with non-metformin treated controls (Figure 4B). While both estrogen and hyperinsulinemia trigger MAPK signaling in obese animals (Figure 5), the exogenous estrogen was insufficient to overcome the reduction IGF1R and IR signaling in response to metformin.
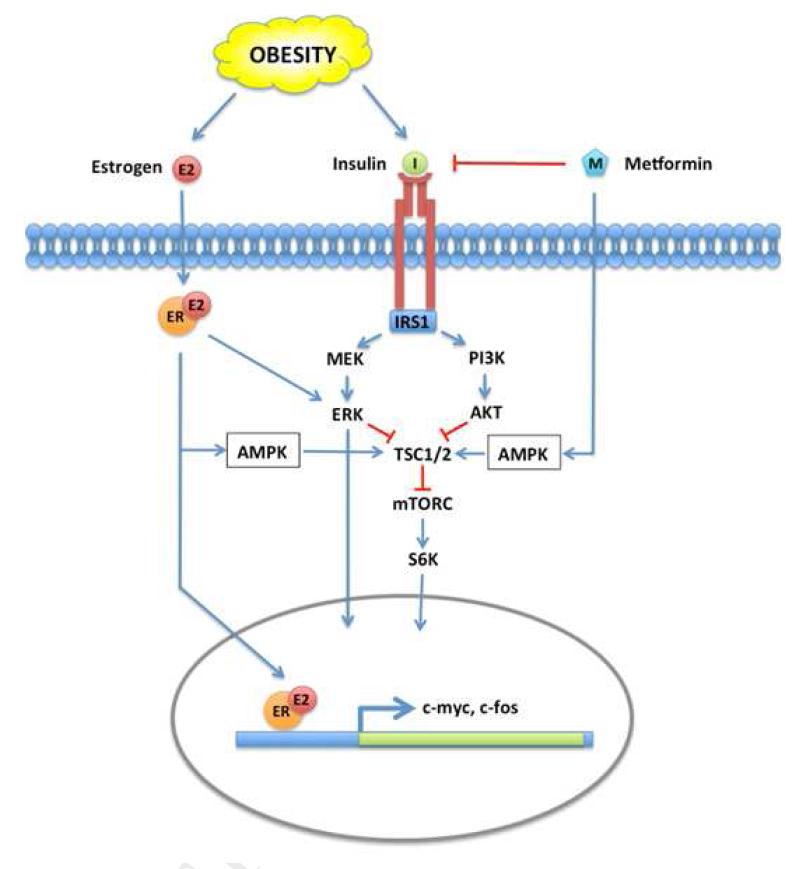
Figure 5.
A model: How metformin attenuates estrogen and insulin/IGF signaling in the obese endometrium.
mTOR and MAPK signaling are activated by insulin and estrogen through their receptors. In the obese rat endometrium, high circulating insulin levels induce accelerated proliferation following estrogen stimulation, and promote pronounced expression of several pro-proliferative genes, including c-myc and c-fos. Metformin inhibits MAPK and mTOR signaling locally via activation of AMPK and also systemically, by lowering circulating insulin level, thereby attenuating estrogen-induced proliferative gene expression.
Effect of metformin on AMP Kinase signaling
Metformin is thought to exert its effect locally by activation of the anti-proliferative AMPK pathway11. We explored the effect of metformin on AMPK activity in rat endometrium by examining the phosphorylation of the AMPK substrate, acetyl-CoA carboxylase (ACC).
Following estrogen treatment, immunohistochemical staining of endometrial tissues with anti-phospho-ACC demonstrated an increase in phospho-ACC in both lean and obese rat endometrium. Phospho-ACC was significantly elevated in 8 of 11 (73%) of the estrogenized lean rat endometrial tissues as compared to 3 of 12 (25%) of the obese rat endometrium (p<0.05), indicating that estradiol induced AMPK activity in lean rat endometrium (Figure 4C). Estradiol has been previously shown to activate AMPK in muscle 15, 16, 17. Given the elevated levels of phospho-AMPK present in response to estrogen, metformin did not further elevate AMPK signaling in obese rat endometrium.
The PI3K, MAPK and AMPK signaling pathways intersect at a critical signaling node, the tuberous sclerosis complex (TSC1/2 complex; Figure 5). Phosphorylation of TSC2 following insulin or IGF1 receptor-mediated activation of the MAP and PI3K kinase pathways promotes dissociation of the TSC complex and stimulates mTOR signaling resulting in the phosphorylation of S6K and changes in gene transcription. Conversely, AMPK phosphorylates TSC2 and prevents dissociation from the TSC complex, thereby suppressing mTOR signaling 18, 19. In vitro, metformin treatment clearly prevents phosphorylation of S6 ribosomal protein (Ser235/236), the downstream target of S6K (Figure 1). Immunohistochemical staining for pS6R was used to monitor the effects metformin on mTOR signaling in obese, estrogenized endometrium. Although not statistically significant, a trend of increased pS6R was associated with obesity; 8 of 13 (62%) obese endometria vs. 4 of 12 (33%) lean endometria (p=0.24). Metformin reduced pS6R in obese animals to levels observed in lean animals; 4 of 13 metformin treated estrogenized obese rats stained positively as compared to 8 of 13 obese animals treated with E2-alone (31% vs. 62%; p=0.21) (Fig 4d). Taken together, our data indicate that metformin therapy attenuates pro-proliferative signaling via IGF1R and MAPK in vivo. While direct effects on endometrial epithelial cells are obvious in vitro, the direct effects of metformin on the activation of the anti-proliferative AMPK pathway are less apparent in vivo.
Comment
Our previously study demonstrated that estrogen-driven proliferative signals in the endometrium are potentiated in an obese, insulin-resistant animal model. We hypothesized that modulation of insulin levels and insulin sensitivity in these animals should blunt this response.
As a proof-of-principle, we initially eliminated insulin production using streptozotocin, a drug toxic to pancreatic beta cells, and confirmed the importance of insulin on estrogen-driven endometrial proliferation. Lack of circulating insulin in STZ-treated animals convincingly hindered estrogen-induced endometrial proliferation. Due to pancreatic beta cell toxicity, this approach does not represent a practical therapeutic strategy in humans; therefore, we investigated whether metformin, an insulin-sensitizing agent commonly used to treat type 2 diabetes, could similarly attenuate estrogen-associated endometrial proliferation in obese, insulin-resistant rats.
Levels of phospho-IGF1R and IR were decreased in the endometrial tissue of obese estrogen-treated insulin resistant rats in response to metformin, reflecting a decrease in receptor tyrosine kinase activity. Metformin further down-regulated signaling through the MAPK pathway, as demonstrated by a decrease in phospho-ERK1/2 in estrogen-treated obese rat endometrium. Finally, metformin effectively hindered induction of the estrogen-responsive, pro-proliferative transcription factors c-myc and c-fos in our model system.
We suggest that these effects occur as a consequence of multiple, metformin-induced changes in signaling both upstream and downstream of the insulin and IGF1 receptors. In addition to rapid, systemic changes in glucose and longer-term changes in insulin levels, metformin is thought to mediate direct growth-inhibitory effects on cells via activation of the AMPK pathway 20, 21. When metabolic stress or metformin increases AMP relative to ATP levels in the cell, AMPK negatively regulates ATP-consuming processes, including cell division.
While normal rat endometrial cells demonstrated a robust AMPK activation in response to metformin in vitro, metformin-induced changes in AMPK activation in vivo were not as pronounced. Decreased levels of IR, IGF1R and MAPK phosphorylation may reflect an overall depletion of ATP in response to metformin.
One of the limitations of this study is the duration of treatment of our in-vivo model. Three weeks of metformin therapy were insufficient to significantly decrease circulating insulin levels in obese animals, and short-term metformin treatment appears to be insufficient to produce significant changes in endometrial proliferation in obese rats. However, our findings hint that growth regulatory pathways are being targeted by metformin. To evaluate the full effects of metformin as a chemopreventive agent, a longer term study is required.
In summary, epidemiologic evidence demonstrates that metformin exerts chemopreventive and anti-proliferative effects for a variety of cancers 8, 9, 10. Our study has shown that metformin modulates insulin receptor and IGF1R autophosphorylation, and attenuates the proliferative pathways of the endometrium in response to estrogen in the context of obesity. Human studies that examine biomarker alteration in the endometrium will be necessary in order to determine whether metformin is a rational and effective approach to the chemoprevention of endometrial cancer in obese women.
Supplementary Material
S1. The effects of STZ on serum insulin levels and cell proliferation in obese and lean Zucker rats.
Animals were fasted overnight before glucose challenge (2g/kg body weight).
Plasma insulin and glucose levels were determined at 30min after glucose challenging. n=3-5.
S2A. The effect of metformin on serum insulin levels and cell proliferation in Zucker obese and lean rats.
Animals were fasted overnight prior to glucose challenge (2g/kg body weight). Plasma insulin and glucose level was determined 45min after glucose challenging. n=3-5.
S3. The effect of metformin on endometrial cell apoptosis.
The effect of metformin on endometrial cell apoptosis was evaluated by immunohistochemical staining for caspase 3. The average number of positively stained cells in 10 high-power microscopic fields were counted per slide, and calculated as: 200× (numbers of stained endometrial cell/total endometrial number) (n=12-13) scale bar=50 μm.
Acknowledgment
The RT-qPCR assays and all runs were done in the Quantitative Genomics Core Laboratory at the University of Texas Medical School at Houston. We thank Dr. Gregory L. Shipley and Dr. Peter J.A. Davies for their assistance with this project.
The project described was supported in part by Grant Number P50CA098258 from the National Cancer Institute, and also in part by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant CA016672.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmay be discovered which could affect the content, and all legal disclaimers that apply to thejournal pertain
The authors report no conflict of interest.
References
- 1.Haslam DW, James WP. Obesity. Lancet. 2005;366:1197–209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 2.Yach D, Stuckler D, Brownell KD. Epidemiologic and economic consequences of the global epidemics of obesity and diabetes. Nat Med. 2006;12:62–6. doi: 10.1038/nm0106-62. [DOI] [PubMed] [Google Scholar]
- 3.Sassi F, Devaux M, Cecchini M, et al. The Obesity Epidemic: Analysis of Past and Projected Future Trends in Selected OECD Countries. OECD Publishing; 2009. p. 45. [Google Scholar]
- 4.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–91. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Q, Shen Q, Celestino J, et al. Enhanced estrogen-induced proliferation in obese rat endometrium. Am J Obstet Gynecol. 2009;200:186 e1–8. doi: 10.1016/j.ajog.2008.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen-Yamamoto L, Deal CL, Finkelstein JA, Van Vliet G. Hormonal control of growth in the genetically obese Zucker rat. I. Linear growth, plasma insulin-like growth factor-I (IGF-I) and IGF-binding proteins. Endocrinology. 1994;134:1382–8. doi: 10.1210/endo.134.3.7509740. [DOI] [PubMed] [Google Scholar]
- 7.Argiles JM. The obese Zucker rat: a choice for fat metabolism 1968-1988: twenty years of research on the insights of the Zucker mutation. Prog Lipid Res. 1989;28:53–66. doi: 10.1016/0163-7827(89)90007-6. [DOI] [PubMed] [Google Scholar]
- 8.Del Barco S, Vazquez-Martin A, Cufi S, et al. Metformin: multi-faceted protection against cancer. Oncotarget. 2011;2:896–917. doi: 10.18632/oncotarget.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dowling RJ, Goodwin PJ, Stambolic V. Understanding the benefit of metformin use in cancer treatment. BMC Med. 2011;9:33. doi: 10.1186/1741-7015-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engelman JA, Cantley LC. Chemoprevention meets glucose control. Cancer Prev Res (Phila) 2010;3:1049–52. doi: 10.1158/1940-6207.CAPR-10-0178. [DOI] [PubMed] [Google Scholar]
- 11.Cantrell LA, Zhou C, Mendivil A, Malloy KM, Gehrig PA, Bae-Jump VL. Metformin is a potent inhibitor of endometrial cancer cell proliferation--implications for a novel treatment strategy. Gynecol Oncol. 2010;116:92–8. doi: 10.1016/j.ygyno.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen Q, Cline GW, Shulman GI, Leibowitz MD, Davies PJ. Effects of rexinoids on glucose transport and insulin-mediated signaling in skeletal muscles of diabetic (db/db) mice. J Biol Chem. 2004;279:19721–31. doi: 10.1074/jbc.M311729200. [DOI] [PubMed] [Google Scholar]
- 13.Wiehle RD, Helftenbein G, Land H, Neumann K, Beato M. Establishment of rat endometrial cell lines by retroviral mediated transfer of immortalizing and transforming oncogenes. Oncogene. 1990;5:787–94. [PubMed] [Google Scholar]
- 14.Algire C, Zakikhani M, Blouin MJ, Shuai JH, Pollak M. Metformin attenuates the stimulatory effect of a high-energy diet on in vivo LLC1 carcinoma growth. Endocr Relat Cancer. 2008;15:833–9. doi: 10.1677/ERC-08-0038. [DOI] [PubMed] [Google Scholar]
- 15.Rogers NH, Witczak CA, Hirshman MF, Goodyear LJ, Greenberg AS. Estradiol stimulates Akt, AMP-activated protein kinase (AMPK) and TBC1D1/4, but not glucose uptake in rat soleus. Biochem Biophys Res Commun. 2009;382:646–50. doi: 10.1016/j.bbrc.2009.02.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Eon TM, Rogers NH, Stancheva ZS, Greenberg AS. Estradiol and the estradiol metabolite, 2-hydroxyestradiol, activate AMP-activated protein kinase in C2C12 myotubes. Obesity (Silver Spring) 2008;16:1284–8. doi: 10.1038/oby.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cederroth CR, Vinciguerra M, Gjinovci A, et al. Dietary phytoestrogens activate AMP- activated protein kinase with improvement in lipid and glucose metabolism. Diabetes. 2008;57:1176–85. doi: 10.2337/db07-0630. [DOI] [PubMed] [Google Scholar]
- 18.Tzatsos A, Kandror KV. Nutrients suppress phosphatidylinositol 3-kinase/Akt signaling via raptor-dependent mTOR-mediated insulin receptor substrate 1 phosphorylation. Mol Cell Biol. 2006;26:63–76. doi: 10.1128/MCB.26.1.63-76.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gwinn DM, Shackelford DB, Egan DF, et al. Ampk phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–26. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zakikhani M, Dowling R, Fantus IG, Sonenberg N, Pollak M. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 2006;66:10269–73. doi: 10.1158/0008-5472.CAN-06-1500. [DOI] [PubMed] [Google Scholar]
- 21.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–62. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S1. The effects of STZ on serum insulin levels and cell proliferation in obese and lean Zucker rats.
Animals were fasted overnight before glucose challenge (2g/kg body weight).
Plasma insulin and glucose levels were determined at 30min after glucose challenging. n=3-5.
S2A. The effect of metformin on serum insulin levels and cell proliferation in Zucker obese and lean rats.
Animals were fasted overnight prior to glucose challenge (2g/kg body weight). Plasma insulin and glucose level was determined 45min after glucose challenging. n=3-5.
S3. The effect of metformin on endometrial cell apoptosis.
The effect of metformin on endometrial cell apoptosis was evaluated by immunohistochemical staining for caspase 3. The average number of positively stained cells in 10 high-power microscopic fields were counted per slide, and calculated as: 200× (numbers of stained endometrial cell/total endometrial number) (n=12-13) scale bar=50 μm.